Abstract
Background
It is known that respiration modulates cavopulmonary flows, but there is little data comparing mean flows under breath holding and free breathing conditions to isolate the respiratory effects, as well as effects of exercise on the respiratory modulation.
Methods
Real time phase contrast magnetic resonance combined with a novel method to track respiration on the same image acquisition was used to investigate respiratory effects on Fontan caval and aortic flows under breath holding, free breathing and exercise conditions. Respiratory phasicity indices based on beat-averaged flow was employed to quantify the respiratory effect.
Results
Flow during inspiration was significantly higher than expiration under the free breathing and exercise conditions for both inferior vena cava (inspiration/expiration: 1.6±0.5 and 1.8±0.5, respectively) and superior vena cava (inspiration/expiration: 1.9±0.6 and 2.6±2.0, respectively). Changes from rest to exercise in the respiratory phasicity index for these vessels further showed the impact of respiration. Total systemic venous flow showed no significant statistical difference between the breath holding and free breathing conditions. In addition, no significant difference was found between the descending aorta and inferior vena cava mean flows under either resting or exercise conditions.
Conclusions
This study demonstrated that inferior vena cava and superior vena cava flow time variance is dominated by respiratory effects, which can be detected by the respiratory phasicity index. However, the minimal respiration influence on net flow validates the routine use of breath holding techniques to measure mean flows in Fontan patients. Moreover, the mean flows in the inferior vena cava and descending aorta are interchangeable.
Keywords: Fontan, MRI, Exercise, Bioengineering
Completion of Fontan palliation in single ventricle patients usually culminates in the total cavopulmonary connection (TCPC) with either an extra cardiac (EC) or a lateral tunnel (LT) connection [1]. TCPC hemodynamics are affected in a complicated way by several factors including anatomical and flow parameters [2], vascular resistances and compliances [3], exercise, respiration, and peripheral muscular contractions [4]. Further, these complexities appear related to clinical outcomes [5].
Precise flow measurement in various physiologic states, especially respiration, is key for numerically assessing TCPC hemodynamics [6]. Current TCPC flow measurements are commonly acquired either during breath holding or averaged during free breathing (at the expense of image blurring)[7]. Despite the apparent effects of respiration and exercise, few studies have focused specifically on delineating these effects [8,9]. Of those that do, some suggest that because of the high venous capacitance in the lower body, one primary effect of inspiration is to increase (as much as 80%) the flow rate and pulsatility of the inferior vena cava [9–14]. Hjortdal et al. examined respiration effects and used an air-filled belt around the abdomen of patients to monitor their respiration, as their imaging method did not allow direct tracking of respiratory cycles [9]. Additionally, the literature lacks a comprehensive study including a comparison of resting breath holding, resting free breathing, and exercise conditions, in order to isolate respiratory effects while keeping other factors constant.
With the advent of advanced real time phase-contrast magnetic resonance (rtPCMR) imaging technologies and image processing techniques, a detailed analysis of respiration effects during rest and exercise is now feasible. In this study, we utilized rtPCMR to investigate respiration effects during resting free breathing, resting breath hold, and supine exercise conditions. Respiratory patterns and vessel flow rates were simultaneously measured in real time using a novel chest wall tracking method. The adequate flow metrics to accurately quantify respiration effects will also be discussed.
Patients and Methods
Patient Cohort
The patients involved in the current study were identified from our prospective enrolled Fontan database. Eleven consecutive single ventricle patients with TCPC anatomy (males/females=7/4; age=20.7±2.9 years, BSA= 1.8±0.2 m2; LT/EC=10/1; left/right/mixed ventricular morphology = 3/6/2; hypoplastic left heart syndrome (HLHS) = 5; fenestration = 2), who completed rtPCMR at rest and at exercise were included. They all have normal bilateral diaphragm function and had at most mild ventricular dysfunction (EF = 42–73%). The inclusion criteria were: 1) TCPC with no other sources of pulmonary blood flow, 2) the ability to undergo the metabolic exercise stress test, using a stationary cycle ergometer. Patients with pacemakers, or other metal devices producing detrimental imaging artifacts, and significant differences in heart rate between individual vessel flow measurements were excluded. Any heart rate difference affected cardiac output which, in turn, impacted the flow measurements. In this study, 4 patients were excluded because the heart rate difference between the acquisitions of their Ao and DAo were larger than 20% of the Ao heart rate. Patients enrolled in this study had all previously completed a routine maximal metabolic exercise test using a ramp cycle protocol. Ventilatory anaerobic threshold (VAT) was measured by the V-slope method, and body surface area (BSA) of all patients calculated using their measured weight and height. Informed consent was obtained from all patients and all study protocols complied with the Institutional Review Boards of the participating institutions.
Data acquisition protocol
A 1.5 T Avanto Whole Body system (Siemens Medical Solutions, Malvern, PA) was used in anatomic and PCMR imaging. The imaging protocol, which utilized parallel imaging, began with an anatomic survey using static steady state free precession; this data was reformatted to acquire slice orientations and positions perpendicular to flow for rtPCMR acquisitions. The rtPCMR was an echoplanar sequence utilizing shared velocity encoding, the details of which have been described previously [15]. It utilized, in general, the following parameters: Repetition time =9.5 msec, Echo time =4.1 msec, Flip angle of 30 degrees, Field of view=320–400 mm, Slice thickness=8–10 mm, and Bandwidth=2841 Hz/pixel. The acquisition protocol consisted of through-plane PCMR across the superior (SVC) and inferior (IVC) vena cava, ascending (Ao) and descending (DAo) aorta for at least 10 seconds (20 frames per second, approximately). The IVC flow was acquired near the diaphragm but above the hepatic entrance to the IVC. The same imaging protocol was performed under the resting FB and BH conditions. Flows during the breath holding were acquired at the end of expiration, while FB flows include inspiration and expiration.
