Abstract
The goal of this study was to develop appropriate methodology to apply drugs quantitatively to perilymph of the ear. Intratympanic applications of drugs to the inner ear often result in variable drug levels in perilymph and can only be used for molecules that readily permeate the round window (RW) membrane. Direct intracochlear and intralabyrinthine application procedures for drugs, genes or cell-based therapies by-pass the tight boundaries at the round window, oval window, otic capsule and the blood-labyrinth barrier. However, perforations can release inner ear pressure, allowing cerebrospinal fluid to enter through the cochlear aqueduct, displacing the injected drug solution into the middle ear. Two markers, fluorescein or fluorescein isothiocyanate (FITC)-labeled dextran, were used to quantify how much of an injected substance was retained in cochlear perilymph following an intracochlear injection. We evaluated whether procedures to mitigate fluid leaks improved marker retention in perilymph. Almost all procedures to reduce volume efflux, including the use of gel for internal sealing and glue for external sealing of the injection site, resulted in improved retention of the marker in perilymph. Adhesive on the RW membrane effectively prevented leaks but also influenced fluid exchange between CSF and perilymph. We conclude that drugs can be delivered to the ear in a consistent, quantitative manner using intracochlear injections if care is taken to control the fluid leaks that result from cochlear perforation.
Keywords: intracochlear, inner ear drug delivery, perilymph pressure, round window
Introduction
Many therapies for the inner ear are being developed that require the application of drugs (sometimes in the form of nanoparticles), gene therapy agents or stem cells to the ear [Staecker and Rogers, 2013]. Although numerous drugs and viral vectors have been successfully applied intratympanically [Jero et al., 2001; Bowe & Jacob 2010], many substances do not readily penetrate into perilymph at the RW membrane or at other sites. Perilymph levels of drugs and markers measured after intratympanic applications of a few hours duration were highly variable even with well-controlled middle ear levels [Mynatt et al., 2006, Plontke et al., 2007, Plontke et al., 2008]. Perilymph drug levels appear to become less variable when drug is delivered for more prolonged periods [Wang et al., 2009]. However, concentration levels in the basal part of scala tympani (ST) typically reach less than 3% of the applied concentration (summarized in [Hahn et al., 2012]). More consistent and efficient delivery would be expected when the solution was directly injected into perilymph. With applications by intracochlear injection into the cochlear apex [Salt et al. 2006] or by injections through the RW membrane [Salt et al. 2007, Hahn et al. 2012], concentrations in perilymph were found to be higher, with lower variation and the substances were distributed more uniformly along scala tympani. Effective deliveries of viral vectors have been accomplished with single intracochlear injection procedures [Stover et al 1999; Kawamoto et al, 2001; Staecker et al., 2014]. Nevertheless, previous studies have suggested that the efficiency of delivery with injections though the RW membrane will be influenced by fluid leakage at the injection site [Salt et al., 2007].
Injections through the round window in guinea pigs do not damage hearing [Kho et al, 2000]. However, a side-effect of inner ear perforation in guinea pigs, mice and other rodents is the resulting efflux of fluid at the perforation site, driven by CSF entering at the base of scala tympani through the cochlear aqueduct. In guinea pigs the rate of efflux from a perforated cochlea is 0.5 – 1 μL/min [Moscovitch et al., 1973; Salt and Stopp 1979; Ohyama et al., 1988]. Perilymph efflux from the perforated ear occurs at a similar rate in mice [Hirose et al, 2014]. If the volume flow displacing perilymph is not controlled, a substantial proportion of the injected solution will be displaced into the middle ear. In earlier studies, the RW niche was filled with hyaluronate gel before injections in an attempt to limit fluid efflux [Salt et al., 2007, Hahn et al., 2012]. The influence of fluid leakage on perilymph drug levels following injection is likely to be highly dependent on the delivery methods used. Many reports of drug delivery to the cochlea as single direct perilymphatic injections do not report their injection procedures in detail. Of those that have given details, injections into perilymph have been performed with hand-held 30G – 36G needles [Stover et al. 1999; Praetorius et al., 2007] or glass pipettes [Sellick et al., 2008]. In the present study, injections through the RW membrane were performed as rigorously as possible with an electronically controlled micropump, mounted on a micromanipulator and using sharp glass pipettes to minimize the size of the perforation and associated fluid leakage. Even with a high degree of care, the influence of fluid leaks remained. Procedures to control those leaks were therefore evaluated.
Materials and Methods
Animal Preparation
This study used 40 pigmented, NIH-strain guinea pigs of both sexes weighing 400 – 600 g and bred in our own colony. Experimental protocols were in accordance with policies of the United States Department of Agriculture, the National Institutes of Health guidelines for the handling and use of laboratory animals, and were approved by the Animal Care Committee of Washington University under protocol numbers 20100135 and 20130069.
