Abstract
Myocardial infarctions and chronic ischemic heart disease both commonly and disproportionately affect elderly patients more than any other patient population. Despite available treatments, heart tissue is often permanently damaged as a result of cardiac injury. This review aims to summarize recent literature proposing the use of modified autologous adult stem cells to promote healing of post-infarct cardiac tissue. This novel cellular treatment involves isolation of adult stem cells from the patient, in vitro manipulation of these stem cells, and subsequent transplantation back into the patient’s own heart to accelerate healing. One of the hindrances affecting this process is that cardiac issues are increasingly common in elderly patients, and stem cells recovered from their tissues tend to be pre-senescent or already in senescence. As a result, harsh in vitro manipulations can cause the aged stem cells to undergo massive in vivo apoptosis after transplantation. The consensus in literature is that inhibition or reversal of senescence onset in adult stem cells would be of utmost benefit. In fact, it is believed that this strategy may lower stem cell mortality and coerce aged stem cells into adopting more resilient phenotypes similar to that of their younger counterparts. This review will discuss a selection of the most efficient and most-recent strategies used experimentally to enhance the effectiveness of current stem cell therapies for ischemic heart diseases.
Keywords: Heart failure, Myocardial infarction, Stem cell, Cell survival, Cell senescence, Preconditioning, Rejuvenation
Introduction
Adult stem cells are a unique type of cell present in nearly every tissue in the body. These cells retain the ability to remain dormant for unspecified periods of time, and when needed, are capable of differentiation to adopt the precise function necessary to maintain, grow, or repair surrounding tissue [1]. This review focuses on cardiac, mesenchymal, and hematopoietic stem cells, all of which are adult stem cells and can be extracted from a consenting patient through various common clinical methods. They are then isolated, cultured and modified in vitro, and later transplanted back into that patient’s own heart to assist in healing of the damaged post-infarct myocardium [2]. Myocardial infarctions and other ischemic heart conditions are most common in the elderly, and can cause permanent damage to the heart, preventing proper recovery of cardiac tissue and function. Adult stem cells hold great potential to heal the damaged heart tissue in such cases, and thus can be utilized as treatment to accelerate healing and regeneration of myocardial infarction or otherwise damaged cardiac tissue [3]. Many clinicians currently prefer to utilize autologous adult cardiac stem cells due to the relative low cost and safety as compared to embryonic stem cell (ESC) or induced pluripotent stem cell (iPSC) use. Additionally, adult stem cells are not blocked by the various legal, religious, immune rejection, and ethical barriers that inevitably arise with the use of embryonic stem cells, or the high cost associated with the use of iPSCs.
Cardiac diseases disproportionately affect elderly patients rather than younger patients. Stem cells harvested from elderly patients thus tend to originate from aged niches, a direct effect of senescence-related factors collectively exerting negative effects on the environment, thereby slowing down natural cellular mechanisms. Telomeres in the stem cell chromosomes gradually become shorter and are likely to have suffered chemical and/or UV radiation damage throughout the lifetime of the patient [4]. Such age-associated changes within the stem cell negatively alters gene expression, which is crucial to the functional properties and to both the replicative and differentiation potentials of stem cells [5]. It is thought that cells from children and young adults may innately secrete proteins conducive to rapid growth and that such mechanisms may be slowed down or repressed as adult’s age. Thus, it is important to study ways of restoring pro-growth pathways in vitro after extraction from the patient in order to improve overall efficacy of stem cell treatment. There are various strategies currently available for donor stem cell modification, and with the correct combination of techniques, stem cell use may be optimized to provide methods to reduce myocardial infarct scars, promote cell proliferation and healing, and enhance angiogenesis and neovascularization to improve cardiac function in patients.
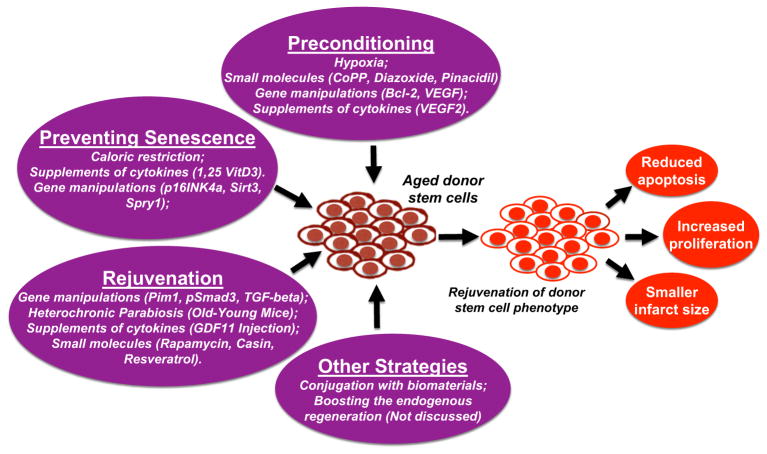
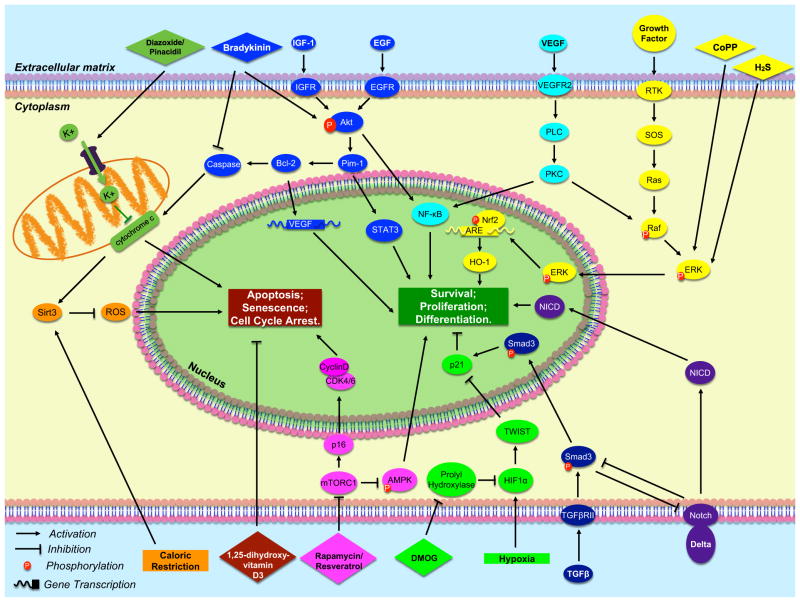
This review is organized in terms of three main strategies commonly utilized to combat the problem of cellular senescence in adult stem cells. These are: cellular preconditioning, prevention of senescence, and rejuvenation of stem cells. We noticed there are additional strategies to improve the effectiveness of stem cell therapy, such as stem cell conjugation with biomaterials [6], boosting the endogenous cardiac regeneration [7, 8], etc., all of which have been recently well-described in many other places [6–8], and will not be discussed here due to the page limitation. Each of these strategies is summarized in Fig. 1. The practice and various methods of cellular preconditioning will be discussed first, as many labs have established that this in vitro preparation of various types of stem cells is highly beneficial for enhancing transplant efficacy either when used alone or in combination with a genetic/pharmacological manipulation. Next we will discuss techniques to delay the onset of senescence in stem cells habituated to the aged cellular niches within the patient’s tissues. Finally, we will review specific ways to coerce aged stem cells to adopt rejuvenated phenotypes resembling those observed in younger, higher functioning stem cells. It is our hope that a comprehensive summary of methods to attempt rejuvenation of aged human cells may aid future scientists in identifying the best combination of techniques to optimize stem cell function and therapy. All related signal pathways for these strategies are summarized in Fig. 2.