After the resting rtPCMR acquisition, the patients were slid partially out from the magnetic resonance imaging bore to perform lower leg exercise using an MRI-compatible supine bicycle ergometer (Lode BV, Groningen, the Netherlands). This ergometer allows revolutions per minute-independent workload ranging from 10 to 250 Watts while the patient maintains their position by bracing themselves with hand grips. The goal was to bring the patients from the resting conditions up to a steady work rate at their VAT as measured in their metabolic exercise test, which was a sustainable work rate for the completion of the PCMR data acquisition. Heart rate was monitored continuously. Initially, the workload was set to 20 watts which was increased progressively at a rate of 20 watts per minute to obtain a heart rate corresponding to that of the heart rate at VAT on their prior metabolic exercise test Exercise was then suspended, their feet quickly removed from the ergometer pedals and they were automatically returned to isocenter for imaging (< 10 seconds). Using this method, rtPCMR measurements of the Ao, DAo, IVC, and SVC were acquired with repeated exercise performed in between for the patient to return to the target heart rate.
Image processing and data analysis
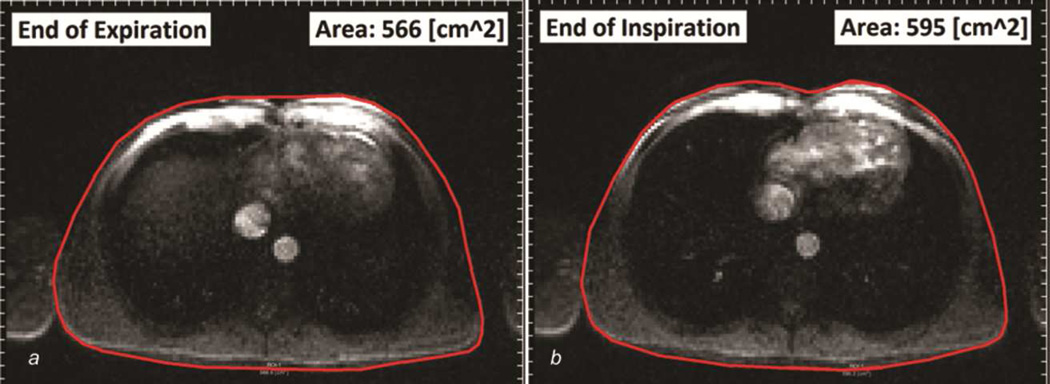
A semi-automatic protocol for image processing, chest wall tracking, and flow segmentation was developed using a freely-available software, Segment (Medviso.com). First, time-varying velocity fields, from through-plane PCMR slices, were integrated spatially over vessel crosssectional areas (e.g. at DAo) to calculate the associated flow (Q) waveforms. Inspiration and expiration periods corresponding to each vessel’s flow waveform were determined by tracking chest wall motion in each vessel’s corresponding magnitude image and measuring the temporal variations in cross-sectional area of the thoracic cavity (Figure 1). The inspiration period was identified by the time points from the local minimum to local maximum chest wall cross-sectional area, and the expiration period was from local maximum to local minimum. This approach, which strongly synchronizes the measurements of respiration and vessel’s flow, provides better investigation of respiratory effects by avoiding inscrutable time delays between the patient’s respiratory movement and the appearance of the respiratory signal [9]. The number of respiratory and cardiac cycles in the acquisition period and the average respiratory and heart rates (RR and HR, respectively) were also calculated with this method.
Figure 1.
Chest wall motion tracking, showing the minimum and maximum areas of the thoracic cavity at the end of a) expiration, and b) inspiration
Time averaged flow rates during inspiration and expiration were calculated using the corresponding times determined from chest wall motion tracking. Since no respiration is expected under the breath holding condition, the time durations to obtain the time-averaged vessel flow rates under breath holding were identical to those under free breathing in the same acquisition. Systemic return (Qs) was calculated by adding IVC and SVC flow rates. All flow rates were normalized by patient BSA.