Animals were initially anesthetized with sodium thiobutabarbital (Inactin, Sigma, St Louis, MO) 100 mg/kg and then maintained on isofluorane (0.8 to 1.2 %) in oxygen using a tracheal cannula and mechanical ventilation, adjusted to maintain a 5 % end-tidal CO2 level measured with a CapnoTrue AMP (Bluepoint Medical, The Netherlands). A pulse-oximeter (Surgivet, Waukesha, WI) was used to monitor heart rate and blood oxygen saturation and a DC- powered thermistor-controlled heating blanket was used to maintain body temperature at 38°C. The head of the animal was held rigidly using ear and bite bars. The auditory bulla was exposed by a ventral approach and opened widely to expose the cochlea. The cochlear apex was first prepared for sequential perilymph sampling, as described in detail [Salt et al. 2006]. Marker solution containing sodium fluorescein or FITC-labeled dextran was injected into perilymph through the RW membrane, either with or without procedures to limit fluid leakage at the injection site. After a delay of 40 min, scala tympani perilymph was collected from the apex using a sequential sampling technique. Only one injection and sampling procedure was performed in each animal.
Solutions
The injected solution consisted of artificial perilymph containing (in mM) NaCl (125), KCl (3.5), NaHCO3 (25), CaCl2 (1.3), MgCl2 (1.2), NaH2PO4 (0.75) and Dextrose (5) to which fluorescent marker was added. Initial experiments used 20 mM sodium fluorescein but the high sensitivity of measurement assays allowed the use of 1 mM sodium fluorescein for most of the experiments. In 8 experiments FITC-labeled Dextran (FD4, Sigma, St Louis, FW 3000 – 5000) was used as the marker, added to artificial perilymph at a concentration of 2.5 mM.
Injections into Scala Tympani
Solutions were injected through the RW membrane either manually (n=6) or with a digitally controlled pump (n=34).. For manual injections a 10 μL Hamilton gas-tight syringe fitted with a 30 G stainless steel hypodermic needle (320 μm outer diameter) was either hand-held or held in a manipulator. The tip of the needle was bent at a 90° angle approximately 2 mm from the tip to allow it to enter scala tympani from the RW niche. A 5 μL volume of fluorescein solution was injected over a 20 s period. This was the method described by Stover et al. [1999].
For pumped injections, a 100 μL gas-tight syringe (1710TLL Hamilton) was mounted on the pump (Ultrapump, World Precision Instruments, Sarasota, FL) which was held in a micromanipulator. The syringe was coupled to a 1 mm diameter glass injection pipette with a plexiglass coupler (MPH6S10, World Precision Instruments, Sarasota, FL). The glass injection pipette was pulled in an electrode puller and then beveled at a 60° angle (Narishige EG-40 beveler), to a tip diameter of 10 – 25 μm. Due to the taper of the pipette, RW perforation was estimated to be 20 – 40 μm diameter, depending on the insertion depth. Injections were performed at a rate of 0.4 μL/min for 7.5 min, injecting a total of 3 μL into ST, which is approximately half the volume of the guinea pig ST [Thorne et al., 1999].
In addition to pumped injections with no attention to sealing (“No Remedy”, n=5), a number of different methods were used to control fluid leakages at the injection site, as shown schematically in Figure 1. One of two gels, 1% sodium hyaluronate (Healon®, Abbott Medical Optics, Santa Ana, CA, n=3), or 17% poloxamer 407 (Spectrum Chemicals, Gardena, CA, n=3) were used as “internal sealing” agents when used as the background medium for the injection solution. “External sealing” was provided by a medical grade biologically-compatible cyanoacrylate adhesive, Tissumend® (Veterinary Products Laboratories, Phoenix, AZ, n=4) which is approved for use in animals. Either before or after the injection through the RW membrane, fluid was wicked away and ~ 1 μL of adhesive was applied to fill the bony annulus of the RW. In 4 additional experiments Healon and Tissumend were combined.
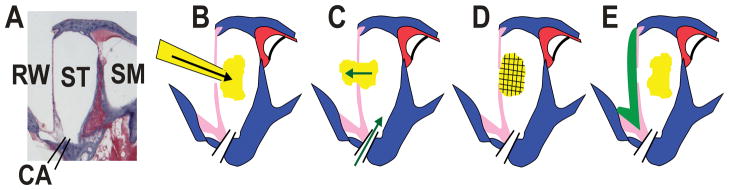
Figure 1.
