Abstract
Background
Specialized endoderm derived epithelial cells, i.e. enteroendocrine cells (EECs), are widely distributed throughout the gastrointestinal (GI) tract. EECs form the largest endocrine organ in the body and play a key role in the control of GI secretion and motility, the regulation of food intake, postprandial glucose levels and metabolism. EECs sense luminal content and release signaling molecules that can enter the circulation to act as classic hormones on distant targets, act locally on neighboring cells and on distinct neuronal pathways including enteric and extrinsic neurons. Recent studies have shed light on EEC sensory transmission by showing direct connections between EECs and the nervous system via axon-like processes that form a well-defined neuroepithelial circuits through which EECs can directly communicate with the neurons innervating the GI tract to initiate appropriate functional responses.
Purpose
This review will highlight the role played by the EECs in the complex and integrated sensory information responses, and discuss the new findings regarding EECs in the brain-gut axis bidirectional communication.
Keywords: gut chemosensing, afferent neurons, peptides
INTRODUCTION
The endocrine system is distributed throughout the human body, providing a constant control of physiological and homeostatic functions by secreting hormones, which act on different cell targets. The largest endocrine system in terms of number of cells is located in the gastrointestinal (GI) tract, where it is comprised of individually scattered enteroendocrine cells (EECs) distributed along the entire GI mucosa in the crypts and villi representing 1% of the total gut epithelium cell population.1-3 EECs are specialized cells capable of sensing luminal content, producing and releasing hormones/signaling molecules and modulating a variety of physiological GI and homeostatic functions. Despite their well-established role, the molecular sensing mechanisms and the modalities of transmission are not completely understood. However, there is increasing evidence that EECs are equipped with a chemosensory machinery to initiate appropriate functional responses through gut-brain and brain-gut interaction and that transmission involves at least in part direct EEC-neurons connections via basal cytoplasmic processes. The first report of EEC basal processes was in 1979 when Larsson et al. described basal cytoplasmic prolongation in somatostatin containing EEC that appeared to contact other cells thus providing evidence for a paracrine function of somatostatin D cells.4 Similar processes of various length have been reported by other investigators in L cells containing polypeptide YY (PYY) in addition to somatostatin D cells.5-7 Whereas basal processes are prominent in L and D cells, in other types of EECs such as I cells secreting cholecystokinin (CCK) basal processes are typically short.8 Independently of the prominence of the basal processes, direct or indirect communication of EECs with nerves is a major mechanism underlying EEC function in the gut mucosa through peptide release.9-11
Recently, there has been considerable interest in the role of EECs in gut-brain/brain-gut communication and the reader is referred to a series of excellent review articles.12-14 The present review focuses on EECs function in nutrient sensing and food intake regulation emphasizing their role in brain-gut bidirectional communication.
EECs: MORPHOLOGY, LOCALIZATION AND FUNCTION
EECs are specialized endoderm-derived epithelial cells that differentiate from pluripotent stem cells at the base of intestinal crypts and migrate up the crypt-villus axis.15 The wide distribution of EECs in the villi and crypts, where they are interspersed between non endocrine cells, is regulated by an interplay of different factors including the cell surface protein ‘notch’ and the basic helix-loop-helix transcription factor family, which control terminal cell differentiation.15,16 Depending on their individual morphology and position in the GI mucosa, EECs are divided into “open type” with a bottle neck shape and an apical prolongation with microvilli facing towards the intestinal lumen or “closed type” that are located close to the basal membrane, do not reach the lumen of the gut and lack microvilli.3,17,18 The open type EECs directly detect luminal contents through the microvilli reaching the lumen, whereas the close types are believed to be activated by luminal content indirectly either through neural or humoral pathways. Both open- and closed-type cells accumulate their secretory products in cytoplasmatic granules and release them by exocytosis at the basolateral membrane upon mechanical, chemical or neural stimulation. The secretory products, mainly peptides/hormones, can act locally in a paracrine manner, activating other EECs and other cell types in the mucosa, reach distant targets through release in the bloodstream or act directly on nerve endings close to the site of release.11,17 Evidence for direct EEC-nerve transmission has been provided by Bohorquez et al.19,20 who have combined high resolution imaging with transgenic mice with EECs expressing green fluorescent protein (GFP) and shown that basal processes of I and L cells have synaptic features, are accompanied by glia cells and contact nerves innervating the mucosa forming neuroepithelial circuits.
Distinct types of EECs expressing more than 20 peptides/hormones have been identified along the GI mucosa (Fig. 1) and it is now clear that many EECs contain more than one signaling molecule.1,10,11 Evidence for a complex phenotype of EECs is supported by studies using transgenic CCK-eGFP mouse, which showed the broad co-expression of six functionally different peptides (CCK, GIP, secretin, glucagon-like peptide-1 [GLP-1], PYY and neurotensin) in intestinal mucosa EECs.21 Other studies combining transcriptional profiles, fluorescence-activated cell sorting analysis and cell culture systems have shown that cells expressing the same peptide/hormone vary depending on the region of the intestine where they are located.22 The complexity of EEC phenotypes has been further confirmed by a study using triple labeling immunohistochemistry which has reported that in both mouse and pig small intestine and colon, a subset of L and K cells overlaps (hence also referred to as K/L or L/K EECs) with different gradients of hormonal marker co-localization (i.e. GLP-1, PYY and gastric inhibitory peptide [GIP]).23 These findings clearly indicate that EEC type classification based on the content of the cytoplasmic granules/vesicles is flawed and that a nomenclature review is necessary. However, the factors that determine how the EECs differentiation process occurs are still largely unknown and there is also evidence of plasticity of the EEC system.18 In this review, we have elected to use the classic nomenclature based on the major hormone secreted by the different EEC subtypes throughout the gut mucosa.
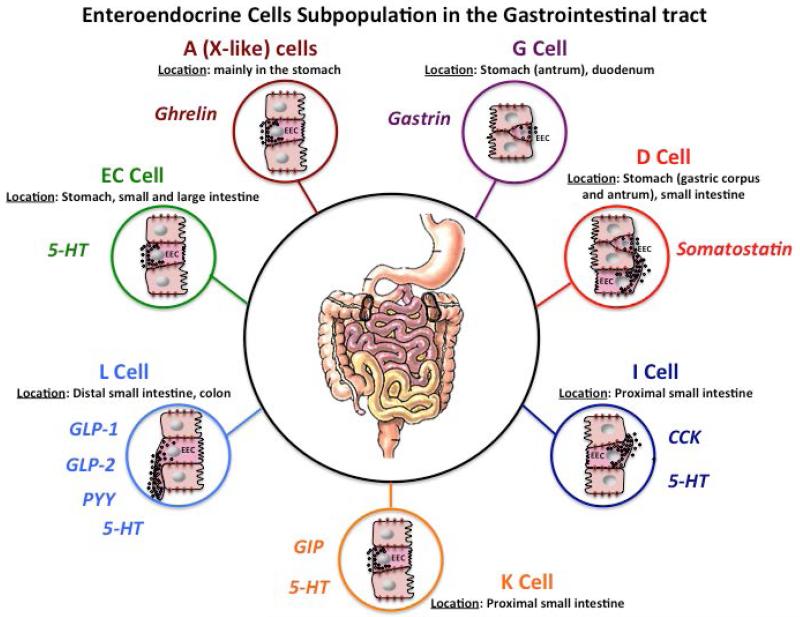
Figure 1. Enteroendocrine cells (EECs) Subpopulations in the Gastrointestinal Tract.