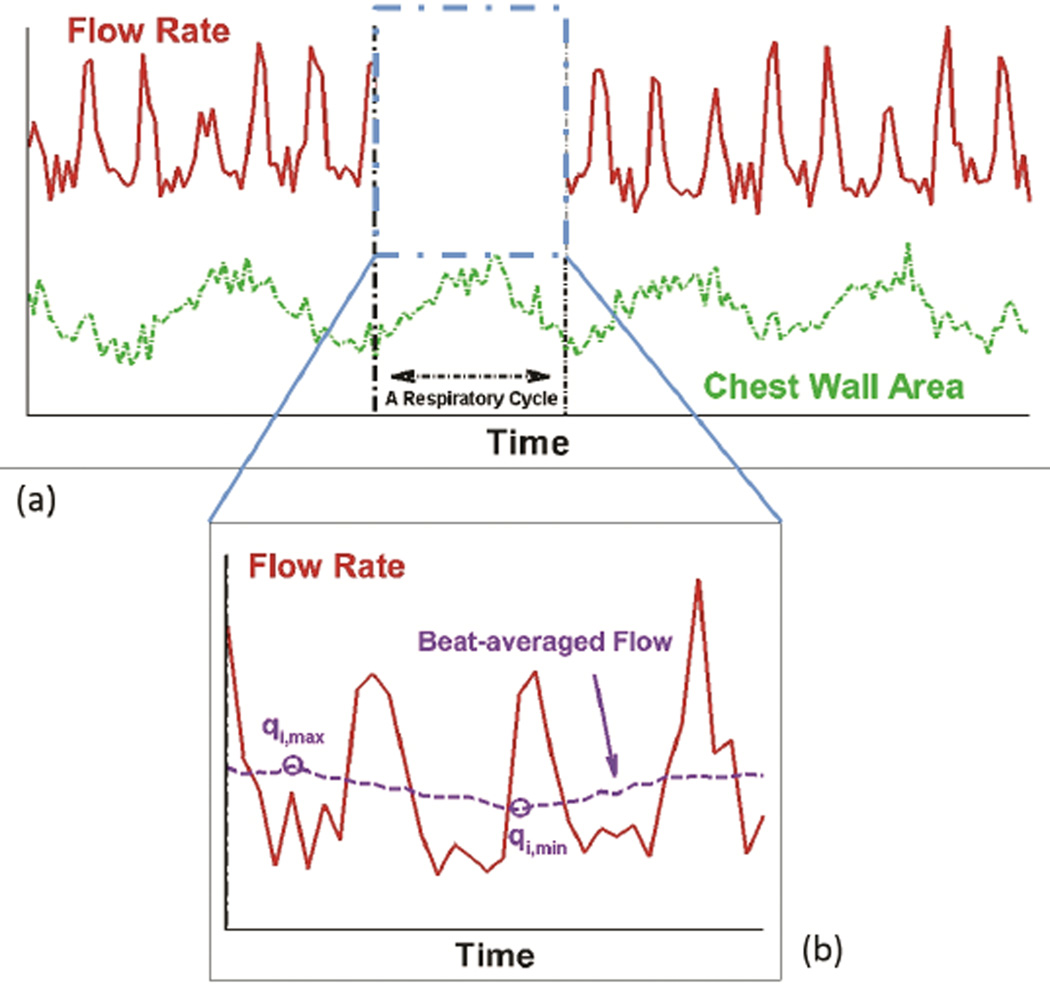
General pulsatility index (PI) was calculated to quantify the overall flow pulsations (caused by both cardiac and respiratory effects) across each vessel i as:
| (1) |
where Qi,mean was the mean (time-averaged) flow rate over the respiratory cycle, and Qi,min, and Qi,max were the minimum and maximum instantaneous flow rates during the respiratory cycle (Figure 2a).
Figure 2.
Schematic showing the flow rates used for calculating the flow overall pulsatility index (PI) and the respiratory phasicity index (PIresp)
A respiratory phasicity index (PIresp) was defined to quantify the flow pulsations caused only by respiratory effects. To calculate PIresp in the free breathing condition, the respiratory cycle was first divided into cardiac cycle timeframes based on the tracked chest wall motion (Figure 2a). The beat-averaged flow is obtained by averaging the flow rate, as shown in Figure 2b, based on the cardiac cycle timeframe, which was calculated as the total acquired flow data time in a respiratory cycle divided by the total number of heart beat in this respiratory cycle. The heart beat is distinguished by apparent peaks of instantaneous flow rate, i.e. 3.5 peaks in Figure 2b. These beat-averaged flows were then used to calculate PIresp as:
| (2) |
where qi,max was the highest beat-averaged flow rate over the respiratory cycle, and qi,min was the minimum beat-averaged flow rate. PIresp in the breath holding condition, which quantifies the small temporal variations in vessel flow rates, was calculated in a time frame equal to the respiratory cycle in the free breathing condition.
Statistical analysis
All variables are presented as mean values with standard deviations, unless otherwise stated. In all comparisons, a p-value<0.05 was considered significant [16]. Most comparisons of this study involve three different conditions of the same set of patients (n=11). Therefore, the one-way ANOVA with Tukey test was employed for normally distributed datasets, and the Kruskal-Wallis with Dunn test was utilized for non-normal distribution. On the other hand, for comparisons only involving two conditions, a paired two-tailed test was utilized for normally distributed datasets, and the Mann-Whitney test was employed for sets with a non-normal distribution.
Results
The measured flow and respiration parameters during resting FB, resting BH, and exercise conditions are summarized in Tables 1 and 2.
Table 1.
Analyzed patient data (n=11) during breath holding free breathing, and exercise
| Resting BH | Resting FB | Exercise | |
|---|---|---|---|
| Heart rate (1/min) | 71.2±12.8 | 69.2±12.1 | 111.4±18.4*† |
| Respiratory rate (1/min) | - | 17.5±4.6 | 28.0±13.1† |
| Cardiac cycles per respiratory cycle | - | 4.2±1.3 | 4.4±1.1 |
| Ao flow (L/min/m2) | 3.3±1.0 | 3.4±0.8 | 6.0±1.6*† |
| DAo flow (L/min/m2) | 1.7±0.4 | 1.7±0.4 | 3.8±0.9*† |
| IVC flow (L/min/m2) | 1.5±0.6 | 1.6±0.5 | 3.9±0.8*† |
| SVC flow (L/min/m2) | 0.5±0.2 | 0.6±0.2 | 1.0±0.6*† |
| Qs = SVC + IVC flow (L/min/m2) | 2.0±0.7 | 2.1±0.6 | 4.8±0.8*† |
p<0.05 in comparison with the breath holding condition;
p<0.05 in comparison between the resting FB and exercise conditions
Table 2.
