Abstract
Thymidine phosphorylase (TP) is a nucleoside metabolism enzyme that plays an important role in the pyrimidine pathway.TP catalyzes the conversion of thymidine to thymine and 2-deoxy-α-D-ribose-1-phosphate (dRib-1-P). Although this reaction is reversible, the main metabolic function of TP is catabolic. TP is identical to the angiogenic factor platelet-derived endothelial-cell growth factor (PD-ECGF). TP is overexpressed in several human cancers in response to cellular stressful conditions like hypoxia, acidosis, chemotherapy and radiotherapy. TP has been shown to promote tumor angiogenesis, invasion, metastasis, evasion of the immune-response and resistance to apoptosis. Some of the biological effects of TP are dependent on its enzymatic activity, while others are mediated through cytokines like interleukin 10 (IL-10), basic fibroblast growth factor (bFGF) and tumour necrosis factor α (TNFα). Interestingly, TP also plays a role in cancer treatment through its role in the conversion of the oral fluoropyrimidine capecitabine into its active form 5-FU. TP is a predictive marker for fluoropyrimidine response. Given its various biological functions in cancer progression, TP is a promising target in cancer treatment. Further translational research is required in this area.
Keywords: Thymidine phosphorylase, Hallmarks of cancer, Angiogenesis, Predictive biomarkers
Introduction
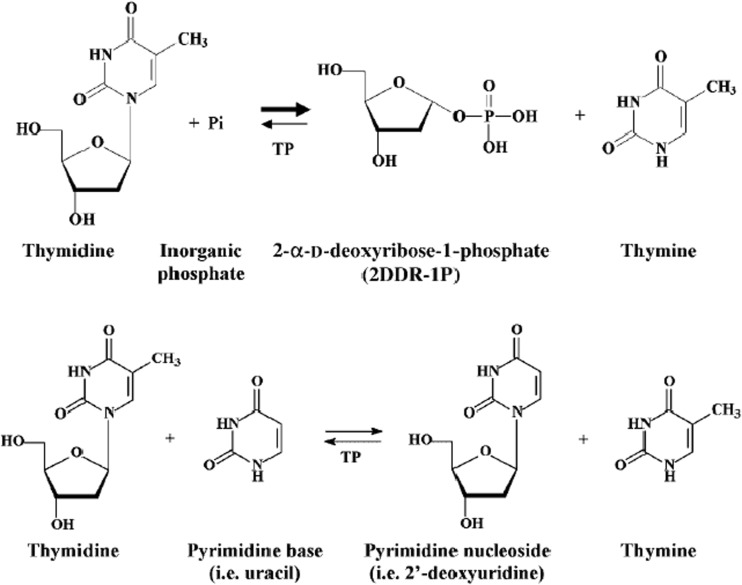
Thymidine phosphorylase (TP) is a nucleoside metabolism enzyme that plays an important role in the pyrimidine salvage pathway. It was first described in 1953 when it was found that TP catalyses the reversible conversion of thymidine (TdR) to thymine and 2-deoxy-α-D-ribose-1-phosphate (dRib-1-P) [1], and the phosphorolysis of deoxyuridine to uracil and 2-deoxy-α-D-ribose-1-phosphate. Although this reaction is reversible the main function of TP is catabolic [2–5]. TP and the angiogenic factor platelet-derived endothelial-cell growth factor (PD-ECGF) are, in fact, different names for the same molecule [3, 5, 6].
In addition to its metabolic function, TP plays a key role in angiogenesis [3, 4]. 2-deoxy-D-ribose (D-dRib) is an endothelial chemoattractant which acts as a downstream mediator of TP function, promoting angiogenesis and chemotactic activity of endothelial cells [7]. Studies in the past 10 years have shown that TP plays an anti-apototic role in cancer cells. There is accumulating evidence that suggests that TP is key factor in determining response to some chemotherapy agents. Moreover, TP is overexpressed by several human cancers and is reported to be associated with poor outcome.
In their landmark paper hallmarks of cancer: the next generation, Hanahan and Weinberg described eight hallmarks that explain the behaviour of cancer cells; self-sufficiency in growth signals, resistance to antigrowth signals, evasion of apoptosis, limitless replicative capability, reprograming of cellular metabolism, evasion of elimination by immune system, invasion and angiogenic capabilities [8]. Herein, we present a review of the accumulating evidence of the role of TP in some of the hallmarks of human cancer. While TP has well established role in promoting some of these hallmarks, like angiogenesis, more work is needed to explore if TP plays a role in other. We further discus the complexity of the dual role that TP plays in cancer: aiding cancer progression on one hand and determining response to some therapeutic agents on the other.
TP in Normal Tissue
The human TP gene is located on chromosome 22q13 [9]. TP is a homodimer, consisting of two identical subunits that are non-covalently associated with a dimeric molecular mass of 51 kD [4, 9]. Each subunit is composed of a small α-helical domain that contains the thymidine-binding site and a large α/β domain that contains the phosphate binding site [10]. High levels of TP are expressed in macrophages, stromal cells, glial cells and some epithelia [11]. It is expressed in the nucleus where it modulates the pool of pyrimidine nucleosides for DNA synthesis and also in the cytoplasm where it also displays its enzymatic functions [11].
Most TP activity present in normal human blood resides in the cytoplasm of platelets [12]. This suggests that blood platelets play an important role in thymidine metabolism. It has been suggested that this role is part of the physiological functions of platelets [13]. Activated platelets release TP to stimulate angiogenesis by enhancing endothelial cell motility and proliferation [13]. The physiological role of TP in angiogenesis extends to the female menstrual cycle. In the normal proliferative endometrium, TP is found exclusively in the basal layer and the inner third of the functionalis; expression is cytoplasmic in glandular epithelium and nuclear in stromal cells. At the end of the menstrual cycle, TP becomes expressed uniformly in the epithelium of all endometrial glands, with nuclear and cytoplasmic expression. At this stage there is very little stromal expression of TP [14, 15]. A more detailed description of the role of TP in angiogenesis is provided below.
Transcriptional Control of TP and its Upregulation in Cancer
TP is overexpressed in oral squamous carcinoma [16], oesophageal [4], gastric [17], breast [18], lung [19–21], colorectal [22], bladder [23], and cervical cancer [24]. TP is expressed by tumour cells and in the tumour microenvironment by fibroblasts, tumour associated macrophages (TAMs) and lymphocytes [3, 4, 17–23, 25]. Several studies have shown that TP is elevated in cancer patients’ plasma [25].
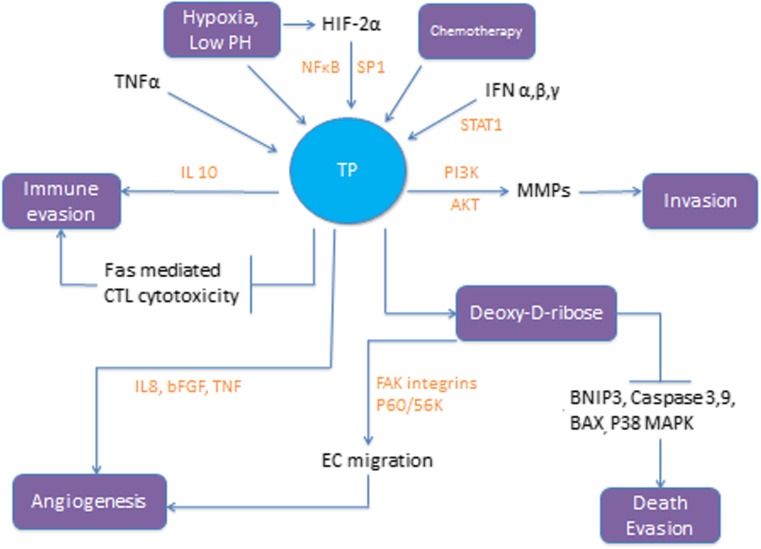
Factors that lead to TP overexpression in tumour cells include inflammatory cytokines, cellular stress like hypoxia and low PH, chemotherapy and radiotherapy (Figs.1 and 2). Of the inflammatory cytokines tumour necrosis factor α (TNFα) plays an important role in the regulation of TP expression. The TP gene has a transcriptional factor SP1 binding site within its promoter region [26]. TNFα activates SP1 which in turn binds to the promoter region of the TP gene leading to expression of the TP protein. Activation of NFκB is another mechanism by which TNFα leads to TP overexpression in cancer [25, 27]. Interferon (IFN) α, β and γ activate the IFN-stimulated response element (ISRE) and the signal transduction and activator of transcription 1 (STAT1) causing expression of TP [25, 28]. Other inflammatory factors that have been reported to upregulate TP include hypoxia inducible factor 2α (HIF-2α), interleukin 1 (IL-1) and interleukin 10 (IL-10) [3, 25]. IFNα/γ stabilizes TP mRNA levels leading to a rise in TP enzyme activity. Epigenetic modulation regulates TP expression at the transcriptional level in breast cancer and colon cancer [25].
