Abstract
Cervical spinal cord injury (SCI) interrupts descending neural drive to phrenic motoneurons causing diaphragm muscle (DIAm) paralysis. Recent studies using a well-established model of SCI, unilateral spinal hemisection of the C2 segment of the cervical spinal cord (SH), provide novel information regarding the molecular and cellular mechanisms of functional recovery after SCI. Over time post-SH, gradual recovery of rhythmic ipsilateral DIAm activity occurs. Recovery of ipsilateral DIAm electromyogram (EMG) activity following SH is enhanced by increasing brain-derived neurotrophic factor (BDNF) in the region of the phrenic motoneuron pool. Delivery of exogenous BDNF either via intrathecal infusion or via mesenchymal stem cells engineered to release BDNF similarly enhance recovery. Conversely, recovery after SH is blunted by quenching endogenous BDNF with the fusion-protein TrkB-Fc in the region of the phrenic motoneuron pool or by selective inhibition of TrkB kinase activity using a chemical-genetic approach in TrkBF616A mice. Furthermore, the importance of BDNF signaling via TrkB receptors at phrenic motoneurons is highlighted by the blunting of recovery by siRNA-mediated downregulation of TrkB receptor expression in phrenic motoneurons and by the enhancement of recovery evident following virally-induced increases in TrkB expression specifically in phrenic motoneurons. BDNF/TrkB signaling regulates synaptic plasticity in various neuronal systems, including glutamatergic pathways. Glutamatergic neurotransmission constitutes the main inspiratory-related, excitatory drive to motoneurons, and following SH, spontaneous neuroplasticity is associated with increased expression of ionotropic N-methyl-D-aspartate (NMDA) receptors in phrenic motoneurons. Evidence for the role of BDNF/TrkB and glutamatergic signaling in recovery of DIAm activity following cervical SCI is reviewed.
Keywords: Main Heading, Neurotrophin and Glutamatergic Signaling in Phrenic Motoneurons
1. Introduction
Cervical spinal cord injuries (SCIs) account for over 50% of all injuries in the United States each year (National Spinal Cord Injury Statistical Center, 2015). SCIs cause significant diaphragm muscle (DIAm) paralysis, an increase in the use of mechanical ventilation to sustain breathing, and leads to significant morbidity and mortality (Devivo, 2012). Respiratory complications (mostly related to inadequate pulmonary clearance and subsequent infection) are the primary cause of death after SCI (Brown et al., 2006; Linn et al., 2000; Winslow and Rozovsky, 2003), likely due to a deficiency in DIAm force generating capacity needed for higher-force expulsive behaviors. Thus, recovery of DIAm activity and the ability to generate the forces necessary for ventilatory and non-ventilatory behaviors is important to ameliorate the morbidity and mortality associated with cervical SCI. Recent studies show that neurotrophins play a significant role in modulating functional neuroplasticity following SCI (Weishaupt et al., 2012). In particular, brain-derived neurotrophic factor (BDNF) acting through the tropomyosin-related kinase receptor subtype B (TrkB) is both necessary and sufficient for spontaneous recovery of rhythmic DIAm activity after high cervical SCI (Gransee et al., 2013, 2015; Mantilla et al., 2013a; Mantilla et al., 2014a; Sieck and Mantilla, 2009). Evidence for a role of BDNF/TrkB and glutamatergic signaling in phrenic motoneurons in functional recovery after cervical SCI will be reviewed in the following sections (Fig. 1).
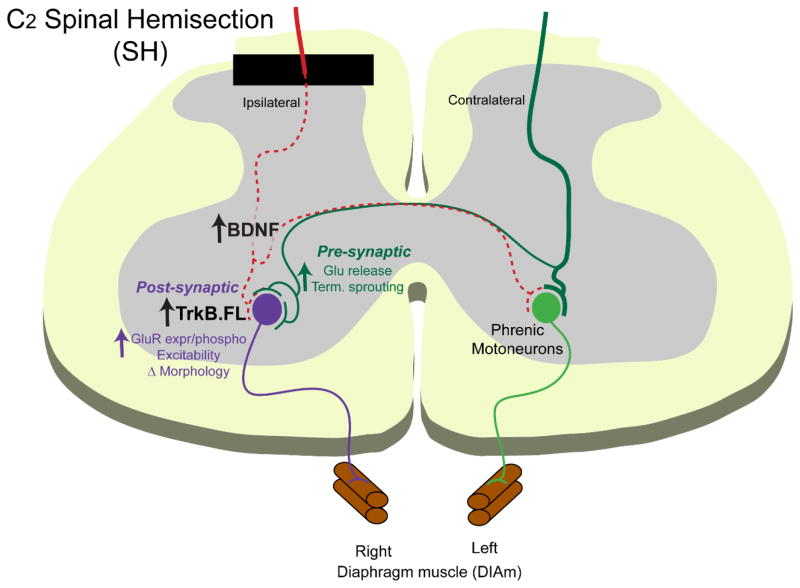
Figure 1.
A commonly used model of spinal cord injury (SCI), unilateral spinal hemisection (SH) at the C2 segment of the cervical spinal cord abolishes descending neural drive to phrenic motoneurons interrupting ipsilateral phrenic motoneuron activity and results in DIAm paralysis. Increasing BDNF/TrkB.FL signaling at phrenic motoneurons strengthens synaptic plasticity following SH and enhances recovery of ipsilateral DIAm activity. Pre-synaptically, (shown in green) BDNF may promote axonal sprouting of spared, contralateral excitatory (mostly glutamatergic) descending inputs, enabling glutamate release and recovery of rhythmic DIAm activity. Post-synaptically, (shown in purple) BDNF/TrkB signaling may impact glutamatergic receptor expression or activity (e.g., via phosphorylation), motoneuron morphology (e.g., increased dendritic branching) and increase motoneuron excitability, thus enhancing synaptic transmission and DIAm recovery.
2. Recovery of Rhythmic Diaphragm Activity after Cervical Spinal Cord Injury
Respiratory deficits following cervical SCI have been studied using various animal models that impair ventilation without the need for chronic mechanical ventilatory support, including midline (el-Bohy et al., 1998; Golder et al., 2011; Lane et al., 2012) vs. unilateral contusion (Alvarez-Argote et al., 2015; Baussart et al., 2006; Nicaise et al., 2013; Nicaise et al., 2012a; Nicaise et al., 2012b), or transection (Imagita et al., 2015). A well-established model of SCI, spinal hemisection of the C2 segment of the cervical spinal cord (SH) abolishes descending neural drive to ipsilateral phrenic motoneurons, transiently interrupting ipsilateral phrenic motoneuron activity and inducing DIAm paralysis (Fuller et al., 2006; Goshgarian, 2003; Gransee et al., 2013, 2015; Mantilla et al., 2013a; Mantilla et al., 2013b; Porter, 1895). However, some studies report long-lasting disruption of ipsilateral phrenic nerve or DIAm activity lasting up to 12 weeks in some animals following SH (Fuller et al., 2008; Vinit et al., 2006).
Using chronic electromyogram (EMG) recordings in SH animals, a physiologically complete lesion can be verified by the absence of ipsilateral DIAm EMG activity immediately after SH and 3 days after injury (Sieck and Mantilla, 2009). Recovery of rhythmic ipsilateral DIAm activity after SH is evident in a subset of animals, such that the proportion of adult male rats displaying recovery is ~20% at 7 days after SH and ~40% at 14 and 42 days after SH (Gransee et al., 2013, 2015; Mantilla et al., 2013a) (Fig. 2). The amplitude of the ipsilateral DIAm EMG activity during eupnea is relatively low compared to pre-injury levels, consistent with a limited contribution of the ipsilateral side to overall ventilatory activity post-SH (Gransee et al., 2013, 2015; Mantilla et al., 2013a; Mantilla et al., 2013b). Midcervical contusion injuries reportedly reduce ventilation and DIAm EMG activity (el-Bohy et al., 1998; Nicaise et al., 2013), although the time course of such reductions and recovery has not been systematically explored in most studies. In a recent study, we did not find consistent changes in ventilation or diaphragm EMG activity following unilateral C3 or C5 contusion (Alvarez-Argote et al., 2015). Of note, the amplitude of ipsilateral phrenic nerve or DIAm EMG activity increases progressively after SH (Fuller et al., 2006; Gransee et al., 2013, 2015; Mantilla et al., 2013a; Mantilla et al., 2014a; Mantilla et al., 2013b), and thus this model has been used to explore neuroplasticity at the ipsilateral phrenic motoneuron pool, which include strengthening of contralateral spared pathways over time through pre- and/or post-synaptic mechanisms.
