Abstract
High levels of reactive oxygen (ROS) and nitrogen (RNS) species can lead to the destruction of extracellular matrix facilitating tumor progression. ROS can activate matrix metalloproteinases (MMP), damage DNA and RNA. Therefore, the levels of MMP, ROS and RNS can serve as additional prognostic markers and for the estimation of the effectiveness of tumor therapy. Concerning gastric cancer, the prognostic role of MMP, its connection with the cancer staging remains controversial and correlations between the activity of MMP with the ROS and RNS levels are insufficiently confirmed. Superoxide generation rates, nitric oxide (NO) levels, concentrations of active forms of matrix metalloproteinases MMP-2 and MMP-9 in tumor and adjacent tissues of patients with stomach cancer at different disease stages were measured by electron spin resonance (ESR) including spin-trapping and polyacrylamide gel zymography. It is shown that the activity of MMP-2 and MMP-9 in tumor tissue correlate with the superoxide radicals generation rate and NO levels (r = 0.48÷0.67, p < 0.05). The activity of MMP-2 and MMP-9 in tumor tissues and superoxide radical generation rates correlate positively with the stage of regional dissemination (r = 0.45 and 0.37, correspondingly, p < 0.05), but MMP-2 and MMP-9 activity inversely depends on distant metastatic degree of stomach cancer (r = 0.58; p < 0.05). Additionally, the feasibility of ESR to locally determine oxidative stress is demonstrated.
Keywords: Reactive oxygen species (ROS), Matrix metalloproteinases, Stomach cancer, Gastric cancer, Electron spin resonance (ESR), Electron paramagnetic resonance (EPR), Metastasis
Introduction
Despite the reduction in incidence, stomach (gastric) cancer (SC) is still the second most frequent cause of cancer related death worldwide [1]. According to the Russian national statistics, 38,318 new SC cases were diagnosed in 2011, and the most of them on the latest disease stages III-IV [2]. In Ukraine in 2013 SC took the second and third places in the structure of men and women cancer related mortalities, correspondingly, though only the fourth (men) and the eights (woman) incidence places in the hierarchy of tumor diseases [3]. Diagnosis of gastric cancer in the early stages is difficult because of the lack of simple and cheap methods of inspection and specific markers of gastric cancer while the symptoms of the disease are vague and tend to overlap with other common and benign conditions [4]. It is expected that better diagnostic tools combined with advances in molecular and genetic analysis will allow better tumor characterization and more individualized treatment planning. Patients can then be evaluated with the optimal modalities for their particular type of tumor and be given the best multimodality treatment [4].
Reactive oxygen and nitrogen species (ROS/RNS) are considered as potential diagnostic and prognostic markers in many types and stages of cancer and targets for the therapeutic treatments [5–9]. For example, depending on the generation rate, duration of exposure, localization, and types of the metabolites that can be produced, superoxide radicals (O2•) can provoke a wide range of cells’ responses [10]. At low levels the superoxide radicals function as mitogenes, they promote cell poliferation and survival. At the intermediate levels O2• can cause a short stopover or a complete arrest of the cell cycle and induce cellular differentiation [11]. At high generation rate O2• can easily react with the membrane lipids causing membrane permeability changes, genomic instability as a result of the genome oxidative modification, etc. Proteins’ modifications can lead to the lowering or changing the catalytic activity of some ferments and sensitivity of the proteins to the proteolytic degradation. In that case the superoxide radicals initiate cells apoptosis or necrosis [12, 13]. At the rates of the superoxide radical generation for which the vital activity of the cells irreversibly alters, O2• can act as a messenger in some intracellular signaling cascades leading to cancer progression.
The effects of nitric oxide (NO) and superoxide anion radicals in the enhanced permeability and retention (EPR) effect for the tumor-selective delivery of macromolecular agents (nanomedicines) are reported and used [14]. In cancer tissues and in carcinomatosis infection stimulates O2• generation via activation of xanthine oxidase while generating NO by inducing NO synthase. These chemicals function in mutation and carcinogenesis and promote inflammation, in which peroxynitrite (a product of O2• and NO) activates matrix metalloproteinases (MMP), damages DNA and RNA.
