Abstract
Some individuals who try electronic cigarettes (e-cigarettes) continue to use long-term. Previous research has investigated the safety of e-cigarettes and their potential for use in smoking cessation, but comparatively little research has explored chronic or habitual e-cigarette use. In particular, the relationship between e-cigarette cues and craving is unknown. We sought to bridge this gap by developing a novel set of e-cigarette (salient) and electronic toothbrush (neutral) videos for use in cue-reactivity paradigms. Additionally, we demonstrate the utility of this approach in a pilot fMRI study of 7 experienced e-cigarette users. Participants were scanned while viewing the cue videos before and after 10 minute use of their own e-cigarettes (producing an 11.7 ng/ml increase in plasma nicotine concentration). A significant session (pre- and post-use) by video type (salient and neutral) interaction was exhibited in many sensorimotor areas commonly activated in other cue-reactivity paradigms. We did not detect significant cue-related activity in other brain regions notable in the craving literature. Possible reasons for this discrepancy are discussed, including the importance of matching cue stimuli to participants’ experiences.
Keywords: e-cigarette, e-cigs, fMRI, stimulus’, cue, craving
1. Introduction
Electronic cigarettes (e-cigarettes) are an electronic nicotine delivery system. Rather than burning tobacco to produce inhalable nicotine (as in traditional cigarettes), e-cigarettes use battery power to apply an electrical current that produces heat to aerosolize a liquid solution that usually contains nicotine. Though only introduced into United States and European markets in 2007 (Noel et al., 2011), e-cigarettes have seen a dramatic increase in popularity and use. In the United States alone, e-cigarette sales more than doubled between 2012–2013 (Giovenco et al., 2014).
E-cigarettes are perceived by many to be a safer alternative to smoking cigarettes, presumably because e-cigarettes do not produce the tobacco smoke linked to various cancers and other negative health outcomes (e.g., Hecht and Hoffmann, 1988; Hecht, 2002, 1999; Pfeifer et al., 2002). Indeed, one of the major reasons adult e-cigarette users cited for their use is the view that e-cigarettes are less toxic than combustibles (Etter and Bullen, 2011). The potential harm reduction benefits of e-cigarettes and their potential for use as smoking cessation aids are two of the dominant themes in e-cigarette marketing (Grana and Ling, 2014), and this seems to be reflected in public perception. Perhaps because of this emphasis, much of the research on e-cigarettes to date has focused on two general questions: Are e-cigarettes safer than their traditional counterparts, and can e-cigarettes be used to aid smoking cessation (e.g., Biener and Hargraves, 2015; Bullen, 2014; Bullen et al., 2013; Caponnetto et al., 2013)?
However, it is becoming increasingly clear that e-cigarette use is not restricted to short-term use as an aid to smoking cessation. While the majority of regular users are current or former smokers, most estimates also show an increasing number of never-smokers trying e-cigarettes, particularly among younger users (Carroll Chapman and Wu, 2014; King et al., 2013). Among those who try e-cigarettes to aid quitting, some continue to use long-term. For example, Etter and Bullen (2011) reported that a large proportion of ex-smokers (79%) feared they would relapse if they discontinued e-cigarette use and other studies (e.g., Foulds et al., 2015) have found that many e-cig users continue use for well beyond six months and over 90% of experienced users report strong cravings to use their e-cig. Collectively, these data suggest that for many individuals, e-cigarettes are being used as nicotine delivery systems on a long-term basis.
With much of the research to date directed at understanding the toxicity of e-cigarettes or their potential to aid cessation, we are left with a knowledge gap surrounding real-world use for a significant portion of e-cigarette users who have become chronic and perhaps addicted users.
Considering that e-cigarettes and combustible cigarettes share nicotine as the main addictive agent, it is tempting to use models of cigarette addiction as a framework for understanding habitual e-cigarette use. However, there are substantial differences between the two forms that make it impossible to equate the neurocognitive effects of using traditional and electronic cigarettes.
One major difference is how the nicotine is packaged and delivered. E-cigarettes dispense nicotine in an aerosol instead of smoke, and the design elements that effect this change are not consistent across devices. The myriad devices therefore differ in their effectiveness at administering nicotine (Brown and Cheng, 2014; Williams and Talbot, 2011). Initial reports indicated that e-cigarettes were not delivering nicotine to the bloodstream (Bullen et al., 2010; Vansickel et al., 2010). These experiments used first generation (less efficient) devices and participants who were not experienced with them. Recent studies demonstrated that experienced users with more advanced devices are capable of delivering physiologically active doses of nicotine to the blood, more comparable with absorption from traditional cigarettes (Farsalinos et al., 2014; Vansickel and Eissenberg, 2013), and that e-cigarette users can remain addicted to nicotine--though this does vary substantially based on device and usage characteristics (Etter and Eissenberg, 2015; Foulds et al., 2015; Yingst et al., 2015). Although some types of e-cigarettes appear capable of delivering nicotine with the same speed and dose as a combustible cigarette (Spindle and Breland, 2014), many e-cigarettes provide much less nicotine per ―puff‖ than combustible cigarettes (Dawkins and Corcoran, 2014; Farsalinos et al., 2014; Nides et al., 2014; Schroeder and Hoffman, 2014; Yan and D’Ruiz, 2015).
The generally slower and smaller amplitude of e-cigarette nicotine delivery for some models, combined with the reusable form factor of e-cigarette devices and looser laws governing their use, may contribute to different usage patterns compared to cigarette smoking. Whereas traditional cigarettes are smoked in measurable quantities (e.g., one at a time) and in discrete periods (e.g., upon waking, smoke breaks at work, after dinner), e-cigarettes may be used more continuously throughout the day. It is possible that this variability in device manufacturing, device efficacy, nicotine concentration, and usage patterns presents a different psychophysiological experience from e-cigarette use as compared with cigarette use. However, few studies have attempted to characterize e-cigarette addiction outside of defining it (see Foulds et al., 2015).
One aspect of e-cigarette addiction that may be especially important to investigate is the relationship between e-cigarette cues, craving, and use behaviors. The study of craving has been a major theme in addiction research across drug types (Robinson and Berridge, 1993; Tiffany, 1990). In cigarette smoking more specifically, exposure to implicit and explicit cigarette cues produces neural, physiological, and cognitive responses that lead to increased desire to smoke (i.e., urges or craving) and subsequent smoking behavior (Abrams et al., 1988; Droungas et al., 1995; Due et al., 2014; Franklin et al., 2007; Janes et al., 2010; McBride et al., 2006; McClernon et al., 2005)--though craving and using do not always go hand-in-hand (Kassel and Shiffman, 1992; Shiffman, 2000; Tiffany, 1990). Given some of the unique characteristics of e-cigarette use compared to cigarette smoking noted above (e.g., more variable nicotine delivery, ability to use more frequently throughout the day, etc.), it is unclear to what extent craving, and more specifically cue-induced craving, play a role in e-cigarette use. We aimed to address this knowledge gap in two important ways: 1) develop a set of stimuli that could be used in e-cigarette research, and 2) apply neuroimaging methods to examine the cue response to those developed stimuli in a pilot study of experienced e-cigarette users. We specifically tested a novel set of video cues, as drug-related videos have proven effective at evoking robust subjective, peripheral physiological, and neurobiological responses in prior work on cigarette craving (e.g., Brody et al., 2007; Tong et al., 2007). We focused on examining neural responses to these videos given the contributions neuroscience has already made in craving research (Wilson and Sayette, 2015), as well as the observation that neural responses to drug cues yield clinically meaningful information that is not captured by other methods (e.g.,Versace et al., 2014). We sought to investigate the extent to which e-cigarette and well-matched neutral videos would elicit different patterns of neural activity in established e-cigarette users. We further sought to investigate the potential utility of combining these videos with fMRI by examining if neural responses to the stimuli are moderated by whether or not participants have recently used an e-cigarette - a state-dependent variable that is highly relevant for characterizing factors that shape use, such as craving and satiety. To our knowledge, this study represents the first demonstration of an exclusively e-cigarette stimuli set and the first application of fMRI to e-cigarette users.
