Abstract
Presence of tumor initiating cells and a proper niche is essential for metastatic colonization. SLUG and SOX9 transcription factors play essential roles in induction and maintenance of tumor initiating capacity in breast cancer cells. On the other hand, Tenascin-C and Periostin are crucial factors in metastatic niche that support tumor initiating capability in breast cancer. In this study, regulatory effect of SLUG and SOX9 transcription factors on the expression of Tenascin-C and Periostin was examined. SLUG and SOX9 were overexpressed and knocked-down in MCF7 and MDA-MB-231 cells, respectively. The cells as little and highly invasive breast cancer-derived cells were infected by inducing and shRNA lentivirus constructs. Then, Tenascin-C and Periostin as well as SLUG and SOX9 expression levels were measured in the cells via Real-Time PCR. Simultaneous overexpression of SLUG and SOX9 significantly induced Tenascin-C and Periostin expression. SLUG and SOX9 knock-down also significantly reduced the expression of Tenascin-C and Periostin. In this analysis Periostin showed the most deviation in both up- and down-regulation levels. This regulatory effect might shed light to a crosstalk between factors involved in the tumor initiating capacity and metastatic niche of the breast cancer.
Keywords: Breast cancer stem cell, Persiostin, Tenascin-C, Slug, Sox9
Introduction
Breast cancer metastasis is the cause of most cancer-related deaths among women [1]. This high mortality rate is due to lack of understanding the disease molecular mechanisms, which leads to the lack of proper treatment. Breast tumor metastasis is shown to be associated to well-orchestrated programs of induction and maintenance of tumor initiating capacity in a spatiotemporal manner. In the highly complex journey of tumor cells from primary to distant site, the most important part could be delivered by competent tumor initiating cells (TICs) that survive in the circulation, reach the proper secondary tissue and grow to a full-blown secondary tumor [2, 3]. Accordingly, successful metastasis relies on competent tumor cells seeded in a fertile microenvironment, the fact that recalls “seed and soil” hypothesis [4].
In breast cancer, TICs might show stem-like characteristics at some levels, depending on expression of essential regulatory factors in tumor cells, as well as in surrounding microenvironment. SLUG (SNAI2) and SOX9 transcription factors (TFs) are considered as major regulators of stemness in mammary cells and also in breast tumor cells [5]. In mouse models of overexpressed Slug and Sox9, differentiated luminal cells could convert to mammary stem cells with the ability to produce full ductal tree structure in another mouse. MCF7 cells normally do not form macrometastases when transplanted into mammary fat pad. However, simultaneous overexpression of these two TFs in transplanted MCF7 cells led to formation of several full-blown metastases in lungs of mice. This shift in metastasis formation property was also confirmed by knocking-down of SLUG and SOX9 in invasive MDA-231 cells and showing a dramatic reduction in lung colonization, subsequently upon injection through tail vain [5].
Tenascin-C (TNC) and Periostin (POSTN), on the other hand, are extracellular matrix (ECM) factors that induce and maintain the tumor initiating capacity in breast tumor cells [6, 7]. In mouse model with Postn deficiency, metastatic colonization in lungs was ablated [6]. Moreover, TNC is essential for pulmonary metastasis, as its knock-down in MDA-231 cells impedes metastatic colonization in lungs [7].
Tremendous similar effects have been independently observed by ectopic and knocked-down distinct expression of SLUG, SOX9, TNC and POSTN in the processes of induced and prevented breast cancer metastasis, respectively. So by considering above-mentioned facts, we hypothesized that there might be a regulatory connection between these major players and factors in breast cancer metastasis [8]. Having these seminal previous works as backbone, in this study we show that expression of TNC and POSTN is correlated and consistent with that of SLUG and SOX9, when the latter are simultaneously expressed.
Materials and Methods
Cell Culture
HEK293T, MCF7 and MDA-MB-231 cell lines were obtained from National Cell Bank of Pasture Institute of Iran. HEK293T cells were grown in DMEM and MCF7/MDA-MB-231 cells in DMEM/F12 (1:1) medium with L-glutamine (Gibco), with 10 % fetal bovine serum and 1 % antibiotic (penicillin–streptomycin) at 37 °C with 5 % CO2. Media were changed twice a week.
Lentivirus Production and Infection
Recombinant lentivirus stocks were produced and concentrated as previously reported [9], Transfer vectors pTK380-Slug, pWPXL-Sox9, pLKO.Sh-SLUG and pLKO.Sh-SOX9 (Addgene), were respectively used for the coding sequences of mouse Slug and Sox9, and human shRNAs targeting SLUG and SOX9. Infected cells were expanded and used for RT-PCR.
