Abstract
Background:
Candidiasis is one of the most prevalent and important opportunistic fungal infections of the oral cavity caused by Candida yeast species like Candida albicans, C. glabrata, and C. krusei. In addition, several bacteria can cause oral infections. The inhibition of microbial biofilm is the best way to prevent oral infections.
Objectives:
The aim of the present study is to evaluate the antifungal, antimicrobial, and anti-biofilm properties of ginger (Zingiber officinale) extract against Candida species and some bacterial pathogens and the extract’s effects on biofilm formation.
Materials and Methods:
Ginger ethanolic extract as a potential mouthwash was used to evaluate its effect against fungi and bacteria using the microdilution method, and biofilm was evaluated using the crystal violet staining method and dead/alive staining. MTT assay was used to evaluate the possible cytotoxicity effects of the extract.
Results:
The minimum inhibitory concentrations (MICs) of ginger extract for evaluated strains were 40, 40, 20, 20, 20, 20, 10, and 5 mg/mL for Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Bacillus cereus, Acinetobacter baumannii, C. albicans, and C. krusei, respectively. Ginger extract successfully inhibited biofilm formation by A. baumannii, B. cereus, C. krusei, and C. albicans. MTT assay revealed no significant reduction in cell viability after 24 hours. The minimum inhibitory biofilm concentrations (MIBCs) of ginger extract for fungi strains (C. krusei and C. albicans) were greater than those of fluconazole and nystatin (P = 0.000).
Conclusions:
The findings of the present study indicate that ginger extract has good antifungal and antibiofilm formation by fungi against C. albicans and C. Krusei. Concentrations between 0.625 mg/mL and 5 mg/mL had the highest antibiofilm and antifungal effects. Perhaps, the use of herbal extracts such as ginger represents a new era for antimicrobial therapy after developing antibiotic resistance in microbes.
Keywords: Biofilms, Antifungal, Antimicrobial, Candida albicans, C. krusei, Zingiber officinale
1. Background
Candidiasis is one of the most prevalent and important opportunistic fungal infections of the oral cavity caused by Candida yeast species like Candida albicans, C. glabrata, and C. krusei. Different forms of candidiasis, in acute and chronic forms, affect various parts of the body, such as the oral and genital mucosa, skin, bronchus, gastrointestinal tract, and lungs. These infections usually diffuse in immunosuppressant status and afflict the internal organs, such as the kidneys and liver (1-3).
Antifungal drugs, in various formulations, are being used topically (like nystatin and clotrimazole) and systemically (azoles and amphotericin B) for the treatment of candidiasis. However, in recent years, numerous studies have reported the failure of treatment in patients with different clinical types of candidiasis. The long-term consumption of antifungal agents caused adverse effects and drug resistance, thereby limiting the use of these therapeutics (4, 5).
The number of infections caused by Candida species is increasing, and these microorganisms now respond poorly to azole treatments, such as fluconazole, which is the best-in-class therapy for the treatment of fungal infections. This drug resistance leads to the increased prevalence of infection. Moreover, concomitant side effects of the common antifungal agents include nausea, vomiting, hepatic dysfunction, arrhythmias, and neuropathies, among others (1, 6). Therefore, recent researches are directed to find more effective antifungal agents with natural origins and fewer side effects. Among herbal extracts, the inhibitory effect of ginger (Zingiber officinale) extract on microorganisms has been well documented. Previous studies revealed the antimicrobial effects of ginger extract against Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli (7, 8). Furthermore, it has been demonstrated that ginger extract has potent antifungal properties against fluconazole-resistant C. albicans species isolated from patients with genital candidiasis (9). Indeed, in traditional medicine, ginger is administrated to cure movement inabilities, nausea, and vomiting during pregnancy. More importantly, apart from sedation and drowsiness, there is no report of any side effects for ginger (10, 11).
Multiple lines of evidence favor the antimicrobial, antifungal, and anti-biofilm effects of ginger extract against some bacterial and fungal species, particularly against C. albicans, and there are a few studies on other Candida species like C. glabrata and C. krusei, but none of them have evaluated this effect in mouthwash form.
2. Objectives
This study aimed to evaluate the antifungal, antimicrobial, and anti-biofilm property of a ginger extract prepared in mouthwash form against C. albicans, C. krusei, and some bacterial pathogens as well as its effect on their biofilm formation.