2. Method
2.1 Development of e-cigarette stimuli
Cigarette research has greatly benefited from well-known sets of cigarette-related pictures or videos (Carter et al., 2006; Gilbert and Rabinovich, 1999; Tong et al., 2007). These generally contain both salient (cigarette) and neutral cues and are usually employed to elicit craving in cigarette smokers experimentally. One major obstacle to studying craving in e-cigarette users has been the lack of any standardized e-cigarette-specific stimuli. In order to rectify this deficit, we developed 12 e-cigarette (salient) and 12 electronic toothbrush (neutral) videos.
2.1.1 Filmed actors
Twelve graduate students at a major research university in the Northeastern US agreed to be filmed using an e-cigarette and an electronic toothbrush. There were an equal number of males and females (n = 6 each) and all appeared to be young adults. The actors were predominantly white (n = 9). Aside from demographic information, personal data were not collected or recorded during this stage. However, while conversing during filming some of the actors voluntarily reported experience with cigarette use; most of the actors reported having never used an e-cigarette previously. Regardless of presumed cigarette/e-cigarette history, all actors were shown YouTube video clips of experienced e-cigarette users and instructed in common technique prior to filming (e.g., taking quick, short draws before longer ones). The actors were not compensated for their participation.
2.1.2 Film design and filming
One goal in creating the video sets was to reduce the potential for confounding effects between salient and neutral videos. Accordingly, rather than filming in diverse “real-world” environments as has been done with other stimulus sets, the same studio location was used for all videos. Additionally, electronic toothbrushes were selected for the neutral videos because of their great degree of physical and conceptual overlap with e-cigarettes (e.g., both are placed in the mouth, hand-held, and battery powered). Each actor was filmed using an e-cigarette and an electronic toothbrush in the same pose (though these poses varied across actors, described next) to maintain consistency between video sets.
Twelve different poses/lighting combinations were designed (four featuring the actor’s left profile, four of the actor’s right profile, and four straight-on). All films were shot against a nondescript gray background and were framed to emphasize the actor’s face or mouth area. Snapshots from one of the e-cigarette videos and the corresponding neutral video are displayed in Figure 1. Three different first generation e-cigarettes (Greensmoke, NJOY, and Firelight Fusion) were used an equal number of times across actors; these devices in particular were selected because of their prevalent marketing, perceived popularity and widespread availability at the time the films were being conceptualized and developed. Five different electronic toothbrushes were randomly assigned to the actors. When branding was apparent on a device, it was either physically or digitally obscured.
Figure 1.
Depiction of e-cigarette video (left) and electronic toothbrush video (right).
Films were shot with a Canon EOS 550D digital camera and Canon EF f/1.4 50mm USM lens in full high-definition resolution (1920×1080 pixels), but were later cropped and/or downsampled to a resolution of 922×622 pixels to work better with the presentation hardware used in the pilot experiment. Each film was trimmed to a length of 30 seconds. Additional postprocessing conducted in Adobe Premiere Pro CS6 (Adobe Systems Incorporated, San Jose, CA, USA) software mainly consisted of color correction and sharpening to ensure a consistent and professional appearance across videos. The end result was twelve e-cigarette and twelve electronic toothbrush videos each of 30 second duration, with each actor featured in the same pose in both the e-cigarette and toothbrush video set.
2.2 Pilot fMRI study of cue response
The videos described in Section 2.1 were used in a small pilot study to assess the neurocognitive response to e-cigarette cues in long-term e-cigarette users. In this study, participants viewed half of the stimulus videos (both types intermixed) in the MRI while abstinent from nicotine for at least 14 hours, provided blood samples while using their e-cigarette, and then viewed the other half of the videos in the MRI. This allowed for a comparison of brain reactivity to the cues while abstinent and sated.
2.2.1 Participants
Participants were recruited from an online survey advertised on several websites, including www.webMD.com/ and many e-cigarette forums. The survey asked respondents about their smoking history, e-cigarette use, and device preferences. Additional details about the survey are reported in Foulds et al. (2015) and Yingst et al. (2015). Survey data were collected and managed using REDCap electronic data capture tools hosted at the Penn State Milton S. Hershey Medical Center and College of Medicine (Harris et al., 2009). Survey data were entered anonymously, but participants who were interested in additional research opportunities were invited to provide contact information. Two research labs (Penn State University, College of Medicine in Hershey, PA, and Penn State University in University Park, PA) collaborated on the study. Those who lived near one of the labs were contacted and screened for eligibility for the pilot study. Because of inconsistency in scanning parameters used between the sites, we report here only those participants that attended in Hershey.
Participants in the present analysis needed to be between the ages of 18 and 60 years old, used an e-cigarette for at least one month (including 20 of the last 28 days), and used a nicotine concentration in their liquid of at least 12 mg/mL. Exclusionary criteria included being a current daily smoker, experiencing a chronic health condition (e.g, diabetes, hypertension, cancer) or cardiovascular or respiratory illness, current psychopathology or prescribed psychiatric medication, current drug or alcohol abuse, current pregnancy, difficulty donating blood in the past, or any other safety concern that would preclude entering an MRI scanner (e.g., permanent piercings or other metal that cannot be removed from the body).
Eight individuals were eligible and completed the lab portion of the study, but one participant was excluded due to excessive head motion during scanning. Demographic information for the remaining seven participants is presented in Table 1. Devices used by participants were all considered ―advanced‖ devices, as determined by an algorithm (factors that were considered in the algorithm included whether the device was larger than a typical cigarette and whether it had a manual button to press). Specifically, these devices were: ProVape ProVari, Vision Spinner/Spinner 2/X.Fire, SmokTech T-Dux 3.0, Innokin iTaste MVP, and Aspire. Participants were paid $150US for their involvement in the study.
Table 1.
Participant Demographics and Smoking/E-cigarette History
| Characteristic | % (n) |
|---|---|
| Male | 28.6 (2) |
| White | 100.0 (7) |
| Former Smoker | 100.0 (7) |
| Using device larger than cigarette and with a button to press prior to inhalation |
100.0 (7) |
| Characteristic | M (SD) |
| Age | 36.6 (14.0) |
| Time since quitting cigarettes (in days) | 235 (251.3) |
| Time using e-cigarettes (in days) | 381.4 (450.2) |
| Number of days used e-cigarette in the past 28 days | 26.9 (3.0) |
| E-cigarette use times per day* | 17.9 (14.4) |
Note Each “time” consisting of around 15 puffs or lasting around 10 minutes, as per Foulds et al. (2015)
2.2.2 Materials
As noted in Section 2.2.1, participants were drawn from a pool of respondents to a large online survey that asked about smoking history, e-cigarette use, and device preferences. The survey can be accessed at: https://redcap.ctsi.psu.edu/redcap/surveys/?s=v94cbA. Participants again answered a subset of these questions during their lab visit to document any changes that may have occurred between survey completion and the lab visit.
Several measures were administered to gauge baseline dependence and withdrawal symptoms. Germane to this report is the Penn State Electronic Cigarette Dependence Index (PSECDI; Foulds et al., 2015). The PSECDI is a 10-item questionnaire (range 0–20, higher scores indicate greater dependence) that is meant to be comparable to the Penn State Cigarette Dependence Index, but for e-cigarette users (see Foulds et al., 2015 for details).