Summary of injection, leaks and sealing procedures. A: Section of the basal turn of scala tympani from a guinea pig with fluid compartments labeled. Abbreviations are RW: round window; ST: scala tympani; SM: scala media; CA: cochlear aqueduct. B: Schematic showing injection through the RW membrane. The size of the injection pipette has been grossly exaggerated for clarity. C: Injected solution is displaced through the RW membrane perforation made by the injection pipette, driven by CSF entering through the cochlear aqueduct (green arrows). D: Internal sealing may be provided if the marker is injected as a viscous gel, which plugs the perforation site preventing fluid efflux. E: External sealing is achieved by applying adhesive (green) to the exterior surface of the RW membrane.
In 15 experiments, an identical injection protocol was used with the delivery pipette inserted into ST through a fenestration of the bony wall. The injection pipette was sealed into the bone with cyanacrylate and silicone adhesives [Salt et al., 2006]. This method prevented all fluid leakage at the injection site.
Sequential Sampling Of Perilymph From The Cochlear Apex
Before injection, the cochlear apex was prepared for perilymph sampling as detailed elsewhere (Mynatt et al, 2006). The mucosa was removed and the bone was allowed to dry. A layer of thin cyanoacrylate (Permabond 101, Permabond LLC, Pottstown, PA) was applied followed by layers of two-part silicone adhesive (Kwik-Cast, World Precision Instruments, Sarasota, FL). The silicone was applied thinly on the apical bone but multiple layers were built up at the edges to form a hydrophobic cup. At the time of sampling, a 30° House stapes pick (N1705 80, Bausch and Lomb Inc.) was used to perforate the adhesives and bone at the apex. The emerging fluid accumulated in the silicone cup, isolated from the mucosa of the bulla. Fluid was collected in hand-held, blunt tipped capillary tubes (VWR 53432-706) marked for a nominal 1 μL volume. Each 1 μL sample took 40 s to 60 s to collect. The volume of each sample was determined by length measurement under a calibrated dissecting microscope. Ten samples were collected and each analyzed separately. As the ST perilymph volume is approximately 5 μL in the guinea pig, the total fluid volume collected (10 μL) substantially exceeded ST volume, with later samples containing CSF that has passed through ST [Mynatt et al. 2006].
Sample handling and analysis
Perilymph samples and three samples of the injected solution were each diluted in 150 μL of artificial perilymph. Reference standards were made by performing 5 serial 10 X dilutions of the injected solution in artificial perilymph. Diluted fluid samples and equal volumes of standards were transferred to a 96 well plate and read in a Synergy HT plate reader (Bio Tek, Winooski, VT, USA). The Solver capability of Microsoft Excel was used to fit a sigmoid (Hill) function to the fluorescence vs concentration data from the standards and subsequently used to convert brightness readings to concentration. Perilymph concentrations were calculated using individual sample and dilutent volumes. Sample concentration data were normalized with respect to the injection concentration of the experiment.
One useful metric was to calculate how much of the injected marker was recovered in all of the samples collected in each experiment. The amount of marker (concentration x volume) was summed across the 10 samples and expressed as a percentage of the total marker injected. Fluid leakage to the middle ear at the RW membrane perforation results in marker loss. Loss also occurs by other processes, some driven into CSF during the injection, some diffusing into adjacent fluid and tissue compartments, and some lost by elimination to blood. Statistical differences between experimental groups were established by one-way ANOVA using Sigmaplot v13 (Systat Software Inc., San Jose, CA).
Results
Injections through the Round Window Membrane
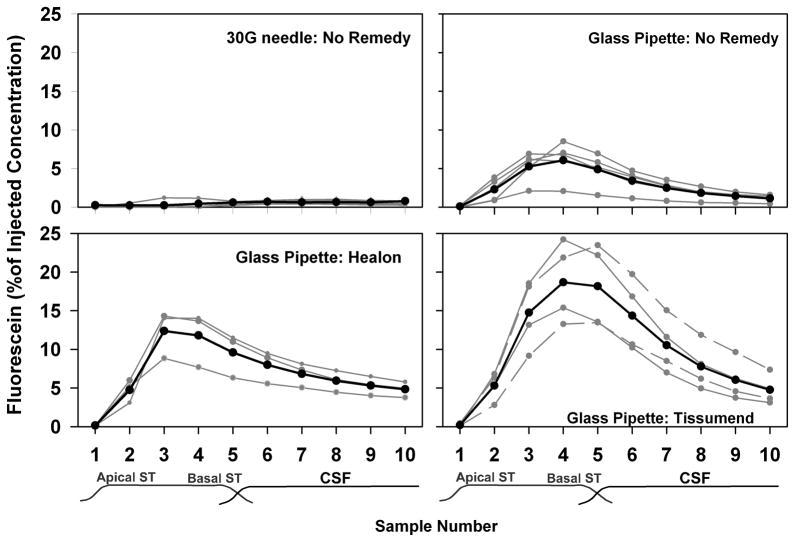