Fig. 1.
Summary of strategies utilized to enhance the efficacy of adult stem cell therapy for heart disease. Endogenous resident cardiac stem cells are surgically isolated most commonly from the myocardium of an aged, injured heart, and must be manipulated in vitro to prevent post-transplantation apoptosis, and increase cell proliferation. Such modifications are characterized here by their function: preconditioning of stem cells before transplantation, prevention of senescence onset, and rejuvenation of post-senescent stem cells. Other strategies, including conjugation with biomaterials and boosting the endogenous regeneration, are not discussed here. Successful techniques performed on aged donor stem cells are expected to rejuvenate cell phenotype and provide optimal regeneration of cardiac tissue through reduced apoptosis, increased proliferation, and smaller infarct size
Fig. 2.
Molecular signal pathways that are associated with enhancing stem cell therapeutic effectiveness. The following is the summary of major pathways mentioned in the text, and described in counterclockwise order. Dark green: Diazoxide and pinacidil cause an influx of potassium into the mitochondria, preventing the release of cytochrome c and thus inhibiting apoptosis; Orange: Upregulation of Sirt3 by caloric restriction decreases oxidative stress by inhibiting ROS production; Maroon: 1,25-dihydroxyvitamin D3 delays the onset of senescence; Magenta: Rapamycin and resveratrol suppress the mTORC1 complex, which inhibits the AMPK growth pathway and the upregulation of CDK inhibitor p16INK4a; Bright green: Hypoxia upregulates HIF1α, which upregulates TWIST, and inhibits the CDK inhibitor p21; Purple & Navy Blue: Delta binding to Notch causes NICD to translocate into the nucleus to activate gene expression. Notch and pSmad3 mutually inhibit each other; Yellow: CoPP preconditioning activates ERK, ERK activates Nrf2 which binds to the ARE promoter to induce transcription of pro-survival antioxidant genes such as HO-1. Light blue: Growth with supplemental VEGF activates the PLC pathway leading to activation of pro-growth NF-kB protein; Blue: Supplemental growth factors lead to Akt expression which activates Pim-1 kinase, and its downstream effectors STAT3 and Bcl-2, which leads to downstream activation of VEGF transcribers
Strategy #1: Preconditioning
The cellular environment in the interior of an infarcted heart is neither as ideal nor as nurturing as that of a petri dish in a laboratory. Massive stem cell death usually occurs almost immediately when transplanted into a damaged patient heart without any prior “priming.” In fact, the few cells that do survive tend not to integrate very well with the host tissue [9]. One method for improving this dismally low survival rate is to precondition the stem cells by exposure before transplantation to in vitro conditions that mimic the harsh environment of an ischemic heart. Numerous labs have demonstrated the success of preconditioning in various types of adult stem cells including bone marrow derived mouse stem cells [10], mesenchymal stem cells, human cardiac progenitor cells [11], and human endothelial progenitor cells [12]. Preconditioning can be induced by a variety of techniques including (but not limited to): in vitro hypoxic shock, pharmacological manipulation, and molecular modification by anti-aging compounds [13]. These methods enhance post-transplantation survival capabilities and stem cell therapeutic potential in areas of ischemic damage in the heart [14]. Preconditioned stem cells are known to adhere better to their post-transplant niche, and in the case of stem cells transplanted into cardiac tissue, fare significantly better at inducing vital angiogenesis to the ischemic area [10].
One condition specific to the infarcted heart is hypoxia, defined as the lack of ample oxygen within the cellular environment. Prior exposure of a stem cell culture to brief periods of low oxygen levels has shown to greatly improve the chances of survival after transplantation back into the damaged tissue [15]. Stem cells cultured only in normoxia (normal oxygen levels) before implantation undergo more apoptosis than stem cells that have been previously exposed to brief periods of hypoxic shock. However, this technique must be carefully controlled, as extraneous oxygen deprivation can be detrimental. Numerous papers have been published on the topic of hypoxic preconditioning, suggesting that brief periods of hypoxia may activate certain pro-survival pathways within stem cells [15]. One pathway associated with hypoxia preconditioning is the HIF1α/TWIST/p21 axis [12] (Fig. 2, light green pathway). Endothelial progenitor cells (EPCs) cultured in hypoxia appear to upregulate Hypoxia-Inducible-Factor-1-α (HIF1α), which subsequently upregulates HIF1α’s target molecule TWIST. TWIST inhibits p21, whose primary function is to activate apoptotic pathways and cell-cycle inhibitors, indirectly causing the onset of cellular senescence. In addition to suppressing p21, hypoxia-exposed EPCs were observed to produce more growth factor proteins, cyclins, and cyclin-dependent kinases. This contributes to increased angiogenesis and capillary density, along with enhanced blood flow after transplantation into an ischemic mice hindlimb [12]. These results confirm that hypoxic preconditioning in vitro is indeed beneficial to enhancing stem cell therapy.
Hypoxia-like conditions may also be stimulated with the use of pharmacological agents. Prolyl hydroxylase inhibitors work exceptionally well for this function, as the prolyl hydroxylase enzymes naturally inhibit HIF1α, granting p21 the ability to activate pro-apoptotic pathways [16]. Dimethyloxalylglycine (DMOG) is an example of a prolyl hydroxylase inhibitor that allows for the HIF1α pathway to flourish (Fig. 2, light green pathway), leading to transcription of survival genes and growth factors. Liu et al. preconditioned rat bone marrow mesenchymal stem cells (BMSCs) with DMOG and found that this treatment mimicked the effects of hypoxic preconditioning by increasing expression of various growth factors and increasing cell survival in vitro through activation of the PI3K/Akt pathway. The preconditioned BMSCs also showed decreased caspase-3 activation, providing a secondary mechanism of apoptosis blockage [16]. After transplantation into myocardially infarcted rat hearts, the DMOG-preconditioned BMSCs showed less cell death, smaller infarct size, and enhanced cardiac function, suggesting that pretreatment with DMOG and possibly other types of prolyl hydroxylase inhibitors may be a useful, efficient way to induce effects similar to those caused by hypoxic preconditioning, but without having to perform the time and energy consuming hypoxic procedures.