EECs comprise different subgroups producing and releasing a variety of hormones under appropriate stimulation. EECs are largely distributed in the gut. The stomach corpus is rich in A cells secreting ghrelin (dark red circle), the antrum is populated by G cells secreting gastrin (purple circle), while in the pylorum there is a high density of D cells secreting somatostatin (red circle). D cells are also present in the proximal segment of the small intestine. I (dark blue circle) and K (orange circle) cells, which secrete CCK and GIP, respectively, are located in the small intestine and L cells secreting GLP-1, GLP-2 and PYY (light blue circle) are widely distributed in the distal small intestine and in the colon (mainly in the proximal portion). Up to 95% of the total 5-HT is produced in the GI tract. EC (green circle) are situated in the pylorus, small and large intestine. 5-HT is also released by I cells (dark blue circle) located in the distal portion of the small intestine and L cells (light blue circle) in the large intestine. Only some example of EECs subgroups and relative hormones secreted are represented in the figure. Abbreviations: EC, enterochromaffin cell; 5-HT, 5-hydroxytryptamine; CCK, cholecystokinin; GLP-1, glucagon like peptide-1; GLP-2, Glucagon like peptide-2; PYY, peptide YY; GIP, gastric inhibitory peptide.
EEC with short or long basal cytoplasmic processes.
EEC secretory products are released in response to diverse types of stimuli and influence a variety of physiological functions. For instance, gastrin, secreted by open-type G cells of the gastric antrum and pylorus in response to luminal aminoacids and calcium, controls gastric acid secretion by acting on closed-type enterochromaffin-like cells of the gastric corpus, which release histamine. In turn, histamine activates parietal cells to secret gastric acid.24 Somatostatin, which is released by closed-type D cells of the gastric corpus in response to intestinal hormones such as CCK or transmitters, inhibits gastric acid secretion by direct inhibition on parietal cells and through inhibition of histamine release.24 By contrast, somatostatin released from open type D cells in the antrum inhibits gastric acid secretion through inhibition of the production and release of gastrin.25 5-HT is a polyfunctional signaling molecule contained in the closed-type enterochromaffin cells (EC) and in a subgroup of open type I and L cells as well as in enteric neurons, mostly interneurons.26,27 5-HT has been regarded as playing a major role in promoting intestinal motility through a combination of neuronal and mucosal mechanisms. The classic notion that 5-HT released from ECs upon chemical stimulation or mechanical distortion initiated the peristaltic reflex and propulsion has been challenged.26-29 Investigators have suggested that 5-HT-neurons play a more important role in gut motility than 5-HT-EC cells though other studies are in disagreement with this conclusion27 .However, other functions have been recently ascribed to mucosal (EC-derived) 5-HT, including worsening inflammation in a rat model of experimental colitis, promoting hepatic regeneration, lowering bone mass by inhibition of osteoblast proliferation and serving as a growth factor.27,30 The satiety hormone, leptin secreted by gastric chief cells and open-type P cells31, and the orexigenic hormone, ghrelin secreted by closed- and open-type X/A-like cells, regulate appetite.31,32 Ghrelin cells are mostly abundant in the stomach, where they have the morphology of the closed-type, whereas in the small and large intestine there are both types with an increasing gradient of open-type cells toward the large intestine, though the physiological significance of this finding is unknown.33 GLP-1, glucagon like peptide-2 (GLP-2) and PYY, are contained in open type L cells and are released in response to ingested nutrients, including carbohydrates and fat. GLP-1 regulates postprandial glucose levels, promotes satiety and has an inhibitory effect on energy intake.34 GLP-2 regulates intestinal lipid absorption, promotes epithelial growth and exerts mucosal defense.35-37 PYY3-36 inhibits food intake and induces food aversion,38,39 and in combination with GLP-1 plays a major role in the ileal brake, a physiological mechanism delaying gut motility and transit in order to allow for nutrient (mainly lipids) absorption.32,40-42 GIP secreted by open type K cells as well as by L cells containing GLP-1 and/or PYY leads to the release of insulin in the postprandial phase of digestion thus participating in the postprandial glucose level control.32,40,43 It is important to keep in mind that, as stated above, subsets of EECs overlap in term of their secretory products supporting that different peptides may affect each other function once released in response to luminal changes.22,23
EECs AS KEY REGULTORS OF GUT CHEMOSENSING, FOOD INTAKE AND ENERGY HOMEOSTASIS
EECs respond to a wide range of substances present in the lumen, classifiable as nutrients, non-nutrient chemicals, food-born toxins, and microorganisms.10,44 EECs represent the first level of integration of the information from the gut lumen and upon stimulation release signaling molecules that activate neuronal circuits, which send information to different regions of the brain to generate appropriate functional responses.1-3 Chemosensing of luminal contents elicits a variety of functions, including digestion and absorption of nutrients as well as defense responses against harmful/toxic substances such as vomiting, diarrhea and food aversion. A major function of EEC-secreted peptides/hormones is to coordinate the response of the gut to ingested nutrients. This includes induction of GI, pancreatic and biliary secretion, and modulation of GI motility to facilitate digestion and absorption as well as other physiological activities such as tissue growth and repair, and increase in the intestinal barrier function through the activation of local and neuronal pathways and the brain-gut axis.3,10,11 By contrast, potentially harmful substances in the lumen are likely to initiate a protective response possibly via EEC activation to reduce, reject or avoid the threat by delaying gastric emptying, increasing intestinal secretion, inducing vomiting, diarrhea or food aversion presumably through the activation of vagal afferents and neurons of the caudal (visceral) nucleus of the solitary tract (NTS), parabrachial nucleus, central nucleus of the amygdala, and paraventricular nucleus of the hypothalamus as shown by intraluminal administration of bitter tastants.45-48 For instance, rotavirus encoded enterotoxin, a major cause of gastroenteritis in children, is likely to cause emesis, a hallmark of the virus-induced illness, by acting on EC cells with subsequent release of 5-HT, which activates areas of the NTS and area postrema in the brain stem associated with nausea and vomiting via vagal activation.49