Comparison between patient (n=11) vessel flow waveforms during resting breath holding and free breathing, and exercise
| Ao | DAo | IVC | SVC | ||
|---|---|---|---|---|---|
| Ratio of inspiration to expiration flow rates (Qinsp/Qexpr) | FB | 1.1±0.1 | 1.2±0.3 | 1.6±0.5* | 1.9±0.6* |
| Exercise | 0.9±0.2 | 1.0±0.3 | 1.8±0.5* | 2.6±2.0* | |
| General pulsatility ndex (PI) | BH | 510±155 | 569±202 | 123±72 | 204±116 |
| FB | 454±116 | 589±303 | 231±120* | 264±160 | |
| Exercise | 430±113 | 357±157*† | 259±98* | 320±174 | |
| Respiratory phasicity index (PIresp) | BH | 72±56 | 62±35 | 36±38 | 49±25 |
| FB | 67±56 | 52±30 | 120±59* | 134±69* | |
| Exercise | 53±28 | 75±37 | 119±48* | 166±73* |
p<0.05 in comparison with the breath holding condition;
p<0.05 in comparison between resting FB and exercise conditions;
Respiratory effects at the resting conditions
As presented in Table 1, heart rate did not change significantly between the resting BH and FB conditions. Each respiratory cycle at free breathing included, on average, 4.2±1.3 cardiac cycles. Total systemic venous flow did not show significant statistical difference from the BH to FB conditions (2.0±0.7 L/min/m2 vs. 2.1±0.6 L/min/m2, respectively).
As presented in Table 2, only IVC and SVC flow rates were significantly higher during inspiration than during expiration at the resting free breathing condition (Qinsp/Qexpr=1.6±0.5 for IVC and 1.9±0.6 for SVC).
The general pulsatility index at the free breathing condition was significantly higher than the breath holding condition in only the IVC. Respiratory phasicity index was significantly higher in both the IVC and SVC at resting free breathing compared with the breath holding condition.
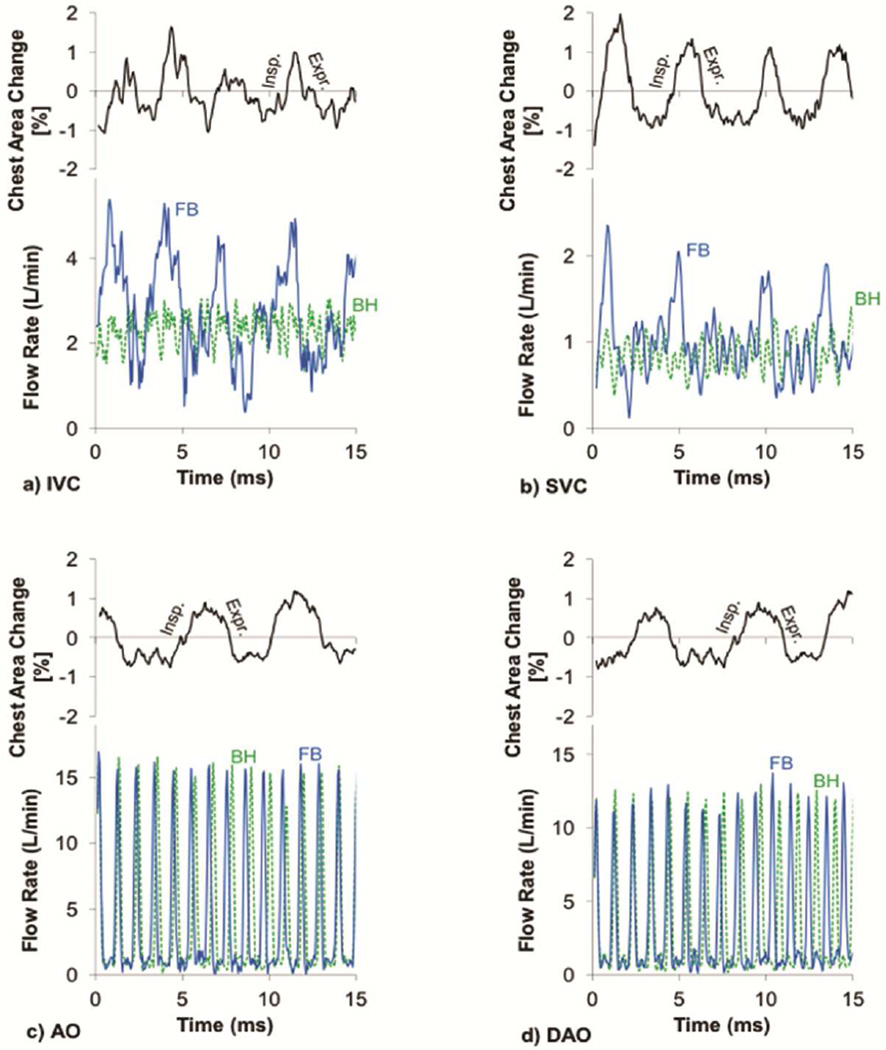
Time varying resting (BH and FB) vessel flow waveforms and corresponding respiratory cycles of a representative patient (female, 21 years old, EC, BSA=1.7 m2) are presented in Figure 3.
