Abstract
Relapse following removal of an alternative source of reinforcement introduced during extinction of a target behavior is called resurgence. This form of relapse may be related to relapse of drug taking following loss of alternative non-drug reinforcement in human populations. Laboratory investigations of factors mediating resurgence with food-maintained behavior suggest higher rates of alternative reinforcement produce faster suppression of target behavior but paradoxically generate more relapse when alternative reinforcement is discontinued. At present, it is unknown if a similar effect occurs when target behavior is maintained by drug reinforcement and the alternative is a non-drug reinforcer. In the present experiment three groups of rats were trained to lever press for infusions of cocaine during baseline. Next, during treatment, cocaine reinforcement was suspended and an alternative response was reinforced with either high-rate, low-rate, or no alternative food reinforcement. Finally, all reinforcement was suspended to test for relapse of cocaine seeking. Higher rate alternative reinforcement produced faster elimination of cocaine seeking than lower rates or extinction alone, but when treatment was suspended resurgence of cocaine seeking occurred following only high-rate alternative reinforcement. Thus, although higher rate alternative reinforcement appears to more effectively suppress drug seeking, should it become unavailable, it can have the unfortunate effect of increasing relapse.
Keywords: resurgence, relapse, drug self-administration, alternative-reinforcement rate, extinction, operant behavior
Alternative-reinforcement based treatments are often used to eliminate problematic human behavior such as pathological drug taking. In these treatments, non-drug rewards are made available for engaging in behavior unrelated to drug taking or abstaining from drug taking [1][2][3]. Though these treatments often suppress unwanted behavior while enforced, lapses in treatment fidelity (e.g., interruptions in or cessation of alternative-reinforcer delivery) have been shown to produce relapse [3][4]. Relapse following omission of an alternative source of reinforcement is termed resurgence [5][6][7].
In the laboratory, resurgence in non-humans typically is studied using a three-phase preparation [8][9]. In Phase 1 (baseline), a target behavior produces reinforcement. During Phase 2 (treatment), reinforcement for the target response is discontinued while an alternative source of reinforcement is made available. Finally, the alternative source of reinforcement is discontinued in Phase 3 to test for resurgence of target responding. Using these procedures, resurgence of ethanol [5][10] and cocaine [11][12] seeking has been demonstrated in rats when alternative food reinforcement is discontinued. Given these findings, some have suggested resurgence might provide a useful model of the effects of loss of non-drug reinforcement on drug seeking following treatments using alternative non-drug reinforcement [13][14][15].
In laboratory examinations of resurgence of food-maintained target behavior in animals, higher rates of alternative reinforcement have been shown to produce faster and more complete suppression of target responding during Phase 2 but greater relapse when alternative reinforcement is discontinued in Phase 3 [9][16][17]. It is unclear, however, if these effects also occur when target behavior is maintained by drug reinforcement. The present experiment thus aimed to determine if rate of alternative non-drug reinforcement has similar effects on cocaine-maintained responding in rats.
Fifteen male Long-Evans rats (Charles River, Portage, MI) were used. Rats were 71–80 days old upon arrival and ~450 g at the time of jugular-catheterization surgery (see below). Rats were housed in a temperature-controlled colony room with a 12:12-hr light/dark cycle (lights on at 7:00 AM) and had free access to water in their home cages. Rats were allowed free access to rat chow in their home cages until they reached their pre-surgery weight following surgery, then they were food restricted to 80% of their free-feeding body weight.
Prior to the experiment, rats underwent jugular-catheterization surgery that was preceded by injections of antibiotic (gentamicin, 2.0 mg/kg, IP) and analgesic/anti-inflammatory (flunixin meglumine, 1.1 mg/kg, SC) drugs. Rats were anesthetized using isoflurane. An indwelling, back-mounted cannula (Plastics One, Roanoke, VA) attached to a silastic catheter (SAI-Infusions, Lake Villa, IL) was inserted through an incision in the rat’s lower back and fed subcutaneously to a 0.5-cm incision centered 2 cm below the rat’s shoulder blades. The catheter was fed subcutaneously from the upper-back incision to an incision made in the right ventral surface of the neck, then inserted into the right jugular vein. Following surgery, rats were given injections of flunixin meglumine (1.1 mg/kg, SC), gentamicin (2.0 mg/kg, IP), and electrolyte solution (Ringer’s, 5 cc, SC) every 12 hr for 2–5 days. Rats began the procedure detailed below after reaching their 80% free-feeding weights. Cocaine hydrochloride was dissolved in a sterile 0.9% saline solution to a concentration 2.56 mg/ml. Drug doses were adjusted by changing the activation duration of the fixed-speed (0.0527 ml/s) syringe pump.
