Abstract
Background
For older adults, falls are a serious health problem with over 30% of people over 65 suffering a fall at least once a year. One element often overlooked in the assessment of falls is whether a person’s balance, walking ability and overall falls risk is affected by performing activities of daily living such as walking.
Objective
This study assessed the immediate impact of incline walking at a moderate pace on falls risk, leg strength, reaction time, gait and balance in 75 healthy adults from 30 to 79 years of age. Subjects were subdivided into five equal groups based upon their age (Group 1, 30–39 years; Group 2, 40–49 years; Group 3, 50–59 years; Group 4, 60–69 years; Group 5, 70–79 years).
Methods
Each person’s falls risk (using the Physiological Profile Assessment), simple reaction time, leg strength, walking ability and standing balance were assessed prior to and following a period of incline walking on an automated treadmill. The walking task consisted of three 5-minute trials at a faster than preferred pace. Fatigue during walking was elicited by increasing the treadmill incline in increments of 20 (from level) every minute to a maximum of 80.
Results
As predicted, significant age-related differences were observed prior to the walking activity. In general, increasing age was associated with declines in gait speed, lower limb strength, slower reaction times and increases in overall falls risk. Following the treadmill task, older adults exhibited increased sway (path length 60–69 yrs; 10.2±0.7 to 12.1±0.7 cm: 70–79 yrs; 12.8±1.1 to 15.1±0.8 cm), slower reaction times (70–79 yrs; 256±6 to 287±8 ms), and declines in lower limb strength (60–69 yrs; 36±2 to 31±1 kg: 70–79 yrs; 32.3±2 to 27±1 kg). However, a significant increase in overall falls risk (pre; 0.51±0.17: post; 1.01±0.18) was only seen in the oldest group (70–79 years). For all other persons (30–69 years), changes resulting from the treadmill-walking task did not lead to a significant increase in falls risk.
Conclusions
As most falls occur when an individual is moving and/or fatigued, assessing functional properties related to balance, gait, strength and falls risk in older adults both at rest and following activity may provide additional insight.
Introduction
For older adults, the likelihood of suffering a fall is a major risk that can have dramatic implications for overall health and wellbeing1. This increased risk is tied, in part, to the general age-related decline in physiological processes integral to the control of balance and gait, with decrements in neuromuscular function, strength, sensation, and cognitive processing all being key factors2–4. The consequence of the declines in these physiological processes is that the older individual is less able to respond to everyday challenges when performing many activities of daily living. A general feeling of being fatigued or tired has also been linked to an increased likelihood of suffering a fall4–6, as the person who is fatigued may be less able to respond appropriately and/or in a timely fashion to challenges when performing daily activities of a dynamic nature (e.g., walking outside, climbing stairs).
Fatigue has been broadly described as a transient decrease in the ability to perform physical activities7 and an overwhelming sustained sense of exhaustion and decreased capacity for physical and mental work. Most commonly, this decrement in movement performance over the time period of the activity can be linked to an inability to maintain a desired force level required for the given task8, with the overall rate of decline dependent on the type of activity, intensity, and duration9. In regards to those mechanisms that contribute to optimal balance, fatigue has a wide range of impacts, leading to increases in postural sway10–14, declines in obstacle avoidance15, stepping16 or general walking ability17–19, decreased muscle function and strength20–22, and reduced proprioception and/or sensation23–25. The consequence of fatigue are particularly pronounced for older adults, with these persons often citing increased levels of fatigue and tiredness as one reason for reducing their levels of physical activity.
There is no doubt that there is a strong association between fatigue and a number of factors that underscore falls risk in older adults. However, falls are a multidimensional problem, with over 400 risk factors being linked with these events26. Our understanding of falls is further complicated by the fact that they can be considered a very individual event; that is, no two people fall for the same reason, under the same conditions and suffer the same consequences. As a result, it is possible that, while fatigue may affect individual physiological components essential for optimal balance control, it is still unclear whether the summative effect on fatigue leads to an actual increase in overall risk. A secondary component often overlooked in the assessment of falls risk is how those selected metrics of balance and falls risk change as fatigue is induced during the performance of activities of daily living like walking. Although most falls occur during movement, the majority of fall risk assessments are performed under resting conditions that fail to take this dynamic into account.
The aim of the current study was to assess the immediate impact of fatigue on overall falls risk, walking ability, balance, reaction time and lower limb strength in 75 adults ranging from 30 to 79 years of age. It was predicted that the effects of fatigue (induced by performing a walking task) would only manifest as an increased falls risk in the older individuals.
Methods
Participants
Seventy five healthy individuals of both sexes (age range 30–79 years) were recruited from the local community to participate in this study. Subjects were subdivided into five equal groups based upon their age by decades: Group 1, 30–39 years; Group 2, 40–49 years; Group 3, 50–59 years; Group 4, 60–69 years; and Group 5, 70–79 years. All individuals were questioned regarding their current level of exercise/activity and the number of falls over the previous 12 months. All participants reported to be physically active. Exclusion criteria included any history of any neurological/cognitive disorders, neuromuscular injury, significant cardiovascular disease, unstable proliferative retinopathy, end-stage renal disease, uncontrolled hypertension or lower limb arthritis that could influence movement performance27. A full physical evaluation which included examination of central nervous system function including coordination, neuropathy and cerebella function, tests of balance and stability and review of current medications was also performed28. General demographics for each age group and the number of previous falls (per age group) are shown in Table 1. Participants provided informed consent prior to inclusion and all procedures complied with University IRB guidelines.
Table 1.
Resting subject characteristics
| Age Group (years) | |||||
|---|---|---|---|---|---|
| 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | |
| N (M/F) | 8/7 | 7/8 | 7/8 | 7/8 | 7/8 |
| Age (years) | 34.7 ± 0.4 | 45.1 ± 0.4 | 54.3 ± 0.4 | 64.2 ± 0.4 | 74.5 ± 0.3 |
| Height (cm) | 170.0 ± 3.1 | 169.3 ± 3.1 | 171.5 ± 2.3 | 168.9 ± 2.9 | 162.6 ± 7.3 |
| Weight (kg) | 92.2 ± 0.2 | 92.8 ± 0.2 | 97.5 ± 0.3 | 84.5 ± 0.2 | 86.4 ± 0.3 |
| Number of persons who fell in the past year | 1/15 | 2/15 | 2/15 | 6/15 | 4/15 |
Experimental Design
Participants attended the laboratory on one occasion to be evaluated on the tests underlying the falls risk assessment (physiological profile assessment, PPA), reaction time, gait, and balance. This evaluation was followed by the walking-exercise fatigue protocol. Immediately afterwards, all subjects were reassessed on all measures.