A novel method of preconditioning involves the exposure of stem cells to the small molecule cobalt-protoporphyrin (CoPP). Our lab has shown that preconditioning human cardiac stem cells with CoPP, an inducer for Heme Oxegenase-1 (HO-1), significantly enhances in vitro cell survival [17], and results in greater improvement in LV remodeling and in indices of cardiac function after infarction [18]. This process is associated with the activation of multiple signaling pathways, including the ERK/NRF2 pathways (Fig. 2, yellow pathway), which both lead to transcription of the gene for HO-1 [17]. In addition, CoPP-induced up-regulation of HO-1 in cultured cardiomyocytes is shown to inhibit the depolarization of the mitochondrial membrane, providing an indirect anti-apoptotic pathway by blocking cytochrome c release [19]. Such increases in survival and cytoprotection of the cardiac stem cells are especially beneficial in later transplantation into the harsh ischemic environment of an infarct heart in an immunodeficient murine model [18]. Other small molecules such as bradykinin (BK) can be used for preconditioning stem cells as well. BK is an important metabolite that holds cytoprotective functions within the ischemic heart, and thus is an ideal candidate for molecular preconditioning in vitro [20]. Sheng et al. showed that BK suppresses apoptosis by reducing caspase-3 activation, leading to increased cell survival both in vitro and in vivo [20]. Akt and VEGF expression levels were upregulated as well (Fig. 2, blue pathway), and the infarct size of the left ventricle in the mouse model was significantly reduced after transplantation with BK-preconditioned human endothelial progenitor cells (hEPCs). Cardiac function also improved, and the heart weight/body weight ratio was lower in the preconditioned group of mice as compared to the control groups. These results suggest that small molecules such as CoPP/bradykinin may be a resource for pretreatment to improve stem cell therapies in the heart.
A variety of medications and specific pharmacological treatments may also be utilized to improve stem cell performance and activate cytoprotective responses in the cell’s own mechanisms. Diazoxide and pinacidil are two ATP channel openers that have been successful in enhancing survival of stem cells. These drugs work primarily by influencing mitochondrial membrane ATP channels to stay open when the cell is under oxidative stress, allowing for more potassium molecules to enter the mitochondria while simultaneously preventing dangerous depolarization of the membrane [21]. The influx of potassium prevents the release of the pro-apoptotic complex cytochrome c, effectively blocking the activation of programmed cell death that is ubiquitous in stem cell transplants (Fig. 2, dark green pathway). Thus, these drugs may be used in proper doses as a technique to increase cell survival and proliferation in vivo. The use of these pharmacological agents acting upon the mitochondria is extremely significant as recent evidence has suggested that the mitochondria may play a bigger role than previously anticipated in the failing heart.
Sildenafil (Viagra) is a pharmacological agent that inhibits the enzyme phosphodiesterase-5 (PDE-5), and has been shown to improve cardiac function and cell survival in mice. Phosphodiesterase-5 is intrinsically involved in contraction of vascular smooth muscle and thus plays a large role in heart functionality [22]. Hoke et al. preconditioned adipose tissue derived stem cells (ASCs) with sildenafil, transplanted those cells intramyocardially, and found that these ASCs increased cell survival and growth factor transcription [22]. Cardiac function was significantly improved 4 weeks after transplantation of the preconditioned ASCs, and increased angiogenesis was observed. Thus, pharmacological inhibitors of PDE-5 may also be optimal for preconditioning stem cells for myocardial transplantation.
Histone deacetylase (HDAC) inhibitors such as Trichostatin A (TSA) also have been used as a method of preconditioning to achieve similar effects. Zhang et al. found that TSA increased cardiomyocyte proliferation and enhanced functional recovery of the infarcted myocardium, but only in c-kit positive stem cells [23]. The effects of TSA were found to be null in cells that had a c-kit mutation (classified as KitW/KitW-v). In the c-kit+ stem cells, angiogenesis was enhanced and the infarct scar of the mouse heart was greatly reduced upon transplantation of c-kit+ CSCs treated with the HDAC inhibitor. These results suggest that the HDAC inhibitor pathway, while cytoprotective, may be dependent on c-kit signaling. This would limit the field of possible candidates of cardiac stem cell therapy to only those stem cells which are c-kit positive.
H2S has similarly been used to precondition human adipose derived stem cells (hASCs) in co-culture with rat cardiomyoblasts. This treatment provided significant cytoprotective effects to the stem cells, such as upregulation of cellular antioxidant defense pathways, as well as increased survival rates of both types of cells within the co-culture after a simulated myocardial ischemia-reperfusion injury. Levels of MAPK (ERK1/2) were elevated in the preconditioned-hASCs (Fig. 2, yellow pathway) and cell proliferation increased as a result of H2S preconditioning, and decreased when a H2S inhibitor was used. In the human body, H2S is also known to inhibit cytochrome c oxidase and help open mitochondrial potassium channels to prevent mitochondrial membrane depolarization. This, in effect, provides similar cytoprotective effects as the aforementioned drugs diazoxide and pinacidil. Each of these endpoints were tested in vitro, and suggest that H2S may be an example of yet another small molecule that may provide success in preconditioning human stem cells for better survival and proliferation after transplantation.
It is also possible to enable transcription of a multitude of protective genes that can be related to a variety of growth factor proteins, RNAs, and other signaling molecules to enhance cell survival (Fig. 2, blue pathway). An example is the molecular modification of cells to overexpress anti-apoptotic oncogenes. Veis et al. observed that mice deficient in Bcl-2 incurred extensive, abnormal cell death in certain organs [24]. Bcl-2 is known to inhibit activation of the pro-apoptotic caspase cascade [25], so a modification for Bcl-2 overexpression is likely to benefit cell survival. Kutschka et al. demonstrated this by showing that silenced Bcl-2 in rat cardiomyoblasts leads to frequent cell death while overexpression leads to higher stem cell survival post-transplantation [26]. Murphy et al. suggested that a reason for such pro-survival effects may be Bcl-2’s role in regulation of mitochondrial membrane actions, such as calcium ion uptake [27]. To test this, Li et al. injected human mesenchymal stem cells with Bcl-2 overexpression into the anterior wall of the left ventricles of acute myocardially infarcted rats, and found highly enhanced secretion of VEGF in these modified hMSCs as compared to the wild-type cells, suggesting that overexpression of the Bcl-2 gene may lead to downstream activation of VEGF transcribers [28]. This is highly significant, as VEGF has been known to enhance engraftment of donor stem cells into surrounding recipient tissue [10].