Sensory receptors expressed on EECs
EECs have a key role in gut chemosensing and are equipped with a wide array of receptors expressed on the luminal side of the gut mucosa, many of which are G protein coupled receptors (GPCRs) and respond to selective luminal substances.10,11
Taste Receptors
EEC sensory receptors include taste receptors (TRs) and taste signaling molecules implicated in taste transduction originally described in taste buds of the mouth where initial gustatory processing occurs.3,50-54 These include two major families of taste detecting GPCRs, the T1Rs sensing sweet and umami, and the T2Rs detecting bitter taste.3,52,55 The T1R family is composed of three isoforms that function as heterodimers, the T1R1-T1R3 dimer detects umami taste, while the T1R2-T1R3 dimer senses sweet taste, including glucose and artificial sweeteners.53,55,56 Gut T1Rs recognize ingested nutrients such as carbohydrates (T1R2-T1R3) and amino acids (T1R1-T1R3). Studies indicate that the gut T1R2-T1R3 may control glucose homeostasis through a combination of GLP-1 release and glucose transporter activation,53 however there is still controversy about the exact role of T1Rs in glucose homeostasis.11 T1R1-T1R3 have been implicated in appetite/satiety regulation by releasing CCK through a pathway that might involve the gut–brain axis.57 By contrast, T2Rs, a large family of receptor subtypes (more than 25 different genes in humans and more than 30 in rodents)58 detect a large group of bitter substances and are believed to play a role in defending the body from external threats.59 Distinct subtypes of T2Rs have been localized in the GI mucosa, including subpopulations of EECs.52,54 Intraluminal bitter tastants have been shown to induce release of ghrelin by EECs45 and activate vagal neurons expressing CCK1 and Y2 receptors which bind to CCK and PYY, respectively, thus suggesting release of these peptides in response to bitter tastants.46
Amino acids Receptors
Different types of amino acids activate distinct receptors on EECs in addition to the umami-T1R1-T1R3 sensing receptors2; examples include the Gq-coupled calcium sensing receptor (CaSR) that detects aromatic and basic aminoacids and is located in G, D and I cells,60 and GPRC6A and GPR92 that are activated by basic and neutral aminoacids or protein hydrolysates, respectively, which are predominantly expressed by G and D cells.57,61
Free fatty acid receptors (FFARs)
GPCRs which bind free fatty acid are involved in sensing medium and long chain fatty acids and are associated with different types of EECs, such as X/A, P, I, K, and L cells as well as EC-containing 5-HT.62-64 In the colon, non-digestible carbohydrates are processed by bacterial fermentation with production of short chain fatty acids (SCFA) detected by FFARs.65 Activation of FFAR1, 2, 3 (also known as GPR40, 43 and 41, respectively) and CaSR induce release of CCK to increase satiety and delay gastric emptying. GLP-1 and PYY are also secreted in response to FFAR1,2,3, activation.66-69 Thus, nutrients and non-nutrient tastants are detected by EECs in the entire GI system from the proximal stomach to the rectum. In addition, recent data indicate that also the ‘gut microbiota’, i.e. the myriad of bacteria - as well as viruses and fungi – present in diverse abundance throughout the gut lumen, contribute to gut chemosensitivity.70,71 Indeed, bacterial products including SCFA metabolites act as a ligand and activate specific receptors such as GPR41 and GPR43 located on EECs, triggering the release of GI hormones (e.g. GLP-1, GLP-2 ad PYY) and a subsequent regulation of physiological, homeostatic and barrier functions.70,72 Bacterial metabolites are also recognized by toll-like receptors, some of which have been localized to the GI mucosa.10
The understanding of the multiple pathways and systems involved in nutrient sensing can provide opportunities to develop strategies for the treatment of food associated disorders by targeting specific EEC receptors.
EECs AND THE BRAIN-GUT AXIS
Brain-Gut Axis Crosstalk
The relationship between the brain and the gut represents an important area of investigation in neurogastroenterology. The brain receives constant input of different stimuli/signals from the GI tract and initiates the appropriate integrated response to target cells in the GI tract.73 The information is predominantly carried from the gut to the brain via vagal and spinal afferent neurons, immune mediators, gut hormones and microbiota-related signaling molecules, while information from the brain to the gut is via autonomic neurons and neuroendocrine factors.10,73 Although it is now well established that there is bidirectional crosstalk between the brain and the gut (Fig. 2) many questions remain unsolved such as how the interplay between the central nervous system (CNS) and the periphery is perturbed in pathophysiological states. A typical example of brain-gut axis dysfunction is apparent in functional GI disorders, such as the irritable bowel syndrome (IBS) and functional dyspepsia.74
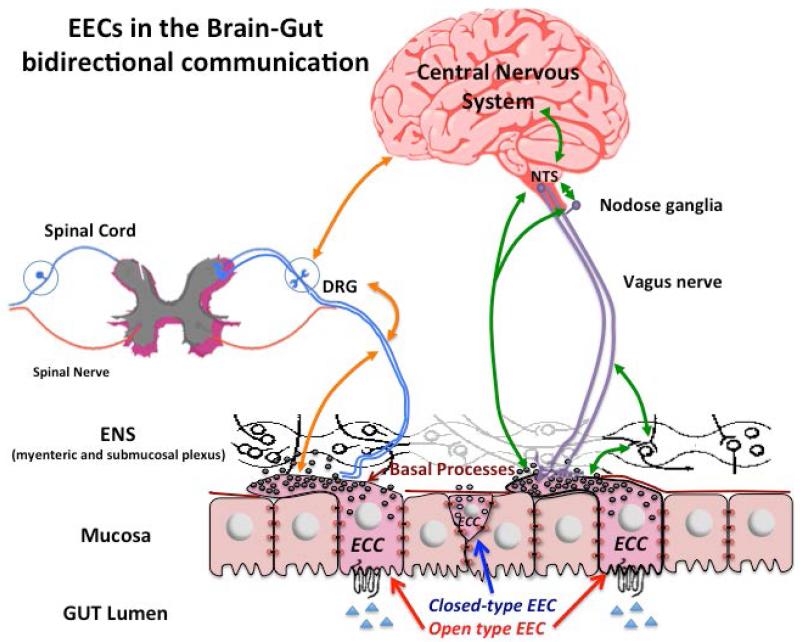
Figure 2. EECs in the Brain-Gut Axis Bidirectional Communication.
Green and Orange arrows indicate the bidirectional interplay referred to brain-gut axis, which include the mucosal EECs, ENS, vagus nerve, spinal cord and CNS. The basal cytoplasmic processes have a role in activating intrinsic and extrinsic sensory neurons. The activation of EECs may leads to sensitization via direct cell to neurons contact. Hormones released by EECs upon stimulation act on receptors located on the vagal and spinal neurons and influence neuronal pathways in the bidirectional brain-gut communication. Open type EECs sense luminal content and make contact with enteric nervous system (black), vagal nerve (afferent and efferent pathways, violet lines) and spinal nerve (afferent and efferent pathways, blue lines), closed-type EEC also contribute to activate these feedback signals from the gut to the brain. Together, this information sends positive or negative feedback to the brain (CNS), which modulates functions of the GI tract, glucose homeostasis and satiety. Moreover, impairment in the brain-gut axis pathways could be implicated in obesity, visceral hypersensitivity, intestinal disorders such as irritable bowel syndrome and inflammatory disorders such as inflammatory bowel disease. Abbreviations: NTS, nucleus tractus solitarii; DRG, dorsal root ganglia; ENS, enteric nervous system (myenteric and submucosal plexus); EEC, enteroendocrine cell.