Falls Risk Assessment
An indication of overall falls risk was determined using the long-form physiological profile assessment (PPA). The PPA consists of 15 different physiological assessments, covering visual function, lower limb sensation, proprioception, lower-limb strength, reactions, general balance and an assessment of postural coordinated stability. Values from five of the 15 measures (i.e., hand reaction time, proprioception, knee extension strength, edge contrast sensitivity, sway on foam surface with eyes open) were used to generate an overall falls risk score (range +4 to −2) with lower values denoting a lower risk of falling27,29.
Reaction Time
All participants completed a simple reaction time (RT) task where upper limb (index finger) and lower limb (foot) responses were collected. After completing 5 practice trials, each person completed 20 trials with each segment (the initial 10 trials were used within the PPA design for derivation of falls risk). Participants responded to a visual cue by depressing a timing switch with either their foot or finger. For the foot RT, participants had their distal end of their foot positioned over a pedal switch placed on the floor.
Gait Assessments
Walking performance was assessed using a 20 ft straight GAITRite pressure sensitive walking surface (CIR Systems Inc, Havertown PA). Individuals were instructed to look straight ahead and walk at their preferred walking pace. Three walking trials were performed (sample frequency 120 Hz). The GAITRite data were assessed using the Protokinetics PKMAS software (ProtoKinetics LLC). Specific spatio-temporal variables assessed included step/stride time (sec), gait velocity (cm/sec) and cadence.
Balance Assessments
These were performed while individuals stood on a Bertec balance plate (model BP6040, sample rate: 100 Hz). This device provides information about center of pressure (COP) excursions in the anterior-posterior (AP) and medio-lateral (ML) direction. Postural motion was collected for the following four conditions: 1) eyes open/firm surface, 2) eyes closed/firm surface, 3) eyes open/foam surface, and 4) eyes closed/foam surface. The foam surface was 15 cm thick and of medium density. COP data were filtered using a second-order low-pass Butterworth filter (cutoff frequency 50 Hz). The dependent measures determined for postural sway included total path length, mean COP velocity, mean, SD, and range of COP excursion in the ML and AP directions. Analyses of the COP data were performed using Matlab software (Mathworks R14).
Exercise-Induced Fatigue
All participants completed an exercise session on an instrumented treadmill (h/p/Cosmos Mercury 4.0). Older individuals wore a safety harness attached to an overhead suspension system. Starting with a treadmill speed of 1.33 m/s, participants walked at faster speeds until they were forced to break into a jog to keep up with the treadmill speed. The treadmill speed used during fatiguing bouts was the fastest speed observed in the period while walking was maintained30. This determination period doubled as a warm-up.
For the walking task, three 5-minute trials were performed. Fatigue during walking was elicited by increasing the treadmill incline in increments of 20 (from level) every minute to a maximum of 80. Participants were allowed to rest for five minutes between each the three fatiguing periods of walking. Immediately following this exercise routine, subjects were reassessed for reaction times, balance, strength, gait, and falls risk.
During the treadmill walking test, both heart rate and RPE values were attained. Heart rate (HR) was recorded using a Polar® monitor (Polar, Inc.). Selected heart rate measures (i.e., maximum heart rate and overall change in HR (maximum HR − baseline HR)) were used to determine the physiological effort for the walking tasks. A rating of perceived exertion (RPE) was obtained at the beginning and end of each fatigue walking trial using a modified Borg 10-point scale (1 as “little or no exertion” to 10 as “maximal effort”). The final RPE and the average change in RPE (i.e. RPE at the end of each fatigue trial minus the baseline RPE) were determined.
Statistical Analysis
All analyses were performed using repeated measures, mixed generalized linear models (GLM) with age group (5 levels) as the between-group factor and exercise session (pre/post-training) as the within-group factor. Planned contrasts were used for any post-hoc evaluations. All statistical analyses were performed using SAS statistical software (SAS Institute Inc., NC), with the risk of Type I error set at p <0.05.
Results
Pre-Exercise Differences
Falls Risk
At baseline, significant age-related differences were observed with regard to falls risk (F4,70 = 6.00; p<0.001). Post hoc analyses revealed differences between group 1 and the three older groups (3, 4, and 5). Significant differences were also found between the oldest group (5) and groups 2 and 3.
A significant difference was found between the different age groups for lower limb proprioception (F4,70 = 2.54; p<0.05). Post hoc analyses revealed differences between group 1 and the three older groups (3, 4, and 5). Significant differences were also found between the oldest group (5) and groups 2 and 3. A significant age-related difference was found for postural coordination task (F4,70=9.11; p<0.001) whereby the two older groups (4 and 5) recorded a significantly greater number of errors compared to the three younger groups (1–3) when performing this tracking task.
Reaction Time
Prior to the exercise intervention, a significant age effect was observed for both hand RT (F4,70 = 10.19; p<0.0001) and foot RT (F4,70 = 2.55; p<0.05). For the hand RT values, differences were between the oldest group (5) and the three younger groups (1, 2 and 3). For the foot RT values, differences were seen between the youngest group 1 and the oldest group 5.
Gait
Significant age effects were found for a number of walking metrics including gait velocity (F4,70 = 2.65; p<0.05), step length (left F4,70 = 2.80; right F4,70 = 2.88; p’s<0.05) and stride length (left F4,70 = 2.85; right F4,70 = 2.93; p’s<0.05). For all gait measures, differences were between the oldest group and the three younger groups (1, 2, and 3).
Balance
Initial analyses revealed a strong effect for the different conditions for the majority of COP variables. Consequently, inferential analyses were performed for each for the four postural conditions separately to more clearly discern differences related to age group and/or fatigue. Overall, the majority of age-related differences were seen for the more challenging balance conditions (i.e., performed wither with the eyes closed or closed on the foam surface). For the eyes open/foam surface condition, significant effects were seen for motion in the ML direction (range F4,70=3.04; mean F4,70=2.98; p’s<0.05), path length (F4,70=4.99; p<0.05) and COP velocity (maximum F4,70=3.41; mean F4,70=4.98; p’s<0.05). Post hoc revealed the main differences were between the youngest and the oldest groups (1 vs. 5).