Some types of stem cells can also be grown with access to extra growth factors in vitro, and cause similar pro-survival effects to take place. This is the case with endothelial progenitor cells (EPCs) that were grown in media containing supplemental VEGF2 [29]. The EPCs showed levels of apoptosis inversely proportional to the dosage of supplemental VEGF2 in each experimental group (Fig. 2, light blue pathway). Thus, it may be worthwhile to consider adding supplement growth factors such as VEGF to stem cells in vitro, and also to induce overexpression of known anti-apoptotic genes such as Bcl-2 to optimize the effects of preconditioning on stem cells being primed for transplantation of hCSCs in the future.
There are a variety of ways in which to grow and culture the stem cells in vitro prior to transplantation into the patient. Each technique may not be optimal for each type of stem cell, and so it is crucial to differentiate between the different possibilities that exist for each purpose. But overall, preconditioning is the basis from which all in vitro methods can begin to combat the problem of senescence. Once stem cells have attempted adaptation to harsh ischemic environments, then only can they endure additional modifications to prevent the onset of cellular senescence and attempt to induce rejuvenation of the aged stem cell.
Strategy #2: Preventing Senescence
Once stem cells have been preconditioned in one or more of the various ways mentioned above, they must overcome the natural onset of cellular senescence. Preconditioning gives the cells an advantage in terms of better survival in vivo, but that is not sufficient to accelerate cardiac healing. Stem cells are not required to solely stay alive, but also to proliferate, differentiate, and engraft within the tissue into which they are transplanted. One way to assist this process is to utilize molecules involved in cell adhesion, mobilization, and proliferation. Use of these molecules and paracrine factors to artificially block natural pro-aging pathways is highly useful to achieve maximal success of post-transplant stem cells. Hypoxic, pharmacological, and molecular preconditioning techniques work in some measure to prevent such pathways from advancing, but these in vitro preconditioning treatments cannot be continued post-transplantation. Thus, it is necessary to modify the cells in a permanent way so that the change continues to be in effect in vivo long after transplantation.
Inhibition of senescence-related genes and proteins may be an option to try and improve youthful phenotypes within aged stem cells (Fig. 1). For example, the CDK-inhibitor p16INK4a is a tumor suppressor protein known to cause aging defects and negatively affect stem cell division and proliferation [30]. Expression of this gene is negligible in young cells, but as organisms age or as cells are exposed to high amounts of stress, p16INK4a expression becomes more ubiquitous [31]. It is directly correlated with decreased abilities of stem cells to migrate and engraft within a particular tissue. Mice deficient in p16INK4a have been observed to undergo programmed cell death less frequently than their wild type counterparts, suggesting that up-regulation of this gene could be harmful to the survival of the mesenchymal stem cells [32]. This gene is also present in aged populations of stem cells that tend to be low in number, slow in proliferation, and show decreased stem cell homing ability [33]. Silencing this gene may possibly lead to successful post-infarct healing and tissue regeneration, as well as increased stem cell survival in vivo. This modification holds great potential for rejuvenating cardiac stem cells, and should be considered in future studies, once genetic modification becomes commonly compatible with clinical application.
A similar method to prevent senescence is up-regulation of Sirt3, a well-known mitochondrial protein deacetylase [34]. Sirt3 expression is normally decreased in patients with Type II diabetes, and can be regulated by caloric restriction. When Sirt3 levels drop significantly, there is a decline in oxygen consumption and an increase of oxidative stress in skeletal muscle [35]. These factors contribute greatly to premature cellular aging by slowing down cellular metabolisms (Fig. 2, orange pathway). Jing et al. postulated that up-regulation of Sirt3 would decrease the amount of ROS production and oxidative stress within the cell, and stated that caloric restriction (CR) is one possible method to up-regulate Sirt3 [35]. CR is a technique known to prevent in vitro glucose-induced senescence, which Stolzing et al. demonstrated by growing rat mesenchymal stem cells (MSCs) in cultures supplemented with varying amounts of glucose [36]. MSCs grown in environments with 25–60 % less calories than the control encountered less programmed cell death, more proliferation, and bigger size of the cells within the colonies in vitro. This was attributed to controlled levels of glucose allowing the EGFR signaling pathway to flourish and lead to cell growth, proliferation, and successful in vitro cell culture expansion [36]. In contrast, the cells grown in media supplied with the most glucose showed massive apoptosis and shortest life spans [36]. Cells exposed to CR show suppression of pro-apoptotic proteins, as well as decreased ROS generation [37]. Reactive oxygen species are produced in high quantities when glucose is ubiquitous, as the glucose leads PKC to activate NADPH oxidase, which then up-regulates ROS production [38]. Based on these results, it is highly likely that CR may be an effective method to prevent senescence and improve stem cell therapeutic efficiency.
It is also necessary to ensure that a portion of the modified stem cells retains their multi-potency after transplant into the recipient, as maintenance of a healthy, undifferentiated stem cell pool in the heart is crucial for long-term repair and regeneration of the cardiac tissue. One technique for accomplishing this is the treatment of cells in vitro with 1,25-dihydroxyvitamin D3 [39]. Klotz et al. showed that treatment of human mesenchymal stem cells with 1,25-dihydroxyvitamin D3 (1,25D3) delays signs of senescence while simultaneously helping the stem cells retain their multipotent capacities (Fig. 2, maroon pathway). The senescence marker β-galactosidase was stained significantly less in 1,25D3 treated cells than in the untreated cells, meaning that the untreated population contained more senescent cells than the treated population. Additionally, the treated cells showed down-regulated expression of the aging-related gene p16INK4a, and significantly less cell death after 72 h when compared to the control groups. However, the 1,25D3 treatment simply delayed senescence while allowing the cells to retain their multi-potent qualities; it did not influence the cells to differentiate into any specific lineage, so if this treatment is to be used, it must be used in conjunction with a differentiating agent [39]. To retain multi-potency, it is essential to conserve a population of quiescent cells so that in case of future injury, these cells can readily contribute to the healing process. Quiescent cells are a type of backup population of cells that are activated in extreme cases of inflammation or injury, by mechanisms that receive feedback from damage of the activated cells [40].