Fig. 1.
Enzymatic reactions catalysed by TP. TP catalyses the reversible conversion of thymidine (TdR) to thymine and 2-deoxy-α-D-ribose-1-phosphate (dRib-1-P), and the phosphorolysis of deoxyuridine to uracil and 2-deoxy-α-D-ribose-1-phosphate. Although this reaction is reversible the main function of TP is catabolic
Fig. 2.
TP is overexpressed in response to cellular stress conditions like hypoxia, low PH and exposure to chemotherapy; TP then exerts its different biological effects. TP = Thymidine Phosphorylase. TNFα = Tumor necrosis factor α. HIF2α = Hypoxia inducible factor 2α. IFN = Interferon. MMP = Matrix metalloprotease. EC = Endothelial cells. FAK = Focal adhesion kinase. bFGF = b fibroblast growth factor. IL = Interleukin. BNIP3 = BCL2/adenovirus E1B 19 kDa protein-interacting protein. BAX = Bcl-2-associated X protein
Chemotherapy agents like docetaxel, paclitaxel, cyclophosphamide, and oxaliplatin can increase TP levels possibly through the induction of inflammatory cytokines like INF and TNF [25, 29].
There is little evidence for TP gene mutation in tumour cells. The fact that TP levels are elevated in non-malignant cells in the tumour microenvironment (TME) suggests that raised TP expression is influenced by local inflammation and stress conditions like hypoxia in the TME rather than genetic changes. This is supported by the observation of increased TP levels in non-malignant chronic inflammatory conditions like arthritis and psoriasis [27].
The Role of TP in Angiogenesis
Angiogenesis is the formation of new blood vessels from pre-existing vasculature. It is essential for tumour growth and cancer progression. TP’s function as an angiogenic factor is well established. It enhances angiogenesis within the tumour by two mechanisms; (i) by stimulating endothelial cell migration and (ii) by stimulating the release of angiogenic factors from malignant cells and stromal cells in the tumour microenvironment.
The angiogenic function of TP is related to its enzymatic activity. It has been shown that blocking the enzymatic function of TP abolishes its angiogenic function [30, 31]. The effects of TP on angiogenesis are mediated through the thymidine metabolite deoxy-D-ribose which results from dephosphorylation of 2-deoxy-D-ribose-1-phosphate and is released out of the cell [30–33]. 2-deoxy-D-ribose stimulates endothelial cell migration by several mechanisms. Firstly, it stimulates the phosphorylation of tyrosine 397 of the focal adhesion kinase (FAK), which is a non-receptor tyrosine kinase that has a role in cell migration and cell death [32, 34]. Subsequently, 2-deoxy-D-ribose activates cell surface integrins leading to focal adhesion formation [34]. Secondly, 2-deoxy-D-ribose activates p70/s6k, an important downstream kinase of the mTOR pathway regulating cell proliferation and angiogenesis [34]. A study of migration of human umbilical vein endothelial cells (HUVECs) found that 2-deoxy-D-ribose-mediated angiogenesis is inhibited by rapamycin through blocking p70/s6k kinase activation [4]. Lastly deoxy-D-ribose provides an energy source for migrating endothelial cells and induces pseudopodia formation [32].
TP stimulates angiogenesis indirectly by enhancing the release of angiogenic factors [34, 35]. Bijnsdorp and colleagues studied TP effects by exposing human umbilical vein endothelial cells (HUVECs) to conditioned medium derived from human colon cancer cell lines with high and no TP expression [34]. They found that TP-expressing cells secreted angiogenic factors (IL-8, bFGF and TNFα) that stimulated endothelial cells migration and invasion, but not proliferation. Inhibition of only one of these factors using blocking antibodies resulted in the inhibition of the angiogenic effects, suggesting that a combination of these angiogenic factors might be contributing to the angiogenic effects of TP. An interesting finding from this study was that TP did not increase the invasiveness or migration capacity of the cancer cell. An important finding reported was that VEGF secretion was not increased with high TP expression. This finding is consistent with results of an earlier retrospective study of TP and VEGF expression in 223 tumour specimens from lung cancer patients [36]. In the latter study evaluation of TP and VEGF expression in cancer cells revealed a weak positive correlation between the two angiogenic factors. A similar observation has been reported in colorectal cancer [36]. However, other reports show increased VEGF amounts in high TP-expressing tumours [34]. VEGF is known to be expressed in response to hypoxia inducible factor-1α (HIF-1α). HIF-1α is upregulated in response to hypoxia; it forms a dimer with HIF-1β increasing the expression of VEGF. On the other hand, TP expression has been linked to HIF-2α in several human cancers. Xian-hua et al. recently published a study on the correlation of HIF-1α and HIF-2α with angiogenic factors in non-small cell lung cancer (NSCLC) [37]. Tumour samples from 140 patients with NSCLC were retrospectively assessed for expression of HIF-1α, HIF-2α, TP, VEGF and cyclooxygenase (COX)-2. A significant correlation was detected between HIF-1α and VEGF expression while no correlation was shown between HIF-2α and VEGF expression. The correlation between HIF-2α and TP was statistically significant while no correlation was found between TP and HIF-1α expression. Authors have hypothesized that increasing oxygen supply from the newly formed blood vessels reduces the degree of hypoxia in the tumour microenvironment leading to rapid downregulation of HIF-1α and upregulation of HIF-2α, which in turn promotes activation of TP and COX-2 to maintain angiogenesis. In this model, VEGF plays a dominant role in the initiation of the angiogenic switch whereas TP contributes to the maintenance of angiogenesis. It is important to note that some tumours have high microvascular density and do not express TP or VEGF [36]. Clearly other mechanisms of angiogenesis are involved in these tumours. Nonetheless, it is now well established that high levels of TP contribute to malignant tumour vasculature [29].
TP and Evasion of Apoptosis
TP inhibits hypoxia-induced apoptosis and protects cancer cells from apoptosis induced by chemotherapy agents such as cisplatin and antimircotubular drugs. Mechanisms involved in the antiapoptotic role of TP include; (1) inhibition of mitochondrial release of cytochrome c [38], (2) upregulation of Bcl-2 and Bcl-xL [38], (3) suppression of the phosphorylation of p38 MAPK and (4) inactivation of caspase 3 and 9 [39].
Mitogen-activated protein kinase (MAPK) cascades are key signalling pathways involved in the regulation of normal cell proliferation, survival and differentiation. Aberrant regulation of MAPK pathway contributes to cancer and other disease. The p38 MAPK pathway plays a dual role depending on the stimulus, mediating either cell survival or death through different mechanism including apoptosis. It mediates apoptosis by phosphorylation of the proapoptotic protein BAX with subsequent release of mitochondrial cytochrome c and activation of caspases [40]. Ikeda et al. examined the role of the TP metabolite, 2-deoxy-D-ribose, in preventing hypoxia induced-apoptosis in human leukaemia cell lines HL-60 and Jurkat cells [41]. Under normoxic conditions, no apoptotic cells were observed in untreated Jurkat cells and treatment with 2-deoxy-D-ribose had no effect on cell morphology. However, under hypoxic conditions chromatin condensation was observed in Jurkat cells. When cells were treated with 2-deoxy-D-ribose, this hypoxia-induced morphological change was not observed. Furthermore, authors found that under hypoxic conditions the activation of p38 MAPK promoted translocation of Bax from the cytosol to the mitochondria and HL-60 cell death. 2-deoxy-D-ribose reduced the level of Bax attached to mitochondria in hypoxic conditions resulting in inhibition of apoptosis. The authors concluded that 2-deoxy-D-ribose inhibits hypoxia-induced apoptosis by suppressing the phosphorylation of p38 MAPK. This study did not show the mechanisms by which 2-deoxy-D-ribose prevents the phosphorylation of p38 MAPK. Another study by the same group examined the effects of TP on key regulators of hypoxia-induced apoptosis, HIF-1α, caspase 3 and BNIP3 [39]. This in vitro study showed that under hypoxic conditions HIF-1α, caspase 3 and BNIP3 were suppressed in TP-overexpressing cells compared to control cells.