EECs: Role in Brain-Gut Axis Communication
The brain-gut axis is a multi-level system to manage various aspects of GI physiology including nutrient signals and related central processing. EECs contribute to these mechanisms by exerting regulatory effects on this bidirectional communication between the brain and the gut. Vagal afferent terminals innervate the wall of the GI tract, reaching the mucosa and the lamina propria and are in close proximity to the mucosal epithelium.75 Peptides/hormones secreted by EECs act on receptors located along the vagal afferent fibers. Vagal afferent pathways convey stimuli generated by EECs to the brainstem, specifically the NTS, and the nodose ganglion represents an intermediate station in the brain-gut axis bidirectional communication. Therefore, vagal afferent pathways activated by peptides released by EEC mediate numerous functions including the regulation of food intake, motility, secretion, inflammatory response and mucosal defence.73,76 Below are some examples of EEC-brain communication.
EECs secreting Cholecystokinin
CCK is mainly secreted in the upper small intestine in response to fat and proteins.3,77 The postprandial release of CCK activates intestinal feedback through extrinsic neural (vagal) pathway to control GI functions such as short-term inhibition of gastric emptying and acid secretion, through CCK1 receptors expressed by vagal afferent terminals that run in the lamina propria adjacent to the mucosal epithelium.75 CCK can transmit sensory signals from the gut lumen by direct EEC-nerve communication and/or activation of vagal pathways through a paracrine mechanism.21,75 Exogenous administration of CCK activates CCK1 receptors on vagal terminals and induces satiation and reduction of meal size, while the administration of CCK1 receptor antagonist reverses these effects. Furthermore, exogenous administration of CCK or nutrients, such as protein or fat, in CCK1 receptor null mice does not affect inhibition of food intake and gastric emptying, thus supporting the role of CCK/CCK1 receptor in mediating these specific effects.78 Moreover, in CCK1 receptor null mice there was a strong decrease in activation of the vagal afferent pathways in response to intestinal lipids compared to wild type mice, confirmed by lack of firing NTS neurons.78 The effect of CCK in the peripheral regulation of food intake is enhanced by leptin. When co-administered, CCK and leptin increase the excitatory response on vagal afferents, which leads to an exaggerated inhibition of gastric emptying and food intake.79 Moreover, the expression of leptin receptors on vagal afferent neurons increase and decrease respectively in fasting or re-feeding states.80 In addition to satiety, the exogenous administration of CCK-8 in rats contributes to lower glucose production.81 The role of CCK1 receptor on vagal afferent terminals innervating the small intestine is fundamental to activate the protein kinase A signaling pathways involving the N-methyl-d-aspartate (NMDA) receptor, which mediates neuronal transmission in the NTS to regulate glucose homeostasis.82
EEC secreting PYY
PYY is a 36 amino acid peptide produced mainly in the ileum and colon in response to feeding especially lipids, but also carbohydrates and proteins.32 PYY is a member of the neuropeptide Y (NPY) family of peptides which are expressed in different areas of the CNS, and five different NPY receptors (Y1,Y2,Y3,Y4,Y5) have been characterized along the brain-gut axis.83 PYY positive neurons are found in the rodent NTS, and nerve terminals containing PYY are localized in the pons, hypothalamus (key energy balance regulating area in the brain) and spinal cord.84 The role of PYY in reducing food intake is mainly due to the activation of Y2 receptor in the hypothalamus.85 However, exogenous administration of PYY3-36 has been shown to exert effects on gastric emptying at least in part via the activation of vagal afferent pathways.86 The localization of Y2 receptor on vagal afferent neurons support that PYY neurons and nerve terminals in the vagus nerve convey visceral sensory information from the gut to the brain and provide a central and peripheral control of food intake.87
EEC secreting GLP-1
GLP-1, an incretin hormone, is released in the small intestine in response to food ingestion, especially carbohydrates and fats, and its main effect is on energy balance and blood glucose homeostasis.32 GLP-1 receptor (GLP-1R) is peripherally distributed in the gut, pancreatic β-cells, kidney and in the vagus nerve as well as centrally in the NTS and hypothalamus.88 Exogenous stimulation of GLP-1R, peripherally or centrally, results in different physiological responses, such as inhibition of gastric emptying,89 glucose-stimulated insulin secretion90 and reduced food intake.87 However, the short half-life of GLP-1 due to the rapid breakdown results in higher hormone concentration close to the site of secretion, and in pig intestine only a small part of that reaches the systemic circulation before it is degraded by Dipeptidyl peptidase-IV (DPPIV).91 Thus, a question that arises, is whether GLP-1 physiological levels (bloodstream) in human are capable of reaching and activating receptors in the hypothalamus and brainstem. GLP-1, secreted by EECs, acts as an endocrine substance and also in a neural manner by activating receptors on the vagal afferent terminals close to the site of secretion, or receptors expressed by the ENS. Indeed, the inhibition of food intake due to an intraperitoneal administration of GLP-1 in rodents is attenuated by the ablation of vagal-brainstem-hypothalamic pathways.87 Prominent basal processes are frequently described in L-type EEC,20 which are likely involved in driving the GLP-1 secretion directly on nerve terminals receptors as “real time” response to the ingested nutrient present in the gut lumen.
Taken together, these data provide evidence supporting the concept that hormones released from the EECs activate vagal afferent pathways in a paracrine manner or via direct EECs-sensory neuron communication, and therefore influence gut activity (e.g. intestinal motility, acid secretion, pancreatic enzyme production and secretion and gallbladder contraction) through the brain-gut axis.
EECs: Role in Sensory Neural Regulation
A significant challenge in the study of EECs and their connection with epithelial cells and nerves is their sparse and irregular localization in the mucosal epithelium. Indirect methods, such as the measurement of plasma hormone levels in response to drugs or meals, result in indirect evidence to support the physiological role(s) of EECs and are not helpful when addressing mechanisms of hormone release.76 EEC lines are a useful tool to assess their role and function, but an obvious limitation of isolated cell lines is that they are not fully representative of the more complex response seen in vivo.76 The development of transgenic mice tagged for hormone promoters driving the expression of GFP provides an excellent means to study the relationship of EECs with other cells, including sensory neurons. These studies have discovered that basal cytoplasmic processes in CCK- and PYY-GFP cells resemble axon with synaptic-like ends containing most of the cell secretory vesicles, many of which are distributed to the tips thus suggesting they guide hormone secretion.8,20,92 These basal cytoplasmic processes, which have been named “neuropods”, contain intermediate filaments, are surrounded by glia19 and appear to make a direct connection with nerves, including sensory nerve endings.20 Furthermore, in vitro study showed a strong attraction and subsequently connection between enteroendocrine CCK-GFP cells and sensory neurons enzymatically dissociated from the trigeminal nerves and from the dorsal root ganglion of wild type mouse.20 Although these findings do not exclude other mechanisms of action of EECs such as paracrine or through the bloodstream, they provide evidence for a direct connection between EEC basal processes and mucosal nerves supporting the dual function of EECs as nutrient chemosensing at the top of the cell, while conveying electrochemical information with cell-to cell and/or cell to nerve connections at the bottom of the cell.