One such damage-sensing mechanism involves the proteins Wnt, BMP, and Noggin. In mesenchymal stem cells, the central marrow region harbors stimulatory signals of the Wnt/BMP pathway, while the endostea bone marrow region contains inhibitory signals for the same pathway [41]. This is consistent with the idea of separate zones being maintained by expression of different proteins, and helps spatially segregate niches of the active and non-active zones. This zoning also helps the quiescent population replace itself faster through regeneration while preventing mutations or tumor formation [41]. Proteins expressed in the aged niche have a disruptive effect on the quiescent populations of stem cells by influencing them to become active at inopportune times, which eventually leads to unnecessary depletion of the reserve zones [40]. FGF2 is one of those proteins, and was found to be upregulated in the niche of the aged mouse stem cell population. Its high expression seemed to negatively affect cellular self-renewal ability, by causing a rapid loss of quiescence and simultaneous large-scale entry into the cell cycle [40]. Accelerated growth is beneficial to the organism, but quickly becomes harmful when the quiescent stem cell population becomes fully depleted. The tissue then has no way to protect itself and heal in case of future ischemia, injury, or inflammation. To prevent this fiasco, over-expression of Spry1 can be used to maintain the quiescent population, as it inhibits the pro-cycling FGF pathway [40]. Further study and over-expression of Spry-1 and similar function genes may be a unique method to prevent loss of quiescence and onset of senescence.
Complete cell cycle arrest leads to senescence, which is detrimental to stem cell treatment efficacy, but over-cycling can lead to tumor formation and depletion of the quiescent population of stem cells, thus harming treatment efficacy. It is essential to find the precise balance of gene expression that will regulate the cycle and block aging-related phenotypes while maintaining a still thriving population of stem cells within the patient’s injured tissue. This may be done by combining one of the aforementioned techniques to prevent the onset of senescence with an additional in vitro manipulation that will simultaneously rejuvenate stem cells.
Strategy #3: Rejuvenation
The last and perhaps most significant strategy for improving stem cell treatment is the act of restoring the phenotypes of aged stem cells so that they more closely resemble their younger counterparts (Fig. 1). In such a scenarios, growth factors, cytokines, and chemokines are made to be expressed in similar levels to young cells. This seems to make the old stem cells behave “younger” in that they are able to regain their regenerative and proliferative potentials. Studies that manipulate gene and/or protein levels within aged stem cells have been successful in neurons [42], liver cells [5], and skeletal muscle of mice [43], and cardiac progenitor cells [44]. Such studies have shown promising results in up-regulating cell proliferation, differentiation, and engraftment for senescent stem cells.
The membrane protein Notch is essential for tissue and muscle repair, and helps enhance regeneration properties within stem cells [5]. It is usually highly activated in younger stem cells, and loss of Notch has been shown to cause massive death of the stem cells in skeletal muscle [5]. One of Notch’s main mechanisms of action is to bind its extracellular ligand known as Delta (Fig. 2, purple pathway). When this binding occurs, Notch is intracellularly cleaved by γ-secretase into NICD (Notch Intracellular Domain), which translocates into the nucleus to activate proliferative gene expression [45]. Loss of Notch leads to an increase in pSmad3, a protein that up-regulates CDK inhibitors to block growth and proliferation of stem cells, leading to the premature onset of senescence [46]. Carlson et al. postulates that when conditions are ideal (i.e., in young, healthy organisms), Notch and pSmad3 levels show a natural abundance of Notch and scarcity of pSmad3 [46]. Healthy cells maintain a precise balance between Notch and pSmad3 in order to facilitate cell proliferation in favorable conditions and halt it when conditions are not ideal (i.e., disease or cancer). This regulation is disrupted in senescent organisms, and thus it was hypothesized that it is the imbalance in molecular levels of these proteins (specifically, the loss of Notch) that causes premature aging [47]. When Notch is lost, as is the case with senescent stem cells, TGFβ/pSmad3 and their downstream CDK inhibitors such as p27, p21, p15 (Fig. 2, navy blue pathway) are upregulated, furthering the aging process and in the replicative decline of aged stem cells [46]. Knockout of TGFβ has shown restoration of proliferative potential, and attenuation of pSmad3 has shown an increase in Notch levels and subsequent regeneration ability of older muscle cells [46]. This lines up with the hypothesis that an ideally balanced Notch pathway will exhibit high Notch and low TGFβ/pSmad3 (Fig. 2, purple & navy blue pathway). Thus, manipulation of this pathway by knocking out either TGFβ or pSmad3 may potentially serve as a convenient tool to rejuvenate the Notch balance, and therefore the aged stem cells themselves.
Another protein associated with the rejuvenation of senescent stem cells is Pim-1 kinase [44]. It has been shown that Pim-1 kinase enhances cell proliferation [48], and is involved in neovasculogenesis [49], that forms a basis of cardiac repair and regeneration response. Pim-1 also serves as a prosurvival factor by preserving mitochondrial integrity with Bcl-2 activation and upregulation of other known cell survival proteins such as STAT3 [48] (Fig. 2, Blue pathway). Recent studies from the Sussman group indicate that transplantation of human cardiac progenitor cells (hCPCs) modified with Pim-1 kinase resulted in the superior regeneration and functional improvement in a murine myocardial infarction model by using cells isolated from an aged patient with heart failure at the time of left ventricular assist device (LVAD) implantation [50]. Pim-1 is known to preserve telomere length and telomerase activity consistent with a youthful cellular phenotype [50], and genetic modification of Pim-1 kinase showed amelioration of the senescence characteristics of hCPCs. This resulted in the rejuvenation of phenotypic and functional properties [44], indicating that the use of Pim-1 modification should be incorporated into cell-based therapeutic approaches to overcome the challenges that are associated with the senescent phenotype of aged hCPC.
Because the environment surrounding the stem cells is usually what contributes to exposure (or lack thereof) of growth factors, it is extremely critical that the correct proteins are expressed within these environments. Disruption of protein levels in the stem cell niche can inhibit optimal function of the cells, but in the case of aged patients, it may be a plethora of proteins that require manipulation. Rather than trying to modify expression levels for multiple proteins at once, one could simply modify the entire extracellular environment by “replacing” the cell niche entirely. When performed in the circulatory system, such a technique is known as parabiosis, and has been used to rejuvenate aged stem cells [5]. When a shared circulatory system was created between two aged mice or between two young mice (known as isochronic parabiosis), there was no difference between the rates of cellular proliferation that the cells in each animal expressed. However, when a single circulatory system was connected between one young mouse and one aged mouse (heterochronic parabiosis), a significant increase in proliferation and regeneration in the stem cells of the aged mouse was detected, along with a slight decrease in the regeneration capabilities of the younger mice [5]. This suggests that it is indeed the cellular environment (the blood) of an organism that heavily influences its satellite stem cell survival abilities. Notch ligand Delta activation was thought to be involved in this process, as upregulated Delta in the extracellular environment increases its binding to Notch, leading to induced expression of proliferative genes as explained above [45]. This heterochronic parabiosis repeatedly showed the importance of enhancing stem cell niches in regulating cell functions and behaviors, and can serve as a significant method for rejuvenation of stem cells.
