Abstract
This study introduces the use of multivariate linear regression (MLR) and support vector regression (SVR) models to predict postoperative outcomes in a cohort of patients who underwent surgery for cervical spondylotic myelopathy (CSM). Currently, predicting outcomes after surgery for CSM remains a challenge. We recruited patients who had a diagnosis of CSM and required decompressive surgery with or without fusion. Fine motor function was tested preoperatively and postoperatively with a handgrip-based tracking device that has been previously validated, yielding mean absolute accuracy (MAA) results for two tracking tasks (sinusoidal and step). All patients completed Oswestry disability index (ODI) and modified Japanese Orthopaedic Association questionnaires preoperatively and postoperatively. Preoperative data was utilized in MLR and SVR models to predict postoperative ODI. Predictions were compared to the actual ODI scores with the coefficient of determination (R2) and mean absolute difference (MAD). From this, 20 patients met the inclusion criteria and completed follow-up at least 3 months after surgery. With the MLR model, a combination of the preoperative ODI score, preoperative MAA (step function), and symptom duration yielded the best prediction of postoperative ODI (R2 = 0.452; MAD = 0.0887; p = 1.17 × 10−3). With the SVR model, a combination of preoperative ODI score, preoperative MAA (sinusoidal function), and symptom duration yielded the best prediction of postoperative ODI (R2 = 0.932; MAD = 0.0283; p = 5.73 × 10−12). The SVR model was more accurate than the MLR model. The SVR can be used preoperatively in risk/benefit analysis and the decision to operate.
Keywords: Cervical spondylotic myelopathy, Multivariate linear regression, Support vector regression, Surgical outcomes
1. Introduction
Cervical spondylotic myelopathy (CSM) is characterized by spondylosis leading to compression of the spinal cord [1]. When conservative therapy fails, surgical management involving anterior cervical discectomy, with or without fusion, is the most common approach. While treatment is normally effective, results can vary [2]. Ebersold et al. [2] reported long term functional decline or no improvement in 45% of patients undergoing anterior cervical discectomy with fusion (ACDF) for CSM and Chang et al. [3] found a lack of improvement in 14.5% of patients undergoing laminectomy and fusion for CSM.
While prognostic factors for patients undergoing surgery for CSM have been investigated, predicting outcomes remains challenging. Age, symptom duration and preoperative neurological function have been reported as prognostic indicators [4–6]. These findings are frequently limited by bias towards specific surgical procedures and non-standardized evaluations of clinical outcomes [7].
Multivariate regression models have been used as prediction mechanisms for postoperative outcomes in conditions such as spinal cord injury [8], stroke [9], and Parkinson’s disease [10]. However, to the best of our knowledge, this approach has not been applied to CSM. The goal of this study was to investigate two different regression models, multivariate linear regression (MLR) and support vector regression (SVR), in a cohort of patients receiving decompressive surgery for CSM to develop a mathematical tool for predicting postoperative outcomes. An accurate predictive model of functional outcomes would allow for optimal patient selection for surgery, realistic postoperative goals for patients, planning for environmental adjustments to accommodate the patient’s postoperative functional status, and preparation for appropriate rehabilitation procedures [9, 11].
2. Methods
2.1. Patient selection and chart review
This study was approved by the authors’ Institutional Review Board and all patients provided consent to participate in the study. All patients were enrolled through a spine clinic and a single neurosurgeon performed all procedures. Patients with coexisting neuromuscular or other spinal cord conditions were excluded. Patient charts were reviewed for information regarding pathology including the level of the spinal cord lesion, symptom duration, and the narrowest spinal cord diameter. Additionally, we investigated whether the patient was rehospitalized subsequent to the hospitalization for the procedure.
2.2. Functional outcome
Patients’ disability levels were measured by administering the Oswestry disability index (ODI) questionnaire [12] during the preoperative consultation and at a minimum of 3 months postoperatively. ODI version 2.0 [13] was used, and the score was linearly scaled from 0 to 1 with 0 representing no disability and 1 representing maximum disability. In addition to the ODI, patients completed the modified Japanese Orthopaedic Association (JOA) survey, which is modified from the original JOA survey to measure the functional capacity of CSM patients [14].