RNA Extraction and Gene Expression Analysis by Real-Time RT-PCR
Nighty six hours after cell infection, total RNA was extracted using RNX Plus reagent (Sinaclone, Iran). Isolated RNA was diluted in DEPC treated water and the quantity of RNA was measured by NanoDrop spectroscopy (ND-1000, Wilmington, Delaware, USA). 1 microgram of RNA was used for reverse transcription into cDNA using murine leukemia virus (MuLV) reverse transcriptase (Fermentas, Lithuania) in the presence of random hexamers and RNase inhibitor according to manufacturer’s protocol. Primers for RT-PCR were designed based on coding sequences, as shown in Table 1. Real time RT-PCR reaction was carried out on Rotor Gen 6000 Corbette detection system using Precision 2X qPCR Mastermix SYBR Green detection kits (Takara Bio) followed by thermocycling conditions Heat hold at 90 °C for 10 min as initial activation followed by 40 cycles of denaturation at 94 °C for 10 s and a combined annealing/extension step at 60 °C for 30 s. Hypoxanthine-guanine phosphoribosyl transferase1 (HPRT1) was amplified as internal control and fold change in relative expression of each target gene was calculated based on the comparative Ct (2-ΔΔCt) method. Melting curve analysis was used to validate whether all primers yield a single PCR product.
Table 1.
Primers for real-time RT-PCR
| Primer Name | Primer Sequence |
|---|---|
| POSTN Fwd | GAAAGGGAGTAAGCAAGGGAG |
| POSTN Rev | TGTCCAGTCTCCAGGTTGTG |
| TNC Fwd | TGTCACCGTGTCAACCTGATG |
| TNC Rev | GTTGGGCTGGTTGTATTGATGC |
| mSlug Fwd | GCACTGTGATGCCCAGTCTA |
| mSlug Rev | CAGTGAGGGCAAGAGAAAGG |
| hSLUG Fwd | CGGACCCACACATTACCTTG |
| hSLUG Rev | GTGCAGGAGAGACATTCTGG |
| mSox9 Fwd | AGGAAGCTGGCAGACCAGTA |
| mSox9 Rev | CGTTCTTCACCGACTTCCTC |
| hSOX9 Fwd | AGAAGGACCACCCGGATTAC |
| hSOX9 Rev | TCGCTTCAGGTCAGCCTTG |
Data Analysis
All data are shown as mean ± (Standard Error of the Mean) SEM. Comparison between groups was made by one-way ANOVA followed by an appropriate post-hoc test. The statistical significances were achieved when: * = P\0.05, ** = P\0.01 and *** = P\0.001. GraphPad (Prism Software Inc.) was used for statistical analyses (Version 5.07, 2007).
Results
Overexpression of Slug and Sox9 induce, while their knock-down suppress, the expression of TNC and POSTN
MCF7 and MDA-231 cell lines were chosen for their contradictory states in terms of expressing SLUG and SOX9; both with low expression in one but high in the other, respectively. Also, these two cell lines are used in the study by Guo et al. [5], in which cooperative roles of SLUG and SOX9 in stemness has been depicted. This further led us to utilize the same system to avoid any possible inconsistency due to different molecular and biologic contexts of other cell lines. To confirm, expression levels of SLUG, SOX9, TNC and POSTN were checked in MCF7 and MDA-231. As expected, expression levels of all above-mentioned genes were higher in MDA-231 compared to MCF7. Among all, POSTN showed the most prominent expression variation between these two cell lines (Fig. 1A). This difference of gene expression between MCF7 and MDA-231 made them as good candidates for gain and loss of function studies of Slug and Sox9, in order to investigate their potential regulatory effects on TNC and POSTN.
Fig. 1.