Participants responded to several visual analogue scales (VAS) at various points during the experiment. For these items, participants were given prompts (e.g., “Rate the degree to which you are looking forward to using your e-cigarette”) and instructed to draw a vertical line across a horizontal line, the ends of which represented minimum quantity (e.g., “Not at all”) or maximum quantity (e.g., “Very much”). These ratings were then quantified by measuring the distance from the base end to the recorded mark and converting that length according to a 0–100 scale. Questions that participants were asked related to level of desire (urge) to use their e-cigarette, withdrawal symptoms, anticipated effects of using their e-cigarette, and observed effects of using their e-cigarette. Importantly, participants responded to 12 VAS items regarding withdrawal symptoms before and after using their e-cigarettes. Participants were also asked to provide a verbal urge rating at various points when they were unable to complete a VAS item (i.e., when they were in the MRI).
Additional measures of working memory and attention were given (rapid visual information processing [RVIP] and N-back tasks) that are outside the scope of the present report. As such, they are not described in detail here.
2.2.3 Procedure
Participants from the online survey that were interested in participating in the lab portion of the study were contacted and screened over the phone according to the study criteria reported in Section 2.2.1. Those that met eligibility requirements were invited to visit the lab. All participants viewed all videos, but the order was counterbalanced to rule out any order effects.
Participants were instructed to remain abstinent from traditional cigarettes for 4 days and from their e-cigarettes (or other nicotine containing products) and caffeine for 14 hours prior to the lab visit. Upon arriving at the lab, participants provided an expired-air carbon monoxide (CO) sample to verify no recent cigarette use (<8 parts per million needed to continue with the protocol). They then completed a series of baseline questionnaires that included demographic and smoking history questions, as well as the PSECDI.
Participants provided a verbal urge rating and practiced the RVIP and N-back tasks before completing the first full RVIP protocol. Participants provided another urge rating as they were placed in the MRI for the first time. In the MRI, participants watched half of the e-cigarette and toothbrush videos. Videos were presented in two sets where salient and neutral videos were intermixed in a pseudorandom fashion. Each participant viewed both sets across the two scan sessions, but the order was counterbalanced by subject (i.e., some participants viewed one set during the first scan, and the remaining participants viewed the other set during the first scan). A variable ISI (ranging from 14–18 seconds) separated each video to allow the hemodynamic response to return to baseline before the next video. Following the video presentation, participants performed the N-back task. The scan session concluded by collecting resting baseline and anatomical images.
Upon removal from the MRI, participants again provided a verbal urge rating and completed several computerized VAS items regarding their anticipation of using their device and any present withdrawal symptoms. Following this, trained nursing staff from the Penn State Clinical Research Center inserted a catheter into the participant’s arm to facilitate frequent blood draws. Next, participants began an e-cigarette use protocol where they were instructed to take one puff on their own e-cigarette every 20 seconds for 10 minutes (30 puffs). Nursing staff sampled blood periodically throughout the protocol and for an additional five minutes. Participants also completed paper-and-pencil VAS questionnaires regarding their desire for and symptoms of using their e-cigarette during this time. At the conclusion of 15 minutes, nursing staff removed the catheter and the participant completed additional summary VAS questionnaires (including urge and the repeated 12 questions related to withdrawal) and the second administration of the RVIP task.
Subsequently, participants were again placed in the MRI. This session was identical to the first scan session, except the participants watched the remaining videos (i.e., those not presented during the first administration). After the second scan session, participants were debriefed and the study concluded.
2.2.4 fMRI data acquisition
Scanning was conducted on a 3-Tesla Siemens MAGNETOM Trio magnet (Siemens Corporation, New York, NY, USA). Scan sequence and settings were identical for both sessions. Functional data were acquired using 34 slice oblique-axial series (3 × 3 × 3 mm voxels), using a one-shot EPI pulse sequence [repetition time (TR) = 2000 ms, echo time (TE) = 25 ms, field of view = 24 cm, flip angle = 80°]. Following the functional runs (stimulus videos, N-back task, and resting baseline), a high-resolution (1 × 1 × 1 mm voxels), 3-dimensional structural volume was acquired using an MPRAGE sequence.
The functional volumes underwent several preprocessing steps, including 3D motion correction, slice timing correction, and correction for signal drift within and between runs. The anatomical images were transformed to the Talairach reference anatomy using a twelve- parameter affine automated algorithm. The transformation parameters were then applied to the functional images. The resulting coregistered functional images were then smoothed with a three-dimensional Gaussian filter (8 mm full width at half maximum). All preprocessing steps and subsequent analyses were performed with BrainVoyager QX 2.8 (Goebel et al., 2006).
fMRI data obtained during the presentation of e-cigarette and neutral videos were analyzed using a random-effects general linear model (GLM) with task-related regressors. Specifically, regressors of interest were created by convolving a box-car function for each video condition with a standard two-gamma hemodynamic response function. These regressors were entered into a GLM to obtain parameter estimates (i.e., beta weights) for each participant. Data from the two fMRI sessions (i.e., pre and post e-cigarette use) were modeled separately. Parameter estimates obtained at the individual participant level were entered into a random effects group analysis. Specifically, we conducted a voxel-wise repeated-measures analysis of variance (ANOVA) with video condition (neutral, e-cigarette) and session (pre, post e-cigarette use) as within-participants factors and participant as a random factor.
The BrainVoyager QX Cluster-Level Statistical Threshold Estimator plug-in (which implements a Monte-Carlo simulation approach to multiple comparisons correction) was used to determine the appropriate threshold for group statistical maps (Goebel et al., 2006). After 1000 iterations, it was determined that a corrected map-wise false positive rate of p < .05 would be obtained by combining a per-voxel threshold of p < .005 with cluster-extent thresholds of 11, 11, and 13 contiguous voxels for the statistical maps corresponding to the main effect of video condition, the main effect of session, and the video condition by session interaction, respectively. Only clusters meeting these criteria were considered significant.
3. Results
3.1 Behavioral results
3.1.1 Biochemical measures
A more detailed description of participants’ nicotine use characteristics is provided in Table 2. The low baseline exhaled CO levels are consistent with claimed non-smoking, and the very low baseline nicotine concentration is consistent with the required 14 hour nicotine abstinence period. The nicotine boost measure provides an estimate of how much nicotine is being absorbed into the blood as a result of e-cig use, and is calculated by subtracting the baseline nicotine concentration level from the maximum, or “peak” recorded value during or after puffing for each individual. A mean blood nicotine boost of almost 12 ng/ml within 2 minutes after completing puffing is nearly comparable to that measured in some laboratory studies of cigarette smoking, albeit typically taking fewer puffs (Cobb et al., 2011). Despite all participants using advanced devices, there was a high degree of variability in the amount of nicotine delivered.
Table 2.
Dependence and Biochemical Measures
| Measure | M (SD) |
|---|---|
| Penn State Electronic Cigarette Dependence Index Score (Foulds et al, 2015) | 7.0 (3.0) |
| Nicotine concentration in liquid (in mg/mL) | 15.4 (3.4) |
| Baseline exhaled CO (in ppm) | 2.9 (1.2) |
| Baseline cotinine concentration (in ng/mL) | 139.2 (116.0) |
| Baseline nicotine concentration (in ng/mL) | 0.5 (0.5) |
| Nicotine boost (in ng/mL) | 11.7 (11.7) |
| Time to peak nicotine concentration from initiating puffing (in minutes) | 11.3 (2.2) |
Note. ppm = parts per million. E-cigarette use protocol consisted of one “puff” every 20 seconds for 10 minutes (30 puffs). Nicotine boost is the estimated increase in blood nicotine serum level, as computed by subtracting the baseline concentration from the maximal recorded concentration. Time to peak represents the time at which participants reached their maximum blood nicotine concentration level.