MMP-2 and MMP-9, also known as gelatinase A and gelatinase B, belong to a zinc-dependent family of endopeptidases implicated in a variety of physiological processes as well as in pathological conditions. They are considered to be the major MMPs involved in invasion and metastasis of cancer because of their capacity to degrade the important components of basement membranes - gelatin, laminin, nidogen, type I and IV collagens [8, 9, 15–19]. Nevertheless, the prognostic role of MMP-2, for example, in gastric cancer and its connection with the cancer staging remains controversial [20]; correlations between the activity of MMP with the NO levels and superoxide radicals generation are insufficiently confirmed. In the recent large-scale study, it was found that he smallest among the matrix metalloproteinases matrilysin (MMP-7) coupled with p53 analysis can serve as promising factor to predict outcome of Stage II/III gastric cancer [21]. On that basis a new staging classification is proposed.
The purpose of this work is to find correlations between the superoxide and nitric oxide generation rates, levels of active forms of MMP-2 and MMP-9 in tumor and adjoining tissues between each other and with the disease stages for SC patients.
Materials and Methods
We have investigated the samples of tumor and adjunct tissues (taken at the distances of 1.5, 2.5 and 5.0 cm from the tumor) of 48 patients with stomach cancer (T2-4, N0-2, M0-1, G1 – G4 according to the Seventh Edition of the American Joint Committee of Cancer classification [22], where T factor is the degree of wall penetration of the primary tumor; N factor is the status of lymph node metastasis; M factor shows the presence of distant metastasis and G describes the grade of the cancer), who were treated at the Kyiv City Clinical Cancer Center. All participants expressed their prior written consent to take part in the research and to use the tissues obtained by surgery for the research purposes. The study was approved and guided under the control of the Bioethical Committee at the R.E. Kavetsky Institute.
The investigated group consisted of 33 men and 15 women with the average age of 58 ± 10.5 year at the next disease stages: stage I - 8 patients, stage II - 12 patients, stage III – 17 patients and stage IV – 11 patients. 24 of them (50 %) had lymph nodes metastasis while 6 (12.5 %) had distant metastasis. According to the G- grade the patients were classified as G1 – 4 patients, G2 – 9, G3 – 22 and G4 – 13 patients. The diagnosis, stage of the disease and presence of metastases were verified morphologically in accordance with the requirements of evidence-based medicine (during the relevant clinical and instrumental examinations).
The tissue species for the investigations were frozen and kept in special molds in liquid nitrogen (T = 77K). Electron spin resonance (ESR) measurements were conducted at room (RT) and liquid nitrogen temperatures on RE-1307 (USSR) and ESP-300 (Bruker, Germany) X-band (9 GHz) spectrometers. (Here we use abbreviation ESR instead the more widely accepted EPR for electron paramagnetic resonance to avoid confusions with the abbreviation for enhanced permeability and retention effect introduced in the previous section). For the RT studies the samples were then unfrozen. The ESR spectrometers were used in continuous-wave (cw) mode by using conventional modulation technique. The spectrometers handling, data collection and analysis were carried out with the home-made personal computer interfaces and self-written software.
Spin traps 1-hydroxy-2,2,6,6-tetramethyl-4-oxo-piperidine hydrochloride (TEMPONE-H, Sigma-Aldrich, USA) and Fe/diethyldithiocarbamate (Fe/DETC) were exploited to extract the rates of superoxide O2• generation and nitric oxide NO concentrations in the investigated tissues, correspondingly, at RT by ESR. The technical details of ESR measurements and sample preparations with spin-traps are given in our previous papers [7, 23, 24].
Concentrations of MMP-2 and MMP-9 in samples both in active and latent forms were determined by gelatine zymography - the polyacrylamide gel electrophoresis-based method with using sodium dodecyl sulfate (SDS). The details of the corresponding analysis are given in [23].
Results and Discussion
It is known [23, 24] that ESR is sensitive to the changes in the mitochondrial electron transport chain (ETC). Signal with g = 1.94 is related to the activity of the iron-sulfur cluster N2 of NADH: ubiquinone oxidoreductase, also called respiratory complex I. Signal with g ≈ 2.00 is usually ascribed to the free radical centers practically completely localized in mitochondria - semiquinones of flavoproteins found in the inner membrane of mitochondria and coenzyme Q semiquinones (ubisemiquinones, USQ). Signal with g ≈ 2.007 characterizes a generation of NO and N types FeS-protein complexes. Signals with g = 2.25 and g = 2.42 are connected with the catalytic cycle of the cytochrome P-450 which serves as a natural detoxifying agent of the body.