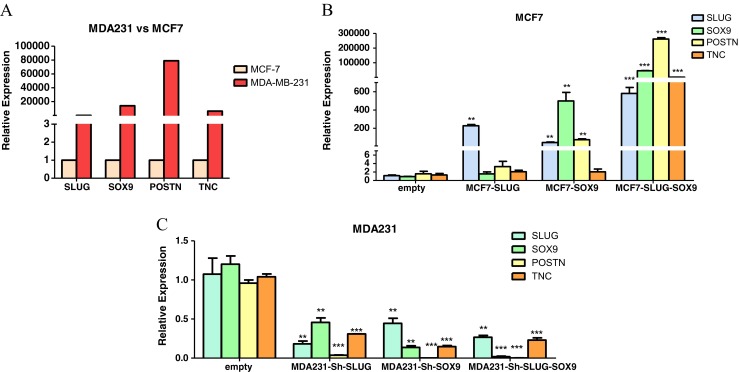
SLUG and SOX9 expression correlates with that of TNC and POSTN. Gene expression analyses have been performed by quantitative real-time RT-PCR. a Relative gene expression comparing between MDA-231 and MCF7 cells showed that all four genes, SLUG, SOX9, POSTN and TNC, are higher in MDA-231 cells. b MCF7 cells have been infected by lentivirus containing transgenes, Slug, Sox9, or both. Significant upregulation of both POSTN and TNC is seen only when both Slug and Sox9 were overexpressed. c SLUG and SOX9 were knocked-down in MDA-231 cells, using infection of lentivirus containing corresponding shRNAs. In all cases both POSTN and TNC were significantly downregulated. Comparison between groups was made by one-way ANOVA followed by an appropriate post hoc test. (* = P\0.05, ** = P\0.01 and *** = P\0.001)
MCF7 cell was infected using Slug and Sox9 constructs in lentiviral system. Lentiviral particles containing the transgene were produced in competent HEK293T cells. Upon overexpression of Slug, no significant change was observed in the expression of TNC or POSTN genes. However, Sox9 overexpression alone resulted in a significant upregulation of Slug, and this had a much stronger effect on Periostin than upregulation of Slug alone, which did not affect Sox9 levels. Intriguingly, simultaneous overexpression of Slug and Sox9 lead to a much more significant up-regulation of both TNC and POSTN. Notably, the expression POSTN was dramatically increased when Slug and Sox9 were overexpressed simultaneously (Fig. 1B).
Next, we generated MDA-231 cells expressing short hairpin RNAs (shRNAs) against SLUG and/or SOX9 to knock-down the expression of corresponding genes. Knocked-down SLUG and/or SOX9 genes in MDA-231 cells also showed consistent correlation with the decreased TNC and POSTN expression. In loss of function experiment, TNC and POSTN genes were downregulated in knock-down of either SLUG or SOX9, or both. Again, POSTN showed the most drastic change by being downregulated more than 90 % in all cases (Fig. 1C).
Discussion
Here we showed that simultaneous expression of SLUG and SOX9 transcription factors correlates with that of ECM factors TNC and POSTN. The strong effect of solely Sox9 upregulation, might be interpreted by Slug upregulation in the cells leading to their synergistic accumulative overexpression of target genes and therefore metastatic capacity [5] in this work. This might shed light to an important molecular link through which TICs can induce the essential niche factors required for maintenance of their stemness characteristics.
Processes that occur in the last steps of metastasis cascade are largely unknown. The complexity of these processes relies on the phenomenal heterogeneity in cancer, and is different in cancer type metastasizing to each distant tissue and different time points. To simplify this enormous complexity to small pieces, we might be able to solve very little problems and then connect them together in order to have a more comprehensive understanding of certain processes involved in metastasis. In breast cancer metastasis it was shown that Slug and Sox9, as well as TNC and POSTN play major roles, mainly through governing tumor initiating properties [5–7].
When Slug and Sox9 were ectopically expressed, they induce stem properties in normal differentiated mammary cells and turning them into mammary stem cells. However, Guo et al. showed that less invasive MCF7 tumor cells overexpressing Slug and Sox9 can form metastatic colonization in lungs. On the contrary, by suppressing SLUG or SOX9 in MDA-231 cells, they showed that macroscopic metastasis did not happen [5]. Likewise, Malanchi et al. and Oskarsson et al. respectively showed necessity of POSTN and TNC, in forming metastatic colonization in lungs [6, 7]. As master TFs are usually rather high in hierarchy of molecular networks, we investigated whether SLUG and SOX9 might induce TNC and POSTN.
SLUG and SOX9 might induce these ECM factors, as well as several other fate-determining factors, through a signaling cascade. Considering a molecular metastatic pathway which has been previously proposed by us, the results of this study may be confirmative. It has been shown that POSTN is induced by TGF-β3 while potentiates WNT signaling [6]. And, TNC, which binds directly to POSTN, induces NOTCH and WNT signaling [7]. On the other hand, SLUG can induce TGF-β3 [10] which together may propose a molecular cascade with TGF-β, WNT and NOTCH interfaces. These signaling pathways are crucial players in the induction and maintenance of TICs and EMT-TFs as autocrine reinforcing signals in metastasis and also stemness [8, 11].