3. Materials and Methods
3.1. Study Design
Ginger ethanolic extract as a potential mouthwash was provided from commercially available ethanolic extract of ginger (Rojin Cosmetic Co, Tabriz, Iran) with an 80 mg/mL concentration. In a 96-well cell culture microplate, 100 µL of sabouraud dextrose medium (2X for fungi strains) (Merck KGaA, Darmstadt, Germany) and Muller hinton broth (2X for bacterial strains) (Merck KGaA, Darmstadt, Germany) were added to each well (a medium quantity was measured to reach the expected optimal concentration). Subsequently, 80 µL of ginger extract was added to the first well and later dilutions were prepared in subsequent wells. Then, 20 µL of a fungal or bacterial specimen (with 0.5 Mcfarland concentrations) was added to each well and after 18 - 24 hours of incubation at 37°C, wells were evaluated with respect to their turbidity. The well before the first turbid well was considered to have the minimum inhibitory concentration (MIC). This was performed in duplicate for all strains. This measurement for MICs and minimum bactericidal concentrations (MBCs) was done as recommended by the Clinical and Laboratory Standards Institute (CLSI) M27-A3 and CLSI M100-S22 (12-14). Different concentrations of ginger extract (80 mg/mL - 0.625 mg/mL) were used. MIC values were estimated using a visual and spectroscopic method by absorbance measurement at 620 nm (OD620 optical density reading at 620 nm) (15). Control tubes with the Muller hinton agar (Merck) (without ginger extract) were used as negative controls and 70% ethanol as positive controls. For the determination of MBCs, sterile swabs were used to inoculate concentrations higher than the MICs into the blood agar plate for 24 hours (16).
All investigated strains were obtained from the iranian persian type culture collection in iranian research organization for science and technology (IROST). Investigated strains were E. coli ATCC 25922, P. aeruginosa ATCC 27853, K. pneumoniae ATCC 700603, S.aureus ATCC 25923, A. baumannii ATCC 19606, B. cereus ATCC 11778, C. krusei DMS 70079, C. albicans ATCC 0231, and two oral isolates of C. albicans.
3.2. Biofilm Assays
Biofilm assays were conducted based on a previously described method (17). For each strain, a few colonies were suspended in physiological saline to 0.5 McFarland and Vortexes for 1 minute. 96 well polystyrene Microtiter plates (Greiner CELLSTAR® flat-bottomed sterile cell-culture Nr. 655180) were filled with 180 µL of trypticase soy broth (TSB) (Merck KGaA, Darmstadt, Germany) + 0.5% glucose (Merck KGaA, Darmstadt, Germany) and 20 µL of bacteria suspension was added to each well (with changing medium dilution final concentrations that were optimized). Four wells per strain were incubated and their mean of absorbance was considered the final absorbance. All plates were done in duplicate. Negative controls (Blank) were TSB + 0.5% glucose alone and were dispensed into 8 wells per tray. After stationary aerobic incubation for 24 hours at 37°C and 5% CO2, the broth was carefully drawn off and the wells were washed 3 times with 300 µL of sterile phosphate buffered saline (PBS, room temperature). Biofilms were fixed with 150 µL of methanol for 20 minutes, flicked, and air dried in an inverted position in the warm room (about 30 minutes). Biofilms were stained with 150 µL of crystal violet solution in water (2%) for 15 minutes at room temperature and the wells were rinsed by placing the plate under running tap water. Microtiter plates were inverted on a paper towel and air dried. To quantify biofilm production, 150 µL of 33% acetic acid was added to each well to destain the biofilms and lidded plates were placed at room temperature for 30 minutes without shaking. Thereafter, the optical density of the resolubilized crystal violet was measured at 570 nm (OD570) using a microtiter plate reader (Multiskan FC® Microplate Photometer, Thermo Scientific, Nr 89087-320). The cut-off optical density (OD) for biofilm formation by isolates was defined as the Optical density upper than OD570 = 0.524 was defined as the cut off optical density for biofilm formation (17).