The fluorescein concentration of samples taken from the apex following fluorescein injections through the RW membrane are summarized for 4 of the experimental conditions in Figure 2. For manual injections with a 30G needle, measured perilymph fluorescein levels were extremely low. For hand-held injections, the mean highest sample concentration was 0.92 % of the injected concentration (SD 0.28, n=3). Very similar results were found with manipulator-held injections from the 30G needle (mean 0.84, SD 0.31, n=3). These data were not significantly different for hand-held and manipulator-held conditions so the data were pooled in the figure. With injections from the much smaller glass pipette, perilymph fluorescein levels were substantially higher than with the 30G needle, even when there was no control of fluid leakage (“Glass pipette: No Remedy” condition). The average highest concentration reached 6.1% of the injected concentration (SD 2.41, n=5). The low initial sample (representing perilymph originating in apical regions) and highest concentration in samples 3 or 4 (representing perilymph originating in the basal turn of ST) confirm the basal-apical concentration gradient that results from the injection of marker into the basal turn of ST through the RW membrane. When fluorescein was injected in a background of Healon gel, higher concentrations were found in the samples, with a mean peak concentration of 12.4% of the injected concentration (SD 3.1, n=3) in sample 3. Similarly, when the injection location in the RW membrane was sealed with Tissumend, sample concentrations were higher with the peak concentration averaging 19.1 % of the injected concentration (SD 5.5, n=4). ANOVA testing (all pairwise, Student-Newman-Keuls) based on the peak sample concentrations of the measured curves showed all four groups to be significantly different from each other (30G vs Glass pipette:No Remedy p=0.014; Tissumend vs No Remedy p<0.001; Tissumend vs. Healon p=0.013; Healon vs No Remedy p=0.015).
Figure 2.
Measured fluorescein concentrations of fluid samples taken from the cochlear apex 40 mins after injection of fluorescein under four delivery conditions. Gray lines indicate results from individual experiments and black lines show the mean for the group. Higher sample concentrations occur when less fluorescein is lost by fluid leaks. Samples taken after injection through a 30G hypodermic needle (“30G needle: No Remedy”; n=6) contained extremely low fluorescein levels. Injections through a 10–25 μm glass pipette (“Glass pipette: No remedy”; n=5) showed higher fluorescein content, with highest concentration in samples 3 or 4, corresponding to those originating from the basal region of scala tympani (ST). When fluorescein was injected in a healon gel matrix (“Glass pipette: Healon”; n=3), sample concentrations in some experiments were higher than with “No Remedy”. When Tissumend was applied to the round window membrane (“Glass pipette: Tissumend”, n=4) either after pipette insertion but before injection (solid lines) or after injection and pipette withdrawal (dotted lines), sample curves were even higher.
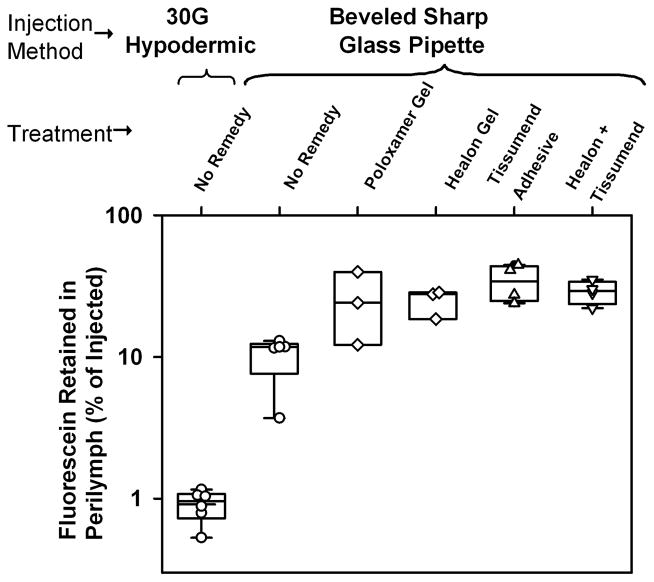
A measure of retention of fluorescein in perilymph was calculated by summing the solute from all 10 samples collected in each experiment and expressing it relative to the total amount injected. The degree of fluorescein retention for the different experimental procedures is summarized in Figure 3. Recovery of fluorescein was extremely low following injection from a syringe with a 30G needle. This procedure generated a perforation of the RW membrane that was clearly visible with the operating microscope and was associated with rapid accumulation of fluid in the RW niche. In contrast, perforations made with the glass injection pipette were not visible with the operating microscope and were only apparent by fluid accumulation on the RW membrane. The difference in fluorescein recovery between injections with a 30G needle (mean 0.92 %, n=6) and injections made with a glass microelectrode (mean 10.3%, n=5) were highly significant (two-tailed t-test, p<0.001)
Figure 3.