2.3. Testing protocol
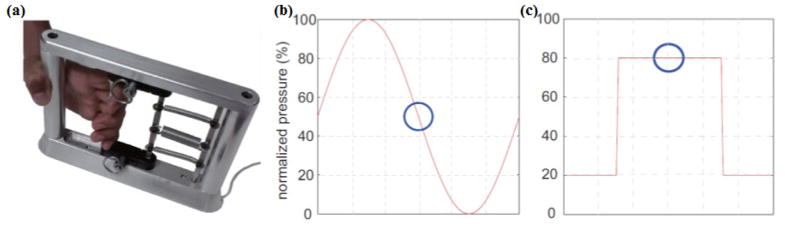
Previously validated handgrip-based tracking tasks [15, 16] were used to quantitatively evaluate fine motor function. The MediSens handgrip device (MediSens Wireless, Santa Clara, CA, USA), a research device developed at the Wireless Health Institute of the University of California Los Angeles, is illustrated in Figure 1a. Using the handgrip protocol described by Getachew et al.[16], fine motor function was quantified by determining the mean absolute error (MAE) during sinusoidal (sine) and step tracking tasks (Fig. 1b). It is computed as the mean value between the target and the patient’s response over the period of the test. The mean absolute accuracy (MAA) was then calculated as 1 − MAE. Each task was performed in triplicate and the average MAA from the three trials was used as the final measure.
Fig. 1.
(a) The handgrip device used to detect grip strength in real-time. (b, c) An illustration of tracking tasks for two different patterns: (b) sinusoidal and (c) step.
2.4. Statistical analyses
We employed two statistical techniques for estimating postoperative ODI scores: MLR and SVR. MLR was used to linearly aggregate prognostic factors to predict the postoperative outcomes. Conversely, SVR allows for a more flexible, non-linear relationship between the prognostic factors and the outcome. This algorithm is constructed upon the statistical learning theory, which generates a hyperplane that best describes correlation among clinical variables to the response variables [17]. Additional information regarding the technique is available [18–22]. The predicted ODI of both regression techniques were compared to the patient’s actual ODI using the coefficients of determination (R2 and adjusted R2), mean absolute difference (MAD), and corresponding p values.
Considering the sample size of this study, we limited the number of predictor variables to three for both MLR and SVR; the maximum number of predictors is approximately 20% of the sample size. This constraint was applied to minimize the chances of over-fitting the regression model.
3. Results
3.1. Patient demographics
A total of 27 patients with CSM met the inclusion criteria and were followed as part of a 24 month cohort trial. Seven patients did not complete follow-up, leaving 20 patients (11 men and 9 women) with a mean age of 62 years (standard deviation 13.8). The average clinical follow-up time was 5.6 months after the surgical procedure. There were 15 patients who underwent laminectomy with fusion, four anterior cervical discectomy and fusion, and one minimally invasive laminectomy without fusion. Of those who underwent fusion, the average number of levels fused was 3.9 (range: 2–7). Out of the entire cohort, the average number of levels decompressed was 4.1 (range: 2–7).
3.2. Univariate correlation to postoperative outcomes
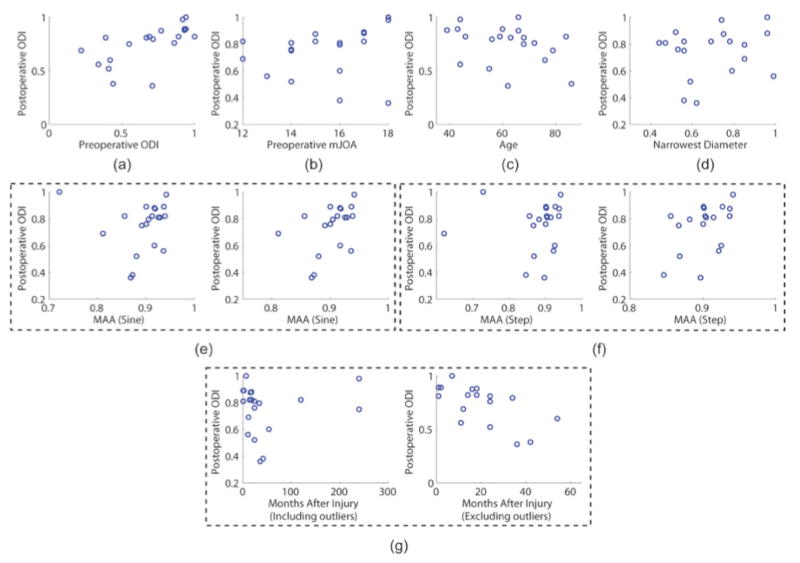
Figure 2 shows the univariate correlations between postoperative ODI and each of the predictor variables: preoperative MAA of sine and step functions, age, narrowest spinal diameter, symptom duration, preoperative ODI, and preoperative modified JOA. Figure 2g displays the correlation between preoperative symptom duration and postoperative ODI, however, the plot on the right is limited to patients with symptom durations < 60 months. The quantitative evaluation (R2, adjusted R2, and p value) of each correlation is provided in Table 1.