3.3. Cell Cytotoxicity Effect
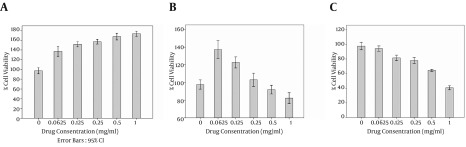
MTT assay is an essential method for evaluating the cytotoxicity of biomaterials in vitro condition. The cytotoxicity effects of ginger extract mouthwash in effective antifungal MICs was investigated by MTT assay on the human gingival fibroblasts cell line. MTT assaytest was evaluated in 24, 48 and 72 hours after adding the mouthwash to the media (Figure 1).
Figure 1. Cell Viability of the Human Gingival Fibroblasts Cell Line After MTT Assay With Ginger Extract After A, 24 hours; B, 48 hours; and C, 72 hours.
3.4. Statistical Analysis
Collected data were first reported using descriptive statistics. Kolmogorov-Smirnov analysis was used to evaluate the data’s normality. SPSS Version 22 (IBM SPSS Statistics, New York, USA) was used for statistical analysis. P values below 0.05 were considered statistically significant.
4. Results
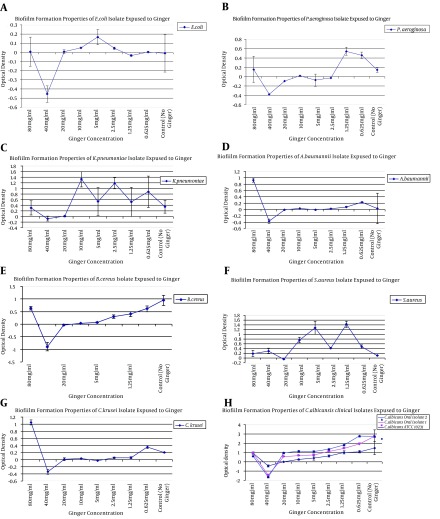
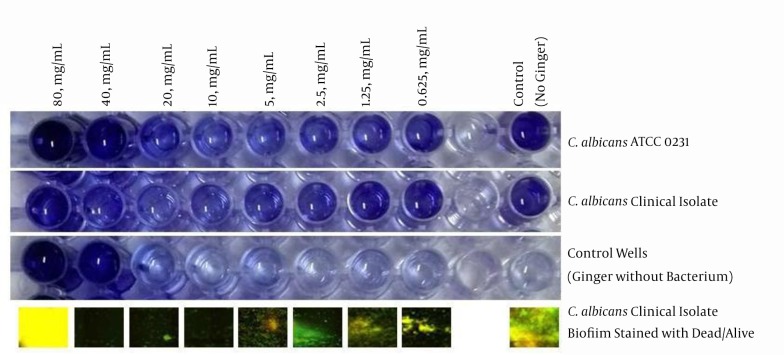
To investigate the effects of antibiotics against the study strains, the MICs for nystatin against C. albicans and C. krusei were 32 µg/mL and 16 µg/mL. The MICs for fluconazole against C. albicans and C. krusei were 16 µg/mL and 16 µg/mL, which suggested that these microorganisms respond poorly to fluconazole and nystatin. To remove the ginger extract background from the wells, a serial dilution without bacteria and fungi was used, and its results were minus from all the results to normalize the final results. The MIC for S. aureus, K. pneumoniae, B. cereus, and A. baumannii was 20 mg/mL and for P. aeruginosa and E. coli it was 40 mg/mL; however, for fungi isolates it was 5 mg/mL for C. krusei and 10 mg/mL for C. albicans. The lowest rates of the anti-biofilm effect of ginger extract were observed against K. pneumoniae, S. aureus, P. aeruginosa, and E. coli respectively, but ginger successfully inhibited the biofilm formation in A. baumannii, B. cereus, C. krusei, and C. albicans (Figure 2). In particular, C. albicans strains of the present study were strong biofilm formers, and ginger extract successfully reduced the biofilm formation from 3 OD to zero or -2OD (in comparison with no microbial load extract). There were fluctuations seen in high concentrations due to high concentrations of the extract; therefore, we used dead/alive staining. The dead/alive staining results indicated that with increasing ginger extract concentration, live cells in biofilm form reduced significantly (Figure 3). MTT assay at 24, 48, and 72 hours with a concentration range of 1 mg/mL revealed no significant reductions in cell viability after 24 hours.