For the eyes closed/foam surface condition, significant effects were seen for motion in the AP direction (range F4,70=3.85; mean COP F4,70=2.89; SD of COP F4,70 =2.82; p’s<0.05), path length (F4,70 =5.21; p<0.05) and COP velocity (maximum F4,70 =3.37; mean F4,70=5.18; p’s<0.05). For all measures, significant differences were found between the youngest and the oldest groups (1 vs. 5). Differences between the 40–49 year old group (2) and the oldest group 5 were also found for path length and COP velocity measures (all p’s<0.05). No differences were seen between the respective age groups during the other postural conditions (eyes open/firm, eyes open/foam).
Exercise Effects
A significant age effect for the treadmill walking speed was observed (F4,70 = 12.23; p<0.001). Planned contrasts revealed that the differences were between the oldest persons (group 5) and the remaining four groups only, with the oldest group walking at a slower speed compared to the other groups during the fatigue-treadmill task.
Heart Rate and RPE
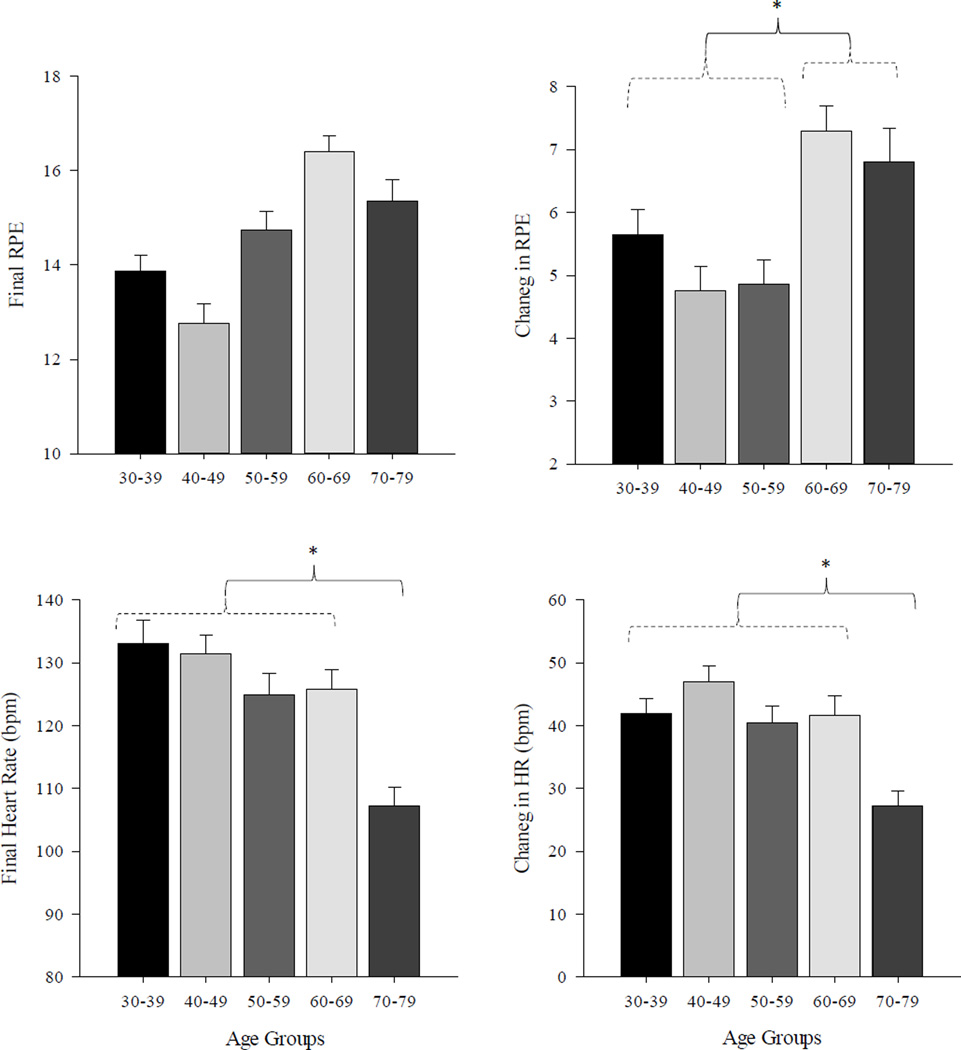
A significant age effect was observed for change in heart rate (F4,70 = 4.33; p<0.01) and overall change in RPE (F4,70 = 4.86; p<0.001). For heart rate, significant differences were found between the oldest group 5 and all other age groups (1–4). For the RPE values, differences were seen between groups 1–3 and groups 4–5. At baseline (prior to the exercise), there were no significant difference in HR between the groups. Figure 1 illustrates the general pattern of differences in RPE values (absolute and change) and heart rate values across the five age groups.
Figure 1.
Differences in RPE values (absolute and change) and heart rate values across the five age groups. Error bars represent one SE of the mean. Significant differences between age groups are denoted by an asterisk (*).