Ten Med Associates (St. Albans, VT) operant chambers were used during the study. Each chamber was equipped with two levers, with stimulus lights above them, on either side of a food receptacle. In this receptacle, 45-mg food pellets (BioServ, Flemington, NJ) could be delivered. A house light was situated at the top-center of this panel was used for general illumination of the chamber. The chamber wall opposite the levers and food aperture housed five small holes equipped with photobeams to detect nose pokes. These holes could be illuminated individually by yellow LEDs. The chambers also were equipped for IV drug self-administration. Tygon tubing encased in a metal-spring tether was inserted through a hole cut in the center of the roof of each camber. At all times, the Tygon tubing and tether were attached to rats’ back-mounted cannulae. The other end of the tubing attached to a stainless-steel swivel (Instech, Plymouth Meeting, PA) suspended above the roof of the chamber. Tygon tubing attached the swivel to a 60-ml syringe mounted on an infusion pump (Med Associates) positioned outside the sound-attenuating cabinet.
Prior to the experiment, rats were trained to consume food pellets from the illuminated food aperture. No stimuli were on during pellet training, and the levers were retracted. Response-independent pellets were delivered into the aperture according to a variable-time (VT) 60-s schedule in four, 45-min sessions (session durations for the remainder of the experiment also were 45 min). Next, rats were trained to self-administer cocaine. These training sessions began with illumination of the house light, insertion of both levers into the chamber, and illumination of the stimulus light above the right (active) lever. Presses to this lever initially produced 1 mg/kg infusions of cocaine, accompanied by a 45-s blackout of the chamber, according to a fixed-ratio (FR) 1 schedule. At all times, presses to the left (inactive) lever were recorded but had no consequences. Across sessions, the ratio requirement was thinned to an FR 4. Then a variable-ratio (VR) 2 schedule replaced the FR 4 schedule. The VR requirement was increased across days in increments of two responses until all rats reliably self-administered 1 mg/kg infusions of cocaine under a VR 20 schedule. Finally, infusions of cocaine were decreased to 0.5 mg/kg then 0.32 mg/kg across sessions [11].
Phase 1 (baseline) began once rats reached the 0.32 mg/kg dose of cocaine. This phase lasted a minimum of five sessions and until no downward trends in responding were observed across the last three sessions of the phase. Rats then were distributed to three groups of five such that mean mg/kg of cocaine infused across the last three sessions of Phase 1 were equivalent between groups. In Phase 2 (treatment), cocaine availability was suspended for all groups and the left-most nose poke aperture was illuminated. For group High-Rate Alternative, pokes into this aperture produced food pellets according to a variable-interval (VI) 15-s schedule. For group Low-Rate Alternative, pokes produced food according to a VI 60-s schedule. Finally, for group Extinction Control, pokes were recorded but had no consequences. This phase lasted for 15 sessions, then Phase 3 (resurgence test) commenced. In Phase 3, alternative food reinforcement was suspended for the High-Rate and Low-Rate groups, while no change in contingencies occurred for the Control group, in a single session. Statistical tests reported below were deemed significant at an α level of .05.
During the last three sessions of Phase 1, cocaine response rates (for the High-Rate, Low-Rate, and Control groups, respectively: M = 7.82, SEM = 0.83; M = 7.82, SEM = 0.88; and M = 7.69, SEM = 0.82 responses per min) and mg/kg of cocaine infused per session (M = 5.53, SEM = 0.55; M = 5.53, SEM = 0.58; and M = 5.31, SEM = 0.51 mg/kg) were similar between groups. Alternative response rates (for the High-Rate, Low-Rate, and Control groups, respectively: M = 0.1, SEM = 0.06; M = 0.63, SEM = 0.46; and M = 0.39, SEM = 0.14 responses per min) and inactive response rates (M = 0.21, SEM = 0.08; M = 0.23, SEM = 0.07; and M = 0.33, SEM = 0.16 responses per min) were near zero for this phase.