Figure 2. Biofilm Formation by E. coli ATCC 25922.
A, P. aeroginosa ATCC 27853; B, K. pneumoniae ATCC 700603; C, A. baumannii ATCC 19606; D, B. cereus ATCC 11778; E, S.aureus ATCC 25923; F, C. krusei DMS 70079; G, C. albicans ATCC 0231, and two oral isolates of C. albicans; H, for ginger extract concentrations ranging from 80 mg/mL to 0.625 mg/mL.
Figure 3. Biofilm Formation Plates of C. albicans ATCC 0231 and Clinical Isolates and Wells Without Microbes and the Dead/Alive Staining of Wells After Biofilm Formation.
Kolmogorov-Smirnov analysis revealed non-parametric data distribution (P > 0.05). The Kruskal-Wallis test indicated that the means of MICs of the tested materials were statistically significant in the case of both fungal species (P = 0.000). The Mann Whitney U-test showed that all pair-wise comparisons were statistically significant (P = 0.000). In other words, the mean MIBC of ginger extract for fungi strains (C. krusei and C. albicans) was greater than fluconazole and nystatin.
5. Discussion
In this study, the antifungal and antimicrobial effects of ginger extract were respectively investigated against disease-causing bacteria and fungi and the effects were compared with two commonly used antifungal agents (fluconazole and nystatin). According to the results of the present study, ginger has a greater antifungal than antimicrobial effect and it had an acceptable anti-Candida effect against strains isolated from patients. In addition, a lower MIC (5 mg/mL) was found against C. krusei than C. albicans (10 mg/mL). Ginger extract had high antibiofilm effect against Candida strains compared to bacterial strains (Figure 2). Biofilm reduction against C. albicans started at a concentration of 0.625 mg/mL, and at 40 mg/mL there was no sign of biofilm formation. These results indicate that concentrations between 0.625mg/mL and 5mg/mL can be used successfully against Candida colonization in the oral cavity. Moreover, MTT assay showed no adverse effects of ginger extract after 24 hours; however, the mouthwash was used for less than a minute. Ginger extract also had powerful effect against bacterial agents, particularly P. aeruginosa and S. aureus. The antifungal effects in the present study were the same for all investigated fungi strains. In many species, ginger was shown to have antifungal properties. A previous study revealed the antifungal effects of the protein in ginger rhizome and its inhibitory effect on some fungi, such as Fusarium oxysporum (18). Taechowisan et al. (19) isolated a material called CMUAC 130 from ginger, which had an inhibitory effect on phytopathogenic fungi growth, like Fusarium. Nguefack et al. (20) indicated that ginger extract could prevent the proliferation of F. moniliforme, Aspergillus flavus, and A. fumigatus in vitro. Ficker et al. evaluated the antifungal properties of 36 herbal extracts on 13 human fungal pathogens and reported that among these extracts, ginger and jipijapa extracts had inhibitory effects on various fungal species. More importantly, it was revealed that ginger extract precluded the growth of fungi that were resistant to amphotericin B and ketoconazole (21, 22). Moreover, Agarwal et al. (23) depicted ginger extract’s inhibitory effect on Spilosoma insect species. Other researchers have evaluated the antifungal effects of ginger’s rhizome and assigned it as an effective extract on Aspergillus and phytopathogens (24). Mohammadi and Moatar (9) assessed ginger’s antifungal properties against clinical isolates of fluconazole-resistant C. albicans. Their findings indicated that ginger extract had an inhibitory effect on all tested species and they declared ginger as an effective agent on C. albicans in a laboratory setting. There have been no studies on the antifungal effect of ginger extract on non-albicans Candida species; our findings indicated a significant antifungal effect of ginger extract against Candida species other than C. albicans. In agreement with previously published studies, our findings also suggested that ginger’s antifungal properties are stronger than fluconazole and nystatin.