Figure 2.
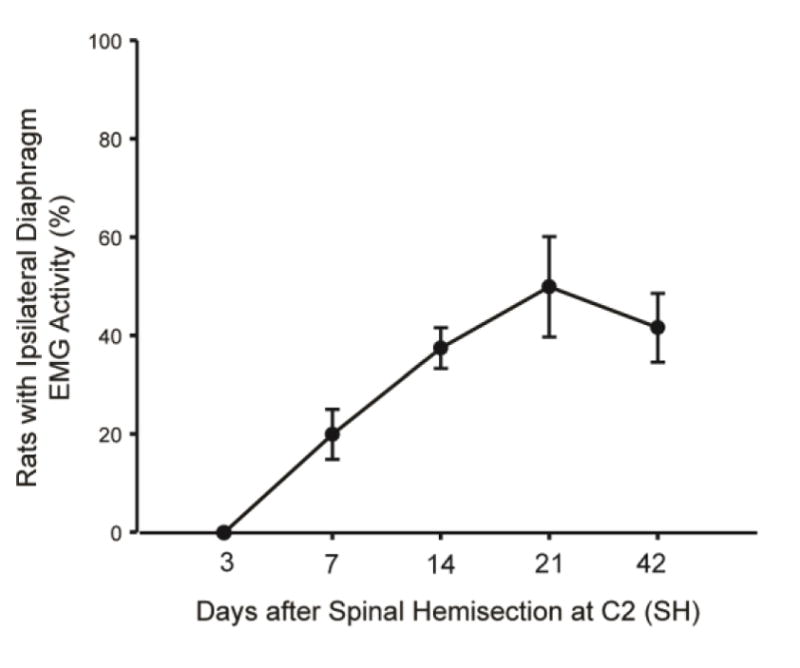
Recovery of rhythmic ipsilateral diaphragm (DIAm) EMG activity after spinal hemisection at C2 (SH) is evident over time post-injury. The percentage of rats displaying recovery of ipsilateral DIAm activity during eupnea is shown at 7, 14, 21 and 42 days after SH (n=6–12 adult male Sprague-Dawley rats/group). Data are summarized from (Gransee et al., 2013, 2015; Mantilla et al., 2012; Mantilla et al., 2013a; Mantilla et al., 2013b).
3. Diaphragm Motor Unit Classification and Recruitment
Motor units are the final element of motor control of skeletal muscles and include a motoneuron and the group of muscle fibers it innervates. Motor units vary in contractile and fatigue properties (Burke et al., 1971). The DIAm comprises all motor unit types (slow-twitch type S, fast twitch-fatigue resistant type FR, fast twitch-fatigue intermediate type FInt and fast twitch-fatigable type FF) (Enad et al., 1989; Fournier and Sieck, 1988; Sieck et al., 1989). Recruitment of DIAm motor units determines the range of motor behaviors that can be accomplished; from lower force, rhythmic ventilatory behaviors to higher-force non-ventilatory behaviors necessary for pulmonary clearance (Greising et al., 2013; Mantilla et al., 2011; Sieck, 1991; Sieck and Fournier, 1989). After cervical SCI, the extensive white matter tract damage and phrenic motoneuron loss prevents sufficient recruitment of DIAm motor units resulting in an inability to sustain ventilation. Consequently, individuals with cervical SCIs often rely on mechanical ventilators, and endure recurrent infections which ultimately cause reduced life expectancy (Devivo, 2012; Winslow and Rozovsky, 2003). For these reasons, interventions designed to enhance DIAm motor unit recruitment after injury would significantly improve the quality of life for individuals with cervical SCI by restoring the ability to generate adequate ventilation as well as the expulsive forces necessary for pulmonary clearance.
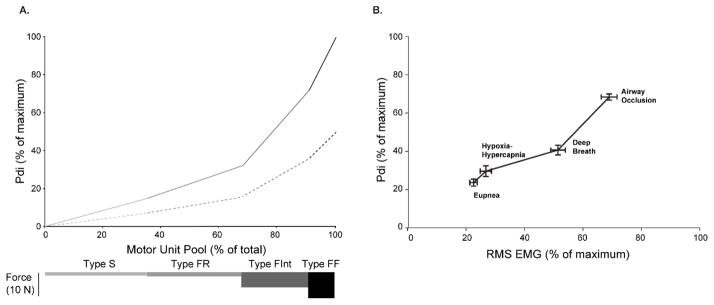
Previous studies have estimated the percentage of the DIAm motor unit pool that needs to be recruited during different ventilatory and higher-force non-ventilatory behaviors (e.g., sneezing, airway occlusion) in various species (Greising et al., 2013; Mantilla et al., 2010; Sieck, 1991, 1994; Sieck and Fournier, 1989) (Fig. 3A). This model of DIAm motor unit recruitment is based on an orderly recruitment based primarily on size and corresponding intrinsic electrophysiological properties of motoneurons (Seven et al., 2014). As such, motor units are recruited in a predictable order: type S, followed by type FR, FInt and lastly, type FF units. This model uses transdiaphragmatic pressures (Pdi) to estimate DIAm forces generated during ventilatory behaviors (e.g. eupnea, exposure to hypoxia (10% O2)-hypercapnia (5% CO2), spontaneous deep breaths), and non-ventilatory behaviors (e.g. airway occlusion, coughing or sneezing). The DIAm has a significant reserve for force generation in rodents, cats and humans (Gill et al., 2015; Greising et al., 2013; Mantilla et al., 2010; Sieck, 1991, 1994; Sieck and Fournier, 1989). For example, in relation to the Pdi generated by bilateral phrenic nerve stimulation (Pdimax), during quiet rhythmic breathing (eupnea) Pdi ranges from 10% to 27% of Pdimax; summarized Pdi data specifically from rats is shown in Figure 3B (Gill et al., 2015; Mantilla et al., 2010). Thus, eupneic Pdi may be achieved by the recruitment of type S and FR motor units alone. Stimulating breathing by exposure to hypoxia–hypercapnia increases Pdi generated during ventilatory behaviors; however, Pdi never exceeds 36% of Pdimax across several species. Conversely, the Pdi generated during higher force, non-ventilatory behaviors is significantly greater with Pdi generated during sustained airway occlusion ranging from 43–70% of Pdimax. Accordingly, this level of Pdi requires additional recruitment of type FInt and some type FF motor units. Only during infrequent expulsive behaviors (e.g., coughing or sneezing) is full recruitment of DIAm motor units necessary.
Figure 3.
A. Model of motor unit recruitment for the rat DIAm. Motor unit recruitment during ventilatory and higher-force non-ventilatory behaviors was modeled based on an orderly recruitment of motor units (type S followed by FR, FInt, and FF) and the forces generated by motor units of each type (bars below). Models were generated for the intact case (solid line) and the case of only 50% motor units (dashed line). B. Transdiaphragmatic pressures (Pdi) generated during various motor behaviors were normalized to maximum generated by bilateral phrenic nerve stimulation. Relative Pdi measurements and peak DIAm EMG RMS amplitude (normalized to maximum) show parallel changes across motor behaviors of varying force (Pearson r2=0.92; p<0.0001; n=14 adult male Sprague-Dawley rats). Data are summarized from (Gill et al., 2015; Mantilla et al., 2010).
In a recent study, we evaluated the impact of complete DIAm hemiparalysis induced by unilateral phrenic denervation in rats. Consistent with other models of DIAm hemiparalysis such as SH (Golder et al., 2001; Goshgarian et al., 1986; Mantilla et al., 2013a), unilateral denervation did not affect the Pdi generated during eupnea or even during exposure to hypoxia-hypercapnia and did not affect overall ventilation (as determined by blood gas levels) (Gill et al., 2015). As expected, following unilateral DIAm paralysis, neural drive to the contralateral DIAm increased significantly during ventilatory behaviors (eupnea or hypoxia-hypercapnia) to compensate for the loss of force contributed by the paralyzed DIAm. These results indicate that the contralateral DIAm force reserve is sufficient to sustain ventilation in the rat even after unilateral DIAm hemiparalysis. In contrast, we found that higher force, non-ventilatory DIAm motor behaviors requiring greater than ~50% Pdimax were compromised. These results indicate that essential airway clearance behaviors (e.g., coughing, sneezing) may be affected when the force reserve of the whole DIAm is compromised and that increased neural drive to the intact DIAm is insufficient to restore these behaviors following injury.