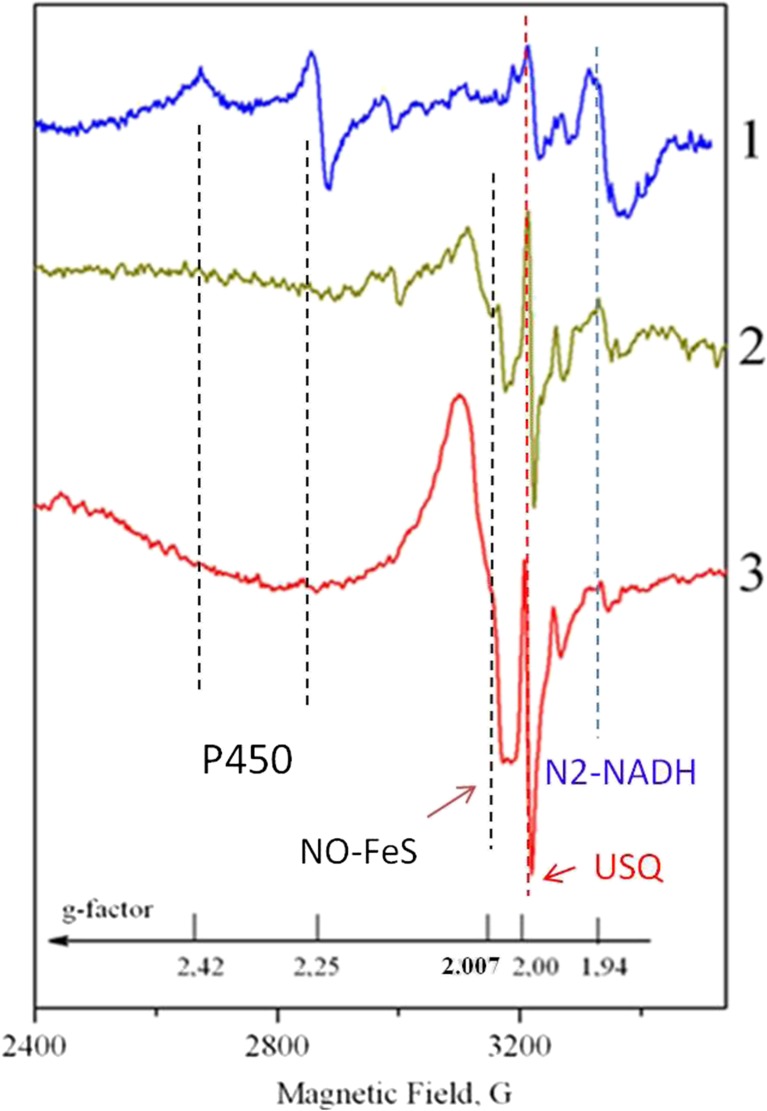
Figure 1 shows typical ESR spectra of tissues taken at different distances from the tumor at T = 77K. The most distant tissue (5 cm from the tumor, curve 1) has a characteristic for the “healthy” tissues set of signals; O2• generation rate is measured to be 0.25 ± 0.18 nmol/g/ min, NO level is 1.5 ± 0.15 nmole/g.
Fig. 1.
ESR spectra of the stomach tissue cells taken at the different distances from the tumor. Curve 1–5 cm from the tumor; curve 2–2.5 cm from the tumor; curve 3 – stomach wall tissues (1.5 cm from the tumor). The magnetic field strength and corresponding g-factors are shown along the x-axis. The dashed lines indicate the ESR features undergoing the prominent transformations. The details of the ESR lines assignment are given in the text
As one approaches the tumor (curve 2 in Fig. 1), ESR signal from cytochrome P-450 disappears. The amplitude of the N2-NADH signal with g = 1.94 decreases (in 3.5 times in Fig. 1) while the amplitudes of ubisemiquinones (at g ≈ 2.00) and NO-FeS complexes (at g ≈ 2.007) grow up significantly (up to 6.7 times in Fig. 1 as compared to curve 1). These indicate that the mitochondrial cells lose their functional activity connected with the oxidative phosphorylation.