Fluorescein retained in perilymph (calculated from the total fluorescein summed across all samples from each animal, relative to the total amount injected) compared for 6 delivery conditions, including the 4 shown in Figure 2. Symbols show individual experiments and box plots show median, 25th and 75th percentile. After injection through a 30G hypodermic needle, only 0.9 % of the fluorescein was retained in perilymph. Injections from a glass pipette with no sealing procedures (No Remedy) produced over 10 times higher recovery. Both internal (gels) and external (adhesive) sealing procedures resulted in significantly greater retention of fluorescein in perilymph relative to the No Remedy condition.
Our results show that gels used as the injection matrix for “internal sealing” or adhesive applied to the RW membrane for “external sealing” both improved the amount of fluorescein recovered in the samples. This finding is consistent with all the treatments reducing loss to the middle ear from fluid leakage. The treatment providing best retention of fluorescein in perilymph was Tissumend adhesive, which allowed 34.3 % of the injected fluorescein to be recovered. The combination of external and internal sealing with Tissumend and Healon did not surpass the effect of Tissumend alone. ANOVA testing (multiple comparisons vs the no Remedy group, Holm-Sidak) showed that Poloxamer (p=0.044), Healon (p=0.026), Tissumend (p=0.002) and Healon+Tissumend (p=0.011) all elevated marker recovery significantly.
Injections through a fenestration in the bony wall of scala tympani
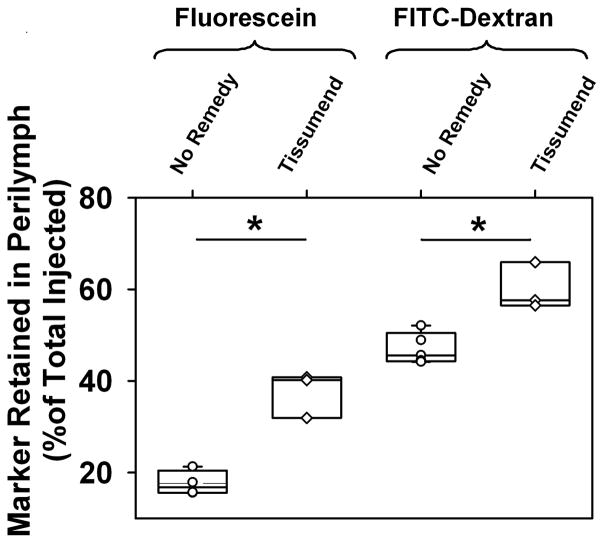
With injections through the RW membrane it was uncertain whether a fluid-tight seal was being achieved by any procedure. We therefore compared perilymph sample concentrations for injections from pipettes sealed into a fenestration of the bony wall of ST, where the absence of fluid leaks could be assured. This experiment was originally intended as a control, to demonstrate that without marker loss through the RW membrane, perilymph concentration would not be influenced by Tissumend on the membrane. Figure 4 shows the measured amounts of marker recovered following fluorescein or FITC-dextran injections when the RW membrane was not manipulated in any way (No Remedy) and when it had been coated with Tissumend. Contrary to our expectations, the retention of marker in perilymph was significantly higher for both markers when Tissumend coated the RW membrane. (Fluorescein t-test, P<0.001; FITC-dextran t-test p=0.0047). FITC-dextran is a much larger molecule (FW ~ 4000) than fluorescein and would not be expected to readily permeate the RW membrane. The higher amount of dextran recovered compared to fluorescein in both conditions is accounted for by lower losses for dextran by other elimination processes, such as to blood. In the dextran studies it was noted that in the No Remedy condition the RW remained clear of fluid so that no dextran loss could have occurred through the membrane. Nevertheless, Tissumend on the RW membrane, significantly increased dextran retention, averaging 60.0 %. Tissumend was therefore elevating the concentration of fluorescein and dextran in perilymph by a mechanism that did not involve marker efflux through the RW membrane in the untreated state.
Figure 4.
Marker retention in perilymph (marker summed across all samples from each animal relative to the total amount injected) for injections from pipettes sealed in the bony wall of scala tympani in the basal turn. Symbols show individual experiments and box plots show median, 25th and 75th percentile. Fluorescein retention was increased when Tissumend was applied to the round window membrane, even though it had not been perforated in these experiments. Similar experiments with fluorescent dextran marker confirmed that retention was higher when Tissumend was applied to the round window membrane.