4. The Extent of Recovery of Diaphragm Activity
Recovery of rhythmic ipsilateral DIAm activity following SH depends on strengthening the residual contralateral premotor input to ipsilateral phrenic motoneurons via latent cross-phrenic pathways (Goshgarian, 2003; Mantilla and Sieck, 2003; Sieck and Mantilla, 2009). While progressive recovery of rhythmic DIAm activity occurs over time following SH, the extent of recovery is minimal and never returns to pre-injury levels (Fuller et al., 2006; Gransee et al., 2013, 2015; Mantilla et al., 2013a; Mantilla et al., 2013b). In a series of rat studies, DIAm root-mean-square (RMS) EMG amplitude during eupnea was used to delineate the degree of recovery after SH (Gransee et al., 2013, 2015; Mantilla et al., 2013a; Mantilla et al., 2013b). All values were normalized to the pre-injury eupneic value for the same animal. Animals that showed recovery of ipsilateral DIAm activity after SH achieved only a fraction (~20%) of the pre-SH eupneic DIAm RMS EMG amplitude by 14 days after injury. Thus, recruitment of a greater proportion of the contralateral phrenic motoneuron pool would be necessary to sustain ventilation resulting in a right-ward shift of the DIAm motor unit recruitment curve (Mantilla and Sieck, 2011) (Fig. 3A). Following acute DIAm hemiparalysis induced by unilateral phrenic denervation (Gill et al., 2015), contralateral DIAm RMS EMG increased only during ventilatory behaviors, but not during higher-force non-ventilatory behaviors. Accordingly, recruitment of all DIAm motor units after SH may not be possible based on contralateral synaptic input alone, unless it is enhanced considerably over time after injury.
While the mechanical properties of rat DIAm motor units remain mostly unchanged at 14 days after SH beyond transient effects immediately post-injury (Gill et al., 2014), it should be noted that a modest selective atrophy of DIAm fibers does occur but only after 6 weeks (Mantilla et al., 2013b). By 14 days after SH, the cross-sectional areas of all types of DIAm fibers are not different from pre-injury values (Miyata et al., 1995). In a study examining the long term effects of SH on DIAm fiber cross-sectional areas, we found that after 6 weeks the cross-sectional area of type IIx and/or IIb DIAm fibers was reduced by 20% and maximal DIAm force was reduced by 40% (Mantilla et al., 2013b). Thus, it will be important to interpret the results of functional recovery after cervical SCI in light of associated changes specific to muscle fiber and motor unit type.
5. Role of Neurotrophins in Motoneuron Neuroplasticity
Neurotrophic factors play a significant role in modulating neuroplasticity in response to CNS injury (Weishaupt et al., 2012). Neurotrophins promote cell survival, axonal growth, and enhance synaptic plasticity in motoneurons (Huang and Reichardt, 2003; Mantilla and Sieck, 2008). Brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), neurotrophin-3 (NT-3) and NT-4 all belong to the neurotrophin family. These neurotrophins signal through interactions with high affinity tropomyosin-related kinase (Trk) receptors that are neurotrophin specific: TrkA for NGF, TrkB for BDNF and NT-4, and TrkC for NT-3; these neurotrophins also interact with a lower affinity p75 receptor. Neurotrophin signaling through these receptors is important for neuroplasticity during development and in the adult CNS, and there is widespread expression of neurotrophins and their receptors, including in motor systems (Garcia et al., 2010; Mantilla and Sieck, 2008). Other trophic factor families may also contribute to plasticity following SCI including glial derived neurotrophic factor, ciliary neurotrophic factor or leukemia inhibitory factor (Harvey et al., 2015; Mantilla and Sieck, 2008), although results are confounded by differences in the site (injury, above, below or remote) and timing of administration as well as the delivery strategies (e.g., viral vs. cell-based) or routes (systemic, intrathecal, peripheral, intramuscular).
Neurotrophins may contribute to recovery of DIAm activity after SH via several possible mechanisms that are both pre- and post-synaptic at the motoneuron level (Fig. 1). Such mechanisms include: 1) enhancement of synaptic input from descending premotor pathways (via increased sprouting of spared (Bregman et al., 1997; Xu et al., 1995), mostly contralateral, axons or regeneration of injured descending projections), 2) strengthening of preexisting synaptic connections (e.g., excitatory neurotransmitter release; (Carvalho et al., 2008)) to motoneurons, and 3) post-synaptic changes that increase excitability of motoneurons (Mantilla et al., 2012) (e.g., changes in morphology such as decreased soma surface area or changes in neurotransmitter receptor expression).
There is now convincing evidence that BDNF availability at motoneuron pools caudal to the injury site is critically important for motoneuron plasticity and recovery after SCI. Indeed, following SH in rats, BDNF administration to region of the phrenic motoneuron pool enhances recovery of ipsilateral DIAm EMG activity when delivered by intrathecal infusion (Mantilla et al., 2013a) or stem cell transplantation (Gransee et al., 2015). Quenching endogenous BDNF by intrathecal infusion of the fusion protein TrkB-Fc in rats (Mantilla et al., 2013a) and inhibiting TrkB kinase activity using chemical-genetic approach in TrkBF616A mice (Mantilla et al., 2014a) prevent spontaneous recovery. Although the specific site(s) of action of BDNF signaling have not been elucidated, several lines of evidence highlight the critical role of signaling at motoneurons in enhancing recovery of rhythmic DIAm EMG activity following SH (Gransee et al., 2013, 2015; Mantilla et al., 2013a; Mantilla et al., 2014a).
Promoting phrenic motoneuron BDNF signaling via the full length TrkB (TrkB.FL) receptor may enhance motoneuron excitability via serotonergic (5-HTR) and/or glutamatergic (e.g., N-methyl-D-aspartate, NMDA) signaling. Serotonergic pathways play a key role in phrenic motor plasticity and recovery from SCI (Basura et al., 2001; Fuller et al., 2005; Hadley et al., 1999; Zhou et al., 2001). Glutamatergic pathways are also essential in the neuroplasticity of phrenic motoneuron output (Alilain and Goshgarian, 2008; McGuire et al., 2008). After SH, 5-HTR and NMDA receptor expression increases within phrenic motoneurons, and the time course of these changes corresponds with the return of spontaneous recovery of rhythmic ipsilateral DIAm activity (Mantilla et al., 2012). Indeed, phrenic motoneuron expression of 5-HTR2a receptors increased 14 days after injury. By 21 days after injury there was a decrease in AMPA receptor expression coupled with an increase in NMDA receptor expression in adult rats (Mantilla et al., 2012), consistent with the time course of recovery (Fig. 4). Future studies should directly examine whether changes in phrenic motoneuron 5-HTR or NMDA receptor expression are modulated by BDNF/TrkB signaling and the contribution of such modulation to functional recovery after SCI.
Figure 4.
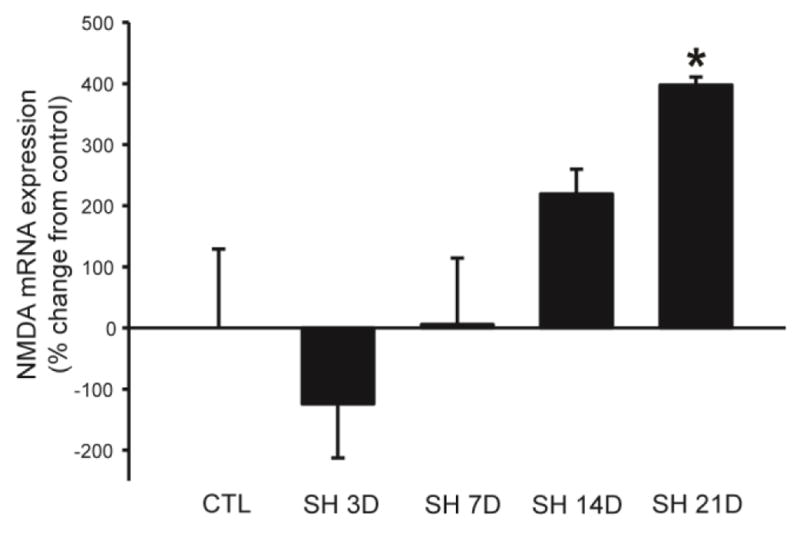
Glutamatergic NMDA receptor expression in microdissected phrenic motoneurons at 7, 14, and 21 days after SH. Data are summarized from (Mantilla et al., 2012) and presented as percent change in mRNA expression from control (relative to the reference gene ribosomal protein S16) across animals (n=6–8 adult male Sprague-Dawley rats/group; mean ± SE). *, p<0.05 vs. other time points (one-way ANOVA; post hoc Tukey-Kramer HSD test).