In the close vicinity to the tumor, the ESR spectra of the adjunct tissues (curves 2 and 3, Fig. 1) are practically identical to the tumor tissue ESR of stage I patients (cf. Fig. 2). The values of the increased ROS and RNS levels depend on the exact distance of the adjunct tissues from the tumor one and in these cases are measured to be in the range from 1.9 ± 0.23 nmol/g /min to 5.0 ± 0.38 nmol/g /min for the O2• generation rate (i.e. up to 20 times higher than in a norm) and from 2.0 ± 0.15 nmol/g till 6.0 ± 0.49 nmole/g (i.e. up to 4 times higher than in a norm) for NO.
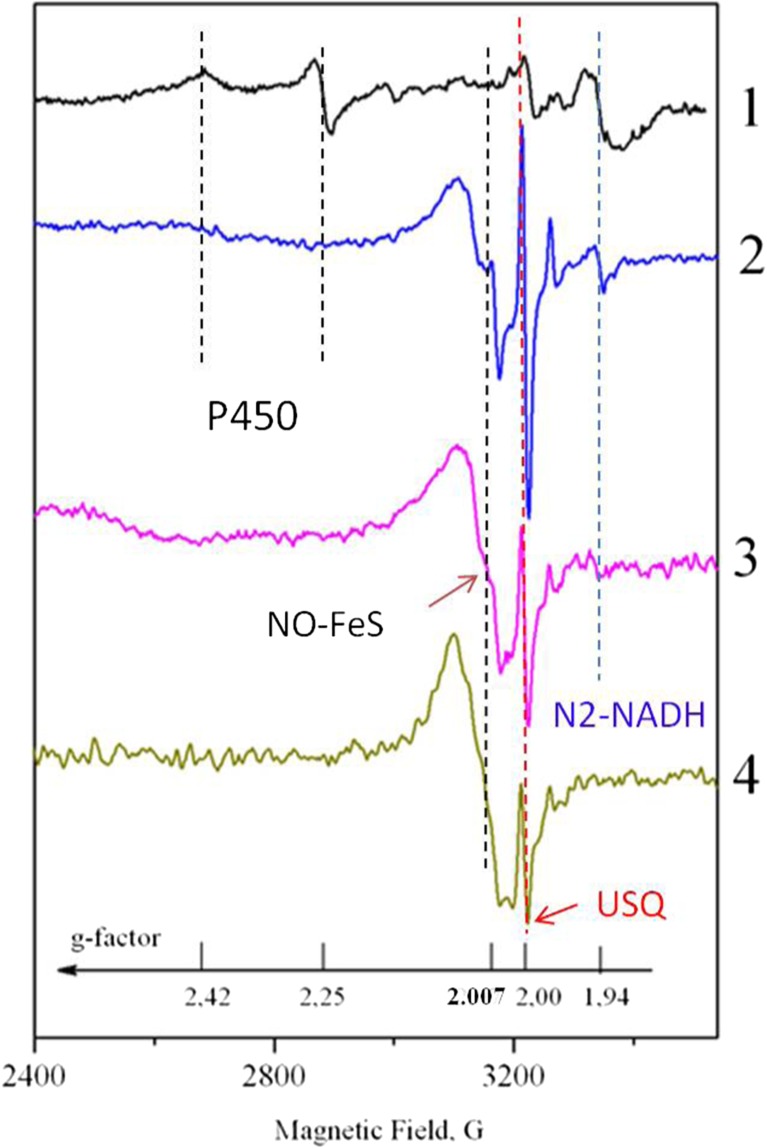
Fig. 2.