Discussion
The retention of drug in the ear following intracochlear injection is demonstrated to be strongly dependent on the application protocol, primarily the size of the perforation made in the RW membrane. Injection from a 30G needle resulted in low marker retention, with less than 1% of the marker recovered in samples taken 40 min after injection. This shows that injections from 30G needles are a very inefficient way to deliver a drug or other substance to the ear. Although some applications of this type may have achieved the intended purpose (Stover et al., 1999), a delivery protocol with higher efficiency would generally be preferable. Delivery through a micropipette provides dramatically better retention, reaching over 10% in this study. The use of gel or adhesive to minimize fluid leakage for injections through the RW membrane is shown to further increase the marker levels retained in perilymph. The amount of drug or marker was typically more than doubled when fluid leakage at the injection site was controlled with gel or glue. The degree of control by such procedures is undoubtedly influenced by the primary delivery method and similar sealing procedures may not be as effective for injections that make substantially larger perforations of the RW membrane. Nevertheless, the present study shows that higher perilymph concentrations can be achieved by intracochlear injections when fluid leakage at the injection site is controlled. With fluid leakage present, the amount required to displace drug solution from ST is extremely small and may not be visible with an operating microscope. This potentially gives a false impression that no leakage is occurring so the problem may be overlooked. Fluorescent markers provide a readily available tool to assess perilymph composition quantitatively, allowing the influence of leaks to be quantified.
The influence of Tissumend adhesive on the RW membrane appears to be more complex than just preventing fluid leaks from the perforation site in the RW membrane caused by pipette insertion. When markers were applied by injections through the bony wall of ST, where the RW membrane was untouched, significantly higher levels of both fluorescein and FITC-dextran were found after the RW membrane was occluded with Tissumend even though there was no possible volume leak at the RW membrane in this condition. Our explanation for this phenomenon is that Tissumend application not only stops leaks but also makes the RW membrane rigid, decreasing its compliance and mechanically affecting fluid physiology. RW compliance of the guinea pig has been estimated to be 0.14 nL/Pa [Décory et al., 1990; Wit et al., 2003]. Considering that respiratory pressure fluctuations in perilymph are 0.3 – 1.0 mm Hg (40 – 133 Pa) [Takeuchi et al. 1991; Böhmer, 1993], each respiratory cycle is calculated to result in a volume displacement across the cochlear aqueduct of 5.6 – 18.6 nL. We incorporated a CSF-perilymph exchange of variable volume into our inner ear fluids simulation program (version 3.083, available for download from our website at http://oto.wustl.edu/cochlea/). A reciprocating volume exchange between perilymph and CSF of just 3 nL per respiratory cycle (3 nL/s) was sufficient to account for the lower marker levels in the “No Remedy” condition and for an increased level of marker when Tissumend was applied (assuming Tissumend abolishes the exchange). It should be emphasized that Tissumend application does not occlude the cochlear aqueduct. The rate at which samples were collected from the apex during sequential perilymph sampling from Tissumend-treated ears was similar to untreated animals. Our data are therefore consistent with an ongoing CSF-perilymph exchange contributing to perilymph status at the base of ST, and to the perilymph concentrations of drugs applied to the normal guinea pig. For injections of drugs and markers through the RW membrane, we conclude that the improved retention achieved with Tissumend likely results from the control of fluid leakage at the injection site and a decreased loss to the CSF compartment from pressure-driven reciprocating fluid oscillation. Further studies are necessary to characterize the kinetics of CSF-perilymph communications in more detail.
Fluid leaks during injection and exchange of fluid between scala tympani and CSF may not occur to the same degree in humans as seen here in guinea pigs. Although the human cochlear aqueduct is thought to be functionally patent in younger humans it may close in some older adults [Marchbanks & Reid, 1990; Wagner & Walsted 2000]. A morphologic study found the minimum diameter aqueduct to average 138 μm in humans, and to be filled with fluid or loose connective tissue in 93% of 101 specimens [Gopen et al., 1997]. Category of patency did not correlate with age in this study. In addition, while intracranial pressure may be positive (7 – 15 mmHg) while supine in normal adults, it becomes negative when upright [Andreson et al., 2015]. Future intracochlear drug and cell based therapies will have to deal with the variations in communication across the cochlear aqueduct across the spectrum of patients that will undoubtedly include children.
It has recently been suggested that microperforations of the RW membrane may allow intratympanically-applied drugs to transiently penetrate into perilymph while limiting volume efflux of the type we have studied here [Kelso et al, 2014]. In vitro measurements made in that study confirmed that perforations made with a 12.5 μm probe (of comparable size to the injection pipettes used in this study) increased diffusion of drug across the membrane. It remains to be demonstrated whether a similar increase in drug entry occurs in vivo, when efflux of perilymph will occur through the perforation as shown by our data. Nevertheless, the possibility that microperforations could allow drugs to be delivered to perilymph more effectively without the need for intracochlear injections, is an exciting possibility.
Future studies also need to investigate how marker retention over longer time periods is influenced by manipulations to suppress fluid leaks. Whether and how fast the perforation heals and whether effective long-term closure is obtained by the procedures that reduce leaks in the short-term must be evaluated. In addition, possible long-lasting effects of sealing procedures on cochlear function and on RW membrane status must be studied. Cai et al. [2013] have shown that RW occlusion with 1 μL of Vetbond (a very similar procedure to that used here) had only minor influence on ABR (13.5 dB elevation) and DPOAE responses measured at 8 weeks after the procedure in rats.