3.1.2 Self-reported dependence, withdrawal, and urge
To determine baseline dependence to e-cigarettes, participants were administered the PSECDI at the beginning of the experiment. Participants endorsed low to medium levels of e-cigarette dependence (M= 7, SD = 3), which is similar to scores of dependence (M = 8.1, SD= 3.5) reported in a larger survey of over 3,600 e-cigarette users (Foulds et al., 2015)
Additional measures of withdrawal symptoms were taken immediately before and after using their e-cigarette devices. The individual VAS questions are presented in Table 3, along with their corresponding means and standard deviations. As can be seen, there was a great deal of variability in the experience of these symptoms. Following the recommendation of Hughes and Hatsukami (1998), we did not generate an overall withdrawal score.
Table 3.
Withdrawal Related VAS Items
| Item # | Item | Pre-Vape M (SD) |
Post-Vape M (SD) |
|---|---|---|---|
| 1. | URGES to vape* | 66.7 (34.7) | 36.4 (32.9) |
| 2. | Irritability/frustration/anger | 16.1 (24.7) | 3.4 (6.1) |
| 3. | Anxious | 19.3 (28.9) | 4.9 (9.1) |
| 4. | Difficulty concentrating | 16.9 (23) | 2.3 (3.9) |
| 5. | Restlessness | 11.7 (24.8) | 7.3 (19.3) |
| 6. | Hunger | 30.1 (26.8) | 28 (20.9) |
| 7. | Constipation | 9.6 (14.6) | 2.4 (6.4) |
| 8. | Impatient | 20.4 (26.1) | 7.9 (14.7) |
| 9. | Craving an e-cig/nicotine | 68.3 (34.3) | 38.1 (39.9) |
| 10. | Drowsiness | 38 (39) | 16.6 (22.3) |
| 11. | Depression/feeling blue | 5.6 (14.2) | 0 (0) |
| 12. | Desire for sweets | 29.0 (38.3) | 12.7 (23.5) |
Note. Each question prompt began with the stem: “Please respond to each word or phrase with how you feel RIGHT NOW.” The word or phrase identified above was depicted below the question prompt. Only pre- and post-vape scores on the first item were tested for differences because that item was of special interest to the investigators.
indicates comparison is significant at p < .05.
To test whether the e-cigarette use protocol was associated with a change in participants’ self-reported urge to use their e-cigarette device, we conducted a two-tailed paired samples t test to compare the urge ratings obtained on the VAS scales before and after use. As expected, urge ratings provided prior to the vaping protocol (M = 66.7, SD = 34.7) were significantly higher than those provided at the conclusion of the protocol (M = 36.4, SD = 36.4), t(6) = 2.96, p = .025, dz = 1.12.
Additionally, we tested whether self-reported urge to use their e-cigarettes changed after witnessing the stimulus videos. Though not statistically significant, verbal urge ratings collected in the scanner were higher in both sessions immediately after viewing the videos than immediately before viewing the videos (two-tailed paired samples t tests; session 1 pre urge rating: M = 58.7, SD = 36.3, session 1 post urge rating: M = 62.9, SD = 34.5, t[6] = −1.3, p = .239, dz = −1.06; session 2 pre urge rating: M = 27.5, SD = 13.3, session 2 post urge rating: M = 33.3, SD = 19.7, t[5] = −1.05, p = .341, dz = −.43). The effect of e-cigarette use in reducing the urge to use the e-cigarette appears larger than the effect of the video in increasing urge to use.
3.2 Imaging results
3.2.1 Main effect of video condition
Brain regions exhibiting a main effect of video condition are reported in Table 4. As presented, activation was greater during the presentation of e-cigarette videos than during neutral videos in a region encompassing portions of the temporal and occipital gyrus in the right hemisphere. Greater activation during neutral videos relative to e-cigarette videos was observed in the right posterior cingulate gyrus and the left middle occipital gyrus.
Table 4.
Brain Regions Exhibiting a Main Effect of Cue
| Talairach Coordinates | M (SD) Beta Weight Estimate | ||||||
|---|---|---|---|---|---|---|---|
| Region | Size (mm3) |
x | y | z | Average F ratio |
E-cig | Neutral |
| Right posterior cingulate gyrus | 513 | 22 | −34 | 31 | 5.26 | −0.03 (0.05) | 0.03 (0.03) |
| Right inferior temporal gyrus, middle temporal gyrus, middle occipital gyrus |
324 | 60 | −60 | 5 | 9.59 | 0.41 (0.2) | 0.23 (0.23) |
| Left middle occipital gyrus, cuneus | 297 | −20 | −81 | 17 | 2.12 | 0.09 (0.11) | 0.14 (0.14) |
Note. Stereotaxic coordinates are given for local maxima of activation clusters in Talairach atlas space. Beta weight estimates are averaged for the whole region per participant, and then averaged for all participants. The estimates were set up to represent percent signal change from baseline.
3.2.2. Main effect of session
A significant main effect of session was observed in the left cuneus (Talairach coordinates: x = −9, y = −84, z = 27; size = 567 mm3; average F ratio = −4.34). Specifically, activation was significantly lower during the pre-use session (M = −0.16, SD = 0.04) than during the post-use session (M = −0.02, SD = 0.04) for this region.
3.2.3. Session X video condition interaction
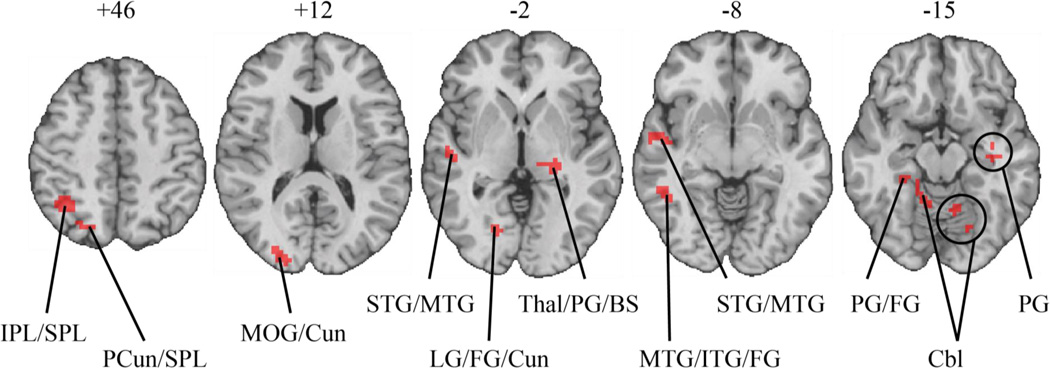
As summarized in Table 5 and depicted in Figure 2, there was a significant session X video cue interaction in several brain areas, including the right temporal gyrus, right parietal lobe, right occipital gyrus, bilateral parahippocampal gyrus, and bilateral cerebellum. To determine the nature of these interaction effects, each region of interest (ROI) was probed to examine the effect of video condition separately for the pre-use session and the post-use session, with results reported in Table 5. As presented in Table 5, activation during neutral videos was greater than activation during e-cigarette videos in the pre-use fMRI session for the majority of the ROIs, whereas the reverse was true in the post-use fMRI session.
Table 5.