Post-Exercise Changes
Falls Risk and PPA
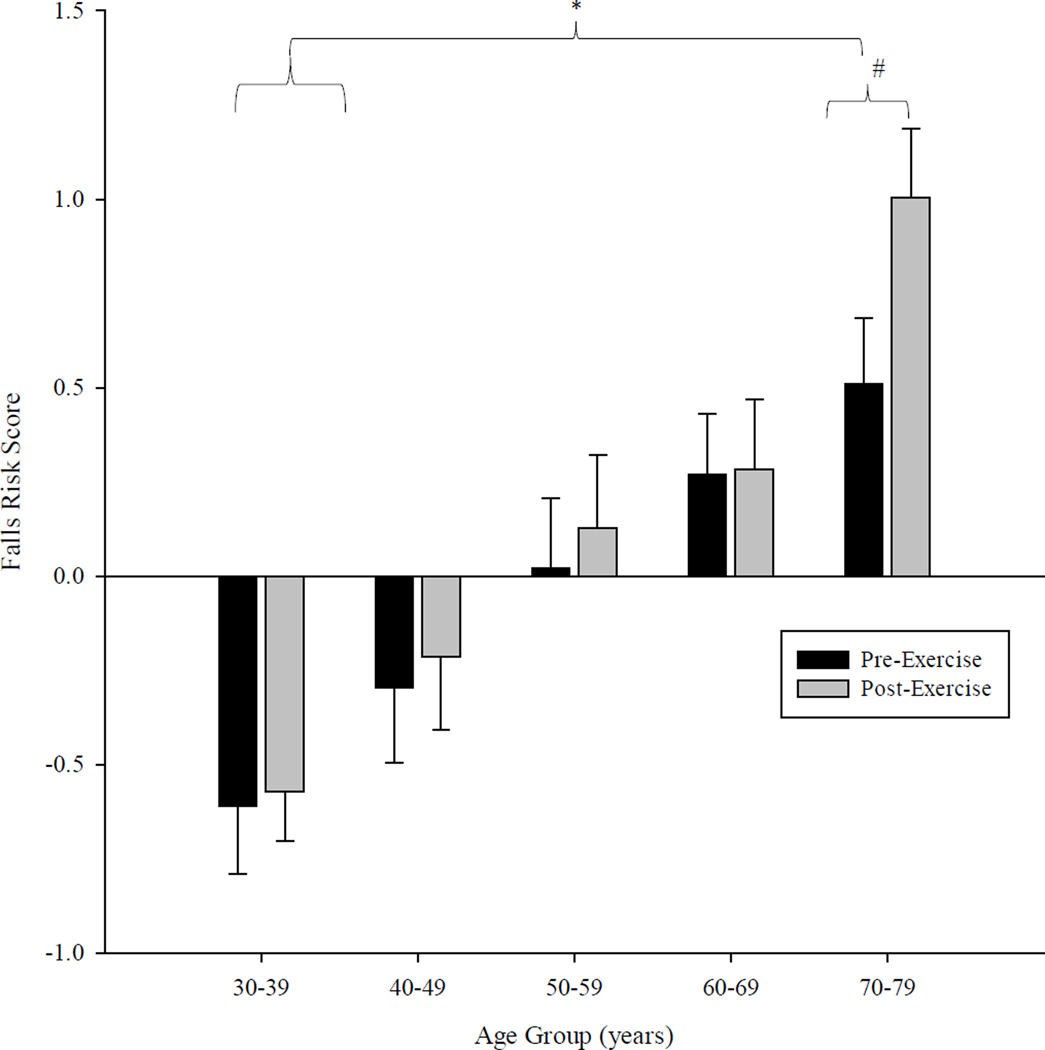
Following exercise, the oldest age group (5) showed a significant increase in overall falls risk (F1,14 = 11.14; p<0.001). For the remaining four age groups, no significant change in falls risk was found as a result of the fatigue activity. The oldest group also exhibited a significant change in proprioception (F1,14 = 5.11; p<0.05) and a significant decrease in quadriceps strength (F1,14 = 6.04; p<0.05) following the exercise activity. For the postural coordination task, the two oldest groups exhibited greater errors following the exercise intervention (Group 5 F1,14 = 4.65; Group 4 F1,14 = 4.01; p<0.05). No significant change in the postural tracking performance for the remaining three age groups was observed following fatigue. Figure 2 illustrates the pattern of change in the overall falls risk between all age groups and as a function of the exercise activity.
Figure 2.
Bar graph depicting differences in overall falls risk (attained from the PPA) as a function of the age and the fatigue intervention. Error bars represent one SE of the mean. Significant differences between age groups are denoted by an asterisk (*) while significant effects due to the fatigue protocol are denoted with a hash mark (#).
Reaction Time
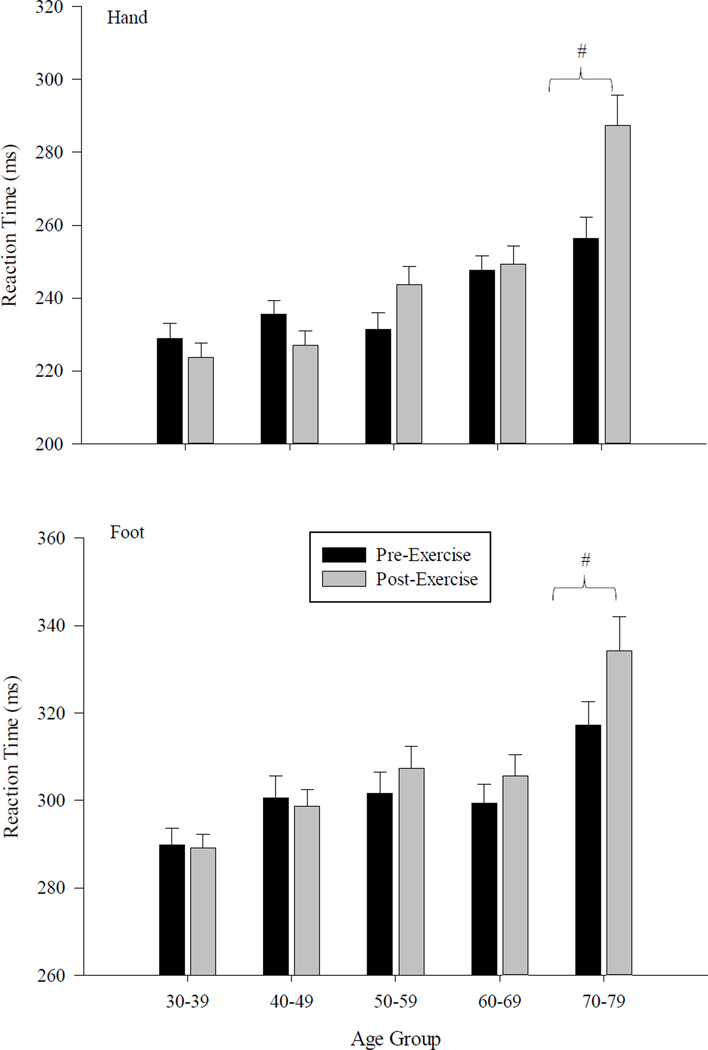
For the oldest group (5), significant increases were found for both hand (F1,14 = 6.11; p<0.0001) and foot reaction times (F1,14 = 5.62;p<0.05) following the exercise intervention. For the next oldest group (4), significant increases in foot reaction time were found (F1,14 = 5.88; p<0.05) while for group 3, hand reaction times increased following the activity (F1,15 = 11.08; p<0.05). No changes in RT values as a function of exercise were found for two younger groups. The general pattern of differences in hand and foot RT between the five age groups are shown in Figure 3. This figure also illustrates the pattern of change as a function of the exercise activity.