An interesting report investigated the effects of TP overexpression on cisplatin-induced apoptosis using human leukaemia Jurkat cell transfected with wild type or mutant TP cDNA [42]. Wild type and mutant TP inhibited a number of steps in the cisplatin-induced apoptotic pathway, activation of caspases 3 and 9 and mitochondrial cytochrome c release. It is important to note that mutant TP lacks enzymatic activity indicating that the ability of TP to inhibit apoptosis induced by cisplatin is independent of its enzymatic activity.
The Role of TP in Invasion and Metastasis
TP plays a role in tumour invasiveness by enhancing the expression of genes involved in the epithelial to mesenchymal transition (EMT) program. Factors reported to be expressed in response to TP include; matrix metalloproteinases (MMP-1, MMP-7, MMP-9), Urokinase-type plasminogen activator (uPA) and VEGF [43]. A possible mechanism by which TP enhances the expression of these genes is via activation of the PI3K-Akt pathway. Akt is a mediator of Activator Protein-1 (AP-1) induction. The AP-1 site is upstream of the transcriptional start sites of MMP-7, MMP-9 and MMP-1, and has long been thought to play a dominant role in the transcriptional activation of the MMP promoters [43]. Inhibition of TP leads to decreased levels of MMP-9 mRNA levels with resultant reduced malignant cells invasiveness [44].
A study has shown that KB cells (human epidermal carcinoma) transfected with TP cDNA had more invasive potential and metastatic phenotype than KB cells transfected with mock vector [45]. In a report by Sato et al. it was found that TP promoted invasive activity and metastatic phenotype in lung adenocarcinoma. This group has reported that inhibition of TP strongly suppressed lung metastasis in nude mice [46].
Moreover, TP increases the chemotactic motility of cancer cells, this effect can be suppressed by the inhibition of TP. It is thought that TP plays a specific role in blood borne metastasis, indeed the inhibition of TP led to reduce metastatic liver burden in animal models [44]. In addition to these mechanisms, TP enhances the metastatic phenotype by its well described function; angiogenesis stimulation.
TP and Cancer Cell Evasion of Immune Cells
The cell-surface Fas receptor (FasR), also termed apoptosis antigen-1 (Apo-1) or cluster of differentiation 95 (CD95), is a member of the tumour necrosis factor (TNF) family of receptors [47]. FasR is a death receptor that leads to programmed cell death (apoptosis). Binding of Fas to its ligand Fas-L leads to activation of the Fas-induced apoptosis pathway. This pathway is one of the pathways by which cytotoxic T-lymphocytes (CTLs) induce cell death as CTLs express Fas-L upon their activation [47]. Several reports have shown that TP overexpression leads to inhibition of Fas induced apoptosis [48, 49]. A study where KB/TP cell lines (a human epidermoid carcinoma KB cell line transfected with TP cDNA and expressing TP) were used to examine the role of TP on Fas-induced cell death showed that TP expression interfered with the release of cytochrome c and caspase-3 cleavage independent of its enzymatic activity. This is in contrast to the angiogenic and chemotactic effects of TP where its enzymatic activity is essential to exert its effects as discussed earlier [48].
It has been reported that TP can suppress the innate immune response to tumour cells via increasing the secretion of IL-10, which inhibits the anti-inflammatory functions of dendritic cells (DC) and macrophages [25]. IL-10 can modulate the expression of other cytokines, soluble mediators and surface receptors by cells of myeloid origin, particularly macrophages and DC. IL-10 is also able to inhibit COX-2 expression, IL-6, IL-12, and TNF-α [25]. This is a possible indirect mechanism of immunomodulation by TP favouring tumour growth and progression.
Slager and colleagues examined the use of TP as a potential target for immunotherapy of cancer [49]. Authors isolated tumour reactive CD8+CTLs clones from peripheral blood of a patient successfully treated with donor lymphocyte infusion (DLI) for relapsed multiple myeloma after HLA-matched allogeneic stem cell transplantation (SCT). These CTLs were directed against TP. The epitope recognized by the CTLs was encoded by a single nucleotide polymorphism (SNP), resulting in a TP-1 minor histocompatibility antigen (mHag). This SNP has been reported previously as a mutation associated with the autosomal recessive disease mitochondrial neurogastrointestinal encephalomyopathy (MNGIE). The fact that T-cells directed against TP were isolated from a patient with multiple myeloma showing complete remission after cellular immunotherapy led authors to conclude that generation of T-cell responses to polymorphisms in TP gene may be a potent combination of direct antitumour immunotherapy and antiangiogenic treatment. The researchers go further to predict that TP can be a potential target not only in haematological malignancies but in solid tumours as well.
Effects on Other Hallmarks of Cancer
TP aids the uncontrolled growth of malignant cells indirectly by enhancing angiogenesis which provides cancer cells with the necessary oxygen, nutrients and mitogens required for growth. Furthermore, TP is a key enzyme in the nucleoside metabolism salvage pathway; it maintains the pool of thymine in the body which is required for DNA synthesis.
Stabilizing telomere length to enable limitless replication of cancer cells and reprogramming the cellular metabolism machinery are other important hallmarks of cancer. Currently there is no evidence for TP contribution to these two hallmarks. However as discussed earlier in this review, hypoxia is an important factor that induces TP expression. Other factors upregulated in hypoxia include HIF-1α which plays a significant role in shifting the cellular metabolism from mitochondrial oxidative phosphorylation to glycolysis.
TP in the Metabolism of Fluoropyrimidines
5-fluoropyrimidine (5-FU) is an antimetabolite drug that works by inhibiting the synthesis of DNA and RNA [50]. The mechanism of 5-FU cytotoxicity has been ascribed to the misincorporation of fluoronucleotides into RNA and DNA and the inhibition of the nucleotide synthetic enzyme thymidylate synthase (TS) [50]. 5-FU is converted intracellularly to several active metabolites; fluorodeoxyuridine monophosphate (FdUMP), fluorodeoxyuridine triphosphate (FdUTP) and fluorouridine triphosphate (FUTP) [50]. These metabolites disturb the action of TS required for DNA synthesis. Since the 1950s 5-FU has been the backbone of chemotherapy in colorectal cancer; it is widely used in cancers like breast carcinoma and head and neck cancers. Following penetration into the cell, 5-FU is metabolised by two routes, which are in competition with each other: the anabolic route, which gives rise to the active metabolites and the catabolic route, which inactivates 5-FU and leads to elimination of the drug from the organism.
The anabolism of 5-FU can be complex, with 5-FU reacting in the following three ways: the first, which is the least important, leads in two stages to the formation of 5-FdUMP. The other two routes both form 5-FUMP, the main mechanism of 5-FU activation. This conversion is catalysed either directly by orotate phosphoribosyltransferase (OPRT) with phosphoribosyl pyrophosphate (PRPP) as the cofactor, or indirectly via fluorouridine (FUR) throught the sequential action of uridine phosphorylase (UP) and uridine kinase (UK) [50]. 5-FUMP then undergoes two successive phosphorylations to give 5-FUDP and then 5-FUTP, which can be incorporated into RNA. 5-FUDP and 5-FdUMP can be transformed into 5-FdUDP, which is then phosphorylated to 5-FdUTP, which acts as a substrate for DNA polymerases and can thus be incorporated into DNA.
More than 80 % of administered 5-FU is degraded by dihydropyrimidine dehydrogenase (DPD) in the liver. Under the action of DPD, 5-FU is reduced to 5,6-dihydro-5-fluorouracil (5-FUH2). This stage is the rate limiting step of 5-FU catabolism and effectively governs the rate at which 5-FU is available for anabolism. 5-FUH2 is then cleaved to give α-fluoro-β-ureidopropionic acid (FUPA), and a third stage leads to the formation of α-fluoro-β-alanine (FBAL), the major catabolite of 5-FU. Most of the catabolic schemes of 5-FU stop at these metabolites.
In the presence of the cofactor 5,10-methylene tetrahydrofolate (MeTHF), serving as the methyl donor, TS and 2’-deoxyuridine-5’-monophosphate (dUMP) form a ternary complex, which enables transfer of a methyl group on carbon 5 of dUMP to form thymidine-5’-monophosphate (dTMP). Following 5-FU exposure and adequate 5-FdUMP formation, the methyl transfer does not take place because the fluorine atom in the C5 position of 5-FdUMP is much more tightly bound than hydrogen. The enzyme is then trapped in a slowly reversible ternary complex. The formation of dTMP is therefore blocked, thereby decreasing the availability of thymidine-5’-triphosphate (dTTP) for DNA replication and repair [51]. Some studies suggest the more prolonged exposure to low doses of 5-FU leads to cell death primarily via the TS-directed mechanism, whereas bolus administration of 5-FU results primarily in an RNA-mediated process of cell death [52, 53].