Brain Regions Exhibiting a Session by Video Condition Interaction
| M (SD) Beta Weight Estimate | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Talairach Coordinates |
Pre-Vaping Session | Post-Vaping Session | |||||||||
| Region | Size (mm3) |
x | y | z | Average F ratio |
E-cig | Neutral | Contrast | E-cig | Neutral | Contrast |
| Right inferior parietal lobe, superior parietal lobe |
1647 | 32 | −55 | 50 | 6.29 | 0.01 (0.25) | 0.05 (0.22) | ns | 0.16 (0.13) | 0.01 (0.09) | *** |
| Right precuneus, superior parietal lobe |
378 | 24 | −70 | 46 | 7.71 | 0.01 (0.16) | 0.09 (0.10) | ns | 0.25 (0.18) | 0.09 (0.14) | * |
| Right middle occipital gyrus, cuneus |
810 | 27 | −87 | 9 | 7.72 | 0.38 (0.19) | 0.53 (0.17) | * | 0.45 (0.24) | 0.39 (0.25) | ns |
| Left thalamus, parahippocampal gyrus, brainstem |
837 | −20 | −25 | 1 | 8.99 | 0.06 (0.10) | 0.17 (0.08) | ** | 0.17 (0.08) | 0.12 (0.10) | * |
| Right lingual gyrus, fusiform gyrus, cuneus |
1026 | 18 | −77 | −3 | 6.08 | 0.09 (0.13) | 0.22 (0.13) | ** | 0.20 (0.16) | 0.13 (0.12) | ** |
| Right superior temporal gyrus, middle temporal gyrus |
729 | 47 | −9 | −4 | −2.26 | −0.08 (0.10) | 0.00 (0.08) | * | 0.00 (0.10) | −0.05 (0.09) | * |
| Right superior temporal gyrus, middle temporal gyrus |
567 | 57 | −16 | −5 | −1.8 | −0.04 (0.09) | 0.03 (0.10) | * | 0.04 (0.06) | −0.01 (0.06) | * |
| Right parahippocampal gyrus, fusiform gyrus |
486 | 33 | −43 | −11 | 7.92 | −0.10 (0.10) | 0.02 (0.10) | ** | 0.02 (0.09) | −0.01 (0.10) | ns |
| Left parahippocampal gyrus | 459 | −36 | −13 | −11 | 7.62 | 0.00 (0.04) | 0.06 (0.02) | * | 0.07 (0.05) | 0.02 (0.09) | ns |
| Right middle temporal gyrus, inferior temporal gyrus, fusiform gyrus |
810 | 47 | −43 | −15 | 8.41 | 0.04 (0.11) | 0.12 (0.16) | ns | 0.21 (0.16) | 0.10 (0.14) | ** |
| Right cerebellum | 351 | 19 | −37 | −13 | −2.59 | −0.11 (0.09) | −0.01 (0.06) | * | −0.01 (0.10) |
−0.08 (0.09) | ns |
| Left cerebellum | 1053 | −19 | −74 | −15 | 4.18 | −0.03 (0.14) | 0.11 (0.17) | ** | 0.14 (0.21) | 0.04 (0.21) | *** |
Note. Stereotaxic coordinates are given for local maxima of activation clusters in Talairach atlas space. ns = not significant
p < .05
p < .01
p < .001.
Beta weight estimates are averaged for the whole region per participant, and then averaged for all participants. The estimates were set up to represent percent signal change from baseline
Figure 2.
Brain regions exhibiting a significant session X video condition interaction. The numbers above each brain slice denote the distance (millimeters) from the anterior commissure–posterior commissure plane in Talairach atlas space. Brain slices are right-left reversed. BS = brainstem; Cbl = cerebellum; Cun = cuneus; FG = fusiform gyrus; IPL = inferior parietal lobe; ITG = inferior temporal gyrus; LG = lingual gyrus; MTG = middle temporal gyrus; PCun = precuneus; PG = parahippocampal gyrus; STG = superior temporal gyrus; SPL = superior parietal lobe; Thal = thalamus.
4. Discussion
We developed a novel set of e-cigarette and neutral comparison videos for use in research experiments. Additionally, we piloted these videos in a small group of experienced e-cigarette users in order to test their utility in eliciting a unique neural and behavioral (i.e., self-reported craving) response. Because this was a pilot study with only 7 participants, we feel it is important to emphasize the general concepts more than the specific results. We will consider those first before offering some interpretations of our findings.
We believe this is the first demonstration of any formal e-cigarette video stimuli developed for use in cue-exposure research. Additionally, this is the first application of neuroimaging techniques to study e-cigarette use. In particular, we were interested in finding out if viewing e-cigarette videos would result in a distinct experience compared with viewing other similar but mundane videos. Consistent with our prediction, e-cigarette users displayed a different brain response to the e-cigarette videos than to the electronic toothbrush videos. Importantly, the brain response seems to have been modulated by recent e-cigarette use/nicotine absorption. This result suggests that the e-cigarette videos were successful in evoking some appetitive processing and highlights their usefulness in understanding how e-cigarette users respond to visual cues.
Because a major focus of this study was to develop stimuli for future use in research, some discussion of the stimuli themselves is warranted. One important facet of research design is the tension between internal and external validity. One such decision was manifested in how we staged our videos. For example, individuals use e-cigarettes in a variety of situations, such as alone, with friends, in the car, at the bar, etc. To be most generalizable, our videos would have depicted such varied usages. Indeed, one could argue that the real neuroscientific question of interest is how the brain responds to such real world vignettes. However, it would have been impossible to adequately represent the myriad situations that individuals might use their e-cigarettes. Because this was an initial attempt at video development, we felt it more important to first establish a basic cue-response relationship in tightly controlled videos. We anticipated that the close-up depiction of e-cigarette use would be the focus of attention and highly activating for users, while other elements of the frame (e.g., the background) would not be salient. To our knowledge, comparisons of more “sterile” versus real-world drug stimuli have not been made, and so it is not clear how, if at all, our results would be different had we used less controlled videos. We believe that exploring the neural response to more ecologically valid stimuli is an important future step.
Similarly, electronic toothbrushes, though visually similar to e-cigarettes, may have been less familiar to participants. It is possible that participants had less experience with electronic toothbrushes and thus there was an added novelty to the neutral videos. This option seems unlikely to us, as our impression of the neutral videos was that they would be boring. Our interpretation is somewhat supported by the reported main effect of video type: The posterior cingulate gyrus showed more activation to the neutral videos than to the salient videos. The posterior cingulate cortex is considered part of the default mode network and shows increased activity during internally-directed cognition, such as daydreaming, and deactivation during attention-heavy tasks (for more detailed reviews, see Leech et al., 2011, and Leech & Sharp, 2014). Thus, this is one clue that participants were not paying more attention to the neutral videos than to the salient ones. Nevertheless, it would be wise to explore additional options for control objects in future stimuli development ventures.
Contrary to our expectations, viewing the e-cigarette videos did not increase self-reported craving significantly. This result may be a limitation of our sample size, as group means of craving increased during both exposures to the videos. Three other possibilities are worth mentioning. One may be that this result represents a functional difference between e-cigarette users and users of other substances. As discussed in Section 1, e-cigarette users generally demonstrate lower dependence to their e-cigarettes than smokers do to traditional cigarettes (Etter and Eissenberg, 2015; Foulds et al., 2015). The participants in this study endorsed low to moderate levels of dependence. It may be that low levels of dependence and/or differences in nicotine delivery (e.g., less nicotine per ―puff‖) result in small degrees of cue-elicited craving.
Another notable possibility is that the salient videos were not sufficient to elicit significant levels of subjective craving in this population. This might arise if the types of e-cigarettes depicted differed from those typically used by participants. One of the challenges in conducting craving research with e-cigarette users is the wide range of devices available and their different potential for delivering nicotine (Brown and Cheng, 2014; Vansickel and Eissenberg, 2013), with advanced devices generally being more capable of delivering larger quantities of nicotine and users finding them to be more satisfying than simpler models (Farsalinos et al., 2014; Schroeder and Hoffman, 2014; Spindle and Breland, 2014; Yingst et al., 2015). All e-cigarettes featured in the piloted films were first generation devices, and all participants reported on here used advanced generation devices at the time of the lab visit. Less than half (N = 3) of our participants reported ever owning a first generation e-cigarette and it is unclear what associations all participants had with these types of devices (e.g., dissatisfaction with prior use, positive or negative reminders of cigarette use, or interest in the different form factor). The many different potential paths that lead to a user’s current preferred device create the potential for highly individualized associations with particular devices. It is possible that the depictions of first generation e-cigarette use were less evocative to these participants. This is an important question that needs to be addressed in future research in order to discern the best way(s) to study cue-reactivity in this emerging population.