Figure 3.
Bar graph depicting differences in mean simple reaction time for the hand and the foot. Differences are shown across the five age groups and as a function of the fatigue intervention. Error bars represent one SE of the mean. Significant differences between age groups are denoted by an asterisk (*) while significant effects due to the fatigue protocol are denoted with a hash mark (#).
Gait
Following the fatigue intervention, significant changes in gait velocity, (F1,14 = 23.54; p<0.05), cadence, (F1,14 = 19.72; p<0.05), stride length (F1,14 = 15.76; p<0.05), and stride time (F1,14 = 6.09; p<0.05) were found for all age groups. Post hoc analyses revealed that all these measures increased following the treadmill fatigue activity (p’s<0.05).
Balance
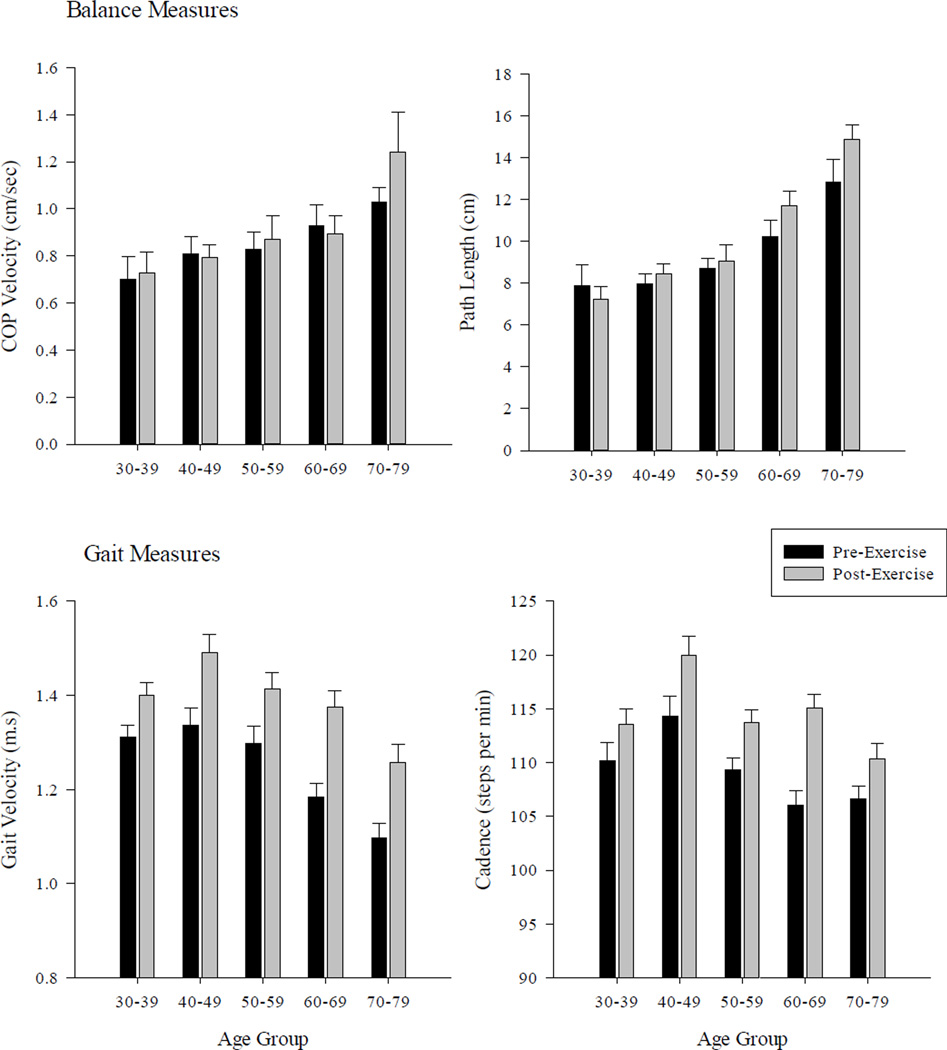
Following the fatigue protocol, persons in the oldest age group exhibited significant changes in specific postural sway metrics (path length, F1,14=10.12; maximum COP velocity F1,14=4.69; mean COP velocity F1,14=9.11; all p’s<0.05) during the eyes open/foam surface condition. Similar changes were found for the same group during the eyes closed/foam surface condition (path length, F1,14=7.82; maximum COP velocity F1,14=3.89; mean COP motion in the ML direction F1,14=4.88; all p’s<0.05). For persons in next oldest group 4, fatigue also had an impact on balance. Significant differences were seen during the eyes open/foam surface condition (path length, F1,14=6.67; COP range in the AP direction F4,70=10.67; p’s<0.05) and during the eyes closed/foam surface condition (Mean COP motion in the AP direction, F1,14=5.43; maximum COP velocity F1,14=3.89; p’s<0.05). No significant changes in the sway metrics following fatigue were found for groups 1–3. Changes in specific gait (i.e., cadence, velocity) and balance (i.e., path length, COP velocity) metrics between the five age groups are shown in Figure 4. This figure also illustrates the changes as a function of the exercise activity.
Figure 4.
Average changes in gait (bottom panels; cadence, velocity) and balance (top panels: path length, COP velocity) across the different age groups as a function of the walking task. For the balance measures, results are shown for eyes open/foam surface postural condition only. Error bars represent one SE of the mean.
Correlation Analysis
To ascertain whether there was any relation between falls risk and other metrics, correlation analysis was performed between falls risk score and selected gait measures (i.e. cadence, gait velocity), PPA measures and COP motion. As the falls risk score is derived from five of the 15 PPA measures, these measures (i.e., hand reaction time, proprioception, knee extension, edge contrast sensitivity, sway on the foam surface/eyes open) were not included in this analysis. For the remaining PPA measures, the only significant relation was for reaction time for the foot (pre-exercise r=0.58, p<0.05) although the strength of this relation dropped following exercise (r=0.44). High correlations for foot RT-falls risk were only found for the older age groups (pre-exercise: 50–59 years, r=0.65; 60–69 years r=0.62; 70–79 years r=0.72) with values tending to decrease following exercise (post-exercise: 50–59 years, r=0.63; 60–69 years r=0.23), only increasing slightly within the oldest group (post-exercise r=0.75). For the other measures which made up the PPA, no significant correlations were observed as a function of age or exercise (pre-exercise range: 0.14–0.38; post-exercise range: 0.11–0.41). Similarly, no significant correlation was found between falls risk score and the COP measures (pre-exercise, r range: 0.18 to 0.48; post-exercise r range: 0.11–0.46) either as a function of age group or exercise.