EECs: Therapeutic Potential
The EEC-sensory neuron connection has thus opened a new exciting prospective on EECs and their role in the communication with ENS and CNS. The direct cell-to-sensory nerves and neurons connections and the presence of presynaptic and postsynaptic genes in the basal cytoplasmic processes20 shed new light on our understanding of the complexity of the bidirectional communication between the brain and the gut. EEC receptors on the luminal side could be a potential target of new drugs to activate hormonal and neuronal pathways providing a novel approach to treat diseases such as diabetes and obesity. Currently, GLP-1R agonist is used to treat diabetes mellitus type 2, based on its function to stimulate insulin secretion from pancreatic β-cells.93 Recently, researchers have highlighted the beneficial role of GLP-1 and PYY3-36 secretion in the reduction of food intake in patients undergoing gastric bypass surgery.94 Thus, PYY and GLP-1 are now targets to treat obesity, a major worldwide public health problem.95 Furthermore, drugs acting to alter EEC functions may participate in the control of depression, anxiety and visceral hypersensitivity, which are key components of functional GI disorders.96 Recently, a significant amount of attention has been given to a subgroup of EECs secreting 5-HT and their role in visceral perception. 5-HT is a key neurotransmitter in the control of nociceptive responses and mood, with receptors located in both the periphery and CNS. Much evidence suggests that activation of 5-HT receptors, specifically 5-HT3 and 5-HT4, sends signals from the gut to the CNS through vagal afferent fibers, which activate multiple pathways including those involved in nociception.97-99
SUMMARY AND CONCLUSION
EECs play an important role in gut chemosensing to orchestrate appropriate functional responses to a variety of stimuli ranging from nutrients, food degradation products, toxic chemicals, microorganisms and bacteria products. This complex balance between maximizing nutrient assimilation and minimizing danger to maintain optimal homeostasis is the result of the interplay of different detection systems including the distinct types of sensory receptors expressed by EECs, the variety of signaling molecules released by EECs and the targets that are activated directly and indirectly such as neighboring cells, distant targets reached via the bloodstream and neuronal pathways. Considerable progress has been made in understanding EECs relationship with neurons of the ENS and CNS and EEC bidirectional communication with the brain. There is now strong evidence that EECs can directly participate in the brain-gut axis bidirectional communication through axon-like processes or basal cytoplasmic processes. The basal processes connect EECs and neurons innervating the gut forming neuroepithelial circuits, which allow sensory transmission from the gut lumen and feedback from the brain to the gut EECs. The activation of afferent and efferent nerve pathways may be explored for the management of diseases related to feeding disorders and GI dysfunction. Alterations in the bidirectional communication between the brain and the gut are likely associated with an impairment of gut functions (e.g. intestinal secretion, motility, blood flow and afferent sensitivity). The modification of the homeostatic reflex might lead to a chronic perturbation of the gut such as visceral hypersensitivity. Therefore, a better understanding of the EEC roles and their relationship with the brain and sensory nerves is an important research target. Future studies are required to fully decipher this complex network. The direct EEC to nerve connection opens a new horizon of research and provides new tools to understand the mechanisms underlying EEC control of food intake and GI dysfunction as well as potential new therapeutic targets.
Key Messages.
Enteroendocrine cells (EECs) located in the entire GI tract are involved in luminal chemosensing and new evidence suggests that they have a role in sensory nerve sensitization.
EECs have direct cell connections to the nervous system. The EEC basal cytoplasmic processes interact with the enteric nervous system (ENS), vagus nerve, and spinal afferent fibers, supporting their participation in the complex and sophisticated activation of brain-gut axis bidirectional pathways.
Perturbation of this critical communication between EECs, ENS and central nervous system might alter the primary physiological and homeostatic gut functions.
AKNOWLEDGEMENTS
The authors would like to thank the following funding agencies for support: National Institutes of Health grants: P30 DK041301 CURE Digestive Diseases Research Core Grant (CS), RO1-DK098447 (CS), UCLA Spitzer award (CS), Department of Veterans Affairs: Research Career Scientist Award to BG-VM and VA Merit BX002188-01 (BG-VM) and VA Merit BX001195-05(BG-VM). R. De G. is supported by grants from the Italian Ministry of Public Health (Ricerca Finalizzata RER2009 - Ita-MNGIE); RFO from the University of Bologna; and “Fondazione Del Monte di Bologna e Ravenna”.
Abbreviations
- 5-HT
5-hydroxytryptamine/serotonin
- CaSR
calcium sensing receptor
- CCK
cholecystokinin
- CNS
central nervous system
- DPPIV
Dipeptidyl peptidase-IV
- EC
enterochromaffin cell
- EECs
enteroendocrine cells
- FFARs
free fatty acid receptors
- GFP
green fluorescent protein
- GI
gastrointestinal tract
- GIP
gastric inhibitory peptide
- GLP-1
glucagon like peptide-1
- GLP-1R
glucagon like peptide-1 receptor
- GLP-2
glucagon like petide-2
- GPCRs
G protein coupled receptor
- IBS
irritable bowel syndrome
- NMDA
N-methyl-d-aspartate receptor
- NPY
neuropeptide Y
- NTS
nucleus of the solitary tract
- PYY
peptide YY
- SCF
short chain fatty acid
- TRs
taste receptors
Footnotes
DISCLOSURE
The authors have no conflicts to disclose related to the contents of this review
Bibliography
- [1].Rehfeld JF. A centenary of gastrointestinal endocrinology. Horm Metab Res. 2004;36(11-12):735–741. doi: 10.1055/s-2004-826154. [DOI] [PubMed] [Google Scholar]
- [2].Janssen S, Depoortere I. Nutrient sensing in the gut: new road to therapeutics? Trends Endocrinol Metab. 2013;24(2):92–100. doi: 10.1016/j.tem.2012.11.006. [DOI] [PubMed] [Google Scholar]
- [3].Sternini C, Anselmi L, Rozengurt E. Enteroendocrine cells: a site of “taste “ in the gastrointestinal chemosensing. Cur Opin in Endocri, Diabet e Obesity. 2008;15(1):73–78. doi: 10.1097/MED.0b013e3282f43a73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Larsson LI, Goltermann N, De Magistris L, Rehfeld JF, Schwarz TW. Somatostatin cell processes as pathways for paracrine secretion. Science. 1979;205(4413):1393–1395. doi: 10.1126/science.382360. [DOI] [PubMed] [Google Scholar]