Fig. 2.
Univariate correlations between postoperative Oswestry disability index (ODI) and each of the considered predictor variables: (a) preoperative ODI, (b) preoperative modified Japanese Orthopaedic Association (mJOA) score, (c) age, (d) narrowest spinal diameter, (e) mean absolute accuracy of sinusoidal tasks (MAA; sine) of the entire dataset (left) and with outliers removed (right), (f) MAA (step) of the entire dataset (left) and with outliers removed (right), (g) symptom duration of the entire dataset and symptom duration < 60 months. This figure is available in color at www.sciencedirect.com.
Table 1.
Quantitative summary of the correlation between postoperative ODI and each of the considered predictor variables
| R2 | Adjusted R2 | p value | |
|---|---|---|---|
| Preoperative ODI | 0.401 | 0.368 | 0.003* |
| Preoperative mJOA | 0.041 | −0.012 | 0.391 |
| Age | 0.102 | 0.052 | 0.171 |
| Narrowest Diameter | 0.012 | −0.043 | 0.643 |
| MAA sine | 7.77 × 10−5 | −0.056 | 0.971 |
| MAA sine (without outliers) | 0.197 | 0.150 | 0.057* |
| MAA step | 0.0025 | −0.053 | 0.836 |
| MAA step (without outliers) | 0.205 | 0.155 | 0.060* |
| Symptom duration (all data) | 0.405 | −0.040 | 0.614 |
| Symptom duration (< 60 months) | 0.401 | 0.368 | 0.003* |
p value at or close to significance (p < 0.05 deemed significant).
MAA = mean absolute accuracy, mJOA = modified Japanese Orthopedic Association scale, ODI = Oswestry disability index, sine = sinusoidal.
Preoperative ODI showed the most significant correlation to the postoperative ODI (p < 0.003). MAA of sine and step tracking tasks (p < 0.057 and p < 0.060, respectively) as well as age (p < 0.171) were associated with the outcome but did not reach statistical significance (Fig. 2e, f). The symptom duration of the entire dataset did not show statistical significance (p < 0.614), but when only patients with symptom duration less than 60 months were considered, it showed a significant correlation (p < 0.003; Fig. 2g).
3.3. Multivariate correlation to postoperative outcomes
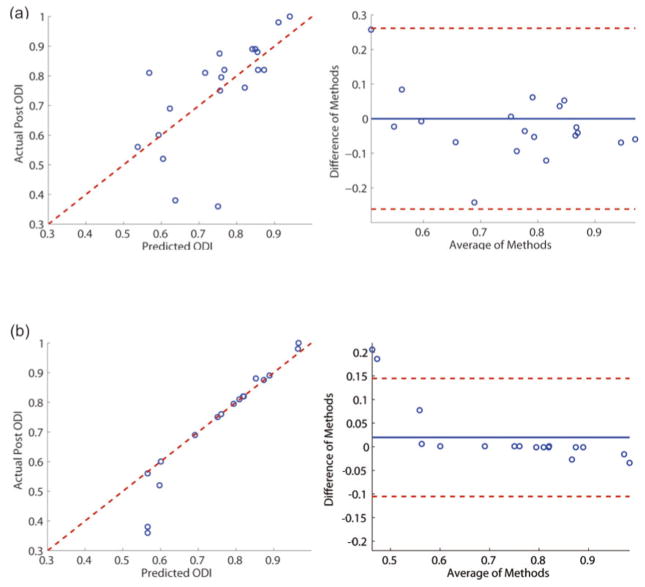
The results of multivariate regressions using MLR and SVR are summarized in Table 2. The regression results and the Bland–Altman plots for both models are provided in Figure 3. In the MLR model, the predictors that produced the best regression results were preoperative ODI, MAA of the step tracking task, and symptom duration. In the SVR model, the best predictors were preoperative ODI, MAA of the sine tracking task, and symptom duration.
Table 2.
Quantitative summary of multivariate regression models: MLR and SVR
| R2 | Adjusted R2 | p value | MAD | Predictors | |
|---|---|---|---|---|---|
| MLR | 0.452 | 0.421 | 1.17 × 10−3 | 0.0887 | Preoperative ODI, MAA step, symptom duration |
| SVR | 0.932 | 0.929 | 5.73 × 10−12 | 0.0283 | Preoperative ODI, MAA sine, symptom duration |
MAA = mean absolute accuracy, MAD = mean absolute difference, MLR = multivariate linear regression, ODI = Oswestry disability index, SVR = support vector regression.