Regarding the gait measures, significant correlations were found between falls risk score and both cadence and gait velocity. Overall, correlation values were lower pre-exercise (cadence r=0.28; velocity r=0.30) and changed significantly following the treadmill activity (cadence r=0.41; velocity: r=0.54, p’s<0.05). The correlation changes were primarily found within the 60–69 year old group (pre-exercise cadence: r=0.06; post exercise r=0.43: pre-exercise velocity: r=0.04; post exercise r=0.38) and the 70–79 year old group (pre-exercise cadence: r=−0.15; post exercise r=0.39: pre-exercise velocity: r=0.18; post exercise r=0.61).
Discussion
The aim of this study was to assess the short-term effects of performing a fatiguing walking activity on falls risk, balance, gait, and general physiological function for healthy, adult individuals aged from 30 to 79 years. As expected, the results revealed significant differences between the respective age-groups prior to the fatiguing exercise, primarily with regards to walking speed, reaction time, quadriceps strength, postural motion, coordination and overall falls risk. These results are consistent with the current literature, which also reported age-related differences in such measures as reaction time, lower limb strength, falls risk, gait, balance (COP) measures and proprioception29,31,32. Following the treadmill intervention, a number of changes were seen, primarily in the older age groups. For example, the oldest individuals; (70–79 years) exhibited slower reaction times, increased postural sway and a higher falls risk. While those in the 60–69 age group showed increases in reaction times and postural sway, the sum of the physiological changes was not enough to lead to an increase in falls risk. Interestingly, all individuals, irrespective of age, walked at a faster gait speed immediately following the treadmill task, with the increased gait speed being correlated with increased falls risk. Overall, the treadmill walking activity had the greatest impact on the older persons in this study, leading to a decline in many of the mechanisms related to balance control and ultimately to increased falls risk for the oldest adults.
A general observation from this study was that the treadmill-fatigue task was perceived as more difficult for the older persons (i.e., groups 4 and 5). When performing this activity, individuals in the oldest age group (70–79 years) self-reported that the task was significantly more difficult (reflected as a higher RPE) compared to the responses of the four other age groups, even though their treadmill walking speed and physiological indices of fatigue (change in HR) were significantly lower than for the other groups. Interestingly, while those in the 60–69 years old group reported that task to be harder as well, there was no significant differences in their treadmill walking speed or indices of fatigue (change in HR) between this group and those 30–59 years of age. This general pattern whereby the greatest impact was observed in the oldest subjects carried over to the other analyses. Of particular note, those in the 70–79 age group experienced a significant increase in their overall falls risk scores (as assessed by the PPA) after performing the fatigue task. For persons of this age, this increased risk was linked to declines in a number of measures, including lower limb strength (primarily knee extension although knee flexion strength declined also), poorer performance in the postural coordination task, declines in proprioception, slower reaction times (for the hand and foot) and increases in the amount of postural motion under more challenging balance conditions. One conclusion that can be reached from these results is that it is the culmination of declines across multiple factors that led to the overall increase in falls risk as this effect was only seen for individuals in the oldest age group. While the fatigue activity led to changes in some selected metrics of other groups (e.g., slower reactions and increased sway in 60–69 year olds), the overall impact was not reflected by an increase in falls risk score. The changes in postural motion and/or reaction time are certainly consistent with the findings of a number of studies reporting that fatigue leads to increased sway, declines in obstacle avoidance ability, changes in sensation and decreases in muscle strength10,12–17,19,33–35.
For the majority of previous studies, fatigue has been achieved through sustained contractions of a specific muscle group5,22,36, a methodological difference which may explain some of the differences. As there are numerous physiological factors that can contribute to falls, stressing one aspect of the system (such as selected muscle groups) would certainly impact of those metrics of balance reliant on this variable, but the overall impact may not be sufficient to lead to an overall increase in falls risk. In this regard, other (non-fatigued) components of the system may be able to compensate for any declines at other levels of the system targeted by the exercise activity as has been previously suggested37,38. A study by Pereria and Goncalves (2011) provides further support to this view. In that study, fatigue was induced in older adults (mean age 67 years) using prolonged walking on a treadmill (20 minutes). However, while changes in the muscle activity of the lower limb were observed following this intervention, no increase in overall falls risk was found18. Similarly, it has been previously reported by Nardone and colleagues (1997, 1998) that while strenuous anaerobic or anaerobic activity can affect various COP measures, the impact of exercise to fatigue on overall control of posture may be minimal as other (non-fatigued) balance mechanisms can be utilized to compensate for any perturbation induced by the exercise37,38. These authors also highlighted an important additional point to consider; namely that the time-course of the effects of fatigue can be very short. Consequently, while fatigue does negatively impact on various metrics of balance and posture5,39,40, these changes are time-dependent and can tend to dissipate quickly. This raises the possibility that a contributing factor to the increased falls risk in the older adults may be their decreased ability to recover as quickly from the fatiguing intervention as individuals in the other (younger) groups41,42. Thus, while all subjects may exhibit declines in function immediately post-exercise, it is the time-course of the recovery period, which may be a determining factor in whether these declines translate to a prolonged increase in falls risk. Together, our results and those of previous studies highlight that while fatigue is certainly a mitigating factor in falls risk, its effects may only be mediated when they affect multiple systems significantly.