- [5].Böttcher G, Sjölund K, Ekblad E, Håkanson R, Schwartz TW, Sundler F. Coexistence of peptide YY and glicentin immunoreactivity in endocrine cells of the gut. Regul Pept. 1984;8(4):261–266. doi: 10.1016/0167-0115(84)90034-x. [DOI] [PubMed] [Google Scholar]
- [6].Keast JR, Furness JB, Costa M. Distribution of certain peptide-containing nerve fibers and endocrine cells in the gastrointestinal mucosa in five mammalian species. J Comp Neurol. 1985;236(3):403–422. doi: 10.1002/cne.902360308. [DOI] [PubMed] [Google Scholar]
- [7].Bohòrquez DV, Chandra R, Samsa LA, Vigna S, Liddle RA. Characterization of basal pseudopod-like processes in ileal and colonic PYY cells. J Mol Histol. 2011;42(1):3–13. doi: 10.1007/s10735-010-9302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chandra R, Samsa L, Vigna S, Liddle R. Pseudopod-like basal cell processes in intestinal cholecystokinin cells. Cell Tis Res. 2010;341(2):289–297. doi: 10.1007/s00441-010-0997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dockray GJ. Enteroendocrine cell signalling via the vagus nerve. Curr Opin Pharm. 2013;13(6):954–958. doi: 10.1016/j.coph.2013.09.007. [DOI] [PubMed] [Google Scholar]
- [10].Furness JB, Riviera LR, Cho HJ, Bravo DM, Challagan B. The gut as a sensory organ. Nat Rev Gastroenterol Hepatol. 2013;10(12):729–740. doi: 10.1038/nrgastro.2013.180. [DOI] [PubMed] [Google Scholar]
- [11].Psichas A, Reimann F, Gribble FM. Gut chemosensing mechanisms. J Clin Invest. 2015;125(3):908–17. doi: 10.1172/JCI76309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Breer H, Eberle J, Frick C, Haid D, Widmayer P. Gastrointestinal chemosensation: chemosensory cells in the alimentary tract. Histochem Cell Biol. 2012;138(1):13–24. doi: 10.1007/s00418-012-0954-z. [DOI] [PubMed] [Google Scholar]
- [13].Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125(3):926–938. doi: 10.1172/JCI76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dockray GJ. Bayliss–Starling prize lecture. Gastrointestinal hormones and the dialogue between gut and brain. J Physiol. 2014;592(14):2927–2941. doi: 10.1113/jphysiol.2014.270850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li HJ, Ray SK, Singh NK, Jhonston B, Leiter AB. Basic helix-loop-helix transcription factors and enteroendocrine cell differentiation. Diabetes, Obesity and Metabolism. 2011;13(1):5–12. doi: 10.1111/j.1463-1326.2011.01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284(5415):770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- [17].Höfer D, Asan E, Drenckhahn D. Chemosensory perception in the gut. News Physiol Sci. 1999;14:18–23. doi: 10.1152/physiologyonline.1999.14.1.18. [DOI] [PubMed] [Google Scholar]
- [18].Gribble FM, Reimann F. Enteroendocrine Cells: Chemosensors in the Intestinal Epithelium. Annu Rev Physiol. 2016;78:3.1–3.23. doi: 10.1146/annurev-physiol-021115-105439. [DOI] [PubMed] [Google Scholar]
- [19].Bohòrquez DV, Samsa LA, Roholt A, Medicetty S, Chandra R, Liddle RA. An enteroendocrine cell-enteric glia connection revealed by 3D electron microscopy. PLoS ONE. 2014;9(2):e89881. doi: 10.1371/journal.pone.0089881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bohórquez DV, Shaid RA, Erdmann A, Kreger AM, Wang Y, Calakos N, Wang F, Liddle RA. Neuroephitelia circuit formed by innervation of sensory enteroendocrine cells. J Clin. Invest. 2015;125(2):782–786. doi: 10.1172/JCI78361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Egerod KL, Engelstoft MS, Grunddal KV, Nøhr MK, Secher A, Sakata I, Pedersen J, Windeløv JA, et al. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology. 2012;153(12):5782–95. doi: 10.1210/en.2012-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Habib AM, Richards P, Cairns LS, Rogers GJ, Bannon CA, Parker HE, Morley TC, Yeo GS, et al. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrynology. 2012;153(7):3054–65. doi: 10.1210/en.2011-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cho H-J, Kosari S, Hunne B, Callaghan B, Rivera LR, Bravo DM, Furness JB. Differences in hormone localisation patterns of K and L type enteroendocrine cells in the mouse and pig small intestine and colon. Cell Tissue Res. 2015;359(2):693–698. doi: 10.1007/s00441-014-2033-3. [DOI] [PubMed] [Google Scholar]
- [24].Dockray GJ, Varro A, Dimaline R. Gastric endocrine cells: gene expression, processing, and targeting of active products. Physiol Rev. 1996;76(3):767–798. doi: 10.1152/physrev.1996.76.3.767. [DOI] [PubMed] [Google Scholar]
- [25].Dockray GJ. The gut endocrine system and its control. Exp Physiol. 1994;79(5):607–34. doi: 10.1113/expphysiol.1994.sp003795. [DOI] [PubMed] [Google Scholar]
- [26].Kending DM, Grider JR. Serotonin and colonic motility. Neurogastroenterol Motil. 2015;27(7):899–905. doi: 10.1111/nmo.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Op Endoc Diab Obes. 2013;20(1):14–21. doi: 10.1097/MED.0b013e32835bc703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Keating DJ, Spencer NJ. Release of 5-Hydroxytryptamine from the mucosa is not required for the generation or propagation of colonic migrating motor complexes. Gastroenterology. 2010;138(2):659–670. doi: 10.1053/j.gastro.2009.09.020. [DOI] [PubMed] [Google Scholar]
- [29].Spencer NJ, Nicholas SJ, Robinson L, Kyloh M, Flack N, Brookes SJ, Zagorodnyuk V, Keating DJ. Mechanisms of underlying distension-evoked peristalsis in guinea pig distal colon: is there a role for enterochromaffin cells? Am J Physiol Gastrointest Liver Physiol. 2011;301(3):G519–G527. doi: 10.1152/ajpgi.00101.2011. [DOI] [PubMed] [Google Scholar]
- [30].Mawe GM, Hoffman JM. Serotonin signaling in the gut-functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10(8):473–486. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev. 2007;8(1):21–34. doi: 10.1111/j.1467-789X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- [32].Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117(1):13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sakata I, Nakamura K, Yamazaki M, Matsubara M, Hayashi Y, Kangawa K, Sakai T. Ghrelin-producing cells exist as two types of cells, closed- and opened type cells, in the rat gastrointestinal tract. Peptides. 2002;23(3):531–536. doi: 10.1016/s0196-9781(01)00633-7. [DOI] [PubMed] [Google Scholar]
- [34].Holst JJ. Incretin hormones and the satiation signal. Int J Obes (Lond) 2013;37(9):1161–1168. doi: 10.1038/ijo.2012.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sigalet DL, Wallace LE, Holst JJ, Martin GR, Kaji T, Tanaka H, Sharkey KA. Enteric neural pathways mediate the anti-inflammatory actions of glucagon-like peptide 2. Am J Physiol Gastrointest Liver Physiol. 2007;293(1):G211–21. doi: 10.1152/ajpgi.00530.2006. [DOI] [PubMed] [Google Scholar]
- [36].Rowland KJ, Brubaker PL. The “cryptic” mechanism of action of glucagon-like peptide-2. Am J Physiol Gastrointest Liver Physiol. 2011;301(1):G1–8. doi: 10.1152/ajpgi.00039.2011. [DOI] [PubMed] [Google Scholar]