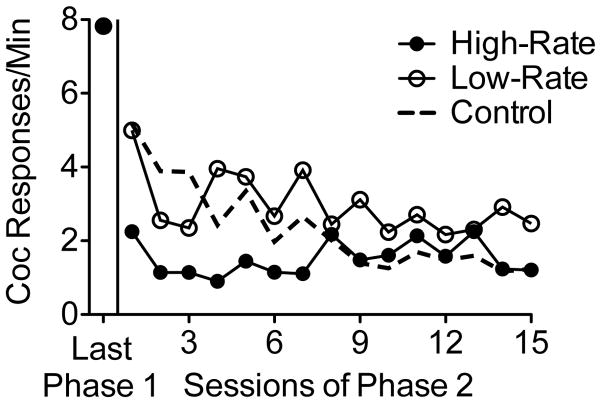
Cocaine seeking decreased across sessions of Phase 2 for all groups (see Figure 1). A 3 × 15 (Group X Session) mixed-model analysis of variance (ANOVA) revealed that these decreases were significant [main effect of Session, F(14, 168) = 4.06, MSE = 5.99] and varied significantly between groups [Group X Session interaction, F(28, 168) = 2.01, MSE = 2.92]; the main effect of Group was not significant, F(2, 12) = 0.99, MSE = 39.61. To isolate the source of the significant Group X Session interaction, follow-up 2 × 15 (Group X Session) ANOVA were conducted for each pairwise group comparison. Cocaine-seeking decreased more rapidly in the High-Rate group than in the Low-Rate, F(14, 112) = 2.31, MSE = 2.23, and Control, F(14, 112) = 2.42, MSE = 4.13, groups. Cocaine-seeking decreased at comparable rates between the Low-Rate and Control groups, F(14, 112) = 1.42, MSE = 2.41. During the final session of Phase 2, cocaine response rates did not differ between groups [one-way ANOVA: F(2, 12) = 1.01, MSE = 2.6]. Thus, cocaine seeking initially decreased most quickly for the High-Rate group, but terminal Phase-2 responding was similar between groups.
Figure 1.
Mean rates of cocaine seeking during the last three sessions of Phase 1 (baseline) and across sessions of Phase 2 (Treatment) for each group.
Alternative responding (i.e., nose poking for food) increased during Phase 2 for the High-Rate and Low-Rate groups. In the final session, an average of 73.97 (SEM = 22.71) and 25.09 (SEM = 6.56) pokes per min, respectively, occurred. Also in this session, an average of 3.42 (SEM = 0.18) and 0.92 (SEM = 0.04) pellets were earned per min by the High-Rate and Low-Rate groups, and alternative responding remained low in the Control group (M = 0.26, SEM = 0.12 responses per min). Further, inactive responding occurred at near-zero rates for the High-Rate, Low-Rate, and Control groups in the final Phase-2 session (respectively: M = 1.02, SEM = 0.89; M = 0.6, SEM = 0.29; and M = 0.53, SEM = 0.4 responses per min).
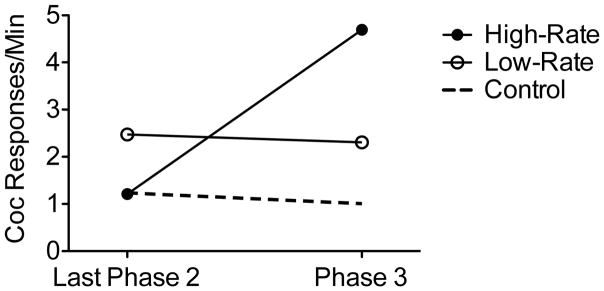
Figure 2 shows response rates from the last session of Phase 2 and during Phase 3 for each group. A 3 × 2 (Group X Phase) mixed-model ANOVA revealed a significant main effect of Phase, F(1, 12) = 6.01, MSE = 7.91, and a significant Group X Phase interaction, F(2, 12) =8.55, MSE = 8.55. The main effect of Group was not significant, F(2, 12) = 1.99, MSE = 8.8. To isolate the source of the significant Group X Phase interaction, follow-up 2 × 2 (Group X Phase) ANOVA were conducted for each pairwise group comparison. The Group X Phase interaction was present when the High-Rate group was compared to the Low-Rate, F(1, 8) = 10.41, MSE = 16.64, and Control, F(1, 8) = 10.14, MSE = 17.26, groups. No interaction was present when the Low-Rate group was compared to the Control group, F(1, 8) = 0.01, MSE = 0.01. Thus, resurgence occurred only for the High-Rate group. Further, resurgence was specific to the lever previously associated with cocaine. Inactive responding remained low for the High-Rate, Low-Rate, and Control groups (respectively: M = 0.87, SEM = 0.61; M = 0.45, SEM = 0.05; and M = 0.45, SEM = 0.2 responses per min) during resurgence testing.
