Abstract
Acceptance and Commitment Therapy (ACT) has been effectively utilized to treat both chronic pain and substance use disorder independently. Given these results and the vital need to treat the comorbidity of the two disorders, a pilot ACT treatment was implemented in individuals with comorbid chronic pain and opioid addiction. This pilot study supported using neurophysiology to characterize treatment effects and revealed that, following ACT, participants with this comorbidity exhibited reductions in brain activation due to painful stimulus and in connectivity at rest.
Keywords: Chronic pain, opioid addiction, Acceptance and Commitment Therapy
1. Introduction
Finding a treatment for the relatively high comorbidity of chronic pain and opioid addiction (OA) is a complex but necessary endeavor (Clark et al., 2008; Højsted and Sjøgren, 2007; Noble et al., 2010; Potter et al., 2008). Acceptance and Commitment Therapy (ACT) has effectively treated patients with either chronic pain or addiction but has not been used to treat their comorbidity (Jensen et al., 2012; Luoma et al., 2012; McCracken and Gutiérrez-Martínez, 2011). Chronic pain and addiction separately affect brain structure, function, and regional connectivity (Lee et al., 2005; Napadow et al., 2010; Smallwood et al., 2013; Upadhyay et al., 2010), and neurophysiological changes can occur in patients across treatments (Napadow et al., 2012). We report the first study to assess ACT in pain and addiction comorbidity using fMRI to evaluate neurophysiological alterations across the treatment. We hypothesize that there will be differences in pain-related activation and in resting state (RS) connectivity between pain regions and the default mode network (DMN) in the ACT group post-treatment compared with pre-treatment and with a control group. With this pilot, we aim to provide proof of concept that ACT induces neural changes in patients with comorbid chronic low back pain (CLBP) and OA.
2. Methods
Twenty-five patients with CLBP persisting >12 months who were enrolled in opioid replacement therapy for OA and met DSM-IV criteria for opioid dependence were randomized into one of two groups for 8 sessions over 4 weeks. Six patients completed the chronic pain-focused ACT group (4 males; mean±SD age: 43.5±11.5 years) and six the health education control (HEC) group (3 males; 49.7±7.1 years). Brain MRI data were obtained at rest and during painful stimulation; pain was delivered via pressure on the thumbnail in eight 5-second triplets at a pressure that subjects rated as 40/100 on a pain scale. The following behavioral variables were collected: Acceptance and Action Questionnaire-II (AAQ-II; Bond et al., 2011) measuring psychological flexibility, Mindfulness Attention Awareness Scale (MAAS; Brown and Ryan, 2003) measuring mindfulness, Roland Morris Disability Questionnaire (RMDQ; Roland and Morris, 1983) measuring disability due to back pain, and three 10-point scale numerical ratings (0–9) – pain intensity, pain interference, and opioid craving. SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) in MATLAB was used to preprocess and analyze fMRI data.
Seed regions of interest (ROIs) for RSfMRI were selected based on published literature to form two networks: regions involved in pain processing and DMN (Watanabe et al., 2013). The Pearson’s correlation coefficients between all ROI time series were transformed into Fisher’s Z for comparison. The following ROIs were seeded as 5mm-radius spheres: insula (Ins), posterior cingulate cortex (PCC), primary somatosensory (S1), secondary somatosensory (S2), amygdala (Amyg), thalamus (Thal), anterior cingulate cortex (ACC), prefrontal cortex (PFC), superior frontal gyrus (SFG), inferior temporal gyrus (ITG), parahippocampal gyrus (Para), and lateral parietal (lat Par). Further localization is specified with right and left (R and L) hemispheric designation, and posterior (p), superior (s/sup), inferior (i/inf), anterior (a), middle (m), anteromedial (am), and ventromedial (vm).