6. BDNF/TrkB Signaling in Phrenic Motoneurons
The central hypothesis guiding our work over the last several years is that recovery of rhythmic DIAm activity after SH may be altered by modulating BDNF/TrkB signaling in phrenic motoneurons after SH. BDNF signaling in the region of the phrenic motoneuron pool is important in the response to respiratory perturbations such as exposure to intermittent hypoxia (Baker-Herman et al., 2004; Golder et al., 2008; Satriotomo et al., 2012). It is our view that targeting specific neuronal populations (e.g., phrenic motoneurons) is essential considering the potentially adverse effects associated with systemic BDNF administration. Indeed, the therapeutic use of intrathecal or systemic delivery of neurotrophins was explored in patients with amyotrophic lateral sclerosis (Beck et al., 2005; Ochs et al., 2000; The BDNF Study Group, 1999) to promote motoneuron survival and mitigate disease progression. Unfortunately, the findings from these studies were disappointing in that patients receiving intrathecal BDNF (from 25μg/day up to 1000 μg/day) exhibited dose-limiting side effects beyond 150 μg/day. Intrathecal BDNF doses in the lower range were expected to produce cerebrospinal fluid levels of ~30 ng/ml (Dittrich et al., 1996). Patients receiving systemic doses (100 μg/kg) reported significant sensory disturbances, agitation and behavioral responses. That being the case, the therapeutic role of BDNF/TrkB signaling in neuroplasticity and recovery of rhythmic DIAm activity after SH may still be exploited locally at the phrenic motoneurons by using a targeted approach (Gransee et al., 2013, 2015).
6.1. Role of BDNF in Recovery of Rhythmic DIAm Activity after SH
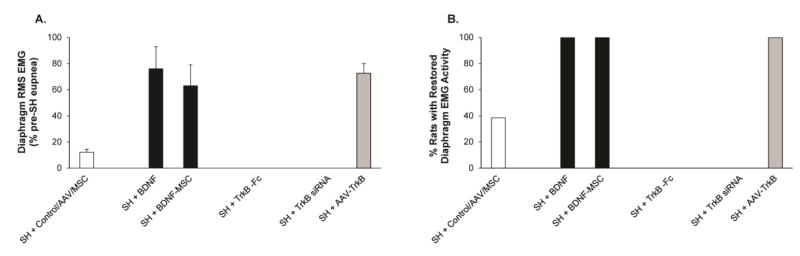
Several studies indicate that neurotrophin (Dougherty et al., 2000; Widenfalk et al., 2001) and Trk receptor expression (King et al., 2000) changes following SCI and that increasing neurotrophin signaling ameliorates functional deficits in various models of SCI (Boyce et al., 2012; Weishaupt et al., 2012). In rats following SH, increasing BDNF availability by intrathecal delivery at the phrenic motoneuron pool enhances the recovery of rhythmic ipsilateral DIAm EMG activity (Fig. 5A) (Mantilla et al., 2013a). Indeed, at 14 days after SH, all rats receiving intrathecal BDNF (180 ng/day) treatment showed recovery of ipsilateral DIAm activity. Furthermore, rats that received BDNF treatment after SH displayed increased DIAm RMS EMG amplitude during eupnea (~75% of the pre-SH RMS EMG value vs. ~5% in untreated rats after SH).
Figure 5.
A. Extent of recovery of rhythmic ipsilateral DIAm EMG activity during eupnea at 14 days post-SH (n=6–12 adult male Sprague-Dawley rats/group). Peak DIAm RMS EMG amplitude was normalized to the pre-SH RMS EMG amplitude for the same animal. Control data include summary of untreated, vehicle-treated, AAV-GFP and wild type MSC treatments. B. Proportion of animals displaying recovery of ipsilateral DIAm EMG activity during eupnea 14 days post-SH. In all animals, absence of DIAm EMG activity was verified at 3 days after SH. Intrathecal BDNF treatment and intraspinal transplantation of mesenchymal stem cells engineered to produced BDNF (BDNF-MSC) increased the extent of recovery (A) and the proportion of animals displaying ipsilateral DIAm EMG activity (B). In contrast, removal of endogenous BDNF with the fusion protein TrkB-Fc prevented recovery. Modifying TrkB receptor expression in phrenic motoneurons by intrapleural TrkB siRNA treatment prevented recovery whereas TrkB-AAV treatment increased the extent of recovery (A) and the proportion of animals displaying recovery (B). Data are summarized from (Gransee et al., 2013, 2015; Mantilla et al., 2013a)
In addition to intrathecal delivery, mesenchymal stem cells (MSCs) may be genetically engineered to produce trophic factors such as BDNF and can thus be used to administer trophic factors locally within the CNS. Furthermore, MSCs may provide regenerative and neurorestorative therapy following SCI because of their multipotent fate (Chopp et al., 2000) and MSCs can be engineered for trophic factor release at high levels for local delivery into the injured spinal cord in order to provide continued release of BDNF (Rooney et al., 2009). In a recent study (Gransee et al., 2015), we localized delivery of BDNF using intraspinal injections near the site of SH injury (C2 level) in rats by utilizing bone marrow-derived adult rat MSCs engineered to produce releasable BDNF by retroviral treatment. All SH animals receiving BDNF-MSCs treatment exhibited recovery of ipsilateral DIAm activity by 14 days post-SH (Fig 5B). In contrast, the percentage of SH animals displaying recovery of rhythmic DIAm EMG activity after 14 days post SH that received non-engineered wild type (WT-MSCs) was no different than the recovery for untreated SH animals. Local delivery of BDNF-MSCs also led to a substantial increase in the amplitude of ipsilateral DIAm RMS EMG activity after SH. These findings support the notion that targeting BDNF to phrenic motoneurons is critical to promoting recovery of rhythmic DIAm EMG activity.
It is important to consider that the genetically engineered MSCs provided a lesser amount of BDNF when compared to intrathecal BDNF infusion after SH (Gransee et al., 2015; Mantilla et al., 2013a). The concentration of BDNF-MSCs injected (total injected volume, 4 μL) in vivo (200,000 cells total) in the study by Gransee et al. (2015) released ~24 ng BDNF per day in SH animals. Conversely, chronic BDNF infusion delivered via intrathecal catheter and implanted mini-osmotic pump distributed ~180 ng BDNF per day (Mantilla et al., 2013a). In each case, BDNF administration yielded recovery of rhythmic DIAm EMG activity in all rats at 14 days after SH. The extent of recovery was also similar between the local, lower dose BDNF administration via BDNF-MSCs compared to the higher dose delivered via intrathecal infusion (~60% vs. ~75% of pre-SH DIAm RMS EMG amplitude, respectively). Taken together, these findings support the efficacy of BDNF administration depending on the route of administration (perhaps reflecting issues related to bioavailability). In a clinical setting, using a lower dose of BDNF in human patients may ameliorate the undesirable effects of a higher doses of BDNF on other systems (e.g., sensory or autonomic). A targeted delivery system that increases BDNF/TrkB signaling selectively at motoneurons may overcome such dosing and administration issues, yet retain therapeutic efficacy in human clinical trials.