Figure 2.
Mean rates of cocaine seeking during the last session of Phase 2 (Treatment) and Phase 3 (Resurgence) for each group.
To summarize, high-rate non-drug alternative reinforcement produced a faster initial decrease in cocaine seeking than low-rate alternative reinforcement or extinction alone. When high-rate alternative reinforcement was suspended, however, resurgence of cocaine seeking was observed. Finally, low-rate alternative reinforcement had no impact on cocaine seeking when compared to extinction alone. These data provide further support for the finding that higher rates of alternative reinforcement produce faster decreases in, and greater resurgence of, behavior that is targeted for elimination [9][16][17]. Further, and most importantly, they extend this finding to drug-maintained behavior, suggesting similar underlying processes are involved in resurgence of drug- and non-drug-maintained responding.
What remains unclear is precisely why higher rates of alternative reinforcement produce faster target response suppression and resurgence. In early treatments of resurgence, some argued that high-rate alternative reinforcement might prevent organisms from engaging in the target behavior and thus prevent extinction [9]. According to this response-prevention hypothesis, resurgence occurs following suspension of alternative reinforcement simply because the target behavior was not sufficiently extinguished. Although the present findings cannot completely rule out this interpretation, it is worth noting that rats in the High-Rate group made more than 1200 cocaine-lever responses on average during Phase 2. Further, the response-prevention hypothesis has generally been rejected [e.g., 7] because resurgence has been shown under conditions where target behavior is fully extinguished prior to introduction of alternative reinforcement [18] and where substantially more target responding occurs with alternative reinforcement than without it [7]. Thus, the magnitude of resurgence appears to be unrelated to the amount of target responding during treatment, and other mechanisms seem to be required to account for the effects of high-rate alternative reinforcement on resurgence.
As one alternative, Shahan and Sweeney’s [6] quantitative model of resurgence suggests alternative sources of reinforcement during treatment (Phase 2) serve two opposing functions. These reinforcers further disrupt target responding during extinction and strengthen the Pavlovian association between reinforcers and the context in which drug taking occurred. This context-reinforcer relation is thought to promote persistence and relapse of drug seeking in a manner positively related to reinforcement rate. When alternative reinforcers are suspended in Phase 3, the additive disruptive impact is removed, but the context-reinforcer relation is unchanged. Thus, their model predicts that higher rates of alternative reinforcement produce faster suppression of drug seeking during Phase 2 and greater resurgence during Phase 3. Although this quantitative model of resurgence accurately predicts that higher-rate alternative reinforcement should produce faster suppression of cocaine seeking, it also predicts that low-rate alternative reinforcement should disrupt target responding. To the contrary, low-rate alternative reinforcement did not suppress drug seeking beyond extinction alone in the present experiment. Further, several other published findings [16][17] have demonstrated contra-therapeutic effects of low-rate alternative reinforcement. That is, low-rate alternative reinforcement can produce more persistent target responding than extinction alone. Thus, this theory does not appear to provide a full account of the present results or resurgence in general.
Alternatively, Bouton and colleagues [7][19] have argued that resurgence results from the context specificity of extinction learning [20]. According to this account, organisms learn to inhibit target behavior during Phase 2 extinction. The change to the novel Phase-3 contingencies produce a change from a context in which inhibitory learning occurred to a context in which inhibitory learning fails to generalize. Presumably, the degree of response suppression engendered by alternative reinforcement should be related to the difference between Phase-1 and -2 reinforcer rates, and the degree of resurgence observed is related to the difference between Phases 2 and 3 rates [17]. Like Shahan and Sweeney’s [6] quantitative account of resurgence, though, Bouton and colleagues’ context hypothesis appears incomplete. For example, similar-fold increases and decreases in reinforcer rates between Phases 1 and 2 should produce equally discriminable Phase-1 and -2 contexts, yet these manipulations do not produce similar decreases in responding [17]. Because the account has not been formalized, however, it is restricted to making qualitative predictions, and it becomes unclear precisely how any given environmental factor (e.g., alternative-reinforcement rate) might be expected to specifically impact the inferred context change [21]. Thus, it can be difficult to know if any given result is consistent with the predictions of the approach or not. Despite these limitations, one appealing feature of the context hypothesis is that it may be used to describe not only resurgence but also other forms of relapse (e.g., renewal, reinstatement, spontaneous recover) in a parsimonious manner. Thus, if the theory were to be formalized such that its predictions could be more clearly specified and tested, it could hold the promise of a formal integrative approach to understanding relapse.