Although the antimicrobial effects of common antimicrobial agents on different microorganisms are compared to the standards deducted by CLSI M27-A3 and CLSI M100-S22, there are no reference standards for other materials, such as herbal extracts. Former studies have merely reported the antifungal properties of these extracts descriptively without comparing them with any references. Herein, we compared the antifungal effects of ginger extract with two commonly used antifungal agents, fluconazole and nystatin, and underscored that its antifungal activity is much greater than fluconazole and nystatin against C. albicans. Because P. aeruginosa and S. aureus have an important role in oral cavity infections, our findings indicate powerful effect of ginger extract against biofilm formation by these strains; however it did not have a significant protective effect against the biofilm formation of E. coli and K. pneumoniae. By using dead/alive staining we found that an increase in absorbance in 80 mg/mL was not related to biofilm formation, and there were no signs of live cells (Figure 3).
In conclusion, the present study’s findings indicate that ginger extract has good antifungal and antibiofilm formation by fungi against C. albicans and C. Krusei. Concentrations between 0.625 mg/mL and 5 mg/mL have the highest antibiofilm and antifungi effect, and it had no adverse effect on MTT assay, which indicates the safety of this extract for use as a mouthwash. In addition, ginger extract was effective against biofilm formation by P. aeruginosa and A. baumannii. Perhaps, the use of herbal extracts such as ginger represents a new era for antimicrobial therapy after developing antibiotic resistance in microbes. Further studies can indicate ginger’s protective effects against other fungi infections.
Acknowledgments
We would like thank all the staff of microbiology laboratory (drug applied research center) for their cooperation.
Footnotes
Authors’ Contribution:Marzieh Aghazadeh carried out the data collection and drafted the manuscript. Abed Zahedi Bialvaei participated in biofilm assay and bacteriological studies. Mohammad Aghazadeh participated in the design of the study. Fahimeh Kabiri participated in MTT assay and the dead/alive staining. Negar Saliani participated in MTT assay. Mehdi Yousefi participated in the design of the study. Hosein Eslami participated in the design of the study Hossein Samadi Kafil participated in the design of the study, antimicrobial study, and drafted the manuscript. All authors read and approved of the final manuscript.
Funding/Support:This study was granted by the drug applied research center, Tabriz University of Medical Sciences for a research project entitled evaluating antibiofilm and antifungal effects of ginger extract.
References
- 1.Berenguer J, Diaz-Guerra TM, Ruiz-Diez B, Bernaldo de Quiros JC, Rodriguez-Tudela JL, Martinez-Suarez JV. Genetic dissimilarity of two fluconazole-resistant Candida albicans strains causing meningitis and oral candidiasis in the same AIDS patient. J Clin Microbiol. 1996;34(6):1542–5. doi: 10.1128/jcm.34.6.1542-1545.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nenoff P, Suss K, Flemming C, Haustein UF. Differentiation of Candida strains by lectin-mediated agglutination kinetics. Mycoses. 2000;43(3-4):101–7. doi: 10.1046/j.1439-0507.2000.00554.x. [DOI] [PubMed] [Google Scholar]
- 3.Rasti S, Asadi MA, Taghriri A, Behrashi M, Mousavie G. Vaginal candidiasis complications on pregnant women. Jundishapur J Microbiol. 2014;7(2):e30167. doi: 10.5812/jjm.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White TC, Holleman S, Dy F, Mirels LF, Stevens DA. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob Agents Chemother. 2002;46(6):1704–13. doi: 10.1128/AAC.46.6.1704-1713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morschhauser J. The genetic basis of fluconazole resistance development in Candida albicans. Biochim Biophys Acta. 2002;1587(2-3):240–8. doi: 10.1016/s0925-4439(02)00087-x. [DOI] [PubMed] [Google Scholar]
- 6.Burket LW. Burket's oral medicine. United States: PMPH-USA; 2003. [Google Scholar]