6.2. Role of TrkB Receptor Activity in Recovery of Rhythmic DIAm Activity after SH
In recent complementary studies, we show that TrkB kinase activity at phrenic motoneurons is necessary and sufficient for spontaneous recovery of rhythmic ipsilateral DIAm activity post-SH (Gransee et al., 2013; Mantilla et al., 2013a; Mantilla et al., 2014a). Targeted delivery of TrkB.FL receptor to phrenic motoneurons using intrapleural delivery of an AAV7 vector enhanced recovery of ipsilateral phrenic motoneuron activity following SH in rats (Gransee et al., 2013). Intrapleural delivery of AAV7-TrkB.FL significantly increased the percentage of animals showing ipsilateral DIAm EMG activity over time such that by 14 days post-SH all animals displayed recovery. Furthermore, ipsilateral DIAm RMS EMG amplitude was ~70% of the pre-SH eupneic value in SH rats receiving AAV7-TrkB.FL treatment compared to ~30% in the untreated SH rats. Motoneurons were targeted utilizing an intrapleural delivery technique (Mantilla et al., 2009) in conjunction with the transduction selectivity of AAV7 (Gransee et al., 2013), which result in evidence of transduction in ~11% of retrogradely-labeled phrenic motoneurons. These results indicate that: 1) retrograde delivery of AAV7 is feasible via intrapleural injection and 2) increasing TrkB.FL expression in phrenic motoneurons via AAV7 is sufficient to enhance recovery of rhythmic DIAm activity after SH.
In contrast, siRNA-induced knock down of TrkB receptor expression in phrenic motoneurons blunts spontaneous recovery of ipsilateral DIAm EMG activity in rats (Mantilla et al., 2013a). TrkB receptor knockdown in phrenic motoneurons was achieved using TrkB siRNA and the effects were compared to animals treated with non-sense siRNA injections. TrkB siRNA administration reduced TrkB mRNA expression ~98% in C6 glioma cells. Daily intrapleural administration of TrkB siRNA resulted in evident retrograde transport to phrenic motoneurons and completely blunted recovery by 14 days post-SH. In contrast, no effect on spontaneous recovery was evident using intrapleural non-sense siRNA treatment. Based on these findings, we subsequently evaluated whether inhibiting TrkB kinase activity would impede recovery of DIAm activity following SH. Utilizing a chemical-genetic approach in TrkBF616A mice (Chen et al., 2005; Greising et al., 2015; Mantilla and Ermilov, 2012; Mantilla et al., 2014b), we found that TrkB kinase activity is essential for the spontaneous recovery of rhythmic ipsilateral DIAm EMG activity following SH (Mantilla et al., 2014a). These genetically-engineered mice (TrkBF616A) express knock-in alleles that permit rapid and selective inhibition of TrkB kinase activity via the phosphoprotein phosphatase 1 inhibitor (PP1) derivative 1NMPP1 (Chen et al., 2005; Greising et al., 2015; Mantilla and Ermilov, 2012; Mantilla et al., 2014b). The TrkBF616A mice develop normally and TrkB kinase activity is only inhibited following 1NMPP1 administration. After SH, two groups of TrkBF616A mice were randomized at 3 days after injury to receive either 11 days of oral 1NMPP1 treatment (25 μM in drinking water) or vehicle treatment (0.3% DMSO in drinking water). At 14 days after SH, ~40% of vehicle-treated mice displayed spontaneous recovery of ipsilateral DIAm activity, results generally consistent with previous studies in rats (Gransee et al., 2013, 2015; Mantilla et al., 2012; Mantilla et al., 2013a). In contrast, only 7% of mice showed spontaneous recovery of rhythmic DIAm EMG activity following inhibition of TrkB kinase activity by oral 1NMPP1 treatment. The considerable reduction in the rate of spontaneous recovery after intrathecal TrkB-Fc or intrapleural TrkB siRNA in rats and after 1NMPP1 treatment in TrkBF616A mice emphasizes the importance of BDNF/TrkB signaling and TrkB kinase activity in facilitating neurotrophin-dependent neuroplasticity following SH.
6.3. Therapeutic Regulation of Phrenic Motoneuron BDNF/TrkB Signaling
Our recent studies examining the role of BDNF/TrkB signaling at phrenic motoneurons in recovery after SH in rodents suggest that there are different windows for therapeutic intervention. Generally similar levels of enhancement of spontaneous recovery of eupneic DIAm activity after SH were evident with AAV-TrkB treatment (delivered 3 weeks pre-SH) (Gransee et al., 2013), with intrathecal BDNF (delivered starting 3 days post-SH) (Mantilla et al., 2013a), and with intraspinal transplantation of MSCs engineered to produce BDNF (delivered at the time of SH) (Gransee et al., 2015). In agreement, disrupting TrkB signaling by intraspinal TrkB-Fc treatment, retrograde delivery of TrkB siRNA to phrenic motoneurons, (Mantilla et al., 2013a) or using 1NMPP1 in TrkBF616A mice (Mantilla et al., 2014a) (all delivered starting 3 days post-SH) similarly prevent spontaneous recovery. These different treatments are expected to result in distinct time courses of therapeutic effects, but in aggregate suggest that enhanced recovery requires increased phrenic motoneuron BDNF/TrkB signaling during a period from 7–14 days post-injury and that recovery does not depend on such signaling during the period immediately following injury (up to 3 days post-SH). Future preclinical studies should directly examine the existence of such therapeutic windows in order to optimize the translational potential of interventions targeting motoneuron BDNF/TrkB signaling in various models of SCI.
7. Role of BDNF/TrkB and Glutamatergic Signaling in Phrenic Motoneurons after SH
The primary excitatory input driving phrenic motoneurons is glutamatergic (McCrimmon et al., 1989). Glutamate NMDA and AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionate) receptors are present on phrenic motoneurons (Chitravanshi and Sapru, 1996; Dong and Feldman, 1999; Robinson and Ellenberger, 1997). A distinguishing feature of ionotropic NMDA receptors is that glutamate binding and post-synaptic depolarization activates the receptor and allows Ca2+ to flow through the cell membrane along with the cations Na+ and K+ (Alpert and Alford). The influence of NMDA receptor activation and Ca2+ influx in sustained long-term potentiation is well established (Bliss and Lomo, 1973), making regulation of the NMDA receptor a prime target of investigation following SCI.
After SH, expression of specific subtypes of glutamatergic receptors increases at phrenic motoneurons (Alilain and Goshgarian, 2008; Mantilla et al., 2012). In a previous study, we examined the time course of changes in mRNA expression of several glutamatergic and 5-HTR receptors after SH by using quantitative real-time RT-PCR in microdissected rat phrenic motoneurons (Mantilla et al., 2012). Indeed, there was a significant increase in NMDA receptor expression in phrenic motoneurons at 21 days post-SH whereas AMPA receptor expression decreased. No change in the expression of metabotropic glutamate receptors mGluR1 or mGluR5 was evident. A transition in ionotropic glutamatergic transmission after SH from AMPA to NMDA receptors may cause an increase in phrenic motoneuron excitability by prolonging excitatory post-synaptic currents and reducing receptor desensitization (Rekling et al., 2000). Accordingly, NMDA upregulation (and simultaneous AMPA down regulation) by 21 days post-SH may be a key factor in the recovery of DIAm activity after SCI. Indeed, in a recent preliminary study in rats, pharmacological inhibition of the NMDA receptor in the phrenic motoneuron pool acutely diminished DIAm activity in animals displaying restored ipsilateral activity 4 weeks post-SH (Mantilla et al., 2014c). Taken together, these findings support the notion that NMDA receptor expression is necessary for recovery of ipsilateral DIAm activity.
The NMDA receptor complex has several distinct subunits: the NR1, NR2A, NR2B, NR2C, NR2D subunits (Monyer et al., 1992). NR2A subunit mRNA levels increase in rats after thoracic (T8) spinal cord contusion and this increase correlates with improved hind limb function (Grossman et al., 2000), suggesting strengthened excitatory synaptic connections on hind limb motoneurons. We previously found evidence of increased NR1 expression in phrenic motoneurons following SH (Mantilla et al., 2012), but a systematic investigation of NMDA subunit expression in microdissected motoneurons has not been performed. Of interest, changes in NR2D expression confer resistance to Mg2+ blockade and may be used therapeutically after SCI (Arvanian et al., 2006; Arvanian et al., 2004). AMPA receptor complexes have four distinct subunits, GluR1–4 (Santos et al., 2009), and GluR2 subunit expression is decreased after thoracic (T8) spinal cord contusion injury (Grossman et al., 1999). Similarly, we found evidence of decreased GluR2 expression in microdissected phrenic motoneurons following SH (Mantilla et al., 2012). The effect of changes in motoneuron BDNF/TrkB signaling on NMDA or AMPA receptor expression has not been directly explored.