Comparison of ESR spectra of tumor tissues (2–4) of patients at different disease stages. Curve 1 – stomach tissue (5 cm from the tumor); 2 – SC in stage I; 3 – SC in stage II; 4 – SC in stage III. The dashed lines indicate the ESR features undergoing the prominent transformations with SC stage. The details of the ESR lines assignment are given in the text
Typical ESR spectra of tumor tissues of patients at different disease stages, which correspond to different invasion and tumor metastasis levels, are shown in Fig. 2. As it was already mentioned above, there are no ESR signals with g = 2.25 and 2.42 that characterize the functioning of Р-450. The intensities of the signals with g = 1.94 and 2.00 which related to the activity of FeS protein in sulfur cluster N2 of NADH: ubiquinone oxidoreductase and mitochondrial ubisemiquinones decreases with the disease stage. The increase of the NO-FeS complexes leads to the growth of signal with g ≈ 2.007 with the disease stage number. The structureless line at g ≈ 2.007 slightly broadens and starts to reveal a resolved three line pattern caused by the influence of nuclear magnetic moment of 14N in NO-FeS complexes. At the same time, the superoxide generation rate in tumor tissues grows up with the disease stage from 0.65 ± 0.19 nmol/g/min (stage I) to 2.5 ± 0.26 nmol/g/min (stage IV) while NO level increases from 2.1 ± 0.22 nmol/g (stage I) to 3.5 ± 0.31 nmol/g (stage IV). In a norm these parameters are 0.25 ± 0.18 nmol/g/min and 1.5 ± 0.15 nmol/g, correspondingly.
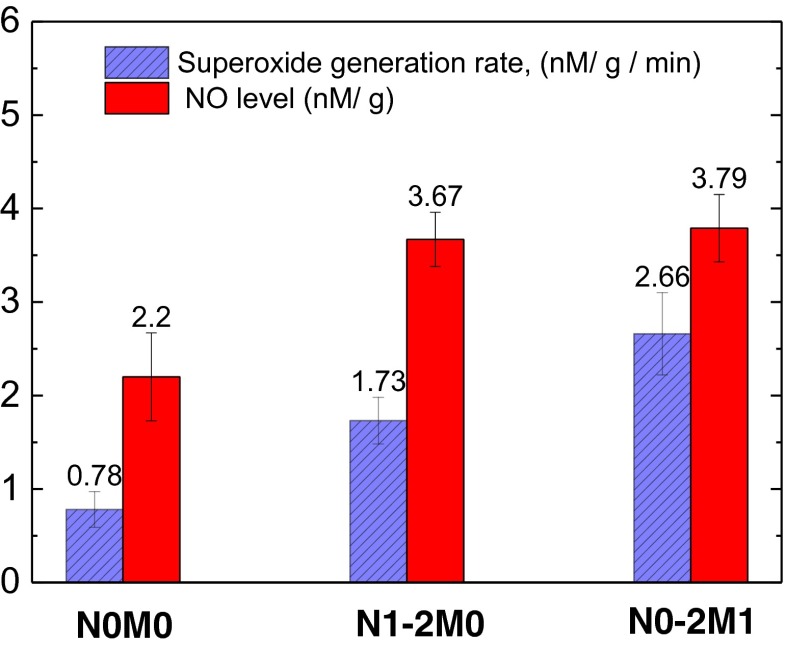
Figure 3 demonstrates that that the tumors of the patients with metastasis (N0-2М1) are characterized by the superoxide generation rates that are in 1.3 times higher (p < 0.05) than the same values in the tumors of patients with metastasis in regional lymph nodes, but without distant metastasis (N1-2М0) and almost 3 times higher than that for the patients without metastasis (N0М0). The same tendency is to observe for NO levels in SC tissues: distant metastasis and regional lymph nodes metastasis increase the NO levels in more than 1.5 times in comparison to the tumor tissue without metastasis (p < 0.05). It is revealed that the superoxide generation rates and NO levels correlate positively with the regional (r = 0.63 and 0.69, correspondingly, p < 0.05) and distant (r = 0.72 and 0.43, correspondingly, p < 0.05) SC metastasis.
Fig. 3.