3. Results
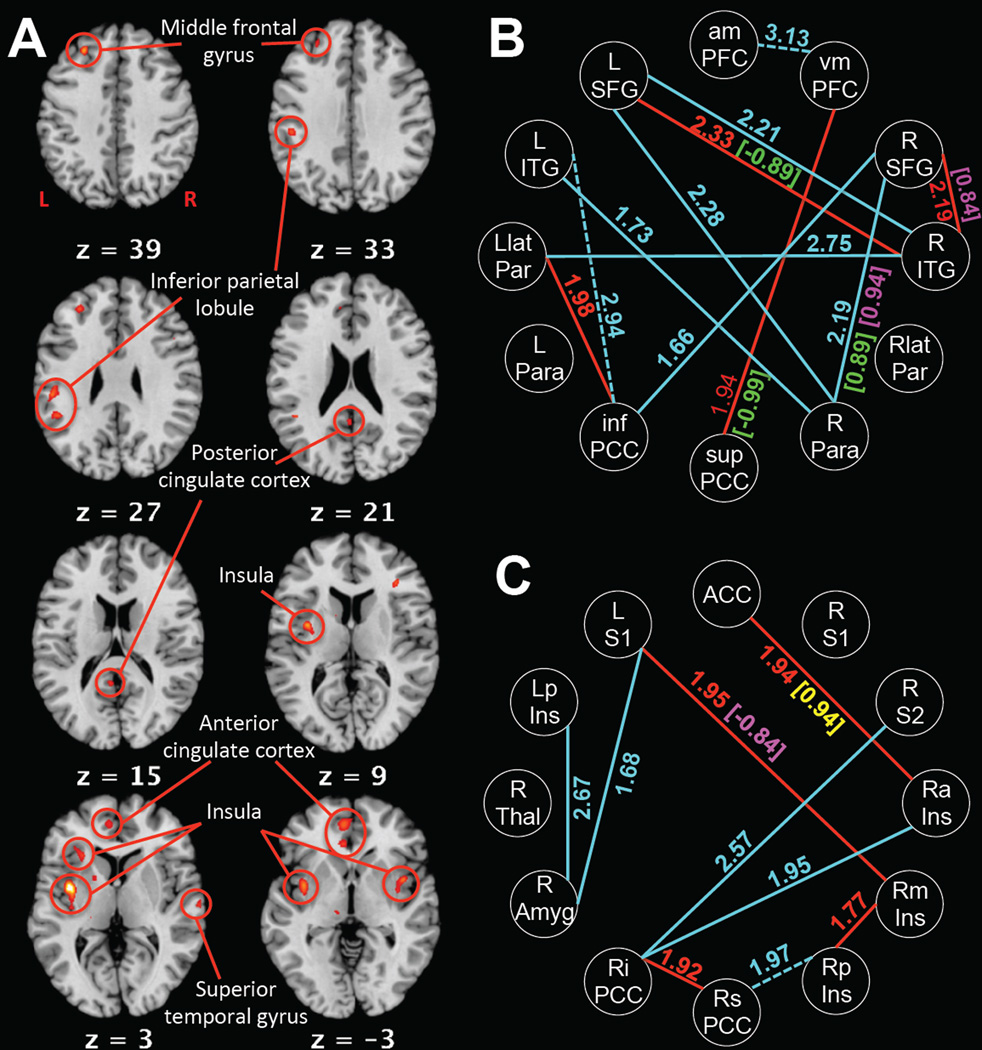
Following treatment, ACT completers exhibited decreased activation during pain compared with HEC completers in middle frontal gyrus, inferior parietal lobule, insula, ACC, PCC, and superior temporal gyrus (Figure 1A, p<0.001 uncorrected). RS connectivity showed alterations in the ACT group from pre-treatment to post-treatment and in the ACT group post-treatment compared with the HEC group post-treatment (Figure 1B&C, p<0.05 uncorrected). The ACT group post-treatment had decreased connectivity compared with pre-treatment and with the HEC group post-treatment, except in three connections that were higher post-treatment than pre-treatment: LITG–infPCC, amPFC–vmPFC, and RsPCC–RpIns.
Figure 1.