Finally, resurgence could result from stress induced by loss of expected reinforcement [11]. Though little is known about the link between stress and resurgence, substantial evidence suggests exposure to acute stressors can produce relapse (i.e., reinstatement) of drug seeking [22]. This proposal also is consistent with evidence showing that clonidine, an α-2 receptor agonist, decreases resurgence of food-maintained behavior [23]. Loss of higher rates of alternative reinforcement in the present experiment might have been more stressful than loss of lower rates of alternative reinforcement, thus generating more resurgence. This hypothesis is speculative because effects of stress-related pharmacological manipulations on resurgence are thus far restricted to resurgence of food seeking. Because stress appears to play a critical roll in other forms of relapse, however, it is plausible stress induced by loss of non-drug reinforcement could provide a neurobiological mechanism for resurgence in general. This possibility deserves considerably more empirical investigation.
Regardless of the biobehavioral processes responsible for resurgence, the present findings suggest that a better understanding of the phenomenon might help to inform substance-abuse treatments employing alternative non-drug reinforcement. For example, consistent with the present finding that higher rates of alternative non-drug reinforcement produced faster reductions in drug seeking, larger magnitude non-drug alternative reinforcers typically produce greater abstinence during treatment and during post-treatment follow-ups in contingency-management interventions [4][24][25]. It is important to note, however, that if abstinence is not achieved during treatment, as is often the case for lower magnitude groups in contingency-management interventions, drug use during follow-ups does not reflect relapse, but rather a continuation of failure to achieve abstinence during treatment. Interestingly, Higgins and colleagues [24] showed that for participants who achieved similar levels of abstinence during treatment with larger and smaller magnitude alternative reinforcers, those who had earned larger alternative reinforcers were less likely to remain abstinent at 9–24 month follow-ups. Although this outcome was unexpected by Higgins and colleagues, it is consistent with the present finding that despite similar levels of drug seeking at the end of treatment, exposure to more alternative reinforcement during treatment generated increased relapse when alternative reinforcement was no longer available. Regardless, the resurgence paradigm is not a direct animal model of contingency management or any other specific treatment for substance abuse. Drug use may cease for any number of reasons including self-attempts at quitting or explicit treatments like contingency management. Loss of alternative non-drug reinforcement might contribute to drug relapse in any of these situations. A better understanding of the behavioral and neurobiological processes responsible for such relapse might inform future attempts to prevent it.
Highlights.
Examined effects of alternative-reinforcer rate on relapse of rats’ cocaine seeking
Higher rate alternative reinforcement suppressed cocaine seeking faster
Higher rate alternative reinforcement generated more resurgence
Treatments using more alternative reinforcement could increase relapse when treatment ends
Acknowledgments
This work was supported by NIH grant 1R21DA037725-01(TAS). The authors thank Kaitlyn O. Browning, Paul J. Cunningham, and Charles C. J. Frye for their assistance in conducting this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Higgins ST, Heil SH, Sigmon SC. Voucher-based contingency management in the treatment of substance use disorders. In: Madden GJ, Dube WV, Hackenberg TD, Hanley GP, Lattal KA, editors. APA handbook of behavior analysis, vol. 2: Translating principles into practice. Washington, DC: American Psychological Association; 2013. pp. 481–500. [DOI] [Google Scholar]
- 2.Meyers RJ, Roozen HG, Smith JE. The community reinforcement approach: An update of the evidence. Alcohol Res Health. 2011;33:380–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Secades-Villa R, Garcia Rodrigez O, Garcia-Fernandez G, Sanchez-Harvas E, Mermandez-Hermida JR, Higgins ST. Community reinforcement approach plus vouchers among cocaine-dependent outpatients: Twelve-month outcomes. Psychol Addict Behav. 2011;25:174–9. doi: 10.1037/a0021451. [DOI] [PubMed] [Google Scholar]