- [37].Hsieh J, Longuet C, Maida A, Bahrami J, Xu E, Baker CL, Brubaker PL, Drucker DJ, et al. Glucagon-like peptide-2 increase intestinal lipid absorption and chylomicron production via CD36. Gastroenterology. 2009;137(3):997–1005. doi: 10.1053/j.gastro.2009.05.051. [DOI] [PubMed] [Google Scholar]
- [38].Batterham RL, Cohen Ma, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349(10):941–8. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- [39].Halatchev IG, Cone RD. Peripheral administration of PYY(3-36) produces conditioned taste aversion in mice. Cell Metab. 2005;1(3):159–68. doi: 10.1016/j.cmet.2005.02.003. [DOI] [PubMed] [Google Scholar]
- [40].Spiller RC, Trotman IF, Higgins BE, Ghetei MA, Grimble GK, Lee YC, Bloom SR, Misiewicz JJ, Silk DB. The ileal brake-inhibition of jejunal motility after ileal fat perfusion in man. Gut. 1984;25(4):365–74. doi: 10.1136/gut.25.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Giralt M, Vergara P. Glucagonlike peptide-1 (GLP-1) participation in ileal brake induced by intraluminal peptones in rat. Dig Dis Sci. 1999;44(2):322–9. doi: 10.1023/a:1026654417697. [DOI] [PubMed] [Google Scholar]
- [42].Van Citters GW, Lin HC. Ileal brake: neuropeptidergic control of intestinal transit. Curr Gastroenterol Rep. 2006;8(5):367–373. doi: 10.1007/s11894-006-0021-9. [DOI] [PubMed] [Google Scholar]
- [43].Daniel H, Zietek T. Taste and move: Glucose and peptide transporters in the gastrointestinal tract. Exp Physiol. 2015 doi: 10.1113/EP085029. doi: 10.1113/EP085029. [DOI] [PubMed] [Google Scholar]
- [44].Dockray GJ. Making sense of gut contents. Scand J Gastroenterol. 2003;38(5):451–455. doi: 10.1080/00365520310000799. [DOI] [PubMed] [Google Scholar]
- [45].Janssen S, Laermans J, Verhulst PJ, Thijs T, Tack J, Depoortere I. Bitter taste receptors and α-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc Natl Acad Sci U S A. 2011;108(5):2094–2099. doi: 10.1073/pnas.1011508108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hao S, Sternini C, Raybould HE. Role of CCK1 and Y2 receptors in activation of hindbrain neurons induced by intragastric administration of bitter taste receptor ligands. Am J Physiol Regul Integr Comp Physiol. 2008;294(1):33–38. doi: 10.1152/ajpregu.00675.2007. [DOI] [PubMed] [Google Scholar]
- [47].Hao S, Dulake M, Espero E, Sternini C, Raybould HE, Rinaman L. Central Fos expression and conditioned flavor avoidance in rats following intragastric administration of bitter taste receptor ligand. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R528–36. doi: 10.1152/ajpregu.90423.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Glendinning JI, Yiin YM, Ackroff K, Sclafani A. Intragastric infusion of denatonium conditions flavor aversion and delays gastric emptying in rodents. Physiol Behav. 2008;93(4-5):757–765. doi: 10.1016/j.physbeh.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hagbom M, Istrate C, Engblom D, Karlsson T, Rodriguez-Diaz J, Buesa J, Taylor JA, Loitto VM, et al. Rotavirus Stimulates Release of Serotonin (5-HT) from human enterochromaffin cells and activates brain structures involved in nausea and vomiting. PLoS Pathol. 2011;7(7):e1002115. doi: 10.1371/journal.ppat.1002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Laffitte A, Neiers F, Briand L. Functional roles of the sweet taste receptor in oral and extraoral tissues. Curr Opin Nutr Metab Care. 2014;17(4):379–85. doi: 10.1097/MCO.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Behrens M, Meyerhof W. Oral and extraoral bitter taste receptors. Results Probl Cell Differ. 2010;52:87–99. doi: 10.1007/978-3-642-14426-4_8. [DOI] [PubMed] [Google Scholar]
- [52].Rozengurt N, Wu SV, Chen MC, Huang C, Sternini S, Rozengurt E. Colocalization of the alpha-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol. 2006;291(5):G792–G802. doi: 10.1152/ajpgi.00074.2006. [DOI] [PubMed] [Google Scholar]
- [53].Margolskee RF, Dyer J, Kokrashvili Z, Salmon SH, Ilegems E, Daly K, Emeline L, Maillet EL, et al. T1R3 and gustducin in gut sense sugar to regulate expression of Na+-glucose cotransporter. Proc Natl Acad Sci U S A. 2007;104(38):15075–80. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Vegezzi G, Anselmi L, Huynh J, Barocelli E, Rozengurt E, Raybould H, Sternini C. Diet-induced regulation of bitter taste receptor subtypes in the mouse gastrointestinal tract. PLoS One. 2014;9(9):e107732. doi: 10.1371/journal.pone.0107732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Li X, Staszewski L, Xu h, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci U S A. 2002;99(7):4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJP, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106(3):381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- [57].Depoortere I. Taste receptors in the gut tune the release of peptides in response to nutrients. Peptides. 2015;66:9–12. doi: 10.1016/j.peptides.2015.01.013. [DOI] [PubMed] [Google Scholar]
- [58].Bachmanov AA, Beauchamp GK. Taste Receptor Genes. Annu Rev nutr. 2007;27:389–414. doi: 10.1146/annurev.nutr.26.061505.111329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses. 2010;35(2):157–170. doi: 10.1093/chemse/bjp092. [DOI] [PubMed] [Google Scholar]
- [60].MacLeod RJ. CaSR function in the intestine: hormone secretion, electrolyte absorption and secretion, paracrine non-canonical Wnt signaling and colonic crypt cell proliferation. Best Pract Res Clin Endocrinol Metab. 2013;27(3):385–402. doi: 10.1016/j.beem.2013.05.005. [DOI] [PubMed] [Google Scholar]
- [61].Haid DC, Jordan-Biegger C, Widmayer P, Breer H. Receptors responsive to protein breakdown products in g-cells and d-cells of mouse, swine and human. Front Physiol. 2012;3:1–15. doi: 10.3389/fphys.2012.00065. doi: 10.3389/fphys.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57(9):2280–2287. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Janssen S, Laermans J, Iwakura H, Tack J, Depoortere I. Sensing of fatty acids for octanoylation of ghrelin involves a gustatory G-protein. PLoS ONE. 2012;7(6):7e40168. doi: 10.1371/journal.pone.0040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11(1):90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- [65].Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278(13):11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- [66].Karaki S, Tazoe H, Hayashi H, Kashiwabara H, Tooyama K, Suzuki Y, Kuwahara A. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J Mol Histol. 2008;39(2):135–142. doi: 10.1007/s10735-007-9145-y. [DOI] [PubMed] [Google Scholar]
- [67].Tazoe H, Otomo Y, Karaki S, Kato I, Fukami Y, Terasaki M, Kuwahara A. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed Res. 2009;30(3):149–156. doi: 10.2220/biomedres.30.149. [DOI] [PubMed] [Google Scholar]