Figure 3.
Resting flow waveforms of a) inferior vena cava (IVC), b) superior vena cava (SVC), c) aorta (Ao), and d) descending aorta (DAo) at the free breathing (solid line) and breath holding (dashed line) conditions along with the corresponding respiratory cycles determined by tracking the chest wall motion for a representative patient
Respiratory effects at the exercise condition
As presented in Tables 1, both HR and RR increased (1.6×) significantly from resting to the exercise condition. However, the number of cardiac cycles per respiratory cycle did not change significantly between the resting FB and exercise conditions (4.4±1.1). Aortic, individual caval, and systemic return flow rates increased significantly in the exercise condition compared to the resting conditions. Similar to the resting condition, only exercise IVC and SVC flow rates were significantly higher during inspiration compared to expiration (Qinsp/Qexpr≈1.8±0.5 for IVC and 2.6±2.0 for SVC) (Table 2).
The IVC general pulsatility and IVC and SVC respiratory phasicity indices under the exercise condition are significantly higher than the breath holding condition. The DAo general pulsatility significantly decreases from resting FB to the exercise condition, while the Ao general pulsatility does not.
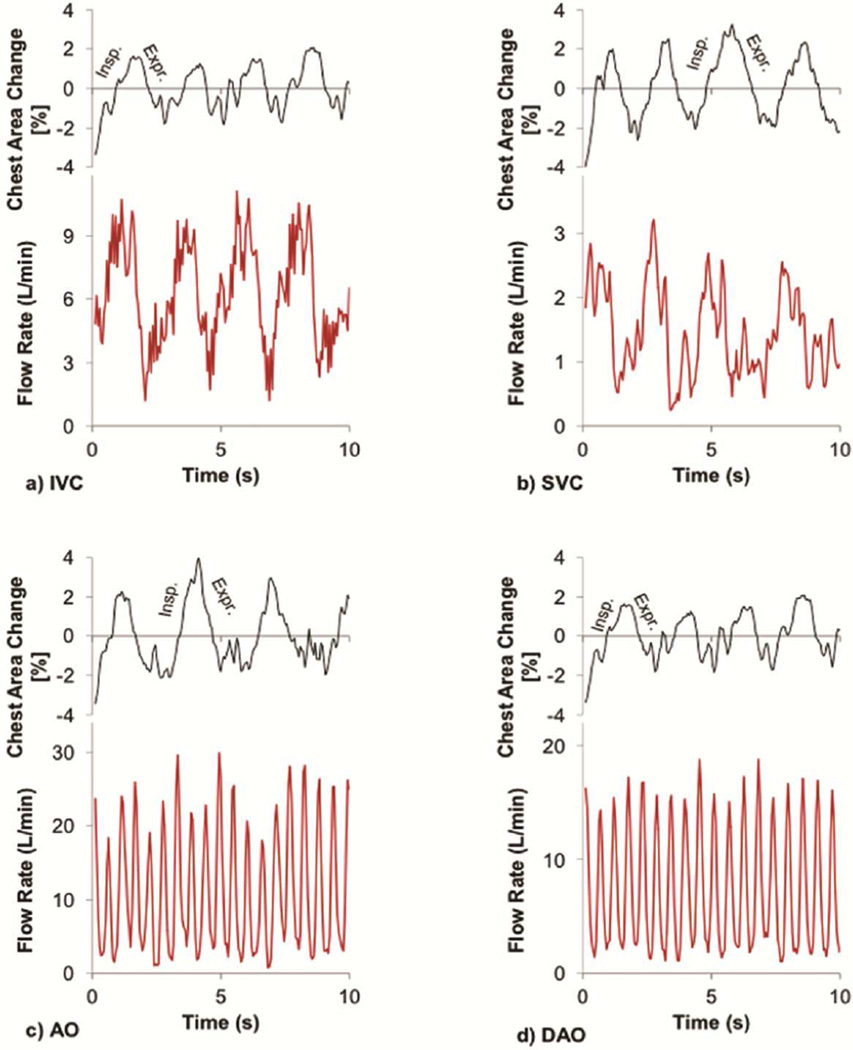
Time varying vessel flow waveforms and corresponding respiratory cycles of a representative patient (female, 21 years old, EC, BSA=1.7 m2) under the exercise condition are presented in Figure 4.
Figure 4.