The rates of superoxide radical generation (left / blue crosshatched columns) and NO level (right / red columns) depending on metastatic index. N0М0 – no metastasis; N1-2M0 - regional lymph nodes metastasis; N0-2M1 – distant metastasis. O2 • generation rate and NO levels correlate positively with the regional and distant (p < 0.05) SC metastasis
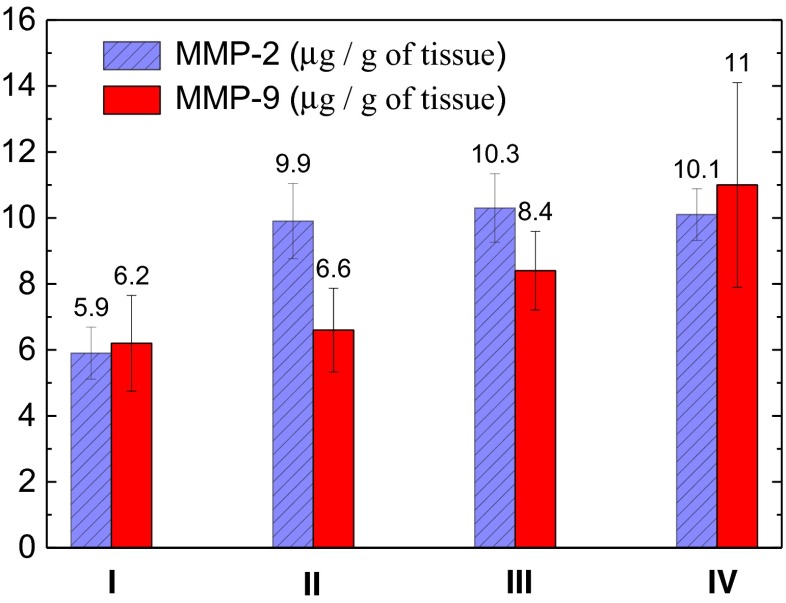
The concentration of the active forms of MMP-2 varies from 0.1 to 52.8 μg/g with the mean value of 8.2 ± 4.9 μg/g. For MMP-9 the corresponding values are in the range from 0.05 to 28.8 μg/g, the mean one is 8.3 ± 5.9 μg/g. No statistically reliable correlations between the activity of both gelatinases with the sex and age of patients were found. No statistically reliable correlations between the activities of both gelatinases with T stage was found. But as Fig. 4 demonstrates, the concentrations of active forms of MMP-2 differ significantly for G1 and G2-G4 stages (p < 0.05) with the maximum at stage G3. Concentration of active forms of MMP-9 slightly grows up according to G grade in 1.7 times but the difference is not statistically reliable (p > 0.05).
Fig. 4.
MMP-2 and MMP-9 activities in SC depending on the stage (I-IV) of disease. The reliable positive correlation (p < 0.05) for the MMP-2 activity growth from stage I to stages II-IV is obtained
We found that the activities of both MMP-2 and MMP-9 in SC tissues correlate with the superoxide radical generation rate and NO levels (r = 0.48 ÷ 0.67, p < 0.05). Besides that, all the values pointed above positively depend on the disease stage (r = 0.59 ÷ 0.71, p < 0.05).
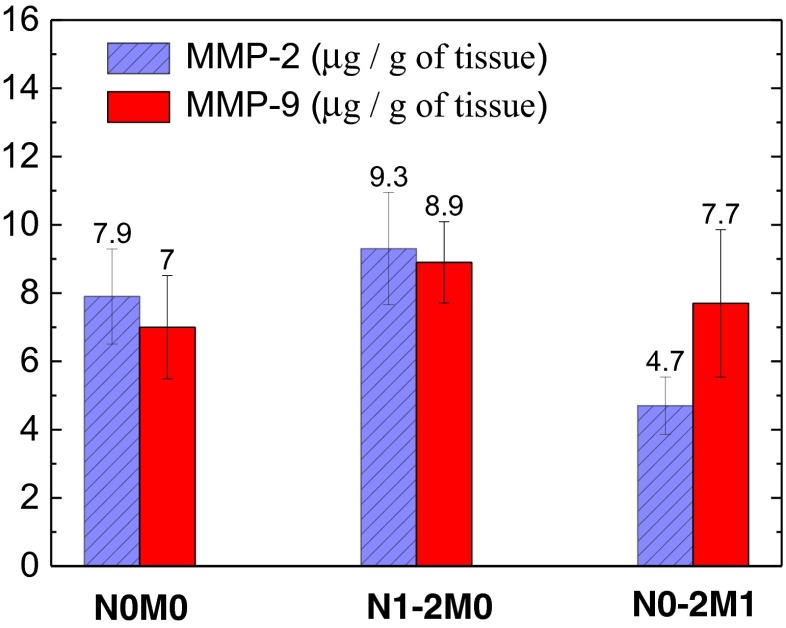
The connection between the MMP-2 and MMP-9 activities in the SC and metastasis level was also analyzed. Though the concentrations of active forms of both gelatinases in tumor tissues for N1-2 are higher than for N0 (see Fig. 5), the statistically reliable difference was not revealed (p > 0.05). At the same time, the MMP-2 activity inversely depends on M category: MMP-2 activity for the patients without distant metastasis is in 2 times higher than that for the patients with metastasis (p < 0.05). The maximal activation of latent forms of MMP-2 in tumor tissues testifies the significant destruction of the extracellular matrix at the disease stages for which the metastases are not revealed clinically, i.e. on the stage of metastases formation or tumor cells dissemination. These results are in accordance with the models suggesting that tumor cells can create favorable for their invasion and subsequent proliferation microenvironment in so-called niches by using appropriate signal pathways [22, 23].