An interesting finding to emerge from this study was that, immediately following the treadmill task, all subjects irrespective of age walked at a faster speed over the GAITRite walking surface (see figure 4). As has been reported in other studies, there were significant differences between the groups in their walking dynamics prior to the treadmill task34,43–45 with the older adults (group 5) walking at the slower speed with shorter strides. After completing the task, increases in walking speed, cadence, stride length and stride time was seen for all groups, irrespective of age. One possible reason to explain these changes is that they simply reflect a transient carry-over effect from walking at a faster pace when performing the treadmill task. However, this result raises an added concern in that, for the older adults, the immediate improvement in walking speed (i.e. increased mobility) were counteracted by a decline in the various metrics related to balance ability (i.e. decreased stability). The results of the correlation analysis supported this finding, showing that for the older individuals (60–69 years and 70–79 years) increases in gait speed and cadence was related to increased fall risk. Consequently, the older persons are possibly at a greater risk of falling immediately post-exercise. Irrespective of the actual mechanism or reason, the declines in all of the specified fall-related metrics observed for the older adults (70–79 years) following the treadmill fatigue task indicates that this activity had a detrimental impact on their ability to maintain optimal balance leading to an increased risk of falling. This result is of particular importance given that, for many older adults, falls can occur when they are moving and/or fatigued. Therefore, a more comprehensive understanding of the problem of falls in older adults can likely be gained from assessing functional properties related to balance and strength both at rest and following activity.
Conclusion
This study assessed the short-term effects of performing a fatiguing walking activity on specific physiological measures, balance and falls risk for individuals from 30 to 79 years of age. Overall, fatigue only affected older adults (60–79 years), who exhibited notable differences in simple reaction time, increased postural sway and a higher falls risk compared to the younger individuals (i.e., 30–39 years). However, the significant increase in overall falls risk was only seen in the oldest individuals (70–79 years). While those in the 60–69 age group showed increases in reaction times and postural sway, the sum of the physiological changes was not enough to lead to an increase in falls risk. As most falls occur when moving and/or fatigued, assessing falls risk and balance following activity may provide a better insight as to overall risk.
Acknowledgments
Funding to support this study was provided by the National Institute of Health (NIA R21 Grant No: 1R21AG037123-01A1, PI Vinik)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berg WP, Alessio HM, Mills EM, Tong C. Circumstances and consequences of falls in independent community-dwelling older adults. Age and Ageing. 1997;26:261–268. doi: 10.1093/ageing/26.4.261. [DOI] [PubMed] [Google Scholar]
- 2.Rubenstein L. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006;35:37–41. doi: 10.1093/ageing/afl084. [DOI] [PubMed] [Google Scholar]
- 3.Tideiksaar R. Falls in Older Persons: Prevention and Management. 2nd. Baltimore: Health Professions Press; 1998. [Google Scholar]
- 4.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. The New England Journal Of Medicine. 1988;319(26):1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 5.Helbostad J, Sturnieks D, Menant J, Delbaere K, Lord S, Pijnappels M. Consequences of lower extremity and trunk muscle fatigue on balance and functional tasks in older people: A systematic literature review. BMC Geriatrics. 2010;10(1):56. doi: 10.1186/1471-2318-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masud T, Morris R. Epidemiology of falls. Age Ageing. 2001;30:3–7. doi: 10.1093/ageing/30.suppl_4.3. [DOI] [PubMed] [Google Scholar]
- 7.Enoka RM. Mechanisms of Muscle Fatigue: Central Factors and Task Dependency. Journal of Electromyography and Kinesiology. 1995;5(3):141–149. doi: 10.1016/1050-6411(95)00010-w. [DOI] [PubMed] [Google Scholar]
- 8.Gandevia SC, Enoka RM, McComas AJ, Stuart DG, Thomas CK. Neurobiology of muscle fatigue. Advances and issues. Advances In Experimental Medicine And Biology. 1995;384:515–525. doi: 10.1007/978-1-4899-1016-5_39. [DOI] [PubMed] [Google Scholar]
- 9.Taylor JL, Gandevia SC. A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J Appl Biomech. 2008;104:542–550. doi: 10.1152/japplphysiol.01053.2007. [DOI] [PubMed] [Google Scholar]
- 10.Caron O. Effects of local fatigue of the lower limbs on postural control and postural stability in standing posture. Neuroscience Letters. 2003;340(2):83–86. doi: 10.1016/s0304-3940(02)01455-6. [DOI] [PubMed] [Google Scholar]
- 11.Corbeil P, Blouin JS, Begin F, Nougier V, Teasdale N. Perturbation of the postural control system induced by muscular fatigue. Gait Posture. 2003;18(2):92–100. doi: 10.1016/s0966-6362(02)00198-4. [DOI] [PubMed] [Google Scholar]
- 12.Springer BK, Pincivero DM. The effects of localized muscle and whole-body fatigue on single-leg balance between healthy men and women. Gait & Posture. 2009;30(1):50–54. doi: 10.1016/j.gaitpost.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Egerton T, Brauer SG, Cresswell AG. The immediate effect of physical activity on standing balance in healthy and balance-impaired older people. Australasian journal on ageing. 2009;28(2):93–96. doi: 10.1111/j.1741-6612.2009.00350.x. [DOI] [PubMed] [Google Scholar]
- 14.Egerton T, Brauer SG, Cresswell AG. Fatigue after physical activity in healthy and balance-impaired elderly. Journal of aging and physical activity. 2009;17(1):89–105. doi: 10.1123/japa.17.1.89. [DOI] [PubMed] [Google Scholar]
- 15.Hatton AL, Menant JC, Lord SR, Lo JCM, Sturnieks DL. The effect of lower limb muscle fatigue on obstacle negotiation during walking in older adults. Gait & Posture. 2013;37(4):506–510. doi: 10.1016/j.gaitpost.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Egerton T, Brauer SG, Cresswell AG. Changes in stepping response to lateral perturbations immediately following a single bout of physical activity. Physiother Res Int. 2011;16(3):141–150. doi: 10.1002/pri.490. [DOI] [PubMed] [Google Scholar]
- 17.Granacher U, Wolf I, Wehrle A, Bridenbaugh S, Kressig R. Effects of muscle fatigue on gait characteristics under single and dual-task conditions in young and older adults. J Neuroeng Rehabil. 2010;7:56. doi: 10.1186/1743-0003-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pereira M, Goncalves M. Effects of fatigue induced by prolonged gait when walking on the elderly. Human Mov. 2011;12:242–247. [Google Scholar]