Exercise flow waveforms of a) inferior vena cava (IVC), b) superior vena cava (SVC), c) aorta (Ao), and d) descending aorta (DAo) along with the corresponding respiratory cycles determined by tracking the chest wall motion for a representative patient
Equivalent mean flow rates between IVC and DAo
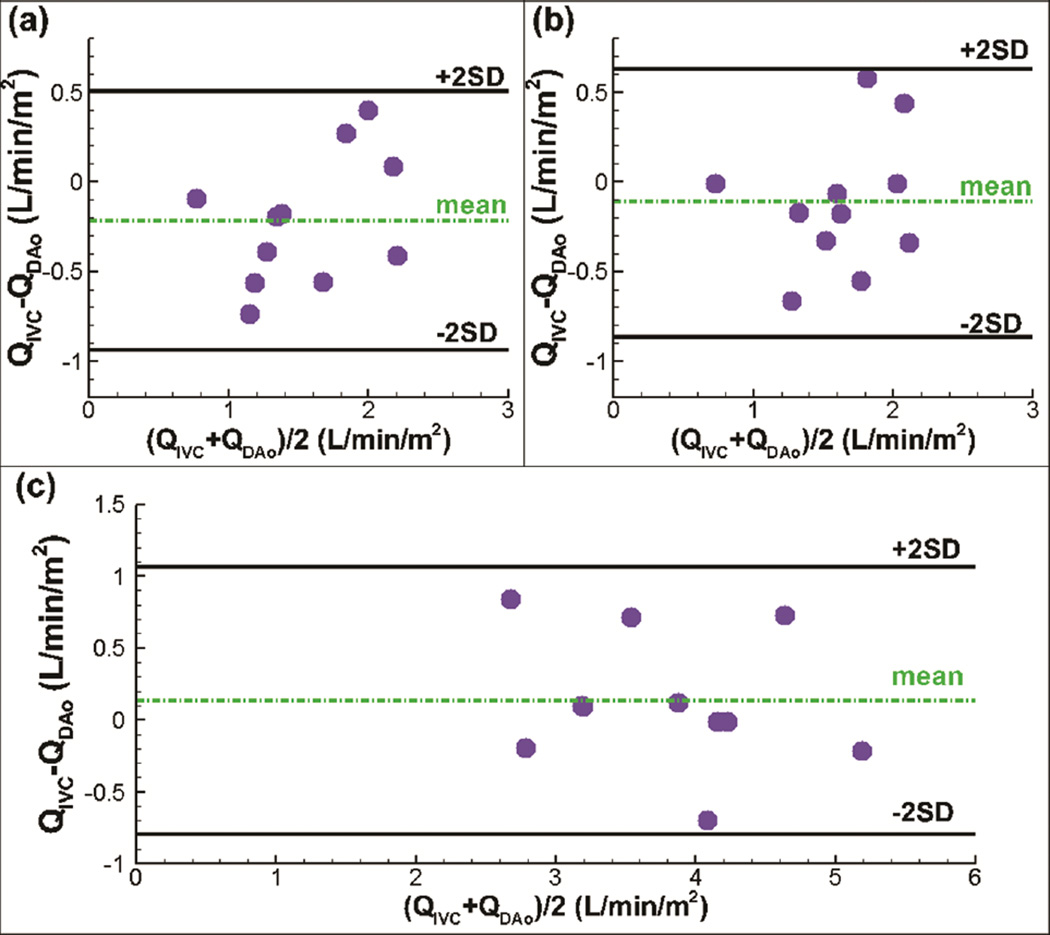
Table 1 demonstrates that the mean flow rates of DAo are 1.7±0.4 L/min/m2, 1.7±0.4 L/min/m2, and 3.8±0.9 L/min/m2 for the resting BH, FB and exercise conditions, respectively. The counterparts of IVC are 1.5±0.6 L/min/m2, 1.6±0.5 L/min/m2, and 3.9±0.8 L/min/m2. Figure 5 shows the Bland-Altman plots of the time-averaged flow rate of the IVC versus the DAo under the resting and exercise conditions. The 95% limits of agreement are indicated by the “2SD” lines. The analyses demonstrate mean difference of 13%, 7%, and 4% corresponding to the mean values of average flow rate in the IVC and DAo under the BH, FB, and exercise conditions, respectively. None of the differences were statistically significant.
Figure 5.
Bland-Altman comparisons of flow rate (Q) between IVC (inferior vena cava) and DAo (descending aorta) during (a) breath holding, (b) free breathing, (c) exercise.
Comment
The TCPC is a passive low pressure system, and therefore any pressure changes in the thoracic cavity (due to respiration) can significantly affect its flow waveforms. On the other hand, external pressure changes have relatively little effect on aortic flows, a higher pressure system. Utilizing rtPCMR, our study demonstrated that respiration does not affect systemic venous flow present in single ventricle patients after TCPC, but exercise affects it. Also, IVC pulsatility indices increased breath holding to free breathing, however, it did not change between free breathing and exercise. The DAo pulsatility index decreases from resting FB to the exercise condition, while the Ao pulsatility index does not. In some patients the AAo may have less pulsatility due to surgically placed non-compliant patch materials. In the current study, 5 of 11 patients were diagnosed with HLHS, and likely had such a patch. Therefore, it is reasonable to find a significant decrease in the general pulsatility index in the DAo between resting FB and exercise conditions, while seeing no such different in the AAo. Furthermore, neither the mean flow nor pulsatility indices of Ao and Dao changes significantly during respiration, confirming that the pulmonary circulation acts as a capacitor to moderate systemic flow even in this passive flow system. Aortic flow was greater that systemic venous return due to systemic to pulmonary collateral flow.
The findings in this study agree with and add to other real-time MRI-based studies. Most notably, Hjortdal et al.[9] report similar increases in IVC and aortic flow between the resting and exercise conditions, the augmentation of only IVC flow during inspiration, and the capacitor-like nature of the pulmonary circulation. However, Hjortdal et al.[9] does not report an increase in SVC flow during exercise, or the augmentation of SVC flow during inspiration seen here. The differences in SVC flow results shown here may be attributed to an older patient cohort (12.4±4.6 years vs 20.0±6.3 years) or of the novel respiration tracking method. Hjortdal et al. admitted that respiratory pressures are distributed to both the IVC and SVC equally, and they possibly see effects only on the IVC because of a less accurate respiration tracking method [9]. This study’s technique employed simultaneous image processing of blood flow and chest wall motion to better align respiration effects.