UP can also catalyse the formation of nucleosides from 5-FU and Rib-1-P. UP activity is usually elevated in various tumour tissues, and UP is the most important phosphorylase identified thus far in the regulation of uridine homeostasis, although TP can also utilise uridine as a substrate to a certain extent [54]. In 2007, Temmink et al. investigated the substrate specificity of TP and UP for thymidine, uridine and 5-FU in colon cancer patients. They concluded that overlapping substrate specificity was found for TP and UP and both enzymes are responsible for converting thymidine and uridine [55]. TP overexpression in cell culture and xenograft models had been shown to increase sensitivity to 5-FU due to enhanced formation of FdUMP [reviewed in 3, 51].
N4-pentyloxycarbonyl-5’-deoxy-5-fluorocytidine, more commonly called Capecitabine or Xeloda® is an oral pro-drug of 5-FU commonly used in colorectal, gastric and breast cancer. Capecitabine was synthesisied in the 1990s by Japanese researchers as an oral formulation designed to circumvent the unacceptable toxicity of 5’-deoxy-5-fluorouridine (5’d5-FUrd) by acting as a pro-drug that could not be metabolised by TP in the intestine. Following oral administration, Capecitabine crosses the gastrointestinal barrier intact and is rapidly and almost completely absorbed. It is subsequently converted to 5-FU in a series of steps: first, it is metabolized by hepatic carboxylesterase into 5’-deoxy-5-fluorocytidine (5’-dFCR), which is then deaminated into 5’d5-FUrd by cytidine deaminase which is located mainly in the liver and in tumour tissue. Finally, 5’d5-FUrd is transformed into 5-FU under the action of UP and TP (which have higher expression in tumour than in normal tissues). Higher levels of 5-FU are thus produced within tumours with minimal exposure of healthy tissue to 5-FU [51]. It is important to note that several studies have underlined the critical role that UP plays in converting 5’d5-FUrd to 5-FU, which some studies have suggested that it might be more important than that of TP [54, 55]. The preferential activation of capecitabine in tumour tissue and its dependence on TP was shown in a study of 19 patients with colorectal cancer requiring surgical resection for primary tumour and/or liver metastases that were treated with capecitabine for 5–7 days prior to surgery [56]. On the day of surgery, samples of tumour tissue, adjacent healthy tissue and blood samples were collected from each patient. Concentrations of 5-FU in various tissues and plasma were measured. The activity of TP was measured in tissue homogenates, by catabolic assays. The ratio of 5-FU concentrations in tumour to adjacent healthy tissue was used as the primary marker for the preferential activation of capecitabine in tumour. In primary colorectal tumours, the concentration of 5-FU was on average 3.2 times higher than in adjacent healthy tissue. The mean liver metastasis/healthy tissue 5-FU concentration ratio was 1.4. Authors concluded that this is explained by the activity of TP in colorectal tumour tissue, which is approximately four times that in adjacent healthy tissue. In the liver, TP activity is approximately equal in metastatic and healthy tissue, which explains the lack of preferential activation of capecitabine in these tissues [56]. Those results are consistent with older reports [57].
TP as a Predictive Biomarker
A recent study determined the gene expression levels of TP by quantitative reverse transcription-PCR in 38 pretreatment biopsies of colorectal tumours from patients who were subsequently treated with 5-FU and leucovorin (LV), it was found that the range of TP gene expression (relative mRNA levels) in those tumours nonresponsive to 5-FU was much broader than that of the responding tumours [58]. Tumours with the highest basal TP expression were nonresponders to 5-FU/LV therapy. The mean TP mRNA level in the nonresponding tumours was 2.6-fold higher than that of the responding patients. TP and TS expressions were found to be independent variables in these tumours, so that low expression levels of both TS and TP in tumours predicted a high response rate to 5-FU/LV as well as a significantly longer survival, whereas none of the patients with high expression of either TP or TS were responders [58]. In another study, Gustavsson et al. retrospectively examined the pretreatment expression of TP and other 17 genes in archival tumour samples from patients with advanced CRC to determine if one or more of the selected genes can be used as either a prognostic or predictive marker of 5-FU-based treatment outcomes [59]. One hundred and forty-four CRC patient samples were analysed via real-time PCR for gene expression. Univariate analyses were used to correlate gene expression with efficacy and time-to-event variables. Low TP gene expression was associated with better time-to-progression in the entire population. Low TP expression was independently associated with improved overall survival. Low TP gene expression was also predictive of response to 5-FU based chemotherapy.
A further study from gastric cancer patients examined TP as a predictive marker for capecitabine response. In a report by Lu and colleagues, using real time quantitative PCR they analysed mRNA expression levels of TP and TS in primary tumours from 57 patients with advanced gastric cancer [60]. It was found that low TP expression and high TS expression in gastric cancer specimens were linked to poor outcome in patients receiving capecitabine-based regimen. In contrast, the subgroup of patients with low TS and high TP expression had the best treatment response. Patients with high TS and low TP expression had poorest response rate (15.8 % vs. 57.9 %) and overall survival (6.6 vs. 13.8 months) compared to the patients with low TS/high TP expression. Authors also studied the correlation between expression of TS and TP and the efficacy of capecitabine combined with different cytotoxic agents (paclitaxel or cisplatin). They found that the expression of TS and TP was associated with a better tumour response. Authors concluded that the combination of TS and TP might add more predictive power than each gene expression alone. Another group of researchers examined TP, DPD and TP/DPD ratio as predictive markers for concomitant capecitabine and radiotherapy in locally advanced rectal cancer in a neoadjuvant setting [61]. Tissue samples, both neoplastic and normal ones, were endoscopically taken from a cohort of 28 patients before treatment for TP and DPD measurement with ELISA. Levels of TP and DPD in tumour tissue did not reveal any statistically significant difference between the patients with proven pathological response and those with no response. However, the median rationof TP to DPD was higher in the response group compared to the no response group. The authors concluded that TP/DPD ratio is a possible predictive factor for tumour response after concomitant preoperative chemoradiation with capecitabine in locally advanced rectal cancer. A multicentre phase II trial evaluated the predictive relevance of several biomarkers, including TP, on the survival of patients with stage III colorectal cancer treated with adjuvant chemotherapy of oral fluoropyrimidine [62]. Patients received oral doxifluridine (5′-deoxy-5-fluorouridine, 5′-dFUR, a fluoropyrimidine derivative) or oral uracil/tegafur for 12 months with a 5-year follow-up. Outcome measures were disease-free survival and tissue markers; TP and DPD protein levels and TP, DPD, TS and orotate phosphoribosyltransferase (OPRT) mRNA levels in tumour samples and TS tandem-repeat type in blood samples. The study showed a significant association between the intratumoural TP/DPD enzyme ratio and disease-free survival. The 5-year disease-free survival rate was statistically significantly higher in patients with high TP/DPD ratios following adjuvant therapy with oral fluoropyrimidines. No significant association was observed between the intratumoural TP/DPD enzyme ratio and the disease-free survival rate in the doxifluridine group. Studies carried out in breast cancer patients strengthen the validity of TP use as a biomarker for oral fluoropyrmidines response [63, 64]. An increase in both relapse-free survival and overall survival was found in TP-positive compared to TP-negative breast tumours in a study of 109 patients treated with adjuvant cyclophosphamide, methotrexate and 5-FU (CMF) [65]. It important to note that no survival difference was found between TP positive and TP negative patients who had not received chemotherapy. There are other papers that have shown that TP/DPD ratio is a predictive biomarker for capecitabine in head and neck cancer [66] and pancreatic adenocarcinoma [67]. In contrast to these findings, a phase II trial found no association between TP/DPD ratio and the efficacy of concomitant chemoradiation with capecitabine in patients with pancreatic adenocarcinoma [68].