Finally, the cue exposure protocol (i.e., 30-second video clips presented intermixed) likely was not an ideal match to the timecourse of phasic urge states (e.g., those induced by cues), which tend to persist over the course of minutes (see Sayette and Wilson, 2015). We elected to use an intermixed, repeated presentation design to increase our power to detect changes in brain activation. Nonetheless, future research using alternative designs that more closely match the expected profile of robust urge states would be useful. It is also important to note that self-reported craving was collected before and after each scan session, whereas our fMRI contrasts were designed to compare salient versus neutral videos. In other words, self-reported craving is presumed to measure a more global change in response to viewing the videos (putatively biased by the salient videos), whereas our fMRI data are an examination of more phasic changes in response to the specific video types.
We discussed several possible reasons why we failed to find a significant effect of e-cigarette videos on self-reported craving. Though discordant with our prediction, this result further highlights the importance of additional research on craving in e-cigarette users. It also reinforces the benefit of applying neuroimaging techniques to the study of this population--despite the lack of significant change in self-reported craving, participants’ brains processed these cues differently than other visually similar videos, and this processing was impacted by recent e-cigarette use.
Acknowledging the limitations inherent in a small pilot study, we restrict our discussion of the neuroimaging findings to the general patterns exhibited, with a focus on the session X video condition interaction. We predicted that e-cigarette videos would provoke a greater response than electronic toothbrush videos when participants were abstinent, but that this effect would diminish following e-cigarette use. Instead, we observed the opposite effect: participants seemed to be more responsive to the e-cigarette videos after using their devices than before. There is some precedence for this in other studies of nicotine use (but see McClernon et al., 2005, 2009 for contrary findings). For example, Bruijnzeel et al. (2014) observed greater brain activation to nicotine administration (compared to saline injection) in rats in brain areas commonly associated with reward processing (e.g., nucleus accumbens, amygdala, and insula) and behavioral planning/imagining (e.g., motor and somatosensory cortices). In abstinent cigarette smokers, Xu et al. (2014) reported that wearing a nicotine patch resulted in greater brain activation to cigarette videos in the bilateral ventral striatum and left amygdala when compared with neutral videos. Notably, the placebo patch did not have such an effect, suggesting that nicotine administration was responsible for these cue-related neural responses. Similarly, David et al. (2007) found greater response to pictures of cigarette use in bilateral ventral striatum (including nucleus accumbens) when participants were not abstinent than when they were abstinent. Consistent with these patterns of brain activation, nicotine administration has also been shown to boost the subjective expectation and experience of pleasure in cigarette smokers (Dawkins et al., 2006).
One interpretation of this pattern is that abstinent users may be “primed” by drug administration--that is, they become more sensitive to drug cues after using.
Phenomenologically, this may be related to increased expectations of use, particularly if they were not fully sated by the drug administration (e.g., see Xu et al., 2014). To explore this option more fully in our participants, we conducted post-hoc analyses to assess the degree of fulfilment our participants felt from the vaping protocol. Five minutes after e-cigarette use ceased, participants responded to several VAS items regarding their experience and desire to use an e-cigarette again. One-sample t tests (two-tailed, H0: M = 0) found that participants rated their e-cigarette use to be satisfying (M = 72, t[6] = 5.7, p = .001, dz = 2.15), pleasant (M = 80.4, t[6] = 6.8, p < .001, dz = 2.57), and endorsed a desire to continue e-cigarette use (M = 53.7, t[6] = 3.9, p = .008, dz = 1.47). These data are consistent with the premise that participants were not fully sated following the vaping procedure.
The above mentioned priming effect may also be explained biochemically. Conditions of abstinence have been shown to reduce dopamine release from the nucleus accumbens and, consequently, lower extracellular levels of dopamine in the striatum for multiple drugs of abuse (e.g., Acquas and Di Chiara, 1992; Rossetti et al., 1992; Zijlstra et al., 2008). Importantly, if mesolimbic dopamine release is impaired, this may limit the ability of the dopamine system to produce acute phasic responses to drug cues (or other rewards)--thus resulting in greater brain activation during conditions of non-abstinence (e.g., see David et al., 2007). Of course, we did not collect measures of dopamine in our participants and so this explanation is necessarily speculative.
One notable difference between our results and those reported above is that we did not detect cue-related activity in many of the brain areas often associated with cue reactivity (e.g., anterior cingulate cortex, ventral striatum, orbitofrontal cortex, medial prefrontal cortex, and amygdala, see Wilson et al., 2004). However, we did find significant activity in other areas also observed in cue reactivity paradigms, such as the fusiform gyrus, inferior temporal gyrus, thalamus, and cerebellum (see Engelmann et al., 2012 for a review). For example, David et al. (2007) reported large bilateral temporal-occipital activation, with peaks in the fusiform gyrus and inferior temporal gyrus, in response to smoking cues. McClernon et al. (2005) observed greater activation in the thalamus to smoking cues in a non-abstinent state than to neutral cues (but not in an abstinent state). The cerebellum has received an increasing amount of attention in craving research (Strick et al., 2009), especially in cocaine abusers (Bonson et al., 2002; Grant et al., 1996; Wang et al., 1999), but also in abstinent alcoholics (Schneider et al., 2014). One possible reason for this cerebellar activity is to facilitate episodic memory recall that involves motor control (i.e., behavioral actions).
The parahippocampal gyrus is another memory-related region that demonstrated greater activation to e-cigarette cues than to neutral cues in the post-vape condition. Park et al. (2007) found that activity in this region was correlated with self-reported craving in abstinent alcoholics following cue exposure. The parahippocampal gyrus provides direct input to the hippocampus (Powell et al., 2004), another structure known to be involved in memory formation and one that is often associated with cue-elicited using behavior (e.g., Davis and Gould, 2008; Everitt and Robbins, 2005; Robbins and Everitt, 2002).
Many of the brain areas indicated in the present investigation relate to visuospatial (e.g., precuneus, cuneus) and auditory (temporal gyri) processing and are also noted in other studies (e.g.,Wilson et al., 2005; see also Hanlon et al., 2014; Yalachkov et al., 2015). In this case, these areas may have been activated to support imaginal elaboration of the e-cigarette cues, especially after being sensitized by in vivo e-cigarette use. We offer these interpretations tentatively, noting the great variability in the location of maximal cue-response (Hanlon et al., 2012) and our limited number of participants. Clearly, this is an area of research that needs additional investigation.
E-cigarette use is gaining popularity in the United States, propelled in part by perceptions of its safety and of its usefulness in aiding smoking cessation (Giovenco et al., 2014; Grana and Ling, 2014). However, it is becoming apparent that a large number of e-cigarette adopters continue to use their device(s) long-term (Etter and Bullen, 2011). A few investigators have begun to examine the potential for continued nicotine dependence with e-cigarettes (Etter and Eissenberg, 2015; Foulds et al., 2015), but so far there has been virtually no research on other elements of compulsive use, such as the relationship between cues, craving, and use. We sought to pave the way for this research by developing a set of stimulus videos and demonstrating the feasibility of combining these video cues with fMRI. In this pilot study, experienced e-cigarette users showed a pattern of cue-reactivity that in many ways resembles patterns seen elsewhere in the literature (with activity in visuospatial, auditory, and memory processing areas), particularly after the e-cigarette use protocol. At the same time, self-reported craving did not significantly increase following exposure to the cues and we did not detect cue-related increases in brain activation in other typical areas, such as anterior cingulate cortex, orbitofrontal cortex, medial prefrontal cortex, and amygdala. Our results suggest that a visual cue-exposure paradigm combined with fMRI can be a useful approach to understanding nicotine dependence in e-cigarette users. We also raise several questions about the best ways to approach such research, including the importance of matching cues to user experiences.