- 4.Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: Effects of reinforcement magnitude. Psychopharmacology. 1999;146:128–38. doi: 10.1037/1064-1297.9.3.317. [DOI] [PubMed] [Google Scholar]
- 5.Podlesnik CA, Jimemez-Gomez C, Shahan TA. Resurgence of alcohol seeking produced by discontinuing non-drug reinforcement as an animal model of drug relapse. Behav Pharmacol. 2006;17:369–74. doi: 10.1097/01.fbp.0000224385.09486.ba. [DOI] [PubMed] [Google Scholar]
- 6.Shahan TA, Sweeney MM. A model of resurgence based on behavioral momentum theory. J Exp Anal Behav. 2011;95:91–108. doi: 10.1901/jeab.2011.95-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winterbauer NE, Bouton ME. Mechanisms of resurgence of an extinguished instrumental behavior. J Exp Psychol Anim Behav Process. 2010;36:343–53. doi: 10.1037/a0017265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leitenberg H, Rawson RA, Bath K. Reinforcement of competing behavior during extinction. Science. 1970;169:301–3. doi: 10.1126/science.169.3942.301. [DOI] [PubMed] [Google Scholar]
- 9.Leitenbert H, Rawson RA, Mulick JA. Extinction and reinforcement of alternative behavior. J Comp Physiol Psychol. 1975;88:640–52. doi: 10.1037/h0076418. [DOI] [Google Scholar]
- 10.Pyszczynski AD, Shahan TA. Loss of nondrug reinforcement in one contest produces alcohol seeking in another context. Behav Pharmacol. 2013;24:496–503. doi: 10.1097/FBP.0b013e328364502a. [DOI] [PubMed] [Google Scholar]
- 11.Quick SL, Pyszczynski AD, Colston KA, Shahan TA. Loss of alternative non-drug reinforcement induces relapse of cocaine-seeking in rats: Role of dopamine D1 receptors. Neuropsychopharmacology. 2011;36:1015–20. doi: 10.1038/npp.2010.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shahan TA, Craig AR, Sweeney MM. Resurgence of sucrose and cocaine seeking in free-feeding rats. Behav Brain Res. 2015;269:47–51. doi: 10.1016/j.bbr.2014.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchant NJ, Li X, Shaham Y. Recent developments in animal models of drug relapse. Curr Opin Neurobiol. 2013;23:675–83. doi: 10.1016/j.conb.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peck JA, Ranaldi R. Drug abstinence: Exploring animal models and behavioral treatment strategies. Psychopharmacology. 2014;231:2045–58. doi: 10.1007/s00213-014-3517-2. [DOI] [PubMed] [Google Scholar]
- 15.Bouton ME, Schepers ST. Resurgence of instrumental behavior after an abstinence contingency. Learn Behav. 2014;42:131–143. doi: 10.3758/s13420-013-0130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sweeney MM, Shahan TA. Effects of high, low, and thinning rates of alternative reinforcement on response elimination and resurgence. J Exp Anal Behav. 2013;100:102–16. doi: 10.1002/jeab.26. [DOI] [PubMed] [Google Scholar]
- 17.Bouton ME, Trask S. Role of the discriminative properties of the reinforcer in resurgence. Learn Behav. 2015 doi: 10.3758/s13420-013-0130-x. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epstein R. Resurgence of previously reinforced behavior during extinction. Behav Anal Lett. 1983;3:391–7. [Google Scholar]
- 19.Bouton ME, Winterbauer NE, Todd TP. Relapse processes after the extinction of instrumental learning: Renewal, resurgence, and reacquisition. Behav Processes. 2012;90:130–41. doi: 10.1016/j.beproc.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouton ME, Todd TP. A fundamental role for context in instrumental learning and extinction. Behav Processes. 2014;104:13–9. doi: 10.1016/j.beproc.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McConnell BL, Miller RR. Associative accounts of recovery-from-extinction effects. Learn Motiv. 2014;46:1–15. doi: 10.1016/j.lmot.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y. Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology. 2016;41:225–56. doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pyszczynski AD, Shahan TA. Examination of the role of Dopamine D2 and Adrenergic α2 Receptors in Resurgence. Behav Brain Res. 2014;271:122–8. doi: 10.1016/j.bbr.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins ST, Heil SH, Dantona R, Donham R, Matthews M, Badger GJ. Effects of varying the monetary value of voucher-based incentives on abstinence achieved during and following treatment among cocaine-dependent outpatients. Addiction. 2007;102:271–81. doi: 10.1111/j.1360-0443.2006.01664.x. [DOI] [PubMed] [Google Scholar]
- 25.Petry NM, Barry D, Alessi SM, Rounsaville BJ, Carroll KM. A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. J Consult Clin Psychol. 2012;80:276–85. doi: 10.1037/a0026883. [DOI] [PMC free article] [PubMed] [Google Scholar]