- [68].Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364–71. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U.S.A. 2008;105(43):16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Cani Pd, Everard A, Duparc T. Gut microbiota, enteroendocrine functions and metabolism. Curr Opin Pharmacol. 2013;13(6):935–40. doi: 10.1016/j.coph.2013.09.008. [DOI] [PubMed] [Google Scholar]
- [71].Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28(2):203–209. [PMC free article] [PubMed] [Google Scholar]
- [72].Nøhr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, Sichlau RM, Grunddal KV, et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs. FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154(10):3552–64. doi: 10.1210/en.2013-1142. [DOI] [PubMed] [Google Scholar]
- [73].Mayer EA. Gut feelings: the emerging biology of gut–brain communication. Nat Rev Neurosci. 2011;12(8) doi: 10.1038/nrn3071. 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Ann Rev Med. 2011;62:381–96. doi: 10.1146/annurev-med-012309-103958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Beyak M, Bulmer DCE, Jiang W, Keating C, Rong W, Grundy D. Extrinsic sensory afferent nerves innervating the gastrointestinal tract. In: Johnson LR, Barrett KE, Ghishan FK, Merchant JL, Said HM, Wood JD, editors. Physiology of the Gastrointestinal Tract. 4th ed. Elsevier; San Diego, CA: 2006. pp. 685–726. [Google Scholar]
- [76].Raybould HE. Gut Chemosensing: interaction between gut endocrine cells and visceral afferents. Auton Neurosci. 2010;153(1-2):41–46. doi: 10.1016/j.autneu.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Dockray GJ. Luminal sensing in the gut: an overview. J Physiol Pharmacol. 2003;54(4):9–17. [PubMed] [Google Scholar]
- [78].Whited KL, Thao D, Lloyd KC, Kopin As, Raybould HE. Targeted distruption of the murine CCK1 receptor gene reduces intestinal lipid-induced feedback inhibition of gastric function. Am J Physiol Gastrointest Liver Physiol. 2006;291(1):G156–G162. doi: 10.1152/ajpgi.00569.2005. [DOI] [PubMed] [Google Scholar]
- [79].Peters JH, Simasko SM, Ritter RC. Modulation of vagal afferent excitation and reduction of food intake by leptin and cholecystokinin. Physiol Behav. 2006;89(4):477–485. doi: 10.1016/j.physbeh.2006.06.017. [DOI] [PubMed] [Google Scholar]
- [80].Buyse M, Ovesjö ML, Goïo H, Guilmeau S, Péranzi G, Moizo L, Walker F, Lewin MJ, et al. Expression and regulation of leptin receptor protein in afferent and efferent neurons of the vagus nerve. Eur J Neurosc. 2001;14(1):64–72. doi: 10.1046/j.0953-816x.2001.01628.x. [DOI] [PubMed] [Google Scholar]
- [81].Cheung GW, Kokorovic A, Lam CK, Chari M, Lam TK. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab. 2009;10(2):99–109. doi: 10.1016/j.cmet.2009.07.005. [DOI] [PubMed] [Google Scholar]
- [82].Rasmussen BA, Breen DM, Luo P, Cheung GWC, Yang CS, Sun B, Kokorovic A, Rong W, et al. Duodenal activation od cAMP-dependent protein kinase induces vagal afferent firing and lower glucose production in rats. Gastroenterology. 2012;142(4):834–843. e3. doi: 10.1053/j.gastro.2011.12.053. [DOI] [PubMed] [Google Scholar]
- [83].Holzer P, Reichmann F, Farzi A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides. 2012;46(6):261–74. doi: 10.1016/j.npep.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ekblad E, Sundler F. Distribution of pancreatic polypeptide and peptide YY. Peptides. 2002;23(2):251–261. doi: 10.1016/s0196-9781(01)00601-5. [DOI] [PubMed] [Google Scholar]
- [85].Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418(6898):650–4. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- [86].Whited KL, Tso P, Raybould HE. Involvement of apolipoprotein A-IV and CCk1 receptor in exogenous PYY3-36-induced stimulation of intestinal feedback. Endocrinology. 2007;148(10):4695–4703. doi: 10.1210/en.2006-1665. [DOI] [PubMed] [Google Scholar]
- [87].Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide (3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044(1):127–131. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- [88].Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- [89].İmeryüz N, Yeğen BC, Bozkurt A, Coşkun T, Villanueva-Peñacarrillo ML, Ulusoy NB. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Physiol. 1997;273(4-1):G920–7. doi: 10.1152/ajpgi.1997.273.4.G920. [DOI] [PubMed] [Google Scholar]
- [90].Nadkarni P, Chepurny OG, Holz GG. Regulation of glucose homeostasis by GLP-1. Prog Mol Biol Transl Sci. 2014;121:23–65. doi: 10.1016/B978-0-12-800101-1.00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Holst JJ, Deacon CF. Glucagone-like peptide-1 mediates the therapeutic actions of PP-IV inhibitors. Diabetologia. 2005;48(4):612–615. doi: 10.1007/s00125-005-1705-7. [DOI] [PubMed] [Google Scholar]
- [92].Bohòrquez DV, Liddle RA. Axon-like processes in enteroendocrine cells: characteristics and potential targets. Clin Transl Sci. 2011;4(5):387–391. doi: 10.1111/j.1752-8062.2011.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Meloni Ar, De Young MB, Lowe C, Parkes DG. GLP-1 receptor activated insulin secretion from pancreatic β-cell: mechanism and glucose dependence. Diabetes Obes Metab. 2013;15(1):15–27. doi: 10.1111/j.1463-1326.2012.01663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Holst JJ. Enteroendocrine secretion of gut hormones in diabetes, obesity and after bariatric surgery. Curr Opin Pharmacol. 2013;13(6):983–988. doi: 10.1016/j.coph.2013.09.014. [DOI] [PubMed] [Google Scholar]
- [95].De Mello AH, Prá M, Cardoso LC, De Bona Schraiber R, Rezin GT. Incretin-based therapies for obesity treatment. Metabolism. 2015 May 23; doi: 10.1016/j.metabol.2015.05.012. pii: S0026-0495(15)00155-9. doi: 10.1016/j.metabol.2015.05.012. [DOI] [PubMed] [Google Scholar]
- [96].Park JH, Rhee P-L, Kim G, Lee JH, KIM Y-H, Kim JJ, Rhee JC, Song SY. Enteroendocrine cell counts correlate with visceral hypersensitivity in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol and Motil. 2006;18(7):539–546. doi: 10.1111/j.1365-2982.2006.00771.x. [DOI] [PubMed] [Google Scholar]
- [97].Greenwood-Van Meerveld B, Mohammadi E, Tyler K, Pietra C, Bee LA, Dickenson A. Synergistic effect of 5-hydroxytryptamine 3 and neurokinin 1 receptor antagonism in rodent models of somatic and visceral pain. J Pharmacol Exp Ther. 2014;351(1):146–52. doi: 10.1124/jpet.114.216028. [DOI] [PubMed] [Google Scholar]
- [98].Gilet M, Eutamene H, Han H, Kim HW, Bueno L. Influence of new 5-HT4 receptor partial agonist, YKP10811, on visceral hypersensitivity in rats triggered by stress and inflammation. 2014. Neurogastrenterol Motil. 2014;26(12):1761–1770. doi: 10.1111/nmo.12458. [DOI] [PubMed] [Google Scholar]
- [99].Keszthelyi Troost FJ, Jonkers DM, Van Eijk HM, Dekker J, Buurman WA, Masclee AAM. Visceral hypersensitivity in irritable bowel syndrome: evidence for involvement of serotonin metabolism- preliminary study. Neurogastroenterol Motil. 2015;27(8):1127–37. doi: 10.1111/nmo.12600. [DOI] [PubMed] [Google Scholar]