Highlights.
We developed novel stimulus videos for use in ecigarette craving research.
We piloted these videos on experienced users in fMRI before and after ecigarette use.
Cue reactivity in sensorimotor and memory brain areas increased after ecigarette use.
Verbal urge ratings did not increase in response to cue exposure.
Match between cues and participants’ own ecigarettes may be especially important.
Acknowledgments
This project was supported by the Penn State Clinical & Translational Research Institute, Pennsylvania State University CTSA (NIH/NCATS Grant Number UL1 TR000127). Additional support was provided by the Penn State Hershey Cancer Institute and the Penn State Social Science Research Institute. JF, JR, SV, JY & SH are primarily funded by the National Institute on Drug Abuse of the National Institutes of Health (NIH-NIDA) and the Center for Tobacco Products of the U.S. Food and Drug Administration (under Award Numbers P50DA03610701, P50DA036105). JF is also supported by research grants from the NIH-NIDA (Award number R21DA038775) and Pfizer. TE’s effort is supported by the NIH-NIDA under Award Number P50DA036105 and the Center for Tobacco Products of the US Food and Drug Administration. SJW is supported by grants from the NIH-NIDA (Award Numbers R21DA038775 and R03DA035929),and the National Cancer Institute (Award Number R21CA190093) of the National Institutes of Health.
The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH, FDA, or any other funding agency.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
JF has done paid consulting for pharmaceutical companies involved in producing smoking cessation medications including GSK, Pfizer, Novartis, J&J, and Cypress Bioscience, and has received a research grant and study drug from Pfizer (not relating to electronic cigarettes).
References
- Abrams DB, Monti PM, Carey KB, Pinto RP, Jacobus SI. Reactivity to smoking cues and relapse: two studies of discriminant validity. Behav. Res. Ther. 1988;26:225–233. doi: 10.1016/0005-7967(88)90003-4. [DOI] [PubMed] [Google Scholar]
- Acquas E, Di Chiara G. Depression of mesolimbic dopamine transmission and sensitization to morphine during opiate abstinence. J. Neurochem. 1992;58:1620–1625. doi: 10.1111/j.1471-4159.1992.tb10033.x. [DOI] [PubMed] [Google Scholar]
- Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob. Res. 2015;17:127–133. doi: 10.1093/ntr/ntu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Scheibal D, London ED, Monterosso JR, Tiffany ST, Korb A, Gan JJ, Cohen MS. Neural substrates of resisting craving during cigarette cue exposure. Biol. Psychiatry. 2007;62:642–651. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CJ, Cheng JM. Electronic cigarettes: product characterisation and design considerations. Tob. Control 23 Suppl. 2014;2:i4–i10. doi: 10.1136/tobaccocontrol-2013-051476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Alexander JC, Perez PD, Bauzo-Rodriguez R, Hall G, Klausner R, Guerra V, Zeng H, Igari M, Febo M. Acute nicotine administration increases BOLD fMRI signal in brain regions involved in reward signaling and compulsive drug intake in rats. Int. J. Neuropsychopharmacol. 2014:18. doi: 10.1093/ijnp/pyu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen C. Electronic cigarettes for smoking cessation. Curr. Cardiol. Rep. 2014;16:538. doi: 10.1007/s11886-014-0538-8. [DOI] [PubMed] [Google Scholar]
- Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, Walker N. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382:1629–1637. doi: 10.1016/S0140-6736(13)61842-5. [DOI] [PubMed] [Google Scholar]
- Bullen C, McRobbie H, Thornley S, Glover M, Lin R, Laugesen M. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tob. Control. 2010;19:98–103. doi: 10.1136/tc.2009.031567. [DOI] [PubMed] [Google Scholar]
- Caponnetto P, Campagna D, Cibella F, Morjaria JB, Caruso M, Russo C, Polosa R. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8:e66317. doi: 10.1371/journal.pone.0066317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll Chapman SL, Wu L-T. E-cigarette prevalence and correlates of use among adolescents versus adults: a review and comparison. J. Psychiatr. Res. 2014;54:43–54. doi: 10.1016/j.jpsychires.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Robinson JD, Lam CY, Wetter DW, Tsan JY, Day SX, Cinciripini PM. A psychometric evaluation of cigarette stimuli used in a cue reactivity study. Nicotine Tob. Res. 2006;8:361–369. doi: 10.1080/14622200600670215. [DOI] [PubMed] [Google Scholar]
- Cobb CO, Shihadeh A, Weaver MF, Eissenberg T. Waterpipe tobacco smoking and cigarette smoking: a direct comparison of toxicant exposure and subjective effects. Nicotine Tob. Res. 2011;13:78–87. doi: 10.1093/ntr/ntq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Munafò MR, Johansen-Berg H, Mackillop J, Sweet LH, Cohen RA, Niaura R, Rogers RD, Matthews PM, Walton RT. Effects of Acute Nicotine Abstinence on Cue-elicited Ventral Striatum/Nucleus Accumbens Activation in Female Cigarette Smokers: A Functional Magnetic Resonance Imaging Study. Brain Imaging Behav. 2007;1:43–57. doi: 10.1007/s11682-007-9004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. Associative learning, the hippocampus, and nicotine addiction. Curr. Drug Abuse Rev. 2008;1:9–19. doi: 10.2174/1874473710801010009. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Corcoran O. Acute electronic cigarette use: nicotine delivery and subjective effects in regular users. Psychopharmacology. 2014;231:401–407. doi: 10.1007/s00213-013-3249-8. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Powell JH, West R, Powell J, Pickering A. A double-blind placebo controlled experimental study of nicotine: I—effects on incentive motivation. Psychopharmacology. 2006;189(3):355–367. doi: 10.1007/s00213-006-0588-8. [DOI] [PubMed] [Google Scholar]
- Droungas A, Ehrman RN, Childress AR, O’Brien CP. Effect of smoking cues and cigarette availability on craving and smoking behavior. Addict. Behav. 1995;20:657–673. doi: 10.1016/0306-4603(95)00029-c. [DOI] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am. J. Psychiatry. 2014 doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, Brown VL, Cinciripini PM. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage. 2012;60:252–262. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter J-F, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106:2017–2028. doi: 10.1111/j.1360-0443.2011.03505.x. [DOI] [PubMed] [Google Scholar]
- Etter J-F, Eissenberg T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015;147:68–75. doi: 10.1016/j.drugalcdep.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, Voudris V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci. Rep. 2014;4:4133. doi: 10.1038/srep04133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J, Veldheer S, Yingst J, Hrabovsky S, Wilson SJ, Nichols TT, Eissenberg T. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking E-cigarette users. Nicotine Tob. Res. 2015;17:186–192. doi: 10.1093/ntr/ntu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O’Brien CP, Detre JA, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Rabinovich NE. Department of Psychology. Southern Illinois University; 1999. International smoking image series (with neutral counterparts), version 1.2. Carbondale, Integrative Neuroscience Laboratory. [Google Scholar]