The neurotrophin BDNF signaling via its high affinity TrkB receptor regulates both pre- and post-synaptic plasticity in various neuronal systems (Carvalho et al., 2008), including the spinal cord (Fig. 1). Pre-synaptically, BDNF enables continued glutamate release during action potentials bursts, enhancing synaptic responses and facilitating an increase in docking of synaptic vesicles to the plasma membrane in hippocampal slices (Jovanovic et al., 2000; Tyler and Pozzo-Miller, 2001). Both pre- and post-synaptically depending on the neuronal system studied, BDNF/TrkB signaling regulates NMDA receptor subunit phosphorylation (Carvalho et al., 2008), and may regulate post-synaptic expression of glutamatergic receptors expression on motoneurons (Fig. 1). Studying the molecular and cellular mechanisms underlying the therapeutic effects of BDNF/TrkB signaling may ultimately permit development of novel single or combination therapies to restore ventilation in patients in SCI, and perhaps even promote the greater force generation needed for expulsive behaviors important for airway clearance.
8. Conclusions
There is now convincing evidence that BDNF signaling via full-length TrkB receptors at motoneuron pools caudal to a cervical SCI is critically important for phrenic motoneuron plasticity and recovery of respiratory function. In a series of complementary studies in rats and mice, targeting BDNF/TrkB signaling was shown to be both necessary and sufficient to enhance recovery of ipsilateral DIAm EMG activity. Although the specific mechanisms of action of BDNF/TrkB signaling have not been elucidated, several lines of evidence highlight the role of glutamatergic neurotransmission at motoneurons in enhancing recovery. Our studies to date have focused on recovery of eupneic DIAm activity and it will be necessary to examine effects on recovery of other motor behaviors requiring higher levels of force generation by the DIAm. Indeed, expulsive behaviors necessary for airway clearance (e.g., coughing, sneezing) may show continued impairment despite effective recovery of eupneic activity. If non-ventilatory DIAm motor behaviors requiring greater than ~50% Pdimax are compromised, increased neural drive to the intact DIAm may be insufficient to accomplish effective airway clearance following injury, placing patients at continued risk for respiratory infections and functional compromise. Future studies should also address the role of BDNF/TrkB signaling in SCI models involving motoneuron loss and tissue damage (e.g., as a result of trauma or compression). Indeed, BDNF effects on motoneuron survival may limit the extent of damage in the vicinity of injury and thus contribute to functional recovery. Combined treatments involving neurotrophins, targeted delivery of TrkB receptors and other therapies (e.g., training or intermittent hypoxia) may also yield beneficial effects. These possibilities remain to be directly examined.
Highlights.
Recovery of rhythmic diaphragm activity after spinal hemisection may be altered by modulating BDNF/TrkB signaling in phrenic motoneurons at critical time points after SH.
TrkB kinase activity at phrenic motoneurons is necessary and sufficient for spontaneous recovery of rhythmic ipsilateral DIAm activity post-SH
BDNF availability at motoneuron pools caudal to the injury site is critically important for motoneuron plasticity and recovery after SCI
BDNF/TrkB signaling may enhance phrenic motoneuron excitability via glutamatergic (NMDA) signaling
Glutamatergic pathways are essential in the neuroplasticity of phrenic motoneuron output
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alilain WJ, Goshgarian HG. Glutamate receptor plasticity and activity-regulated cytoskeletal associated protein regulation in the phrenic motor nucleus may mediate spontaneous recovery of the hemidiaphragm following chronic cervical spinal cord injury. Exp Neurol. 2008;212:348–357. doi: 10.1016/j.expneurol.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert MH, Alford S. Synaptic NMDA receptor-dependent Ca(2)(+) entry drives membrane potential and Ca(2)(+) oscillations in spinal ventral horn neurons. PloS one. 2013;8:e63154. doi: 10.1371/journal.pone.0063154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Argote S, Gransee HM, Mora JC, Stowe JM, Jorgenson AJ, Sieck GC, Mantilla CB. The Impact of Mid-Cervical Contusion Injury on Diaphragm Muscle Function. J Neurotrauma. 2015 doi: 10.1089/neu.2015.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanian VL, Bowers WJ, Petruska JC, Motin V, Manuzon H, Narrow WC, Federoff HJ, Mendell LM. Viral delivery of NR2D subunits reduces Mg2+ block of NMDA receptor and restores NT-3-induced potentiation of AMPA-kainate responses in maturing rat motoneurons. J Neurophysiol. 2004;92:2394–2404. doi: 10.1152/jn.00278.2004. [DOI] [PubMed] [Google Scholar]
- Arvanian VL, Bowers WJ, Anderson A, Horner PJ, Federoff HJ, Mendell LM. Combined delivery of neurotrophin-3 and NMDA receptors 2D subunit strengthens synaptic transmission in contused and staggered double hemisected spinal cord of neonatal rat. Exp Neurol. 2006;197:347–352. doi: 10.1016/j.expneurol.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Basura GJ, Zhou SY, Walker PD, Goshgarian HG. Distribution of serotonin 2A and 2C receptor mRNA expression in the cervical ventral horn and phrenic motoneurons following spinal cord hemisection. Exp Neurol. 2001;169:255–263. doi: 10.1006/exnr.2001.7682. [DOI] [PubMed] [Google Scholar]
- Baussart B, Stamegna JC, Polentes J, Tadie M, Gauthier P. A new model of upper cervical spinal contusion inducing a persistent unilateral diaphragmatic deficit in the adult rat. Neurobiol Dis. 2006;22:562–574. doi: 10.1016/j.nbd.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Beck M, Flachenecker P, Magnus T, Giess R, Reiners K, Toyka KV, Naumann M. Autonomic dysfunction in ALS: a preliminary study on the effects of intrathecal BDNF. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6:100–103. doi: 10.1080/14660820510028412. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce VS, Park J, Gage FH, Mendell LM. Differential effects of brain-derived neurotrophic factor and neurotrophin-3 on hindlimb function in paraplegic rats. European Journal of Neuroscience. 2012;35:221–232. doi: 10.1111/j.1460-9568.2011.07950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregman BS, McAtee M, Dai HN, Kuhn PL. Neurotrophic factors increase axonal growth after spinal cord injury and transplantation in the adult rat. Exp Neurol. 1997;148:475–494. doi: 10.1006/exnr.1997.6705. [DOI] [PubMed] [Google Scholar]
- Brown R, DiMarco AF, Hoit JD, Garshick E. Respiratory dysfunction and management in spinal cord injury. Respir Care. 2006;51:853–868. discussion 869–870. [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Levine DN, Zajac FE., 3rd Mammalian motor units: physiological-histochemical correlation in three types in cat gastrocnemius. Science. 1971;174:709–712. doi: 10.1126/science.174.4010.709. [DOI] [PubMed] [Google Scholar]
- Carvalho AL, Caldeira MV, Santos SD, Duarte CB. Role of the brain-derived neurotrophic factor at glutamatergic synapses. British Journal of Pharmacology. 2008;153(Suppl 1):S310–324. doi: 10.1038/sj.bjp.0707509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, Zhang C, Johnson NM, England PM, Shokat KM, Ginty DD. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46:13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Chitravanshi VC, Sapru HN. NMDA as well as non-NMDA receptors mediate the neurotransmission of inspiratory drive to phrenic motoneurons in the adult rat. Brain Res. 1996;715:104–112. doi: 10.1016/0006-8993(95)01565-5. [DOI] [PubMed] [Google Scholar]
- Chopp M, Zhang XH, Li Y, Wang L, Chen J, Lu D, Lu M, Rosenblum M. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport. 2000;11:3001–3005. doi: 10.1097/00001756-200009110-00035. [DOI] [PubMed] [Google Scholar]
- Devivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord. 2012;50:365–372. doi: 10.1038/sc.2011.178. [DOI] [PubMed] [Google Scholar]