Acquiring data at both the resting BH and FB conditions in the present study provided the framework to independently compare the respiratory effects on mean vessel flows. During resting free breathing the overall flow pulsatility increased significantly compared to breath holding in the IVC (Table 2). This finding confirmed previous observations of the pronounced effect of respiration on the IVC flow waveform [9–11].
Furthermore, as shown in Figure 3, a distinct respiratory pattern was also present in SVC flow waveforms (a similar SVC respiratory pattern may also be recognized [9]). However, this was somewhat masked by the SVC cardiac pulsatility at rest. By defining the respiratory phasicity index (Table 2), this study was better able to quantify the SVC respiratory effect.
While respiration has an effect on both IVC and SVC flow waveforms, the data presented suggests that it has a minimal effect on net flow. The small mean difference between breath holding and free breathing conditions (0.1 L/min/m2) challenges the notion that respiration acts as a significant pump which drives flow through the Fontan physiology [17]. This change in thinking was previously suggested by Fogel et al. [18] when they estimated respiratory effects to be only 30% of the overall driving force in the systemic venous pathway [10]. In addition to challenging the notion of the respiratory pump, the lack of respiration influence on net flow validates the routine use of breath holding techniques to measure mean flows in Fontan patients. However, this minimal effect on mean flow should not lead to false application of time-averaged flow boundary conditions in Fontan circulation computational models. The significant increase in TCPC inlet flow pulsatility from BH to FB conditions will introduce noticeable power loss error in computational simulations if transient boundary conditions are not employed [19]. In addition to this bulk metric, investigating detailed unsteady flow and pressure [13], stagnation points, wall shear stress, etc. undoubtedly requires transient boundary conditions. Ultimately, pulsatile boundary conditions are always recommended for any simulations or in vitro experiments based on FB conditions.
Previous work has demonstrated that it is inappropriate to substitute DAo flow for IVC flow in patients with superior cavopulmonary connections, primarily due to the prevalence of upper body to lower body systemic venous collaterals or veno-veno collaterals. However, systemic venous collaterals may not be a significant issue in the TCPC population for the most part [20]. The interchangeability of the DAo and IVC mean flow rates proved in the current study is an important result because the increased diaphragmatic excursion during exercise conditions makes accurate IVC data acquisition difficult. This conclusion can extend beyond this study when IVC mean flow rate is important information, but its direct acquisition is unreliable.
Conclusion
This study found that the effect of breath holding on mean flow is not significant, supporting the routine use of breath holding in assessing mean flows in Fontan patients via CMR. This is an important finding, since many investigators presume that breath holding affects average Fontan flows when in fact it does not. Therefore, breath holding may, in fact, be a part of a preferred scanning protocol as it reduces image artifacts due to respiratory motion without significantly affecting mean flows. On the other hand, respiratory effects have a considerable impact on IVC and SVC flows, but have relatively little effect on systemic arterial flows in Fontan patients. These findings partially disprove conclusions of Hjortdal et al. [9] – that respiration does not affect the SVC flow. The discrepancy may be induced by the novel method of tracking respiration demonstrated in the current paper. This approach improves the accuracy of obtaining respiration by tracking respiration in the same image acquisition as the rtPCMR records the blood flow. Furthermore, the respiratory phasicity index is found to be a better parameter, compared with the general pulsatility index, to identify respiratory effects. It also demonstrated that mean flows of DAo and IVC are interchangeable with each other, regardless of whether breath holding or free breathing technique is used. This conclusion strengthens the argument that clinicians can make use of DAo mean flow rates as an IVC surrogate, or vice versa. Finally, the present technique of tracking respiration and the presented method for obtaining respiratory cycle and vessel flow rates using the same PCMR slice can simplify similar future studies by eliminating the need for separately monitoring the respiratory cycle.
Acknowledgments
This study was supported by the National Heart, Lung, and Blood Institute Grants HL67622, HL098252, and HL089647.