(A) Regions with greater activation during pain in the HEC group post-treatment than the ACT group post-treatment (p<0.001 uncorrected). Z slice coordinate denoted in MNI coordinates. Laterality specified by R and L on first slice. (B) Default mode network connections during rest compared across the treatment in the ACT group (solid cyan = decreased connectivity post-treatment; dashed cyan = increased connectivity post-treatment) and between the HEC and ACT groups post-treatment (solid red = decreased connectivity in ACT compared with HEC). (C) Pain region connections during rest compared across the treatment in the ACT group (solid cyan = decreased connectivity post-treatment; dashed cyan = increased connectivity post-treatment) and between the HEC and ACT groups post-treatment (solid red = decreased connectivity in ACT compared with HEC). Numbers indicate Fisher’s Z (p<0.05 uncorrected). Numbers in brackets indicate Pearson’s R for significant (p<0.05 uncorrected) correlations in the ACT group post-treatment at rest with behavioral variables: green = pain intensity; magenta = pain interference; yellow = AAQ-II.
4. Discussion
Reduced activation in regions commonly involved in pain processing indicates that ACT may decrease the brain’s responsiveness to painful stimuli in patients with comorbid CLBP and OA. Jensen et al. (2012) found the opposite effect following ACT treatment of chronic pain, with activity increases. However, their diagnosis was fibromyalgia; there are likely distinct pain processing patterns between the two disorders, and addiction comorbidity and daily methadone use further differentiate the groups.
Overwhelmingly, the ACT group had decreased connectivity at rest post-treatment compared with pre-treatment and with the HEC group. They also had more differing connections and stronger differences in those connections in the DMN than in the pain network. This suggests ACT may target DMN function at rest more than connectivity between pain-related regions.
Mindfulness-based approaches to treatment are gaining popularity and are effective at treating a variety of psychological conditions (Khoury et al., 2013). This pilot study is important because it is the first to demonstrate neurophysiological effects following the mindfulness-based treatment ACT in patients with CLBP and OA comorbidity. The primary limitation of this study is the small sample size; because of the low N, the most conservative corrected statistical thresholds could not be used and generalizations are difficult to draw. Yet, our data suggest ACT can successfully alter neurophysiology in patients with comorbid CLBP and OA. Critically, we argue that fMRI can be a powerful tool in determining the neural mechanisms of action of ACT. ACT treatment is promising for improving patient quality of life and understanding how that increase is reflected through changes in brain activity and connectivity in a variety of patient groups.
Highlights.
We implement Acceptance and Commitment Therapy in opioid addicts with chronic pain.
We use functional MRI before and after treatment to investigate neural changes.
Decreases in brain activation during pain induction post-treatment were observed.
Connections between brain regions at rest decreased across treatment.
Acknowledgments
This study was supported by NIH NIDA grant K23DA022297-04 (Potter). The authors thank Harvir Virk for his contributions.
Abbreviations
- ACT
Acceptance and Commitment Therapy
- OA
opioid addiction
- RS
resting state
- DMN
default mode network
- CLBP
chronic low back pain
- HEC
Health education control
- AAQ-II
Acceptance and Action Questionnaire II
- MAAS
Mindfulness Attention and Awareness Scale
- RMDQ
Roland Morris Disability Questionnaire
- Ins
insula
- PCC
posterior cingulate cortex
- S1
primary somatosensory cortex
- S2
secondary somatosensory cortex
- Amyg
amygdala
- Thal
thalamus
- ACC
anterior cingulate cortex
- PFC
prefrontal cortex
- SFG
superior frontal gyrus
- ITG
inferior temporal gyrus
- Para
parahippocampal gyrus
- lat Par
lateral parietal
- sup
superior
- inf
inferior
- am
anteromedial
- vm
ventromedial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ballantyne JC, LaForge KS. Opioid dependence and addiction during opioid treatment of chronic pain. Pain. 2007;129(3):235–255. doi: 10.1016/j.pain.2007.03.028. [DOI] [PubMed] [Google Scholar]