Fig. 5.
MMP-2 (left / blue crosshatched columns) and MMP-9 (right / red columns) activities in SC depending on metastatic index. N0М0 – no metastasis; N1-2M0 - regional lymph nodes metastasis; N0-2M1 – distant metastasis. The reliable negative correlation (p < 0.05) between the MMP-2 activity and M stage is obtained
Our experiments show that MMP-2 and MMP-9 activities in SC tumor (as well as superoxide radical generation rates and NO levels) correlate positively with the regional metastasis (r = 0.45 and 0.37, correspondingly; p < 0.05) but MMP-2 activity in contrast to other values inversely correlate with the distant metastasis (r = 0.58; p < 0.05). In other words, at the presence of distant metastasis the tumor tissue is characterized by high values of NO levels and superoxide generation rates but by the low activities of MMP-2. The nature of this MMP “down-regulation” is still not totally clear. We can suggest that in this case a disintegration of the signalling pathways involved in activation of MMP occurs.
Conclusion
In this work it is shown that the activity of MMP-2 and MMP-9 in tumor tissue correlate with the superoxide radicals generation rate and NO levels (r = 0.48÷0.67, p < 0.05). The activity of MMP-2 and -9 in tumor tissues and superoxide radical generation rates correlate positively with the stage of regional dissemination (r = 0.45 and 0.37, correspondingly, p < 0.05), but MMP-2 and MMP-9 activity inversely depends on distant metastatic degree of stomach cancer (r = 0.58; p < 0,05).
The tumor cells are characterized by the re-programmed mitochondrial metabolism, the high level of cells hypoxia, defective redox system and unbalanced concentrations of molecules that determine the aggressive phenotype of the tumor. It becomes apparent that the proliferation, migration and the tumor invasion correlate with the disease progress. Measuring the redox state of tumor tissue (among them the superoxide generation rate, NO level, activity of MMP-2 and MMP-9) can be used as a supplementary objective tool in diagnostics. Moreover, the effectiveness of the anticancer therapy can be additionally controlled in this way.
Acknowledgments
The work is performed in the framework of the cooperation agreement between R.E. Kavetsky Institute and Kazan Federal University according to the Russian Government Program of Competitive Growth of Kazan Federal University.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
Compliance with ethical standards
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Chissov VI, Starinsky VV, Petrova . Malignant neoplasms in Russia in 2011 (incidence and mortality) Moscow: Hertzen Research Institute of Oncology; 2013. [Google Scholar]
- 3.Fedorenko Z, Goulak LO, Gorokh YL, Ryshov AY, Soumkina OV, Koutsenko LB. Cancer in Ukraine 2013–2014: incidence, mortality, activities of oncological service. Bulletin of national cancer registry of Ukraine vol 16. Kyiv: National Institute of Cancer; 2015. [Google Scholar]
- 4.Sabesan A, Benett JJ. Diagnosis, staging, and workup of gastric cancer. In: Strong VE, editor. Gastric cancer. Switzerland: Springer; 2015. pp. 127–142. [Google Scholar]
- 5.Torti SV, Torti FM. Iron and cancer: more ore to be mined. Nat Rev Cancer. 2013;13:342–355. doi: 10.1038/nrc3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott RL, Head JF. Cancer: tumor iron metabolism, mitochondrial dysfunction and tumor immunosuppression; “a tight partnership—was Warburg correct?”. J Cancer Ther. 2012;3:278–311. doi: 10.4236/jct.2012.34039. [DOI] [Google Scholar]
- 7.Burlaka AP, Ganusevich II, Gafurov MR, Lukin SN, Sidorik EP. Electron paramagnetic resonance study of tumor affected bone marrow. Cancer Microenviron. 2013;6:273–276. doi: 10.1007/s12307-013-0137-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burlaka AP, Sidorik EP, Ganusevich II, Osinsky SP. Effects of radical oxygen species and NO: formation of intra-cellular hypoxia and activation of matrix metalloproteinases in tumor tissues. Exp Oncol. 2006;28:49–53. [PubMed] [Google Scholar]