Based on the available evidence, it appears that TP is a valid predictive biomarker for oral fluoropyrimidines like capecitabine. The evidence is less clear for 5-FU. However, when it comes to making clinical decisions, the available evidence is not sufficient to treat patients based on their TP expression status when being considered for fluoropyrimidine chemotherapy. Firstly, there is lack of prospective randomised data to support such practice. Secondly, current data on the predictive role of TP is not entirely consistent particularly in the case of 5-FU. As discussed earlier in this review TP plays a role in cancer progression and angiogenesis, yet it is important in activating capecitabine to its active form 5-FU. These two contradicting roles, best described by one publication as a Jekyll and Hyde story [69], are independently related to TP expression.
Targeting Thymidine Phosphorylase
The role of TP in cancer progression and angiogenesis, makes it an attractive target for therapy. Of particular interest is the inhibition of its angiogenic effects. The formation of new blood vessels is essential for tumour growth, and despite the clinical success of antiangiogenic agents like bevacizumab, many cancers develop resistance to the currently available antiangiogenic therapies [70]. Resistance to those agents that target VEGF is thought to be due to activation of VEGF-independent angiogenesis [71]. Therefore combining VEGF blockade with agents that target those VEGF-independent angiogenesis like TP is an appealing approach [71]. Another reasonable strategy is the combination of TP-inducing chemotherapy, such as paclitaxel, as discussed earlier in this review, with drugs activated by TP like capecitabine [3]. This strategy has proven to be successful in several human tumours [3].
It is important to note that there are no specific TP inhibitors approved for clinical use at present. [3, 4, 28, 72, 73]. In 2000, Fukushima and his colleagues identified a pyrimidine analogue, 5-chloro-6-(1-[2-iminopyrrolidinyl]methyl)uracil hydrochloride (TPI) as the most potent TP inhibitor to date [72]. They have shown that TPI inhibits several biological functions of TP in mouse models. Since then it has been used in many preclinical experiments and proposed to be an effective antitumor agent. TPI and the antimetabolite α,α,α-trifl uorothymidine (FTD) have been formulated into one novel oral compound: TAS-102. FTD cytotoxic activity is mediated through two mechanisms: inhibition of thymidylate synthase and more importantly, incorporation into DNA, resulting in long lasting DNA damage. TPI is added to TAS-102 to prevent FTD degradation by TP. Recently published results of a randomised phase 2 study of TAS-102 for fluorouracil-refractory colorectal cancer patients, showed an impressive median overall survival of 9·0 months (95 % CI 7·3–11·3) noted with TAS-102, compared with 6·6 months (4·9–8·0) for placebo [74]. These impressive results are not solely due to FTD effects but TPI is likely exerting antitumor and antiangiogenic effects [75]. Other TP inhibitors include purine analogues that have been shown to inhibit the enzymatic activity of TP and TP mediated angiogenesis [73]. Another inhibitor of the enzymatic activity of TP is 2-deoxy-L-ribose which is a stereoisomer of deoxy-D-ribose [73].
A popular therapeutic approach is to combine TP-inducing agents with capecitabine, which is activated by TP, leading to enhanced cytotoxicity of capecitabine [76]. This approach has been recently studied in NSCLC [77]. Emodin, an anthraquinone derivative from the root and rhizome of Rheum palmatum with reported antitumor effects, was used in combination with capecitabine to treat human NSCLC cell lines [77]. It was found that emodin increased the cytotoxic effects of capecitabine through enhancing TP mRNA and protein expression. It is important to note that capecitabine is not used clinically to treat NSCLC hence the novelty of this approach. Another important point is that combining capecitabine with TP-inducing agents like cisplatin or paclixaxel has worked successfully in clinical practise.
A second treatment strategy is based on the finding that TP overexpression has been linked to cisplatin resistance [42]. Therefore, targeting the antiapoptotic effects of TP could enhance cisplatin cytotoxicity. A recent publication examined the effect of TP inhibition, using Heat shock protein 90 (HSP90) inhibitor, on cisplatin cytotoxicity in NSCLC cell lines [78]. HSP90 is a molecular chaperone that is required for conformational folding and maintaining the stability of numerous proteins. HSP90 was inhibited using 17-allylamino-17-demethoxygeldanamycin (17-AAG). That resulted in decreased TP mRNA levels and cellular TP protein which was accompanied by a downregulation of phosphorylated MKK1/2-ERK1/2 and AKT protein levels. The authors found that the 17-AAG treatment disrupted the interaction between HSP90 and TP and triggered TP protein degradation through the ubiquitin-26S proteasome pathway. The net result was inhibition of TP-mediated antiapoptosis leading to enhanced cisplatin cytotoxicity. Similar results were reported by another group using human hepatoma HepG2 cells [79]. Liu et al. found that treating human hepatoma HepG2 cells with cisplatin alone led to increased TP expression through phosphorylation of ERK, p38 and PI3K/AKT pathways. When the cells were pretreated with fucoxanthin, the major non-provitamin A carotenoid found in Undaria pinnatifida, followed by cisplatin, a significant attenuation of cisplatin-induced TP mRNA expression was observed with resulting enhanced cisplatin cytotoxicity.
Another interesting TP targeting molecule is the purine riboside derivative KIN59 (5′-O-tritylinosine) which was identified in 2004 [80]. KIN59 was found to inhibit human and bacterial recombinant TP and TP-induced angiogenesis. In contrast to other TP inhibitors, KIN59 does not compete with the pyrimidine nucleoside or the phosphate-binding site of the enzyme but noncompetitively inhibits TP when thymidine or phosphate is used as the variable substrate [80, 81]. It was shown that KIN59 completely inhibited angiogenesis in a chick chorioallantoic membrane (CAM) assay [80, 82]. Recently KIN59 was shown to inhibit the angiogenic factor fibroblast growth factor-2 (FGF2), suggesting that it might play a role in multitargeted antiangiogenic cancer therapy [83].
Finally, Metformin which is an anti-diabetic drug recently shown to inhibit cancer cell proliferation and growth, was used to treat NSCLC cell lines in an experiment by Ko and colleagues [84]. Metformin treatment decreased cellular TP mRNA and protein levels by down-regulating phosphorylated MEK1/2-ERK1/2 protein levels in a dose- and time-dependent manner. The study proposed that TP downregulation is a mechanism by which metformin exerts its cytotoxic effects.
Conclusion
The complex role of TP in tumour progression and treatment is an example of the complexity of cancer biology. On the one hand TP enhances metastasis, invasion, angiogenesis and cell death evasion. On the other, it is crucial for activation of widely use chemotherapy agents like capecitabine. The malignant process is a complex one involving non-malignant cells and inflammatory factors that are reprogrammed to be in the advantage of cancer cell survival and growth [85].
The clinical importance of TP inhibition is yet to be fully defined. TP is widely expressed in human cancers and is associated with tumour progression. Research has shown that TP has a predictive value particularly in oral flouropyrimidines. Recently TAS-102 (Lonsurf ®) has been approved in Japan for the treatment of refractory metastatic colorectal cancer following the results of a phase III clinical trial which demonstrated a survival benefit of 1.8 months compared to placebo. Phase III trials are currently underway in the United States, Canada and Europe. Further translational research and clinical prospective trials in predictive value and therapeutic targeting of TP are required to expand our knowledge of this promising area.