Fig. 3.
The regression plots (left) and associated Bland–Altman plots (right) for (a) multivariate linear regression and (b) support vector regression. ODI = Oswestry disability index. This figure is available in color at www.sciencedirect.com.
SVR produced superior regression performance (p < 5.73 × 10−12) compared to MLR (p < 1.17 × 10−3; Fig. 3). For the SVR model, the bias and the limit of agreement of the Bland–Altman plot were 0.0198 and 0.125, respectively. The Bland–Altman plot of the SVR results shows that the differences in methods are randomly distributed within the entire range of the averages of the methods (Fig. 3b). There exist two data points exceeding the limit of agreement, which are potential outliers. The Cook’s distances were computed for all data points to identify the outliers using
where n is the total number of data points (20). Variable r̂j represents the regression estimate of the response variable (postoperative ODI) of a data point j, and r̂j−i represents the regression estimate of the data point j when the data point i is removed from the regression analysis. The variable s represents the root mean square of the regression and k represents the number of predictors used in the regression model (k = 3). A data point is defined as an outlier if the following inequality is true:
According to this definition, the two points that exceeded the limit of agreement were defined as outliers. Their Cook’s distances were 0.63 and 1.05 where 4/(n − (k + 1)) = 0.22. These points belonged to the two patients who reported their symptom durations as 20 years and as a consequence, their prediction results were significantly exaggerated by the SVR model (Fig. 3b).
4. Discussion
Predictions of surgical outcomes for CSM remain a challenge and many patients do not benefit from surgery. In a retrospective study by Nirala et al.[23], 18.9% of patients undergoing anterior surgery for CSM did not achieve good or excellent outcomes based on Odom’s criteria. In a retrospective analysis by Carol et al.[24], 27% of patients who underwent ACDF for CSM did not improve, and 32% of patients who underwent laminectomy did not improve. Therefore, optimizing patient selection could reduce the number of poor surgical outcomes.
We investigated preoperative variables that have been shown to have utility in predicting outcomes after surgery for CSM. The inclusion of the narrowest cervical spinal diameter points to evidence that narrow spinal canals contribute to accelerated disease progression [25]. Age and symptom duration have also shown correlations with outcomes in CSM patients [4, 5]. The final variables included were MAA measurements obtained from the handgrip tracking tasks. We hypothesized that these would correlate with postoperative outcomes because they are sensitive measurements of symptom severity [16].
In our MLR model, a combination of the preoperative ODI score, preoperative MAA score for the step tracking task, and symptom duration yielded the best prediction of postoperative ODI. The most accurate SVR model included the preoperative ODI score, MAA score for the sine tracking task and symptom duration. Both models included preoperative ODI as a predictor. This is consistent with the univariate analysis, in which preoperative ODI had one of the most significant correlations with postoperative ODI. Symptom duration also had a strong univariate correlation with outcomes, possibly because a greater amount of time had passed for ischemia and other forms of compressive damage to develop. The selected predictors for the MLR model included MAA score of the step task which had the fourth most significant linear correlation to the postoperative ODI (p < 0.060). Overall, both models suggest that patients with more chronic and severe symptoms do not fare as well postoperatively.
While clinical maneuvers for evaluating CSM patients typically evaluate strength, sensation, or reflexes [26, 27], fine motor function is a factor that is less commonly accounted for. Nonetheless, a reduction in fine motor function is a major complaint of myelopathic patients [28, 29]. Its relation to postoperative ODI indicates that those with poorer fine motor function preoperatively experience worse postoperative outcomes. The presence of myelopathic symptoms may indicate greater spinal cord compression which is less likely to improve after surgery due to ischemia. There is evidence that ischemia may be a secondary cause of CSM, compounding the primary damage done by nerve compression [25, 30]. The importance of preoperative fine motor function in our models is consistent with the survey by Tetreault et al. in which the presence of myelopathic symptoms was considered one of the most important predictive factors of outcomes in CSM patients undergoing surgery [31]. In a case series by Bertalanffy and Eggert, the presence of myelopathic symptoms was inversely correlated with outcomes [32]. In addition, hand muscle atrophy has been found to be predictive of poorer postoperative outcomes[33, 34], further supporting the use of the handgrip device in our assessment. Symptom severity was also regarded as an important prognostic factor in the survey by Tetreault et al.[31], and the MAA score included in our models is a highly sensitive measurement of this [16].