- Dittrich F, Ochs G, Grosse-Wilde A, Berweiler U, Yan Q, Miller JA, Toyka KV, Sendtner M. Pharmacokinetics of intrathecally applied BDNF and effects on spinal motoneurons. Exp Neurol. 1996;141:225–239. doi: 10.1006/exnr.1996.0157. [DOI] [PubMed] [Google Scholar]
- Dong XW, Feldman JL. Distinct subtypes of metabotropic glutamate receptors mediate differential actions on excitability of spinal respiratory motoneurons. J Neurosci. 1999;19:5173–5184. doi: 10.1523/JNEUROSCI.19-13-05173.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty KD, Dreyfus CF, Black IB. Brain-derived neurotrophic factor in astrocytes, oligodendrocytes, and microglia/macrophages after spinal cord injury. Neurobiol Dis. 2000;7:574–585. doi: 10.1006/nbdi.2000.0318. [DOI] [PubMed] [Google Scholar]
- el-Bohy AA, Schrimsher GW, Reier PJ, Goshgarian HG. Quantitative assessment of respiratory function following contusion injury of the cervical spinal cord. Exp Neurol. 1998;150:143–152. doi: 10.1006/exnr.1997.6757. [DOI] [PubMed] [Google Scholar]
- Enad JG, Fournier M, Sieck GC. Oxidative capacity and capillary density of diaphragm motor units. J Appl Physiol. 1989;67:620–627. doi: 10.1152/jappl.1989.67.2.620. [DOI] [PubMed] [Google Scholar]
- Fournier M, Sieck GC. Mechanical properties of muscle units in the cat diaphragm. J Neurophysiol. 1988;59:1055–1066. doi: 10.1152/jn.1988.59.3.1055. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Baker-Herman TL, Golder FJ, Doperalski NJ, Watters JJ, Mitchell GS. Cervical spinal cord injury upregulates ventral spinal 5-HT2A receptors. J Neurotrauma. 2005;22:203–213. doi: 10.1089/neu.2005.22.203. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Golder FJ, Olson EB, Jr, Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol. 2006;100:800–806. doi: 10.1152/japplphysiol.00960.2005. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol. 2008;211:97–106. doi: 10.1016/j.expneurol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia N, Tomas M, Santafe MM, Lanuza MA, Besalduch N, Tomas J. Localization of brain-derived neurotrophic factor, neurotrophin-4, tropomyosin-related kinase b receptor, and p75 NTR receptor by high-resolution immunohistochemistry on the adult mouse neuromuscular junction. J Peripher Nerv Syst. 2010;15:40–49. doi: 10.1111/j.1529-8027.2010.00250.x. [DOI] [PubMed] [Google Scholar]
- Gill LC, Ross HH, Lee KZ, Gonzalez-Rothi EJ, Dougherty BJ, Judge AR, Fuller DD. Rapid diaphragm atrophy following cervical spinal cord hemisection. Respir Physiol Neurobiol. 2014;192:66–73. doi: 10.1016/j.resp.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill LC, Mantilla CB, Sieck GC. Impact of unilateral denervation on transdiaphragmatic pressure. Respir Physiol Neurobiol. 2015;210:14–21. doi: 10.1016/j.resp.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Reier PJ, Bolser DC. Altered respiratory motor drive after spinal cord injury: supraspinal and bilateral effects of a unilateral lesion. J Neurosci. 2001;21:8680–8689. doi: 10.1523/JNEUROSCI.21-21-08680.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci. 2008;28:2033–2042. doi: 10.1523/JNEUROSCI.3570-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Fuller DD, Lovett-Barr MR, Vinit S, Resnick DK, Mitchell GS. Breathing patterns after mid-cervical spinal contusion in rats. Exp Neurol. 2011;231:97–103. doi: 10.1016/j.expneurol.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG, Moran MF, Prcevski P. Effect of cervical spinal cord hemisection and hemidiaphragm paralysis on arterial blood gases, pH, and respiratory rate in the adult rat. Exp Neurol. 1986;93:440–445. doi: 10.1016/0014-4886(86)90206-2. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. Plasticity in Respiratory Motor Control: Invited Review: The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol. 2003;94:795–810. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- Gransee HM, Zhan WZ, Sieck GC, Mantilla CB. Targeted Delivery of TrkB Receptor to Phrenic Motoneurons Enhances Functional Recovery of Rhythmic Phrenic Activity after Cervical Spinal Hemisection. PloS one. 2013;8:e64755. doi: 10.1371/journal.pone.0064755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gransee HM, Zhan WZ, Sieck GC, Mantilla CB. Localized Delivery of Brain-Derived Neurotrophic Factor-Expressing Mesenchymal Stem Cells Enhances Functional Recovery following Cervical Spinal Cord Injury. J Neurotrauma. 2015;32:185–193. doi: 10.1089/neu.2014.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Sieck DC, Sieck GC, Mantilla CB. Novel method for transdiaphragmatic pressure measurements in mice. Respir Physiol Neurobiol. 2013;188:56–59. doi: 10.1016/j.resp.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Ermilov LG, Sieck GC, Mantilla CB. Ageing and neurotrophic signalling effects on diaphragm neuromuscular function. J Physiol. 2015;593:431–440. doi: 10.1113/jphysiol.2014.282244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman SD, Wolfe BB, Yasuda RP, Wrathall JR. Alterations in AMPA receptor subunit expression after experimental spinal cord contusion injury. J Neurosci. 1999;19:5711–5720. doi: 10.1523/JNEUROSCI.19-14-05711.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman SD, Wolfe BB, Yasuda RP, Wrathall JR. Changes in NMDA receptor subunit expression in response to contusive spinal cord injury. J Neurochem. 2000;75:174–184. doi: 10.1046/j.1471-4159.2000.0750174.x. [DOI] [PubMed] [Google Scholar]
- Hadley SD, Walker PD, Goshgarian HG. Effects of serotonin inhibition on neuronal and astrocyte plasticity in the phrenic nucleus 4 h following C2 spinal cord hemisection. Exp Neurol. 1999;160:433–445. doi: 10.1006/exnr.1999.7238. [DOI] [PubMed] [Google Scholar]
- Harvey AR, Lovett SJ, Majda BT, Yoon JH, Wheeler LP, Hodgetts SI. Neurotrophic factors for spinal cord repair: Which, where, how and when to apply, and for what period of time? Brain Res. 2015;1619:36–71. doi: 10.1016/j.brainres.2014.10.049. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Imagita H, Nishikawa A, Sakata S, Nishii Y, Minematsu A, Moriyama H, Kanemura N, Shindo H. Tidal volume and diaphragm muscle activity in rats with cervical spinal cord injury. J Phys Ther Sci. 2015;27:791–794. doi: 10.1589/jpts.27.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci. 2000;3:323–329. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- King VR, Bradbury EJ, McMahon SB, Priestley JV. Changes in truncated trkB and p75 receptor expression in the rat spinal cord following spinal cord hemisection and spinal cord hemisection plus neurotrophin treatment. Exp Neurol. 2000;165:327–341. doi: 10.1006/exnr.2000.7480. [DOI] [PubMed] [Google Scholar]
- Lane MA, Lee KZ, Salazar K, O’Steen BE, Bloom DC, Fuller DD, Reier PJ. Respiratory function following bilateral mid-cervical contusion injury in the adult rat. Exp Neurol. 2012;235:197–210. doi: 10.1016/j.expneurol.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn WS, Adkins RH, Gong H, Jr, Waters RL. Pulmonary function in chronic spinal cord injury: a cross-sectional survey of 222 southern California adult outpatients. Archives Of Physical Medicine and Rehabilitation. 2000;81:757–763. doi: 10.1016/s0003-9993(00)90107-2. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Invited Review: Mechanisms underlying motor unit plasticity in the respiratory system. J Appl Physiol. 2003;94:1230–1241. doi: 10.1152/japplphysiol.01120.2002. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Trophic factor expression in phrenic motor neurons. Respir Physiol Neurobiol. 2008;164:252–262. doi: 10.1016/j.resp.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Zhan WZ, Sieck GC. Retrograde labeling of phrenic motoneurons by intrapleural injection. J Neurosci Methods. 2009;182:244–249. doi: 10.1016/j.jneumeth.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Seven YB, Zhan WZ, Sieck GC. Diaphragm motor unit recruitment in rats. Respir Physiol Neurobiol. 2010;173:101–106. doi: 10.1016/j.resp.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Seven YB, Hurtado-Palomino JN, Zhan WZ, Sieck GC. Chronic assessment of diaphragm muscle EMG activity across motor behaviors. Respir Physiol Neurobiol. 2011;177:176–182. doi: 10.1016/j.resp.