Aged stem cells can also be pharmacologically manipulated to induce expression of proteins for rejuvenation of stem cells. Rapamycin is a drug that can be utilized for such a purpose, as it inhibits the pro-senescence mTOR pathway [51]. When exposed to stem cells, Rapamycin is known to increase cellular lifespan and restore self-renewal [52]. It inhibits the TORC1 complex, effectively pulling aged cells out of senescence and back into the quiescent state for proliferation and growth [53]. An alternate definition of cellular aging can include the hyperactivity of certain cellular signaling processes which create unnecessary cell growth. The mTOR pathway fits these criteria by sensitivity to DNA damage accumulation, which is often induced by over-expression of the protein progerin. The subsequent mTOR pathway response is over-activation of DNA damage control mechanisms [54]. Using Rapamycin has effectively blocked this hyperactivity in multiple organisms, reducing the damaging effects of cellular decline. This drug has also demonstrated an ability to inhibit apoptosis while enhancing self-renewal in multiple types of stem cells. This was measured by a decrease in expression of senescence markers on the stem cells, such as p21 and p16INK4a [55]. Resveratrol can be used in combination with Rapamycin in order to induce maximal rejuvenating effects on cardiac stem cells taken from post-infarct mouse hearts. Resveratrol is similar to Rapamycin as it is also able to suppress TORC1 activity, and therefore can aid in reducing the amount of senescent cells in a population [56]. These two drugs combined are able to activate the AMPK pathway which has been shown to be reduced in aged cells as compared to younger cells (Fig. 2, magenta pathway). Similarly, the two drugs also help inhibit IL-1β over-secretion (an interleukin cytokine involved in inflammation), which is up-regulated in aged cells at a greater level than in young cells [56]. Exposure to these drugs in vitro helps the stem cells regain their abilities to repair a post-infarct heart after transplantation by increasing angiogenesis and decreasing levels of apoptosis of these once-senescent stem cells. In this way, the injury site is eventually reduced in size, and minimal scarring is observed while the number of c-kit positive cardiac stem cells is increased [56]. This double-drug treatment is simple in that it only requires in vitro exposure of the stem cell culture to the drugs and subsequent transplantation back into the infarcted heart. It is also significantly more efficient than the alternative treatments, which often involve complex genetic manipulations of one or more senescence-related pathways in the cells. The ex vivo resveratrol/rapamycin treatment achieves the similar desired effects as genetic manipulation does, but is cheaper, more time efficient, and has fewer potential side effects [56]. Thus, this holds potential for use as a common method for rejuvenation of human cardiac stem cells in vitro.
Whether it be a technique as complex as a heterochronic parabiosis, or as simple as exposure to a pharmacological inhibiting agent, there are a variety of methods to induce cellular rejuvenation phenotypes. Genetic manipulations of the stem cells are the most common, but require extensive studies before they can be directly applied to the clinical treatment of human patients. Each of the discussed experiments provides valuable insight into harnessing the power of natural signaling pathways to rejuvenate stem cells, and should be considered in future studies involving improvement for current stem cell therapies.
Conclusion and Future Directions
This review discusses different novel strategies of improving the use of donor stem cells as a treatment for myocardial infarction and other ischemic heart diseases. Though stem cells certainly harbor the intrinsic ability to accelerate healing by rejuvenation of damaged tissue, it is advisable to perform the preliminary chemical/genetic in vitro modification to enhance this regeneration potential. Once delivered via intracoronary injection, adult stem cells have demonstrated extremely deficient rates of survival in the ischemic, post-infarct heart [57]. Cellular senescence can negatively impact this dismally low rate of transplanted cell survival, and numerous studies have shown that it is possible to improve this rate by priming stem cells prior to transplant. Donor stem cells can be preconditioned with pharmacological agents, molecular manipulations, or periods of oxygen deprivation to trigger activation of cytoprotective pathways. There is also the obstacle of cell integration, as donor stem cells do not usually engraft very well into the host tissue nor are able to generate any significant type of angiogenesis to sustain themselves. To tackle this challenge, stem cells can be further biochemically manipulated in vitro with certain growth factors, or by genetic engineering. Once cells are successfully readied in vitro, they are injected back into the infarct tissue, but must be closely observed to ensure proper survival and function. Intracoronary stem cell infusion to combat heart diseases remain in practice in clinical trials thus far, and have yet to become commonplace as many additional large-scale studies are needed to establish full competence of this procedure [58]. However, this technique has been growing in popularity in recent years, and thus it is of utmost importance to continue efforts for improving effectiveness of this stem cell therapy for the damaged heart.
Not all adult stem cells are created equal; to advance towards the noble goal of finding methods to enhance efficacy of stem cell treatment for healing of the infarcted human heart, we believe it is crucial to optimize and carefully hand-pick the exact combination of techniques that will prepare selected stem cells for maximum in vitro survival and proliferation.
Acknowledgments
This study was supported by NIH Grants R01HL114951 (to C.C.) from the National Institutes of Health, and Research Grant 12BGIA9090005 (to C.C.) from the American Heart Association.
Footnotes
Compliance with Ethical Standards
Conflict of Interest The authors declare no potential conflicts of interest.