- 19.Yoshino K, Motoshige T, Araki T, Matsuoka K. Effect of prolonged free-walking fatigue on gait and physiological rhythm. J Biomech. 2004;37:1271–1280. doi: 10.1016/j.jbiomech.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 20.Adlerton A, Moritz U. How does calf-muscle fatigue and age affect vibration-perturbed one-leg stance? Advances in Physiotherapy. 2001;3:179–187. [Google Scholar]
- 21.Hunter SK, Critchlow A, Shin I-S, Enoka RM. Fatigability of the elbow flexor muscles for a sustained submaximal contraction is similar in men and women matched for strength. J Appl Physiol. 2004;96(1):195–202. doi: 10.1152/japplphysiol.00893.2003. [DOI] [PubMed] [Google Scholar]
- 22.Tejin Y, Bonnie Schlinder D-L, Erin EG, Sandra KH. Age-related muscle fatigue after a low-force fatiguing contraction is explained by central fatigue. Muscle & Nerve. 2008;37(4):457–466. doi: 10.1002/mus.20969. [DOI] [PubMed] [Google Scholar]
- 23.Forestier N, Teasdale N, Nougier V. Alteration of the position sense at the ankle induced by muscular fatigue in humans. Med Sci Sports Exerc. 2002;34(1):117–122. doi: 10.1097/00005768-200201000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Pline K, Madigan M, Nussbaum M, Grange R. Lumbar extensor fatigue and circumferential ankle pressure impair ankle joint motion sense. Neurosci Lett. 2005;390:9–14. doi: 10.1016/j.neulet.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 25.Vuillerme N, Boisgontier M. Muscle fatigue degrades force sense at the ankle joint. Gait & Posture. 2008;28(3):521–524. doi: 10.1016/j.gaitpost.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Close JCT, Lord SL, Menz HB, Sherrington C. What is the role of falls? Best Practice & Research Clinical Rheumatology. 2005;19(6):913–935. doi: 10.1016/j.berh.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn PA. Predictors of future falls in Parkinson disease. Neurology. 2010;75(2):116–124. doi: 10.1212/WNL.0b013e3181e7b688. [DOI] [PubMed] [Google Scholar]
- 28.Rojas-Fernandez C, Dadfar F, Wong A, Brown SG. Use of fall risk increasing drugs in residents of retirement villages: a pilot study of long term care and retirement home residents in Ontario, Canada. BMC research notes. 2015;8:568. doi: 10.1186/s13104-015-1557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lord SR, Menz HB, Tiedemann A. A physiological profile approach to falls risk assessment and prevention. Physical Therapy. 2003;83(3):237–252. [PubMed] [Google Scholar]
- 30.Simoneau M, Bégin F, N T. The effects of moderate fatigue on dynamic balance control and attentional demands. J Neuroeng Rehabil. 2006;3:22. doi: 10.1186/1743-0003-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fozard JL, Vercryssen M, Reynolds SL, Hancock PA, Quilter RE. Age differences and changes in reaction time: The baltimore longitudinal study of aging. Journal of Gerontology. 1994;49:179–189. doi: 10.1093/geronj/49.4.p179. [DOI] [PubMed] [Google Scholar]
- 32.Menz HB, Lord SR, Fitzpatrick RC. A structural equation model relating impaired sensorimotor function, fear of falling and gait patterns in older people. Gait & Posture. 2007;25(2):243–249. doi: 10.1016/j.gaitpost.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Helbostad J, Leirfall S, Moe-Nilssen R, Sletvold O. Physical fatigue affects gait characteristics in older persons. J Gerontol A Biol Sci Med Sci. 2007;62:1010–1015. doi: 10.1093/gerona/62.9.1010. [DOI] [PubMed] [Google Scholar]
- 34.Nagano H, James L, Sparrow W, Begg R. Effects of walking-induced fatigue on gait function and tripping risks in older adults. J NeuroEngineering Rehabil. 2014;11(1):1–7. doi: 10.1186/1743-0003-11-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vuillerme N, Burdet C, Isableu B, Demetz S. The magnitude of the effect of calf muscles fatigue on postural control during bipedal quiet standing with vision depends on the eye-visual target distance. Gait & Posture. 2006;24(2):169–172. doi: 10.1016/j.gaitpost.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Allman B, Cheng A, Rice C. Quadriceps fatigue caused by catchlike-inducing trains is not altered in old age. Muscle Nerve. 2004;30:743–751. doi: 10.1002/mus.20161. [DOI] [PubMed] [Google Scholar]
- 37.Nardone A, Tarantola J, Galante M, Schieppati M. Time course of stabilometric changes after a strenuous treadmill exercise. Archives Of Physical Medicine And Rehabilitation. 1998;79(8):920–924. doi: 10.1016/s0003-9993(98)90088-0. [DOI] [PubMed] [Google Scholar]
- 38.Nardone A, Tarantola J, Giordano A, Schieppati M. Fatigue effects on body balance. Electroencephalogr Clin Neurophysiol. 1997;105:309–320. doi: 10.1016/s0924-980x(97)00040-4. [DOI] [PubMed] [Google Scholar]
- 39.Vuillerme N, Sporbert C, Pinsault N. Postural adaptation to unilateral hip muscle fatigue during human bipedal standing. Gait & Posture. 2009;30(1):122–125. doi: 10.1016/j.gaitpost.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Wilson EL, Madigan ML, Davidson BS, Nussbaum MA. Postural strategy changes with fatigue of the lumbar extensor muscles. Gait & Posture. 2006;23(3):348–354. doi: 10.1016/j.gaitpost.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Dalton B, Power G, Paturel J, Rice C. Older men are more fatigable than young when matched for maximal power and knee extension angular velocity is unconstrained. AGE. 2015;37(3):1–16. doi: 10.1007/s11357-015-9790-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Justice JN, Mani D, Pierpoint LA, Enoka RM. Fatigability of the dorsiflexors and associations among multiple domains of motor function in young and old adults. Experimental Gerontology. 2014;55(0):92–101. doi: 10.1016/j.exger.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Julius LM, Brach JS, Wert DM, VanSwearingen JM. Perceived Effort of Walking: Relationship With Gait, Physical Function and Activity, Fear of Falling, and Confidence in Walking in Older Adults With Mobility Limitations. Physical Therapy. 2012;92(10):1268–1277. doi: 10.2522/ptj.20110326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richardson CA, Glynn NW, Ferrucci LG, Mackey DC. Walking Energetics, Fatigability, and Fatigue in Older Adults: The Study of Energy and Aging Pilot. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2015;70(4):487–494. doi: 10.1093/gerona/glu146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tirosh O, Sparrow W. Age and walking speed effect on muscle recruitment in gait termination. Gait Posture. 2005;21:279–288. doi: 10.1016/j.gaitpost.2004.03.002. [DOI] [PubMed] [Google Scholar]