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Phrenic motor unit recruitment during ventilatory and non-ventilatory behaviors. Respir Physiol Neurobiol. 2011;179:57–63. doi: 10.1016/j.resp.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Bailey JP, Zhan WZ, Sieck GC. Phrenic motoneuron expression of serotonergic and glutamatergic receptors following upper cervical spinal cord injury. Exp Neurol. 2012;234:191–199. doi: 10.1016/j.expneurol.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Ermilov LG. The novel TrkB receptor agonist 7,8-dihydroxyflavone enhances neuromuscular transmission. Muscle Nerve. 2012;45:274–276. doi: 10.1002/mus.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Gransee HM, Zhan WZ, Sieck GC. Motoneuron BDNF/TrkB signaling enhances functional recovery after cervical spinal cord injury. Exp Neurol. 2013a;247C:101–109. doi: 10.1016/j.expneurol.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Greising SM, Zhan WZ, Seven YB, Sieck GC. Prolonged C2 spinal hemisection-induced inactivity reduces diaphragm muscle specific force with modest, selective atrophy of type IIx and/or IIb fibers. J Appl Physiol. 2013b;114:380–386. doi: 10.1152/japplphysiol.01122.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Greising SM, Stowe JM, Zhan WZ, Sieck GC. TrkB Kinase Activity is Critical for Recovery of Respiratory Function after Cervical Spinal Cord Hemisection. Exp Neurol. 2014a;261:190–195. doi: 10.1016/j.expneurol.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Stowe JM, Sieck DC, Ermilov LG, Greising SM, Zhang C, Shokat KM, Sieck GC. TrkB Kinase Activity Maintains Synaptic Function and Structural Integrity at Adult Neuromuscular Junctions. J Appl Physiol. 2014b;117:910–920. doi: 10.1152/japplphysiol.01386.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Zhan WZ, Ermilov LG, Gransee HM, Sieck GC. Impact of glutamatergic NMDA signaling on recovery of phrenic activity after cervical spinal cord injury. Soc Neurosci Abstr 827.25 2014c [Google Scholar]
- McCrimmon DR, Smith JC, Feldman JL. Involvement of excitatory amino acids in neurotransmission of inspiratory drive to spinal respiratory motoneurons. J Neurosci. 1989;9:1910–1921. doi: 10.1523/JNEUROSCI.09-06-01910.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire M, Liu C, Cao Y, Ling L. Formation and maintenance of ventilatory long-term facilitation require NMDA but not non-NMDA receptors in awake rats. J Appl Physiol. 2008;105:942–950. doi: 10.1152/japplphysiol.01274.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata H, Zhan WZ, Prakash YS, Sieck GC. Myoneural interactions affect diaphragm muscle adaptations to inactivity. J Appl Physiol. 1995;79:1640–1649. doi: 10.1152/jappl.1995.79.5.1640. [DOI] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- National Spinal Cord Injury Statistical Center. Spinal Cord Injury, Facts and Figures at a Glance. University of Alabama at Birmingham; Birmingham, AL: 2015. [Google Scholar]
- Nicaise C, Hala TJ, Frank DM, Parker JL, Authelet M, Leroy K, Brion JP, Wright MC, Lepore AC. Phrenic motor neuron degeneration compromises phrenic axonal circuitry and diaphragm activity in a unilateral cervical contusion model of spinal cord injury. Exp Neurol. 2012a;235:539–552. doi: 10.1016/j.expneurol.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Nicaise C, Putatunda R, Hala TJ, Regan KA, Frank DM, Brion JP, Leroy K, Pochet R, Wright MC, Lepore AC. Degeneration of phrenic motor neurons induces long-term diaphragm deficits following mid-cervical spinal contusion in mice. J Neurotrauma. 2012b;29:2748–2760. doi: 10.1089/neu.2012.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicaise C, Frank DM, Hala TJ, Authelet M, Pochet R, Adriaens D, Brion JP, Wright MC, Lepore AC. Early phrenic motor neuron loss and transient respiratory abnormalities following unilateral cervical spinal cord contusion. J Neurotrauma. 2013;30:1092–1099. doi: 10.1089/neu.2012.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs G, Penn RD, York M, Giess R, Beck M, Tonn J, Haigh J, Malta E, Traub M, Sendtner M, Toyka KV. A phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:201–206. doi: 10.1080/14660820050515197. [DOI] [PubMed] [Google Scholar]
- Porter WT. The Path of the Respiratory Impulse from the Bulb to the Phrenic Nuclei. J Physiol. 1895;17:455–485. doi: 10.1113/jphysiol.1895.sp000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D, Ellenberger H. Distribution of N-methyl-D-aspartate and non-N-methyl-D-aspartate glutamate receptor subunits on respiratory motor and premotor neurons in the rat. J Comp Neurol. 1997;389:94–116. doi: 10.1002/(sici)1096-9861(19971208)389:1<94::aid-cne7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Rooney GE, McMahon SS, Ritter T, Garcia Y, Moran C, Madigan NN, Flugel A, Dockery P, O’Brien T, Howard L, Windebank AJ, Barry FP. Neurotrophic factor-expressing mesenchymal stem cells survive transplantation into the contused spinal cord without differentiating into neural cells. Tissue Eng Part A. 2009;15:3049–3059. doi: 10.1089/ten.TEA.2009.0045. [DOI] [PubMed] [Google Scholar]
- Santos SD, Carvalho AL, Caldeira MV, Duarte CB. Regulation of AMPA receptors and synaptic plasticity. Neuroscience. 2009;158:105–125. doi: 10.1016/j.neuroscience.2008.02.037. [DOI] [PubMed] [Google Scholar]
- Satriotomo I, Dale EA, Dahlberg JM, Mitchell GS. Repetitive acute intermittent hypoxia increases expression of proteins associated with plasticity in the phrenic motor nucleus. Exp Neurol. 2012;237:103–115. doi: 10.1016/j.expneurol.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seven YB, Mantilla CB, Sieck GC. Recruitment of Rat Diaphragm Motor Units Across Motor Behaviors with Different Levels of Diaphragm Activation. J Appl Physiol. 2014;117:1308–1316. doi: 10.1152/japplphysiol.01395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol. 1989;66:2539–2545. doi: 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Fournier M, Enad JG. Fiber type composition of muscle units in the cat diaphragm. Neurosci Lett. 1989;97:29–34. doi: 10.1016/0304-3940(89)90134-1. [DOI] [PubMed] [Google Scholar]
- Sieck GC. Neural control of the inspiratory pump. NIPS. 1991;6:260–264. [Google Scholar]
- Sieck GC. Physiological effects of diaphragm muscle denervation and disuse. Clin Chest Med. 1994;15:641–659. [PubMed] [Google Scholar]
- Sieck GC, Mantilla CB. Role of neurotrophins in recovery of phrenic motor function following spinal cord injury. Respir Physiol Neurobiol. 2009;169:218–225. doi: 10.1016/j.resp.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The BDNF Study Group. A controlled trial of recombinant methionyl human BDNF in ALS (Phase III) Neurology. 1999;52:1427–1433. doi: 10.1212/wnl.52.7.1427. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci. 2001;21:4249–4258. doi: 10.1523/JNEUROSCI.21-12-04249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinit S, Gauthier P, Stamegna JC, Kastner A. High cervical lateral spinal cord injury results in long-term ipsilateral hemidiaphragm paralysis. J Neurotrauma. 2006;23:1137–1146. doi: 10.1089/neu.2006.23.1137. [DOI] [PubMed] [Google Scholar]
- Weishaupt N, Blesch A, Fouad K. BDNF: the career of a multifaceted neurotrophin in spinal cord injury. Exp Neurol. 2012;238:254–264. doi: 10.1016/j.expneurol.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Widenfalk J, Lundstromer K, Jubran M, Brene S, Olson L. Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J Neurosci. 2001;21:3457–3475. doi: 10.1523/JNEUROSCI.21-10-03457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil. 2003;82:803–814. doi: 10.1097/01.PHM.0000078184.08835.01. [DOI] [PubMed] [Google Scholar]
- Xu XM, Guenard V, Kleitman N, Aebischer P, Bunge MB. A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp Neurol. 1995;134:261–272. doi: 10.1006/exnr.1995.1056. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Basura GJ, Goshgarian HG. Serotonin(2) receptors mediate respiratory recovery after cervical spinal cord hemisection in adult rats. J Appl Physiol. 2001;91:2665–2673. doi: 10.1152/jappl.2001.91.6.2665. [DOI] [PubMed] [Google Scholar]