References
- 1.Friedkin M, Roberts D. The enzymatic synthesis of nucleosides. I. thymidine phosphorylase in mammalian tissue. J Biol Chem. 1954;207(1):254–256. [PubMed] [Google Scholar]
- 2.Iltzsch MH, El Kouhni MH, Cha S. Kinetic studies of thymidine phosphorylase from mouse liver. Biochemistry. 1985;24(24):6799–6807. doi: 10.1021/bi00345a011. [DOI] [PubMed] [Google Scholar]
- 3.Toi M, Rahman MA, Bando H, Chow LW. Thymidine phosphorylase (platelet-derived endothelial growth factor) in cancer biology and treatment. Lancet Oncol. 2005;6(3):158–166. doi: 10.1016/S1470-2045(05)01766-3. [DOI] [PubMed] [Google Scholar]
- 4.Bronckaers A, Gago F, Balzarini J, Liekens S. The dual role of thymidine phosphorylase in cancer development and chemotherapy. Med Res Rev. 2009;29(6):903–953. doi: 10.1002/med.20159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moghaddam A, Bicknell R. Expression of platelet-derived endothelial cell growth factor in Escherichia coli and confirmation of its thymidine phosphorylase activity. Biochemistry. 1992;31(48):12141–12146. doi: 10.1021/bi00163a024. [DOI] [PubMed] [Google Scholar]
- 6.Usuki K, Saras J, Waltenberger J, Miyazono K, Pierce G, Thomason A, Heldin CH. Platelet-derived endothelial cell growth factor has thymidine phosphorylase activity. Biochem Biophys Res Commun. 1992;184(3):1311–1316. doi: 10.1016/S0006-291X(05)80025-7. [DOI] [PubMed] [Google Scholar]
- 7.Bijnsdorp IV, Peters GJ, Temmink OH, Fukushima M, Kruyt FA. Differential activation of cell death and autophagy results in an increased cytotoxic potential for trifluorothymidine compared to 5-fluorouracil in colon cancer cells. Int J Cancer. 2010;126(10):2457–2468. doi: 10.1002/ijc.24943. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Stenman G, Sahlin P, Dumanski JP, Hagiwara K, Ishikawa F, Miyazono K, et al. Regional localization of the human platelet-derived endothelial cell growth factor (ECGF1) gene to chromosome 22q13. Cytogenet Cell Genet. 1992;59(1):22–23. doi: 10.1159/000133191. [DOI] [PubMed] [Google Scholar]
- 10.El Omari K, Bronckaers A, Liekens S, Pérez-Pérez MJ, Balzarini J, Stammers DK. Structural basis for non-competitive product inhibition in human thymidine phosphorylase: implications for drug design. Biochem J. 2006;399(2):199–204. doi: 10.1042/BJ20060513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox SB, Moghaddam A, Westwood M, Turley H, Bicknell R, Gatter KC, et al. Platelet-derived endothelial cell growth factor/thymidine phosphorylase expression in normal tissues: an immunohistochemical study. J Pathol. 1995;176(2):183–190. doi: 10.1002/path.1711760212. [DOI] [PubMed] [Google Scholar]
- 12.Shaw T, Smillie RH, Miller AE, MacPhee DG. The role of blood platelets in nucleoside metabolism: regulation of platelet thymidine phosphorylase. Mutat Res. 1988;200(1–2):117–131. doi: 10.1016/0027-5107(88)90075-9. [DOI] [PubMed] [Google Scholar]
- 13.Pula G, Garonna E, Dunn WB, Hirano M, Pizzorno G, Campanella M, et al. Paracrine stimulation of endothelial cell motility and angiogenesis by platelet-derived deoxyribose-1-phosphate. Arterioscler Thromb Vasc Biol. 2010;30(12):2631–2638. doi: 10.1161/ATVBAHA.110.215855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sivridis E, Giatromanolaki A, Koukourakis MI, Bicknell R, Harris AL, Gatter KC. Thymidine phosphorylase expression in normal and hyperplastic endometrium. J Clin Pathol. 2000;53(9):704–708. doi: 10.1136/jcp.53.9.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Mackenzie IZ, Rees MC, Bicknell R. Regulation of the expression of the angiogenic enzyme platelet-derived endothelial cell growth factor/thymidine phosphorylase in endometrial isolates by ovarian steroids and cytokines. Endocrinology. 1997;138(11):4921–4930. doi: 10.1210/endo.138.11.5517. [DOI] [PubMed] [Google Scholar]
- 16.Yao L, Itoh S, Furuta I. Thymidine phosphorylase expression in oral squamous cell carcinoma. Oral Oncol. 2002;38(6):584–590. doi: 10.1016/S1368-8375(01)00113-0. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Huang X, Chen Y, Jin X, Li Q, Yi TN. Prognostic value of TP/PD-ECGF and thrombocytosis in gastric carcinoma. Eur J Surg Oncol. 2012;38(7):568–573. doi: 10.1016/j.ejso.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Ruckhäberle E, Karn T, Engels K, Turley H, Hanker L, Müller V, et al. Prognostic impact of thymidine phosphorylase expression in breast cancer-comparison of microarray and immunohistochemical data. Eur J Cancer. 2010;46(3):549–557. doi: 10.1016/j.ejca.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Chujo M, Miura T, Kawano Y, Miyawaki M, Imakiire T, Hayashita Y, et al. Thymidine phosphorylase levels and dihydropyrimidine dehydrogenase levels in non-small cell lung cancer tissues. Oncol Rep. 2006;16(4):777–780. [PubMed] [Google Scholar]
- 20.Yano T, Takeo S. Thymidine phosphorylase activity in nonsmall cell lung carcinoma tissues. Cancer. 2001;92(10):2658–2661. doi: 10.1002/1097-0142(20011115)92:10<2658::AID-CNCR1619>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Brockenbrough JS, Morihara JK, Hawes SE, Stern JE, Rasey JS, Wiens LW, et al. Thymidine kinase 1 and thymidine phosphorylase expression in non-small-cell lung carcinoma in relation to angiogenesis and proliferation. J Histochem Cytochem. 2009;57(11):1087–1097. doi: 10.1369/jhc.2009.952804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadahiro S, Suzuki T, Tanaka A, Okada K, Nagase H, Uchida J. Association of right-sided tumors with high thymidine phosphorylase gene expression levels and the response to oral uracil and tegafur/leucovorin chemotherapy among patients with colorectal cancer. Cancer Chemother Pharmacol. 2012;70(2):285–291. doi: 10.1007/s00280-012-1909-8. [DOI] [PubMed] [Google Scholar]
- 23.Shimabukuro T, Matsuyama H, Baba Y, Jojima K, Suyama K, Aoki A, et al. Expression of thymidine phosphorylase in human superficial bladder cancer. Int J Urol. 2005;12(1):29–34. doi: 10.1111/j.1442-2042.2004.00992.x. [DOI] [PubMed] [Google Scholar]
- 24.Hasegawa K, Okamoto H, Kawamura K, Kato R, Kobayashi Y, Sekiya T, et al. The effect of chemotherapy or radiotherapy on thymidine phosphorylase and dihydropyrimidine dehydrogenase expression in cancer of the uterine cervix. Eur J Obstet Gynecol Reprod Biol. 2012;163(1):67–70. doi: 10.1016/j.ejogrb.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Bijnsdorp IV, de Bruin M, Laan AC, Fukushima M, Peters GJ. The role of platelet-derived endothelial cell growth factor/thymidine phosphorylase in tumour behaviour. Nucleosides Nucleotides Nucleic Acids. 2008;27(6):681–691. doi: 10.1080/15257770802143988. [DOI] [PubMed] [Google Scholar]
- 26.Stark M, Bram EE, Akerman M, Mandel-Gutfreund Y, Assaraf YG. Heterogeneous nuclear ribonucleoprotein H1/H2-dependent unsplicing of thymidine phosphorylase results in anticancer drug resistance. J Biol Chem. 2011;286:3741–3754. doi: 10.1074/jbc.M110.163444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikuta K, Waguri-Nagaya Y, Kikuchi K, Yamagami T, Nozaki M, Aoyama M, et al. The Sp1 transcription factor is essential for the expression of gliostatin/thymidine phosphorylase in rheumatoid fibroblast-like synoviocytes. Arthritis Res Ther. 2012;14(2):R87. doi: 10.1186/ar3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liekens S, Bronckaers A, Pérez-Pérez MJ, Balzarini J. Targeting platelet-derived endothelial cell growth factor/thymidine phosphorylase for cancer therapy. Biochem Pharmacol. 2007;74(11):1555–1567. doi: 10.1016/j.bcp.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 29.De Bruin M, Temmink OH, Hoekman K, Pinedo HM, Peters GJ. Role of platelet derived endothelial cell growth factor/thymidine phosphorylase in health and disease. Cancer Ther. 2006;4:99–124. [Google Scholar]