References
- 1.Dong L, Hao H, Han W, Fu X. The role of the micro-environment on the fate of adult stem cells. Science China. Life Sciences. 2015 doi: 10.1007/s11427-015-4865-9. [DOI] [PubMed] [Google Scholar]
- 2.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 3.Mohsin S, Siddiqi S, Collins B, Sussman MA. Empowering adult stem cells for myocardial regeneration. Circulation Research. 2011;109:1415–1428. doi: 10.1161/CIRCRESAHA.111.243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanada F, Kim J, Czarna A, Chan NY, Signore S, Ogorek B, Isobe K, Wybieralska E, Borghetti G, Pesapane A, Sorrentino A, Mangano E, Cappetta D, Mangiaracina C, Ricciardi M, Cimini M, Ifedigbo E, Perrella MA, Goichberg P, Choi AM, Kajstura J, Hosoda T, Rota M, Anversa P, Leri A. c-Kit-positive cardiac stem cells nested in hypoxic niches are activated by stem cell factor reversing the aging myopathy. Circulation Research. 2014;114:41–55. doi: 10.1161/CIRCRESAHA.114.302500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 6.Cutts J, Nikkhah M, Brafman DA. Biomaterial approaches for stem cell-based myocardial tissue engineering. Biomarker Insights. 2015;10:77–90. doi: 10.4137/BMI.S20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finan A, Richard S. Stimulating endogenous cardiac repair. Frontiers in Cell and Developmental Biology. 2015;3:57. doi: 10.3389/fcell.2015.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nadal-Ginard B, Ellison GM, Torella D. The cardiac stem cell compartment is indispensable for myocardial cell homeostasis, repair and regeneration in the adult. Stem Cell Research. 2014;13:615–630. doi: 10.1016/j.scr.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Liang Y, Van Zant G, Szilvassy SJ. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood. 2005;106:1479–1487. doi: 10.1182/blood-2004-11-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubo M, Li TS, Kurazumi H, Takemoto Y, Ohshima M, Murata T, Katsura S, Morikage N, Furutani A, Hamano K. Hypoxic preconditioning enhances angiogenic potential of bone marrow cells with aging-related functional impairment. Circulation Journal : Official Journal of the Japanese Circulation Society. 2012;76:986–994. doi: 10.1253/circj.cj-11-0605. [DOI] [PubMed] [Google Scholar]
- 11.Hu S, Yan G, Xu H, He W, Liu Z, Ma G. Hypoxic preconditioning increases survival of cardiac progenitor cells via the pim-1 kinase-mediated anti-apoptotic effect. Circulation Journal : Official Journal of the Japanese Circulation Society. 2014;78:724–731. doi: 10.1253/circj.cj-13-0841. [DOI] [PubMed] [Google Scholar]
- 12.Lee SH, Lee JH, Yoo SY, Hur J, Kim HS, Kwon SM. Hypoxia inhibits cellular senescence to restore the therapeutic potential of old human endothelial progenitor cells via the hypoxia-inducible factor-1alpha-TWIST-p21 axis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013;33:2407–2414. doi: 10.1161/ATVBAHA.113.301931. [DOI] [PubMed] [Google Scholar]
- 13.Haider H, Ashraf M. Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. Journal of Molecular and Cellular Cardiology. 2008;45:554–566. doi: 10.1016/j.yjmcc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haider H, Ashraf M. Preconditioning and stem cell survival. Journal of Cardiovascular Translational Research. 2010;3:89–102. doi: 10.1007/s12265-009-9161-2. [DOI] [PubMed] [Google Scholar]
- 15.Rosova I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu XB, Wang JA, Ji XY, Yu SP, Wei L. Preconditioning of bone marrow mesenchymal stem cells by prolyl hydroxylase inhibition enhances cell survival and angiogenesis in vitro and after transplantation into the ischemic heart of rats. Stem Cell Research & Therapy. 2014;5:111. doi: 10.1186/scrt499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai C, Teng L, Vu D, He JQ, Guo Y, Li Q, Tang XL, Rokosh G, Bhatnagar A, Bolli R. The heme oxygenase 1 inducer (CoPP) protects human cardiac stem cells against apoptosis through activation of the extracellular signal-regulated kinase (ERK)/NRF2 signaling pathway and cytokine release. The Journal of Biological Chemistry. 2012;287:33720–33732. doi: 10.1074/jbc.M112.385542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai C, Guo Y, Teng L, Nong Y, Tan M, Book MJ, Zhu X, Wang XL, Du J, Wu WJ, Xie W, Hong KU, Li Q, Bolli R. Preconditioning human cardiac stem cells with an HO-1 inducer exerts beneficial effects after cell transplantation in the infarcted murine heart. Stem cells. 2015 doi: 10.1002/stem.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Issan Y, Kornowski R, Aravot D, Shainberg A, Laniado-Schwartzman M, Sodhi K, Abraham NG, Hochhauser E. Heme oxygenase-1 induction improves cardiac function following myocardial ischemia by reducing oxidative stress. PLoS One. 2014;9:e92246. doi: 10.1371/journal.pone.0092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheng Z, Yao Y, Li Y, Yan F, Huang J, Ma G. Bradykinin preconditioning improves therapeutic potential of human endothelial progenitor cells in infarcted myocardium. PLoS One. 2013;8:e81505. doi: 10.1371/journal.pone.0081505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akao M, Teshima Y, Marban E. Antiapoptotic effect of nicorandil mediated by mitochondrial atp-sensitive potassium channels in cultured cardiac myocytes. Journal of the American College of Cardiology. 2002;40:803–810. doi: 10.1016/s0735-1097(02)02007-7. [DOI] [PubMed] [Google Scholar]
- 22.Hoke NN, Salloum FN, Kass DA, Das A, Kukreja RC. Preconditioning by phosphodiesterase-5 inhibition improves therapeutic efficacy of adipose-derived stem cells following myocardial infarction in mice. Stem Cells. 2012;30:326–335. doi: 10.1002/stem.789. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Qin X, Zhao Y, Fast L, Zhuang S, Liu P, Cheng G, Zhao TC. Inhibition of histone deacetylases preserves myocardial performance and prevents cardiac remodeling through stimulation of endogenous angiomyogenesis. Journal of Pharmacology and Experimental Therapeutics. 2012;341:285–293. doi: 10.1124/jpet.111.189910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, poly-cystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 25.Nunez G, Benedict MA, Hu Y, Inohara N. Caspases: the proteases of the apoptotic pathway. Oncogene. 1998;17:3237–3245. doi: 10.1038/sj.onc.1202581. [DOI] [PubMed] [Google Scholar]
- 26.Kutschka I, Kofidis T, Chen IY, von Degenfeld G, Zwierzchoniewska M, Hoyt G, Arai T, Lebl DR, Hendry SL, Sheikh AY, Cooke DT, Connolly A, Blau HM, Gambhir SS, Robbins RC. Adenoviral human BCL-2 transgene expression attenuates early donor cell death after cardiomyoblast transplantation into ischemic rat hearts. Circulation. 2006;114:I174–I180. doi: 10.1161/CIRCULATIONAHA.105.001370. [DOI] [PubMed] [Google Scholar]
- 27.Murphy AN, Bredesen DE, Cortopassi G, Wang E, Fiskum G. Bcl-2 potentiates the maximal calcium uptake capacity of neural cell mitochondria. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9893–9898. doi: 10.1073/pnas.93.18.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, Furlani D, Piechaczek C, Moebius JM, Lutzow K, Lendlein A, Stamm C, Li RK, Steinhoff G. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007;25:2118–2127. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- 29.Shintani S, Kusano K, Ii M, Iwakura A, Heyd L, Curry C, Wecker A, Gavin M, Ma H, Kearney M, Silver M, Thorne T, Murohara T, Losordo DW. Synergistic effect of combined intramyocardial CD34+ cells and VEGF2 gene therapy after MI. Nature Clinical Practice Cardiovascular Medicine. 2006;3(Suppl 1):S123–S128. doi: 10.1038/ncpcardio0430. [DOI] [PubMed] [Google Scholar]