- 7.Ogodo AC, Ekeleme UG. In-vitro antibacterial activity of garlic cloves and ginger rhizomes on food-borne pathogens. Int J Basic App Sci. 2013;2(4):387–92. [Google Scholar]
- 8.Azu NC, Onyeagba RA. Antimicrobial properties of extracts of Allium cepa (Onions) and Zingiber officinale (Ginger) on Escherichia coli, Salmonella typhi, and Bacillus subtilis. Int J Trop Med. 2007;3(2):8. [Google Scholar]
- 9.Mohammadi R, Moatar F. Antifungal activity of Zingiber officinale Rosc. essential oil against fluconazole resistant vaginal isolates of Candida albicans. J Med Plants. 2007;4(24):22–7. [Google Scholar]
- 10.White B. Ginger: an overview. Am Fam Physician. 2007;75(11):1689–91. [PubMed] [Google Scholar]
- 11.Riazipour M, Tavakoli HR, Fooladi AAI. Assessment of carboxyl esterase activity in clinical isolates of Candida albicans. Jundishapur J Microbiol. 2011;4(1):43–8. [Google Scholar]
- 12.Kafil HS, Mobarez AM, Moghadam MF. Adhesion and virulence factor properties of Enterococci isolated from clinical samples in Iran. Indian J Pathol Microbiol. 2013;56(3):238–42. doi: 10.4103/0377-4929.120375. [DOI] [PubMed] [Google Scholar]
- 13.Kafil HS, Asgharzadeh M. Vancomycin-resistant enteroccus faecium and enterococcus faecalis isolated from education hospital of iran. Maedica (Buchar). 2014;9(4):323–7. [PMC free article] [PubMed] [Google Scholar]
- 14.CLSI. Performance standards for antimicrobial susceptibility testing : 22nd informational supplement.; Clinical and Laboratory Standards Institute.; Wayne. 2014. [Google Scholar]
- 15.Koo H, Gomes BP, Rosalen PL, Ambrosano GM, Park YK, Cury JA. In vitro antimicrobial activity of propolis and Arnica montana against oral pathogens. Arch Oral Biol. 2000;45(2):141–8. doi: 10.1016/s0003-9969(99)00117-x. [DOI] [PubMed] [Google Scholar]
- 16.Dziedzic A, Kubina R, Wojtyczka RD, Kabala-Dzik A, Tanasiewicz M, Morawiec T. The antibacterial effect of ethanol extract of polish propolis on mutans streptococci and lactobacilli isolated from saliva. Evid Based Complement Alternat Med. 2013;2013:681891. doi: 10.1155/2013/681891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kafil HS, Mobarez AM. Assessment of biofilm formation by enterococci isolates from urinary tract infections with different virulence profiles. J King Saud Univ Sci. 2015;27(4):312–7. doi: 10.1016/j.jksus.2014.12.007. [DOI] [Google Scholar]
- 18.Wang H, Ng TB. An antifungal protein from ginger rhizomes. Biochem Biophys Res Commun. 2005;336(1):100–4. doi: 10.1016/j.bbrc.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 19.Taechowisan T, Lu C, Shen Y, Lumyong S. Secondary metabolites from endophytic Streptomyces aureofaciens CMUAc130 and their antifungal activity. Microbiology. 2005;151(Pt 5):1691–5. doi: 10.1099/mic.0.27758-0. [DOI] [PubMed] [Google Scholar]
- 20.Nguefack J, Leth V, Amvam Zollo PH, Mathur SB. Evaluation of five essential oils from aromatic plants of Cameroon for controlling food spoilage and mycotoxin producing fungi. Int J Food Microbiol. 2004;94(3):329–34. doi: 10.1016/j.ijfoodmicro.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Ficker CE, Arnason JT, Vindas PS, Alvarez LP, Akpagana K, Gbeassor M, et al. Inhibition of human pathogenic fungi by ethnobotanically selected plant extracts. Mycoses. 2003;46(1-2):29–37. doi: 10.1046/j.1439-0507.2003.00838.x. [DOI] [PubMed] [Google Scholar]
- 22.Nwosu CO, Djieyep NA. Candidiasis and trichomoniasis among pregnant women in a rural community in the semi-arid zone, north-eastern Nigeria. West Afr J Med. 2007;26(1):17–9. [PubMed] [Google Scholar]
- 23.Agarwal M, Walia S, Dhingra S, Khambay BP. Insect growth inhibition, antifeedant and antifungal activity of compounds isolated/derived from Zingiber officinale Roscoe (ginger) rhizomes. Pest Manag Sci. 2001;57(3):289–300. doi: 10.1002/ps.263. [DOI] [PubMed] [Google Scholar]
- 24.Endo K, Kanno E, Oshima Y. Structures of antifungal diarylheptenones, gingerenones A, B, C and isogingerenone B, isolated from the rhizomes of Zingiber officinale. Phytochemistry. 1990;29(3):797–9. [Google Scholar]