- 30.Hemalatha T, Tiwari M, Balachandran C, Manohar BM, Puvanakrishnan R. Platelet-derived endothelial cell growth factor mediates angiogenesis and antiapoptosis in rat aortic endothelial cells. Biochem Cell Biol. 2009;87(6):883–893. doi: 10.1139/O09-056. [DOI] [PubMed] [Google Scholar]
- 31.Pula G, Mayr U, Evans C, Prokopi M, Vara DS, Yin X, et al. Potential of colony-forming units and endothelial progenitor cell cultures proteomics identifies thymidine phosphorylase as a key regulator of the angiogenic. Circ Res. 2009;104:32–40. doi: 10.1161/CIRCRESAHA.108.182261. [DOI] [PubMed] [Google Scholar]
- 32.Brown NS, Bicknell R. Thymidine phosphorylase, 2-deoxy-d-ribose and angiogenesis. Biochem J. 1998;334(Pt 1):1–8. doi: 10.1042/bj3340001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bijnsdorp IV, Vrijland K, Vroling L, Fukushima M, Peters GJ. Increased migration by stimulation of thymidine phosphorylase in endothelial cells of different origin. Nucleosides Nucleotides Nucleic Acids. 2010;29(4–6):482–487. doi: 10.1080/15257771003730201. [DOI] [PubMed] [Google Scholar]
- 34.Bijnsdorp IV, Capriotti F, Kruyt FA, Losekoot N, Fukushima M, Griffioen AW, et al. Thymidine phosphorylase in cancer cells stimulates human endothelial cell migration and invasion by the secretion of angiogenic factors. Br J Cancer. 2011;104(7):1185–1192. doi: 10.1038/bjc.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown NS, Jones A, Fujiyama C, Harris AL, Bicknell R. Thymidine phosphorylase induces carcinoma cell oxidative stress and promotes secretion of angiogenic factors. Cancer Res. 2000;60(22):6298–6302. [PubMed] [Google Scholar]
- 36.O'Byrne KJ, Koukourakis MI, Giatromanolaki A, Cox G, Turley H, Steward WP, et al. Vascular endothelial growth factor, platelet-derived endothelial cell growth factor and angiogenesis in non-small-cell lung cancer. Br J Cancer. 2000;82(8):1427–1432. doi: 10.1054/bjoc.1999.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu XH, Qian C, Yuan K. Correlations of hypoxia-inducible factor-1α/hypoxia-inducible factor-2α expression with angiogenesis factors expression and prognosis in non-small cell lung cancer. Chin Med J. 2011;124(1):11–18. [PubMed] [Google Scholar]
- 38.Ikeda R, Furukawa T, Kitazono M, Ishitsuka K, Okumura H, Tani A, et al. Molecular basis for the inhibition of hypoxia-induced apoptosis by 2-deoxy-D-ribose. Biochem Biophys Res Commun. 2002;291(4):806–812. doi: 10.1006/bbrc.2002.6432. [DOI] [PubMed] [Google Scholar]
- 39.Ikeda R, Tajitsu Y, Iwashita K, Che XF, Yoshida K, Ushiyama M, et al. Thymidine phosphorylase inhibits the expression of proapoptotic protein BNIP3. Biochem Biophys Res Commun. 2008;370(2):220–224. doi: 10.1016/j.bbrc.2008.03.067. [DOI] [PubMed] [Google Scholar]
- 40.Trempolec N, Dave-Coll N, Nebreda AR. SnapShot: p38 MAPK signaling. Cell. 2013;152(3):656–656. doi: 10.1016/j.cell.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 41.Ikeda R, Che XF, Ushiyama M, Yamaguchi T, Okumura H, Nakajima Y, et al. 2-Deoxy-D-ribose inhibits hypoxia-induced apoptosis by suppressing the phosphorylation of p38 MAPK. Biochem Biophys Res Commun. 2006;342(1):280–285. doi: 10.1016/j.bbrc.2006.01.142. [DOI] [PubMed] [Google Scholar]
- 42.Ikeda R, Furukawa T, Mitsuo R, Noguchi T, Kitazono M, Okumura H, et al. Thymidine phosphorylase inhibits apoptosis induced by cisplatin. Biochem Biophys Res Commun. 2003;301(2):358–363. doi: 10.1016/S0006-291X(02)03034-6. [DOI] [PubMed] [Google Scholar]
- 43.Gotanda T, Haraguchi M, Tachiwada T, Shinkura R, Koriyama C, Akiba S, et al. Molecular basis for the involvement of thymidine phosphorylase in cancer invasion. Int J Mol Med. 2006;17(6):1085–1091. [PubMed] [Google Scholar]
- 44.Nakajima Y, Haraguchi M, Furukawa T, Yamamoto M, Nakanishi H, Tatematsu M, et al. 2-Deoxy-L-ribose inhibits the invasion of thymidine phosphorylase overexpressing tumors by suppressing matrix metalloproteinase-9. Int J Cancer. 2006;119(7):1710–1716. doi: 10.1002/ijc.22014. [DOI] [PubMed] [Google Scholar]
- 45.Takao S, Akiyama SI, Nakajo A, Yoh H, Kitazono M, Natsugoe S, et al. Suppression of metastasis by thymidine phosphorylase inhibitor. Cancer Res. 2000;60(19):5345–5348. [PubMed] [Google Scholar]
- 46.Sato J, Sata M, Nakamura H, Inoue S, Wada T, Takabatake N, et al. Role of thymidine phosphorylase on invasiveness and metastasis in lung adenocarcinoma. Int J Cancer. 2003;106(6):863–870. doi: 10.1002/ijc.11315. [DOI] [PubMed] [Google Scholar]
- 47.Waring P, Müllbacher A. Cell death induced by the fas/fas ligand pathway and its role in pathology. Immunol Cell Biol. 1999;77(4):312–317. doi: 10.1046/j.1440-1711.1999.00837.x. [DOI] [PubMed] [Google Scholar]
- 48.Mori S, Takao R, Ikeda R, Noma H, Mataki Y, Wang X, et al. Role of thymidine phosphorylase in fas induced apoptosis. Biochem Biophys Res Commun. 2002;295:300–305. doi: 10.1016/S0006-291X(02)00662-9. [DOI] [PubMed] [Google Scholar]
- 49.Slager EH, Honders MW, van der Meijden ED, van Luxemburg-Heijs SA, Kloosterboer FM, Kester MG, et al. Identification of the angiogenic endothelial-cell growth factor 1/thymidine phosphorylase as a potential target for immunotherapy of cancer. Blood. 2006;107(12):4954–4960. doi: 10.1182/blood-2005-09-3883. [DOI] [PubMed] [Google Scholar]
- 50.Longley DB, Harkin DP. Johnston PG 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3(5):330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 51.Malet-Martino M, Martino R. Clinical studies of three oral prodrugs of 5-fluorouracil (capecitabine, UFT, S-1): a review. Oncologist. 2002;7(4):288–323. doi: 10.1634/theoncologist.7-4-288. [DOI] [PubMed] [Google Scholar]
- 52.Aschele C, Sobrero A, Faderan MA, Bertino JR. Novel mechanism(s) of resistance to 5-fluorouracil in human colon cancer (HCT-8) sublines following exposure to two different clinically relevant dose schedules. Cancer Res. 1992;52:1855–1864. [PubMed] [Google Scholar]
- 53.Diasio RB, Johnson MR. Dihydropyrimidine dehydrogenase: its role in 5-fluorouracil clinical toxicity and tumour resistance. Clin Cancer Res. 1999;5:2672–2673. [PubMed] [Google Scholar]
- 54.Cao D, Pizzorno G. Uridine phosphorylase: an important enzyme in pyrimidine metabolism and fluoropyrimidine activation. Drugs Today (Barc) 2004;40(5):431–444. doi: 10.1358/dot.2004.40.5.850491. [DOI] [PubMed] [Google Scholar]
- 55.Temmink OH, de Bruin M, Turksma AW, Cricca S, Laan AC, Peters GJ. Activity and substrate specificity of pyrimidine phosphorylases and their role in fluoropyrimidine sensitivity in colon cancer cell lines. Int J Biochem Cell Biol. 2007;39(3):565–575. doi: 10.1016/j.biocel.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 56.Schüller J, Cassidy J, Dumont E, Roos B, Durston S, Banken L, et al. Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother Pharmacol. 2000;45(4):291–297. doi: 10.1007/s002800050043. [DOI] [PubMed] [Google Scholar]
- 57.Ishikawa T, Sekiguchi F, Fukase Y, Sawada N, Ishitsuka H. Positive correlation between the efficacy of capecitabine and doxifluridine and the ratio of thymidine phosphorylase to dihydropyrimidine dehydrogenase activities in tumors in human cancer xenografts. Cancer Res. 1998;58(4):685–690. [PubMed] [Google Scholar]