- 30.Ohtani N, Yamakoshi K, Takahashi A, Hara E. The p16INK4a-RB pathway : molecular link between cellular senescence and tumor suppression. The Journal of Medical Investigation. 2004;51:146–153. doi: 10.2152/jmi.51.146. [DOI] [PubMed] [Google Scholar]
- 31.Brookes S, Rowe J, Gutierrez Del Arroyo A, Bond J, Peters G. Contribution of p16(INK4a) to replicative senescence of human fibroblasts. Experimental Cell Research. 2004;298:549–559. doi: 10.1016/j.yexcr.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 32.Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE, Scadden DT. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 33.Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nature Medicine. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 34.Sack MN. The role of SIRT3 in mitochondrial homeostasis and cardiac adaptation to hypertrophy and aging. Journal of Molecular and Cellular Cardiology. 2012;52:520–525. doi: 10.1016/j.yjmcc.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jing E, Emanuelli B, Hirschey MD, Boucher J, Lee KY, Lombard D, Verdin EM, Kahn CR. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14608–14613. doi: 10.1073/pnas.1111308108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stolzing A, Coleman N, Scutt A. Glucose-induced replicative senescence in mesenchymal stem cells. Rejuvenation Research. 2006;9:31–35. doi: 10.1089/rej.2006.9.31. [DOI] [PubMed] [Google Scholar]
- 37.Lee JH, Jung KJ, Kim JW, Kim HJ, Yu BP, Chung HY. Suppression of apoptosis by calorie restriction in aged kidney. Experimental Gerontology. 2004;39:1361–1368. doi: 10.1016/j.exger.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 38.Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52:2795–2804. doi: 10.2337/diabetes.52.11.2795. [DOI] [PubMed] [Google Scholar]
- 39.Klotz B, Mentrup B, Regensburger M, Zeck S, Schneidereit J, Schupp N, Linden C, Merz C, Ebert R, Jakob F. 1,25-dihydroxyvitamin D3 treatment delays cellular aging in human mesenchymal stem cells while maintaining their multipotent capacity. PLoS One. 2012;7:e29959. doi: 10.1371/journal.pone.0029959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490:355–360. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, Hirshman MF, Lebowitz J, Shadrach JL, Cerletti M, Kim MJ, Serwold T, Goodyear LJ, Rosner B, Lee RT, Wagers AJ. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–652. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohsin S, Khan M, Nguyen J, Alkatib M, Siddiqi S, Hariharan N, Wallach K, Monsanto M, Gude N, Dembitsky W, Sussman MA. Rejuvenation of human cardiac progenitor cells with Pim-1 kinase. Circulation Research. 2013;113:1169–1179. doi: 10.1161/CIRCRESAHA.113.302302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou X. Role of Notch signaling in the mammalian. 2014;47:1–10. doi: 10.1590/1414-431X20133177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carlson ME, Hsu M, Conboy IM. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature. 2008;454:528–532. doi: 10.1038/nature07034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshida Y, Hayashi Y, Suda M, Tateno K, Okada S, Moriya J, Yokoyama M, Nojima A, Yamashita M, Kobayashi Y, Shimizu I, Minamino T. Notch signaling regulates the lifespan of vascular endothelial cells via a p16-dependent pathway. PLoS One. 2014;9:e100359. doi: 10.1371/journal.pone.0100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fischer KM, Cottage CT, Wu W, Din S, Gude NA, Avitabile D, Quijada P, Collins BL, Fransioli J, Sussman MA. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation. 2009;120:2077–2087. doi: 10.1161/CIRCULATIONAHA.109.884403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zippo A, De Robertis A, Bardelli M, Galvagni F, Oliviero S. Identification of Flk-1 target genes in vasculogenesis: Pim-1 is required for endothelial and mural cell differentiation in vitro. Blood. 2004;103:4536–4544. doi: 10.1182/blood-2003-11-3827. [DOI] [PubMed] [Google Scholar]
- 50.Mohsin S, Khan M, Toko H, Bailey B, Cottage CT, Wallach K, Nag D, Lee A, Siddiqi S, Lan F, Fischer KM, Gude N, Quijada P, Avitabile D, Truffa S, Collins B, Dembitsky W, Wu JC, Sussman MA. Human cardiac progenitor cells engineered with Pim-I kinase enhance myocardial repair. Journal of the American College of Cardiology. 2012;60:1278–1287. doi: 10.1016/j.jacc.2012.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blagosklonny MV. Aging, stem cells, and mammalian target of rapamycin: a prospect of pharmacologic rejuvenation of aging stem cells. Rejuvenation Research. 2008;11:801–808. doi: 10.1089/rej.2008.0722. [DOI] [PubMed] [Google Scholar]
- 52.Gao XM, Wong G, Wang B, Kiriazis H, Moore XL, Su YD, Dart A, Du XJ. Inhibition of mTOR reduces chronic pressure-overload cardiac hypertrophy and fibrosis. Journal of Hypertension. 2006;24:1663–1670. doi: 10.1097/01.hjh.0000239304.01496.83. [DOI] [PubMed] [Google Scholar]
- 53.Blagosklonny MV. Cell cycle arrest is not yet senescence, which is not just cell cycle arrest: terminology for TOR-driven aging. Aging. 2012;4:159–165. doi: 10.18632/aging.100443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menendez JA, Vellon L, Oliveras-Ferraros C, Cufi S, Vazquez-Martin A. mTOR-regulated senescence and autophagy during reprogramming of somatic cells to pluripotency: a roadmap from energy metabolism to stem cell renewal and aging. Cell Cycle. 2011;10:3658–3677. doi: 10.4161/cc.10.21.18128. [DOI] [PubMed] [Google Scholar]
- 55.Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Science Signaling. 2009;2:ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Avolio E, Gianfranceschi G, Cesselli D, Caragnano A, Athanasakis E, Katare R, Meloni M, Palma A, Barchiesi A, Vascotto C, Toffoletto B, Mazzega E, Finato N, Aresu G, Livi U, Emanueli C, Scoles G, Beltrami CA, Madeddu P, Beltrami AP. Ex vivo molecular rejuvenation improves the therapeutic activity of senescent human cardiac stem cells in a mouse model of myocardial infarction. Stem Cells. 2014;32:2373–2385. doi: 10.1002/stem.1728. [DOI] [PubMed] [Google Scholar]
- 57.Cesselli D, Beltrami AP, D’Aurizio F, Marcon P, Bergamin N, Toffoletto B, Pandolfi M, Puppato E, Marino L, Signore S, Livi U, Verardo R, Piazza S, Marchionni L, Fiorini C, Schneider C, Hosoda T, Rota M, Kajstura J, Anversa P, Beltrami CA, Leri A. Effects of age and heart failure on human cardiac stem cell function. The American Journal of Pathology. 2011;179:349–366. doi: 10.1016/j.ajpath.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frey N, Linke A, Suselbeck T, Muller-Ehmsen J, Vermeersch P, Schoors D, Rosenberg M, Bea F, Tuvia S, Leor J. Intracoronary delivery of injectable bioabsorbable scaffold (IK-5001) to treat left ventricular remodeling after ST-elevation myocardial infarction: a first-in-man study. Circulation Cardiovascular Interventions. 2014;7:806–812. doi: 10.1161/CIRCINTERVENTIONS.114.001478. [DOI] [PubMed] [Google Scholar]