IC by Hsia to come
Abbreviations and Acronyms
- Ao
aorta
- BH
breath Holding
- BSA
body surface area
- DAo
descending aorta
- EC
extra cardiac
- EXE
exercise
- FB
free breathing
- HLHS
hypoplastic left heart syndrome
- HR
heart rate
- IVC
inferior vena cava
- LT
lateral tunnel
- PCMR
phase contrast magnetic resonance
- PI
general pulsatility index
- PIresp
respiratory pulsatility index
- Q
flow rate
- Qexpr
mean flow rate during expiration
- Qinsp
mean flow rate during inspiration
- Qs
systemic return
- RR
respiratory rate
- rtPCMR
real time phase contrast magnetic resonance
- SVC
superior vena cava
- VAT
ventilatory anaerobic threshold
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mair DD, Puga FJ, Danielson GK. The Fontan procedure for tricuspid atresia: Early and late results of a 25-year experience with 216 patients. J Am Coll Cardiol. 2001;37:933–939. doi: 10.1016/s0735-1097(00)01164-5. [DOI] [PubMed] [Google Scholar]
- 2.Dur O, DeGroff CG, Keller BB, Pekkan K. Optimization of Inflow Waveform Phase-Difference for Minimized Total Cavopulmonary Power Loss. J Biomech Eng Asme. 2010;132 doi: 10.1115/1.4000954. [DOI] [PubMed] [Google Scholar]
- 3.Orlando W, Shandas R, DeGroff C. Efficiency differences in computational simulations of the total cavo-pulmonary circulation with and without compliant vessel walls. Comput Methods Programs Biomed. 2006;81:220–227. doi: 10.1016/j.cmpb.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Hsia TY, Khambadkone S, Deanfield JE, Taylor JFN, Migliavacca F, de Leval MR. Subdiaphragmatic venous hemodynamics in the Fontan circulation. J Thorac Cardiovasc Surg. 2001;121:436–447. doi: 10.1067/mtc.2001.112527. [DOI] [PubMed] [Google Scholar]
- 5.Whitehead KK, Pekkan K, Kitajima HD, Paridon SM, Yoganathan AP, Fogel MA. Nonlinear power loss during exercise in single-ventricle patients after the Fontan: insights from computational fluid dynamics. Circulation. 2007;116:I165–1171. doi: 10.1161/CIRCULATIONAHA.106.680827. [DOI] [PubMed] [Google Scholar]
- 6.Tang E, Haggerty CM, Khiabani RH, de Zelicourt D, Kanter J, Sotiropoulos F, et al. Numerical and experimental investigation of pulsatile hemodynamics in the total cavopulmonary connection. J Biomech. 2013;46:373–382. doi: 10.1016/j.jbiomech.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregg CL, Butcher JT. Translational paradigms in scientific and clinical imaging of cardiac development. Birth Defects Res Part C-Embryo Today-Reviews. 2013;99:106–120. doi: 10.1002/bdrc.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsia TY, Khambadkone S, Bradley SM, de Leval MR. Subdiaphragmatic venous hemodynamics in patients with biventricular and Fontan circulation after diaphragm plication. J Thorac Cardiovasc Surg. 2007;134:1397–1405. doi: 10.1016/j.jtcvs.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 9.Hjortdal VE, Emmertsen K, Stenbog E, Frund T, Schmidt MR, Kromann O, et al. Effects of exercise and respiration on blood flow in total cavopulmonary connection - A real-time magnetic resonance flow study. Circulation. 2003;108:1227–1231. doi: 10.1161/01.CIR.0000087406.27922.6B. [DOI] [PubMed] [Google Scholar]
- 10.Fogel MA, Weinberg PM, Hoydu A, Hubbard A, Rychik J, Jacobs M, et al. The nature of flow in the systemic venous pathway measured by magnetic resonance blood tagging in patients having the Fontan operation. J Thorac Cardiovasc Surg. 1997;114:1032–1041. doi: 10.1016/S0022-5223(97)70017-5. [DOI] [PubMed] [Google Scholar]
- 11.Hsia TY, Khambadkone S, Redington AN, Migliavacca F, Deanfield JE, de Leval MR. Effects of respiration and gravity on infradiaphragmatic venous flow in normal and Fontan patients. 102:2000. doi: 10.1161/01.cir.102.suppl_3.iii-148. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen EM, Stenbøg EV, Fründ T, Houlind K, Kromann O, Sørensen KE, et al. Flow during exercise in the total cavopulmonary connection measured by magnetic resonance velocity mapping. Heart. 2002;87:554–558. doi: 10.1136/heart.87.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vukicevic M, Conover T, Jaeggli M, Zhou J, Pennati G, Hsia T, et al. Control of respiration-driven retrograde flow in the subdiaphragmatic venous return of the Fontan circulation. ASAIO J. 2014:21–23. doi: 10.1097/MAT.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vukicevic M, Chiulli Ja, Conover Ta, Pennati G, Hsia T-Y, Figliola RS. Mock circulatory system of the fontan circulation to study respiration effects on venous flow behavior. ASAIO J. 2013;59:253–260. doi: 10.1097/MAT.0b013e318288a2ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin HY, Bender JA, Ding Y, Chung YC, Hinton AM, Pennell ML, et al. Shared velocity encoding: A method to improve the temporal resolution of phase-contrast velocity measurements. Magn Reson Med. 2012;68:703–710. doi: 10.1002/mrm.23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller Da. “Significant” and “highly significant”. Nature. 1966;210:1190. doi: 10.1038/2101190a0. [DOI] [PubMed] [Google Scholar]
- 17.Rowland TW. The circulatory response to exercise: Role of the peripheral pump. Int J Sports Med. 2001;22:558–565. doi: 10.1055/s-2001-18526. [DOI] [PubMed] [Google Scholar]
- 18.Fogel Ma, Weinberg PM, Hoydu aK, Hubbard aM, Rychik J, Jacobs ML, et al. Effect of surgical reconstruction on flow profiles in the aorta using magnetic resonance blood tagging. Ann Thorac Surg. 1997;63:1691–1700. doi: 10.1016/s0003-4975(97)00330-5. [DOI] [PubMed] [Google Scholar]
- 19.Khiabani RH, Restrepo M, Tang E, De Zélicourt D, Sotiropoulos F, Fogel M, et al. Effect of flow pulsatility on modeling the hemodynamics in the total cavopulmonary connection. J Biomech. 2012;45:2376–2381. doi: 10.1016/j.jbiomech.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitehead KK, Gillespie MJ, Harris MA, Fogel MA, Rome JJ. Noninvasive quantification of systemic-to-pulmonary collateral flow: A Major Source of Inefficiency in Patients With Superior Cavopulmonary Connections. Circ Cardiovasc Imaging. 2009;2:405–411. doi: 10.1161/CIRCIMAGING.108.832113. [DOI] [PMC free article] [PubMed] [Google Scholar]