- 58.Metzger R, Danenberg K, Leichman CG, Salonga D, Schwartz EL, Wadler S, et al. High basal level gene expression of thymidine phosphorylase (platelet-derived endothelial cell growth factor) in colorectal tumors is associated with nonresponse to 5-fluorouracil. Clin Cancer Res. 1998;4(10):2371–2376. [PubMed] [Google Scholar]
- 59.Gustavsson B, Kaiser C, Carlsson G, Wettergren Y, Odin E, Lindskog EB, et al. Molecular determinants of efficacy for 5-FU-based treatments in advanced colorectal cancer: mRNA expression for 18 chemotherapy-related genes. Int J Cancer. 2009;124(5):1220–1226. doi: 10.1002/ijc.23852. [DOI] [PubMed] [Google Scholar]
- 60.Lu M, Gao J, Wang XC, Shen L. Expressions of thymidylate synthase,thymidine phosphorylase, class iii β-tubulin, and excision repair cross-complementing group 1predict response in advanced gastric cancer patients receiving capecitabine plus paclitaxel or cisplatin. Chin J Cancer Res. 2011;23(4):288–294. doi: 10.1007/s11670-011-0288-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boskos CS, Liacos C, Korkolis D, Aygerinos K, Lamproglou I, Terpos E, et al. Thymidine phosphorylase to dihydropyrimidine dehydrogenase ratio as a predictive factor of response to preoperative chemoradiation with capecitabine in patients with advanced rectal cancer. J Surg Oncol. 2010;102(5):408–412. doi: 10.1002/jso.21423. [DOI] [PubMed] [Google Scholar]
- 62.Mori T, Ohue M, Takii Y, Hashizume T, Kato T, Kotake K, et al. Factors predicting the response to oral fluoropyrimidine drugs: a phase II trial on the individualization of postoperative adjuvant chemotherapy using oral fluorinated pyrimidines in stage iii colorectal cancer treated by curative resection (ACT-01 Study) Oncol Rep. 2013;29(2):437–444. doi: 10.3892/or.2012.2177. [DOI] [PubMed] [Google Scholar]
- 63.Yang Q, Yoshimura G, Mori I, Sakurai T, Kakudo K. Thymidine phosphorylase and breast carcinoma. Anticancer Res. 2002;22(4):2355–2360. [PubMed] [Google Scholar]
- 64.Yang Q, Barbareschi M, Mori I, Mauri F, Muscarà M, Nakamura M, et al. Prognostic value of thymidine phosphorylase expression in breast carcinoma. Int J Cancer. 2002;97(4):512–517. doi: 10.1002/ijc.1633. [DOI] [PubMed] [Google Scholar]
- 65.Fox SB, Engels K, Comley M, Whitehouse RM, Turley H, Gatter KC, et al. Relationship of elevated tumour thymidine phosphorylase in node-positive breast carcinomas to the effects of adjuvant CMF. Ann Oncol. 1997;8(3):271–275. doi: 10.1023/A:1008280110558. [DOI] [PubMed] [Google Scholar]
- 66.Saito K, Khan K, Yu SZ, Ronson S, Rhee J, Li G, et al. The predictive and therapeutic value of thymidine phosphorylase and dihydropyrimidine dehydrogenase in capecitabine (Xeloda)-based chemotherapy for head and neck cancer. Laryngoscope. 2009;119(1):82–88. doi: 10.1002/lary.20003. [DOI] [PubMed] [Google Scholar]
- 67.Saif MW, Hashmi S, Bell D, Diasio RB. Prognostication of pancreatic adenocarcinoma by expression of thymidine phosphorylase/dihydropyrimidine dehydrogenase ratio and its correlation with survival. Expert Opin Drug Saf. 2009;8(5):507–514. doi: 10.1517/14740330903173217. [DOI] [PubMed] [Google Scholar]
- 68.Saif MW, Black G, Roy S, Bell D, Russo S, Eloubeidi MA, et al. Phase II study of capecitabine with concomitant radiotherapy for patients with locally advanced pancreatic cancer: up-regulation of thymidine phosphorylase. Cancer J. 2007;13(4):247–256. doi: 10.1097/PPO.0b013e31813c12b8. [DOI] [PubMed] [Google Scholar]
- 69.Ciccolini J, Evrard A, Cuq P. Thymidine phosphorylase and fluoropyrimidines efficacy: a Jekyll and Hyde story. Curr Med Chem Anticancer Agents. 2004;4(2):71–81. doi: 10.2174/1568011043482089. [DOI] [PubMed] [Google Scholar]
- 70.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8(8):579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 71.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fukushima M, Suzuki N, Emura T, Yano S, Kazuno H, Tada Y, et al. Structure and activity of specific inhibitors of thymidine phosphorylase to potentiate the function of antitumor 2′-deoxyribonucleosides. Biochem Pharmacol. 2000;59(10):1227–1236. doi: 10.1016/S0006-2952(00)00253-7. [DOI] [PubMed] [Google Scholar]
- 73.Pérez-Pérez MJ, Priego EM, Hernández AI, Camarasa MJ, Balzarini J, Liekens S. Thymidine phosphorylase inhibitors: recent developments and potential therapeutic applications. Mini Rev Med Chem. 2005;5(12):1113–1123. doi: 10.2174/138955705774933301. [DOI] [PubMed] [Google Scholar]
- 74.Yoshino T, Mizunuma N, Yamazaki K, Nishina T, Komatsu Y, Baba H, et al. TAS-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2012;13(10):993–1001. doi: 10.1016/S1470-2045(12)70345-5. [DOI] [PubMed] [Google Scholar]
- 75.Peters GJ, Bijnsdorp IV. TAS-102: more than an antimetabolite. Lancet Oncol. 2012;13(12):e518–e519. doi: 10.1016/S1470-2045(12)70426-6. [DOI] [PubMed] [Google Scholar]
- 76.Sawada N, Ishikawa T, Fukase Y, Nishida M, Yoshikubo T, Ishitsuka H. Induction of thymidine phosphorylase activity and enhancement of capecitabine efficacy by taxol/taxotere in human cancer xenografts. Clin Cancer Res. 1998;4(4):1013–1019. [PubMed] [Google Scholar]
- 77.Ko JC, Tsai MS, Kuo YH, Chiu YF, Weng SH, Su YC, et al. Modulation of Rad51, ERCC1, and thymidine phosphorylase by emodin result in synergistic cytotoxic effect in combination with capecitabine. Biochem Pharmacol. 2011;81(5):680–690. doi: 10.1016/j.bcp.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 78.Weng SH, Tseng SC, Huang YC, Chen HJ, Lin YW. Inhibition of thymidine phosphorylase expression by using an HSP90 inhibitor potentiates the cytotoxic effect of cisplatin in non-small-cell lung cancer cells. Biochem Pharmacol. 2012;84(1):126–136. doi: 10.1016/j.bcp.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 79.Liu CL, Lim YP, Hu ML. Fucoxanthin enhances cisplatin-induced cytotoxicity via NFκB-mediated pathway and downregulates DNA repair gene expression in human hepatoma HepG2 cells. Mar Drugs. 2013;11(1):50–66. doi: 10.3390/md11010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liekens S, Hernández AI, Ribatti D, De Clercq E, Camarasa MJ, Pérez-Pérez MJ, et al. The nucleoside derivative 5′-O-trityl-inosine (KIN59) suppresses thymidine phosphorylase-triggered angiogenesis via a noncompetitive mechanism of action. J Biol Chem. 2004;279(28):29598–29605. doi: 10.1074/jbc.M402602200. [DOI] [PubMed] [Google Scholar]
- 81.Casanova E, Hernandez AI, Priego EM, Liekens S, Camarasa MJ, Balzarini J, et al. 5′-O-tritylinosine and analogues as allosteric inhibitors of human thymidine phosphorylase. J Med Chem. 2006;49(18):5562–5570. doi: 10.1021/jm0605379. [DOI] [PubMed] [Google Scholar]
- 82.Liekens S, Balzarini J, Hernández AI, De Clercq E, Priego EM, Camarasa MJ, et al. Thymidine phosphorylase is noncompetitively inhibited by 5′-O-trityl-inosine (KIN59) and related compounds. Nucleosides Nucleotides Nucleic Acids. 2006;25(9–11):975–980. doi: 10.1080/15257770600888925. [DOI] [PubMed] [Google Scholar]
- 83.Liekens S, Bronckaers A, Belleri M, Bugatti A, Sienaert R, Ribatti D, et al. The thymidine phosphorylase inhibitor 5′-O-tritylinosine (KIN59) is an antiangiogenic multitarget fibroblast growth factor-2 antagonist. Mol Cancer Ther. 2012;11(4):817–829. doi: 10.1158/1535-7163.MCT-11-0738. [DOI] [PubMed] [Google Scholar]
- 84.Ko JC, Huang YC, Chen HJ, Tseng SC, Chiu HC, Wo TY, et al. Metformin induces cytotoxicity by down-regulating thymidine phosphorylase and ERCC1 expression in non-small cell lung cancer cells. Basic Clin Pharmacol Toxicol. 2013 doi: 10.1111/bcpt.12052. [DOI] [PubMed] [Google Scholar]
- 85.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]