The ultimate goal of this work is to devise procedures facilitating drug, gene or cell based therapies of the inner ear through intracochlear and intralabyrinthine applications to the perilymph of humans. Such techniques need to preserve auditory function (i.e. to be safe for hearing) and to be quantitatively consistent in pharmacokinetic terms.
Conclusions
Drugs injected directly into perilymph of the inner ear are rapidly displaced by fluid efflux at the injection site, resulting in inefficient and variable delivery. Fluid efflux can be controlled by “internal sealing” procedures, such as by applying the drug in a gel, or by an “external sealing” procedure, using adhesive. More efficient and consistent delivery is possible when fluid leakage at the injection site is controlled.
Acknowledgments
This work was supported by research grant DC01368 from NIDCD, NIH.
Footnotes
The authors report no conflicts of interest related to this study.
References
- Andresen M, Hadi A, Petersen LG, Juhler M. Effect of postural changes on ICP in healthy and ill subjects. Acta Neurochir. 2015;157:109–113. doi: 10.1007/s00701-014-2250-2. [DOI] [PubMed] [Google Scholar]
- Böhmer A. Hydrostatic pressure in the inner ear fluid compartments and its effects on inner ear function. Acta Otolaryngol Suppl. 1993;507:3–24. [PubMed] [Google Scholar]
- Bowe SN, Jacob A. Round window perfusion dynamics: implications for intracochlear therapy. Curr Opin Otolaryngol Head Neck Surg. 2010;18:377–85. doi: 10.1097/MOO.0b013e32833d30f0. [DOI] [PubMed] [Google Scholar]
- Cai Q, Whitcomb C, Eggleston J, Sun W, Salvi R, Hu BH. Round window closure affects cochlear responses to suprathreshold stimuli. Laryngoscope. 2013;123:E116–121. doi: 10.1002/lary.24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Décory L, Franke RB, Dancer AL. Measurement of the middle ear transfer function in cat, chinchilla and guinea pig. In: Dallos P, Geisler CD, Matthews JW, Ruggero MA, Steele CR, editors. The Mechanics and Biophysics of Hearing, Springer. Berlin, Germany: 1990. pp. 270–277. [Google Scholar]
- Gopen Q, Rosowski JJ, Merchant SN. Anatomy of the normal human cochlear aqueduct with functional implications. Hear Res. 1997;107:9–22. doi: 10.1016/s0378-5955(97)00017-8. [DOI] [PubMed] [Google Scholar]
- Hahn H, Salt AN, Biegner T, Kammerer B, Delabar U, Hartsock JJ, Plontke SK. Dexamethasone levels and base-to-apex concentration gradients in the scala tympani perilymph after intracochlear delivery in the guinea pig. Otol Neurotol. 2012;33:660–665. doi: 10.1097/MAO.0b013e318254501b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Hartsock JJ, Johnson S, Santi P, Salt AN. Systemic lipopolysaccharide compromises the blood-labyrinth barrier and increases entry of serum fluorescein into the perilymph. J Assoc Res Otolaryngol. 2014;15:707–719. doi: 10.1007/s10162-014-0476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jero J, Mhatre AN, Tseng CJ, Stern RE, Coling DE, Goldstein JA, Hong K, Zheng WW, Hoque AT, Lalwani AK. Cochlear gene delivery through an intact round window membrane in mouse. Hum Gene Ther. 2001;12:539–548. doi: 10.1089/104303401300042465. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Kanzaki S, Yagi M, Stöver T, Prieskorn DM, Dolan DF, Miller JM, Raphael Y. Gene-based therapy for inner ear disease. Noise Health. 2001;3:37–47. [PubMed] [Google Scholar]
- Kho ST, Pettis RM, Mhatre AN, Lalwani AK. Cochlear microinjection and its effects upon auditory function in the guinea pig. Eur Arch Otorhinolaryngol. 2000;257:469–72. doi: 10.1007/s004050000280. [DOI] [PubMed] [Google Scholar]
- Kelso CM, Watanabe H, Wazen JM, Bucher T, Qian ZJ, Olson ES, Kysar JW, Lalwani AK. Microperforations Significantly Enhance Diffusion Across Round Window Membrane. Otol Neurotol. 2014:10. doi: 10.1097/MAO.0000000000000629. E-pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchbanks RJ, Reid A. Cochlear and cerebrospinal fluid pressure: their inter-relationship and control mechanisms. Br J Audiol. 1990;24:179–187. doi: 10.3109/03005369009076554. [DOI] [PubMed] [Google Scholar]
- Moscovitch DH, Gannon RP, Laszlo CA. Perilymph displacement by cerebrospinal fluid in the cochlea. Ann Otol Rhinol Laryngol. 1973;82:53–61. doi: 10.1177/000348947308200113. [DOI] [PubMed] [Google Scholar]