These factors could play a role in one of several types of breast cancer metastasis. As was shown by Ghajar and colleagues, POSTN, together with TGF-β1 is one of the secreted factors from endothelial tip cells at the distant organ and can induce metastasis outgrowth [12]. We suggested these factors might trigger an autoregulatory network inducing TICs in order to give rise to a full blow tumor [13]. Also, this network together with other crucial signaling cascades [14] may govern the journey of tumor cells from primary to secondary sites. It would be interesting to consider underlying molecular mechanisms that make TICs as a self-sufficient master key in cancer metastasis. Also regulatory effect of stem-like associated inducing factors on niche elements in a spatiotemporal manner is recommended for further studies.
Acknowledgments
This work was in fulfillment of requirements for a M.Sc. thesis by Hassan Fazilaty. The authors thank Mina Rasoulnezhad and Sina Lasemi for their fantastic helps on experimental procedures. This work was supported by grant # 92-01-117-2178 from Cellular and Molecular Research Center (CMRC) Iran University of Medical Sciences (IUMS), and # 92003378 from Iran National Science Foundation (INSF) to Dr. Babak Behnam.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global Cancer Statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Valastyan S, Weinberg RA. Tumor Metastasis: Molecular Insights and Evolving Paradigms. Cell. 2011;147(2):275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nieto MA. Epithelial Plasticity: A Common Theme in Embryonic and Cancer Cells. Science. 2013;342(6159):1234850. doi: 10.1126/science.1234850. [DOI] [PubMed] [Google Scholar]
- 4.Fidler IJ. The Pathogenesis of Cancer Metastasis: The 'Seed and Soil' Hypothesis Revisited. Nat Rev Cancer. 2003;3(6):453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 5.Guo W, Keckesova Z, Donaher JL, Shibue T, Tischler V, Reinhardt F, Itzkovitz S, Noske A, Zurrer-Hardi U, Bell G, Tam WL, Mani SA, van Oudenaarden A, Weinberg RA. Slug and Sox9 Cooperatively Determine the Mammary Stem Cell State. Cell. 2012;148(5):1015–1028. doi: 10.1016/j.cell.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J. Interactions Between Cancer Stem Cells and Their Niche Govern Metastatic Colonization. Nature. 2012;481(7379):85–89. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 7.Oskarsson T, Acharyya S, Zhang XH, Vanharanta S, Tavazoie SF, Morris PG, Downey RJ, Manova-Todorova K, Brogi E, Massague J. Breast Cancer Cells Produce Tenascin C as a Metastatic Niche Component to Colonize the Lungs. Nat Med. 2011;17(7):867–874. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fazilaty H, Gardaneh M, Bahrami T, Salmaninejad A, Behnam B. Crosstalk Between Breast Cancer Stem Cells and Metastatic Niche: Emerging Molecular Metastasis Pathway? Tumour Biol. 2013;34(4):2019–2030. doi: 10.1007/s13277-013-0831-y. [DOI] [PubMed] [Google Scholar]
- 9.Gardaneh M, Gholami M, Maghsoudi N. Synergy Between Glutathione Peroxidase-1 and Astrocytic Growth Factors Suppresses Free Radical Generation and Protects Dopaminergic Neurons Against 6-Hydroxydopamine. Rejuvenation Res. 2011;14(2):195–204. doi: 10.1089/rej.2010.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medici D, Hay ED, Olsen BR. Snail and Slug Promote Epithelial-Mesenchymal Transition Through Beta-Catenin-T-Cell Factor-4-Dependent Expression of Transforming Growth Factor-beta3. Mol Biol Cell. 2008;19(11):4875–4887. doi: 10.1091/mbc.E08-05-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheel C, Eaton EN, Li SH, Chaffer CL, Reinhardt F, Kah KJ, Bell G, Guo W, Rubin J, Richardson AL, Weinberg RA. Paracrine and Autocrine Signals Induce and Maintain Mesenchymal and Stem Cell States in the Breast. Cell. 2011;145(6):926–940. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY, Chen EI, Lyden D, Bissell MJ. The Perivascular Niche Regulates Breast Tumour Dormancy. Nat Cell Biol. 2013;15(7):807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fazilaty H, Behnam B. The Perivascular Niche Governs an Autoregulatory Network to Support Breast Cancer Metastasis. Cell Biol Int. 2014;38(6):691–694. doi: 10.1002/cbin.10261. [DOI] [PubMed] [Google Scholar]
- 14.Fazilaty H, Mehdipour P. Genetics of Breast Cancer Bone Metastasis: A Sequential Multistep Pattern. Clin Exp Metastasis. 2014;31(5):595–612. doi: 10.1007/s10585-014-9642-9. [DOI] [PubMed] [Google Scholar]