- 9.Burlaka AP, Sidorik EP, Ganusevich II, Leschenko YM, Osinsky SP. High formation of superoxide anion and nitric oxide, and matrix metalloproteinases activity in vascular wall of rectal carcinoma vessels. Exp Oncol. 2006;28:323–325. [PubMed] [Google Scholar]
- 10.Burlaka AP, Sidorik EP. Radical forms of oxygen and nitrogen oxide in the tumors process. Kyiv: Naukova dumka; 2006. [Google Scholar]
- 11.Gauron C, Rampon C, Bouzaffour M, Ipendey E, Teilon J, Volovitch M, Vriz S. Sustained production of ROS triggers compensatory proliferation and is required for regeneration to proceed. Sci Rep. 2013;2084:123–133. doi: 10.1038/srep02084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Kalyanamaran B, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. PNAS. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimokawa H. Reactive oxygen species promote vascular smooth muscle cell proliferation. Circ Res. 2013;113:1040–1042. doi: 10.1161/CIRCRESAHA.113.302049. [DOI] [PubMed] [Google Scholar]
- 14.Maeda H. The link between infection and cancer: tumor vasculature, free radicals, and drug delivery to tumors via the EPR effect. Cancer Sci. 2013;104:779–789. doi: 10.1111/cas.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubben FJ, Sier CF, van Duijn W, Griffioen G, Hanemaaijer R, van de Velde CJ, et al. Matrix metalloproteinase–2 is a consistent prognostic factor in gastric cancer. Br J Cancer. 2006;94:1035–1040. doi: 10.1038/sj.bjc.6603041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peña S. Matrix metalloproteases as molecular markers in gastric cancer. Med Clin. 2010;134:123–126. doi: 10.1016/j.medcli.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 17.Wu CY, Wu MS, Chiang EP, et al. Plasma matrix metalloproteinase-9 level is better than serum matrix metalloproteinase-9 level to predict gastric cancer evolution. Clin Cancer Res. 2007;13:2054–2060. doi: 10.1158/1078-0432.CCR-06-2299. [DOI] [PubMed] [Google Scholar]
- 18.Chu D, Zhang Z, Li Y, et al. Matrix metalloproteinase-9 is associated with disease-free survival and overall survival in patients with gastric cancer. Int J Cancer. 2011;129:887–895. doi: 10.1002/ijc.25734. [DOI] [PubMed] [Google Scholar]
- 19.Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS. 2011;278:16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 20.Shen W, Xi H, Wei B, Chen L. The prognostic role of matrix metalloproteinase 2 in gastric cancer: a systematic review with meta-analysis. J Cancer Res Clin. 2014;140:1003–1009. doi: 10.1007/s00432-014-1630-6. [DOI] [PubMed] [Google Scholar]
- 21.Sawada T, Yashiro M, Sentani K, Oue N, Yasui W, Miyazaki K, et al. New molecular staging with G-factor supplements TNM classification in gastric cancer: a multicen-ter collaborative research by the Japan Society for Gastroenterological Carcinogenesis G-Project committee. Gastric Cancer. 2015;18:119–128. doi: 10.1007/s10120-014-0338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Joint Committee on Cancer . AJCC cancer staging manual. 7. New York: Springer; 2010. [Google Scholar]
- 23.Burlaka AP, Ganusevich II, Lukin SN, Gafurov MR, Sidorik EP. Superoxide-and NO-dependent mechanisms of the reprogramming of bone marrow cells by tumor cells. Appl Magn Reson. 2014;11:1261–1273. doi: 10.1007/s00723-014-0610-y. [DOI] [Google Scholar]
- 24.Burlaka A, Selyuk M, Gafurov M, Lukin S, Potaskalova V, Sidorik E. Changes in mitochondrial functioning with electromagnetic radiation of ultra high frequency as revealed by electron paramagnetic resonance methods. Int J Rad Biol. 2014;90:357–362. doi: 10.3109/09553002.2014.899448. [DOI] [PubMed] [Google Scholar]