- Mynatt R, Hale SA, Gill RM, Plontke SKR, Salt AN. Demonstration of a longitudinal concentration gradient along scala tympani by sequential sampling of perilymph from the cochlear apex. J Assoc Res Otolaryngol. 2006;7:182–193. doi: 10.1007/s10162-006-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama K, Salt AN, Thalmann R. Volume flow rate of perilymph in the guinea-pig cochlea. Hear Res. 1988;35:119–29. doi: 10.1016/0378-5955(88)90111-6. [DOI] [PubMed] [Google Scholar]
- Plontke SK, Mynatt R, Gill RM, Salt AN. Concentration gradient along scala tympani following the local application of gentamicin to the round window membrane. Laryngoscope. 2007;117:1191–1198. doi: 10.1097/MLG.0b013e318058a06b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plontke SK, Biegner T, Kammerer B, Delabar U, Salt AN. Dexamethasone concentration gradients along scala tympani after application to the round window membrane. Otology & Neurotology. 2008;29:401–406. doi: 10.1097/MAO.0b013e318161aaae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius M, Baker K, Brough DE, Plinkert P, Staecker H. Pharmacodynamics of adenovector distribution within the inner ear tissues of the mouse. Hear Res. 2007;227:53–58. doi: 10.1016/j.heares.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Salt AN, Stopp PE. The effect of cerebrospinal fluid pressure on perilymphatic flow in the opened cochlea. Acta Otolaryngol. 1979;88:198–202. doi: 10.3109/00016487909137160. [DOI] [PubMed] [Google Scholar]
- Salt AN, Hale SA, Plonkte SK. Perilymph sampling from the cochlear apex: a reliable method to obtain higher purity perilymph samples from scala tympani. J Neurosci Methods. 2006;153:121–129. doi: 10.1016/j.jneumeth.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt AN, Sirjani DB, Hartsock JJ, Gill RM, Plontke SK. Marker retention in the cochlea following injections through the round window membrane. Hear Res. 2007;232:78–86. doi: 10.1016/j.heares.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellick P, Layton MG, Rodger J, Robertson D. A method for introducing non-silencing siRNA into the guinea pig cochlea in vivo. Journal of Neuroscience Methods. 2008;167:237–245. doi: 10.1016/j.jneumeth.2007.08.026. [DOI] [PubMed] [Google Scholar]
- Staecker H, Rodgers B. Developments in delivery of medications for inner ear disease. Expert Opin Drug Deliv. 2013;10:639–650. doi: 10.1517/17425247.2013.766167. [DOI] [PubMed] [Google Scholar]
- Staecker H, Schlecker C, Kraft S, Praetorius M, Hsu C, Brough DE. Optimizing atoh1-induced vestibular hair cell regeneration. Laryngoscope. 2014;124(Suppl 5):S1–S12. doi: 10.1002/lary.24775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöver T, Yagi M, Raphael Y. Cochlear gene transfer: round window versus cochleostomy inoculation. Hear Res. 1999;136:124–130. doi: 10.1016/s0378-5955(99)00115-x. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Takeda T, Saito H. Pressure relationship between perilymph and endolymph associated with endolymphatic infusion. Ann Otol Rhinol Laryngol. 1991;100:244–248. doi: 10.1177/000348949110000314. [DOI] [PubMed] [Google Scholar]
- Thorne M, Salt AN, DeMott JE, Henson MM, Henson OW, Jr, Gewalt SL. Cochlear fluid space dimensions for six species derived from reconstructions of three-dimensional magnetic resonance images. Laryngoscope. 1999;109:1661–1668. doi: 10.1097/00005537-199910000-00021. [DOI] [PubMed] [Google Scholar]
- Wagner N, Walsted A. Postural-induced changes in intracranial pressure evaluated non-invasively using the MMS-10 tympanic displacement analyser in healthy volunteers. Acta Otolaryngol Suppl. 2000;543:44–47. [PubMed] [Google Scholar]
- Wang X, Dellamary L, Fernandez R, Harrop A, Keithley EM, Harris JP, Ye Q, Lichter J, LeBel C, Piu F. Dose-dependent sustained release of dexamethasone in inner ear cochlear fluids using a novel local delivery approach. Audiol Neurootol. 2009;14:393–401. doi: 10.1159/000241896. [DOI] [PubMed] [Google Scholar]
- Wit HP, Feijen RA, Albers FW. Cochlear aqueduct flow resistance is not constant during evoked inner ear pressure change in the guinea pig. Hear Res. 2003;175:190–199. doi: 10.1016/s0378-5955(02)00738-4. [DOI] [PubMed] [Google Scholar]