- Bond FW, Hayes SC, Baer RA, Carpenter KM, Guenole N, Orcutt HK, Waltz T, Zettle RD. Preliminary psychometric properties of the Acceptance and Action Questionniare - II: A revised measure of psychological flexibility and experiential avoidance. Behav. Ther. 2011;42:676–688. doi: 10.1016/j.beth.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Brown KW, Ryan RM. The benefits of being present: Mindfulness and its role in psychological well-being. J. Pers. Soc. Psychol. 2003;84:822–848. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- Clark MR, Stoller KB, Brooner RK. Assessment and Management of Chronic Pain in Individuals Seeking Treatment for Opioid Dependence Disorder. Ottawa, ON, Canada: Canadian Psychiatric Association; 2008. [DOI] [PubMed] [Google Scholar]
- Højsted J, Sjøgren P. Addiction to opioids in chronic pain patients: A literature review. Eur. J. Pain. 2007;11:490–518. doi: 10.1016/j.ejpain.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Jensen KB, Kosek E, Wicksell R, Kemani M, Olsson G, Merle JV, Kadetoff D, Ingvar M. Cognitive Behavioral Therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgia. Pain. 2012;153:1495–1503. doi: 10.1016/j.pain.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Khoury B, Lecomte T, Fortin G, Masse M, Therien P, Bouchard V, Chapleau MA, Paquin K, Hofmann SG. Mindfulness-based therapy: A comprehensive meta-analysis. Clin. Psychol. Rev. 2013;33(6):763–771. doi: 10.1016/j.cpr.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Lee T, Zhou W, Luo X, Yuen KSL, Ruan X, Weng X. Neural activity associated with cognitive regulation in heroin users: a fMRI study. Neurosci. Lett. 2005;382(3):211–216. doi: 10.1016/j.neulet.2005.03.053. [DOI] [PubMed] [Google Scholar]
- Luoma JB, Kohlenberg BS, Hayes SC, Fletcher L. Slow and steady wins the race: A randomized clinical trial of acceptance and commitment therapy targeting shame in substance use disorders. J. Consult. Clin. Psychol. 2012;80(1):43. doi: 10.1037/a0026070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken LM, Gutiérrez-Martínez O. Processes of change in psychological flexibility in an interdisciplinary group-based treatment for chronic pain based on Acceptance and Commitment Therapy. Behav. Res. Ther. 2011;49(4):267–274. doi: 10.1016/j.brat.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62(8):2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, Kim J, Clauw DJ, Harris RE. Decreased Intrinsic Brain Connectivity is Associated with Reduced Clinical Pain in Fibromyalgia. Arthritis Rheum. 2012;64(7):2398–2403. doi: 10.1002/art.34412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble M, Treadwell JR, Tregear SJ, Coates VH, Wiffen PJ, Akafomo C, Schoelles KM. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst. Rev. 2010;1(1) doi: 10.1002/14651858.CD006605.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter JS, Prather K, Weiss RD. Physical Pain and Associated Clinical Characteristics in Treatment-Seeking Patients in Four Substance Use Disorder Treatment Modalities. Am. J. Addict. 2008;17(2):121–125. doi: 10.1080/10550490701862902. [DOI] [PubMed] [Google Scholar]
- Roland MO, Morris RW. A study of the natural history of back pain. Part 1: Development of a reliable and sensitive measure of disability in low back pain. Spine. 1983;8:141–144. doi: 10.1097/00007632-198303000-00004. [DOI] [PubMed] [Google Scholar]
- Smallwood RF, Laird AR, Ramage AE, Parkinson AL, Lewis J, Clauw DJ, Williams DA, Schmidt-Wilcke T, Farrell MJ, Eickhoff SB, Robin DA. Structural Brain Anomalies and Chronic Pain: A Quantitative Meta-Analysis of Gray Matter Volume. J. Pain. 2013;14(7):663–675. doi: 10.1016/j.jpain.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, Wallin D, Pendse G, McDonald L, Griffin M, Anderson J, Nutile L, Renshaw P, Weiss R, Becerra L, Borsook D. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133(7):2098–2114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Hirose S, Wada H, Imai Y, Machida T, Shirouzu I, Konishi S, Miyashita Y, Masuda N. A pairwise maximum entropy model accurately describes resting-state human brain networks. Nat. Commun. 2013;4(1370):1–10. doi: 10.1038/ncomms2388. [DOI] [PMC free article] [PubMed] [Google Scholar]