- Giovenco DP, Hammond D, Corey CG, Ambrose BK, Delnevo CD. E-Cigarette Market Trends in Traditional U.S. Retail Channels, 2012-2013. Nicotine Tob. Res. 2014 doi: 10.1093/ntr/ntu282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E. Analysis of FIAC data with BrainVoyager QX: from single-subject to cortically aligned group GLM analysis and self-organizing group ICA. Hum. Brain Mapp. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grana RA, Ling PM. “Smoking revolution”: A content analysis of electronic cigarette retail websites. Am. J. Prev. Med. 2014;46:395–403. doi: 10.1016/j.amepre.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc. Natl. Acad. Sci. U. S. A. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Dowdle LT, Naselaris T, Canterberry M, Cortese BM. Visual cortex activation to drug cues: a meta-analysis of functional neuroimaging papers in addiction and substance abuse literature. Drug Alcohol Depend. 2014;143:206–212. doi: 10.1016/j.drugalcdep.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Jones EM, Li X, Hartwell KJ, Brady KT, George MS. Individual variability in the locus of prefrontal craving for nicotine: implications for brain stimulation studies and treatments. Drug Alcohol Depend. 2012;125:239–243. doi: 10.1016/j.drugalcdep.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS. Tobacco smoke carcinogens and breast cancer. Environ. Mol. Mutagen. 2002;39:119–126. doi: 10.1002/em.10071. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Tobacco smoke carcinogens and lung cancer. J. Natl. Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Hoffmann D. Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis. 1988;9:875–884. doi: 10.1093/carcin/9.6.875. [DOI] [PubMed] [Google Scholar]
- Hughes J, Hatsukami DK. Errors in using tobacco withdrawal scale. Tob. Control. 1998;7:92–93. doi: 10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, deB Frederick B, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol. Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassel JD, Shiffman S. What can hunger teach us about drug craving? A comparative analysis of the two constructs. Advances in Behaviour Research and Therapy. 1992;14:141–167. [Google Scholar]
- Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31(9):3217–3224. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain: A Journal of Neurology. 2014;137(Pt 1):12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BA, Alam S, Promoff G, Arrazola R, Dube SR. Awareness and ever-use of electronic cigarettes among U.S. adults, 2010-2011. Nicotine Tob. Res. 2013;15:1623–1627. doi: 10.1093/ntr/ntt013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005;30:1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Lutz AM, Rose JE. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology. 2009;204:25–35. doi: 10.1007/s00213-008-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nides MA, Leischow SJ, Bhatter M, Simmons M. Nicotine blood levels and short-term smoking reduction with an electronic nicotine delivery system. Am. J. Health Behav. 2014;38:265–274. doi: 10.5993/AJHB.38.2.12. [DOI] [PubMed] [Google Scholar]
- Noel JK, Rees VW, Connolly GN. Electronic cigarettes: a new “tobacco” industry? Tob. Control. 2011;20:81. doi: 10.1136/tc.2010.038562. [DOI] [PubMed] [Google Scholar]
- Park M-S, Sohn J-H, Suk J-A, Kim S-H, Sohn S, Sparacio R. Brain substrates of craving to alcohol cues in subjects with alcohol use disorder. Alcohol Alcohol. 2007;42:417–422. doi: 10.1093/alcalc/agl117. [DOI] [PubMed] [Google Scholar]
- Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21:7435–7451. doi: 10.1038/sj.onc.1205803. [DOI] [PubMed] [Google Scholar]
- Powell H, Guye M, Parker G, Symms MR. Noninvasive in vivo demonstration of the connections of the human parahippocampal gyrus. Neuroimage. 2004 doi: 10.1016/j.neuroimage.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol. Learn. Mem. 2002;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Brain Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur. J. Pharmacol. 1992;221:227–234. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Wilson SJ. The measurement of desires and craving. In: Hofmann W, Nordgren L, editors. The Psychology of Desire. Guilford Publications; New York, NY: 2015. p. 104. [Google Scholar]
- Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, Toni I, Sulzbach C, Hönig K, Maier W. Subcortical correlates of craving in recently abstinent alcoholic patients. Am. J. Psychiatry. 2014 doi: 10.1176/appi.ajp.158.7.1075. Others. [DOI] [PubMed] [Google Scholar]
- Schroeder MJ, Hoffman AC. Electronic cigarettes and nicotine clinical pharmacology. Tob. Control. 2014;23(Suppl 2):i30–i35. doi: 10.1136/tobaccocontrol-2013-051469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. Comments on craving. Addiction. 2000;95:S171–S175. doi: 10.1080/09652140050111744. [DOI] [PubMed] [Google Scholar]
- Spindle TR, Breland AB. Preliminary results of an examination of electronic cigarette user puff topography: The effect of a mouthpiece-based topography measurement device on plasma …. Nicotine Tob. Res. 2014 doi: 10.1093/ntr/ntu186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu. Rev. Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol. Rev. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tong C, Bovbjerg DH, Erblich J. Smoking-related videos for use in cue-induced craving paradigms. Addict. Behav. 2007;32:3034–3044. doi: 10.1016/j.addbeh.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE. A Clinical Laboratory Model for Evaluating the Acute Effects of Electronic “Cigarettes”: Nicotine Delivery Profile and Cardiovascular and Subjective Effects. Cancer Epidemiol. Biomarkers Prev. 2010;19:1945–1953. doi: 10.1158/1055-9965.EPI-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob. Res. 2013;15:267–270. doi: 10.1093/ntr/ntr316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Engelmann JM, Robinson JD, Jackson EF, Green CE, Lam CY, Minnix JA, Karam-Hage MA, Brown VL, Wetter DW, Cinciripini PM. Prequit fMRI responses to pleasant cues and cigarette-related cues predict smoking cessation outcome. Nicotine Tob. Res. 2014;16:697–708. doi: 10.1093/ntr/ntt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, Wong CT, Felder C. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64:775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- Williams M, Talbot P. Variability among electronic cigarettes in the pressure drop, airflow rate, and aerosol production. Nicotine Tob. Res. 2011;13:1276–1283. doi: 10.1093/ntr/ntr164. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA. Neuroimaging craving: urge intensity matters. Addiction. 2015;110:195–203. doi: 10.1111/add.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Delgado MR, Fiez JA. Instructed smoking expectancy modulates cue-elicited neural activity: a preliminary study. Nicotine Tob. Res. 2005;7:637–645. doi: 10.1080/14622200500185520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat. Neurosci. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Clark US, David SP, Mulligan RC, Knopik VS, McGeary J, MacKillop J, McCaffery J, Niaura RS, Sweet LH. The Effects of Nicotine Deprivation and Replacement on BOLD-fMRI Response to Smoking Cues as a Function of DRD4 VNTR Genotype. Nicotine Tob. Res. 2014 doi: 10.1093/ntr/ntu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalachkov Y, Kaiser J, Naumer MJ. The Wiley Handbook on the Cognitive Neuroscience of Addiction. John Wiley & Sons, Ltd; 2015. The Role of Sensory and Motor Brain Regions in Drug-Cue Reactivity; pp. 173–194. [Google Scholar]
- Yan XS, D’Ruiz C. Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes. Regul. Toxicol. Pharmacol. 2015;71:24–34. doi: 10.1016/j.yrtph.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Yingst JM, Veldheer S, Hrabovsky S, Nichols TT, Wilson SJ, Foulds J. Factors Associated With Electronic Cigarette Users’ Device Preferences and Transition From First Generation to Advanced Generation Devices. Nicotine Tob. Res. 2015 doi: 10.1093/ntr/ntv052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra F, Booij J, van den Brink W, Franken IHA. Striatal dopamine D2 receptor binding and dopamine release during cue-elicited craving in recently abstinent opiate-dependent males. Eur. Neuropsychopharmacol. 2008;18:262–270. doi: 10.1016/j.euroneuro.2007.11.002. [DOI] [PubMed] [Google Scholar]