The SVR model produced superior correlation results compared to the MLR model (Table 2; Fig. 3). While patients with greater preoperative disability may have more postoperative disability than their less disabled counterparts, their improvement cannot be assumed to be directly proportional. The SVR superiority to MLR for predicting outcomes underscores the variability of the response to surgery. The greater performance of a more flexible predictive model suggests that even with the availability of prognostic factors, patients may not respond to surgery as expected. The variability in response could be a concern to patients and surgeons who are unsure if a surgical approach to CSM should be pursued. Using the SVR model to predict functional status after surgery would aid in the decision making process.
The models we have introduced could easily be implemented in a CSM patient’s preoperative evaluation. Assessing disability with the ODI questionnaire is routinely performed in clinics, and measuring fine motor function with the handgrip is a reliable and rapid clinical test [15]. Furthermore, the models we have constructed predict ODI, which is a single, readily interpretable score [13]. Applying a mathematical model is a more objective approach than analyzing subjective preoperative factors such as T2-weighted signal intensity, which can be difficult to interpret [7] but continues to be a commonly applied prognostic indicator in CSM patients [31]. Our model also has the benefit of yielding a quantitative measurement of outcomess, while others are qualitative [35].
One limitation of this study was the use of ODI as an outcome measurement, while a CSM-specific scale such as the JOA assessment may have been better suited to our patients. However, ODI has been applied to cervical patients before [15], and ODI correlates with the JOA score [36].
5. Conclusion
Currently, the preoperative factors that have been implicated in predicting patient responses to CSM surgery are inconsistent, and the available multivariate regression models are limited in scope. CSM treatment would benefit from our SVR model which can accurately and quantitatively predict postoperative outcomes. Our model would offer the ability to optimize patient selection for surgery, however, further confirmation awaits a larger scale clinical trial.
Highlights.
Two models to predict surgical outcomes for CSM are introduced
Models were multivariate linear regression (MLR), support vector regression (SVR)
Twenty CSM patients were examined before and after surgery
Both models used preoperative data to accurately predict postoperative outcomes
The SVR model was more accurate than the MLR model
Acknowledgments
Funding for this research was made possible by generous support from the J. Yang & Family Foundation and the National Institutes of Health (NIH) funded by the National Institute of Biomedical Imaging and Bioengineering, the National Institute of Neurological Disorders and Stroke, and the National Institute for Communicable Diseases. The research described was conducted at the University of California Los Angeles (UCLA) Clinical and Translational Research Center which was supported by NIH/National Center for Advancing Translational Science (NCATS) UCLA Clinical and Translational Science Institute. D.C.L. is a 1999 Paul & Daisy Soros New American Fellow.
Footnotes
Conflicts of Interest/Disclosures
The authors declare that they have no financial or other conflicts of interest in relation to this research and its publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lebl DR, Hughes A, Cammisa FP, O’Leary PF. Cervical spondylotic myelopathy: pathophysiology, clinical presentation, and treatment. HSS J. 2011;7:170–8. doi: 10.1007/s11420-011-9208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ebersold MJ, Pare MC, Quast LM. Surgical treatment for cervical spondylitic myelopathy. J Neurosurg. 1995;82:745–51. doi: 10.3171/jns.1995.82.5.0745. [DOI] [PubMed] [Google Scholar]
- 3.Chang V, Lu DC, Hoffman H, Buchanan C, Holly LT. Clinical results of cervical laminectomy and fusion for the treatment of cervical spondylotic myelopathy in 58 consecutive patients. Surg Neurol Int. 2014;5:S133–7. doi: 10.4103/2152-7806.130670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chagas H, Domingues F, Aversa A, Fonseca AV. Cervical spondylotic myelopathy: 10 years of prospective outcome analysis of anterior decompression and fusion. Surg Neurol. 2005;64(S1):30–5. doi: 10.1016/j.surneu.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Holly LT, Matz PG, Anderson PA, Groff MW, Heary RF, Kaiser MG, Mummaneni PV, Ryken TC, Choudhri TF, Vresilovic EJ, Resnick DK. Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11:112–8. doi: 10.3171/2009.1.SPINE08718. [DOI] [PubMed] [Google Scholar]
- 6.Suri A, Chabbra R, Mehta VS, Gaikwad S. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003;3:33–45. doi: 10.1016/s1529-9430(02)00448-5. [DOI] [PubMed] [Google Scholar]
- 7.Vedantam A, Rajshekhar V. Does the type of T2-weighted hyperintensity influence surgical outcome in patients with cervical spondylotic myelopathy? A review Eur Spine J. 2013;22:96–106. doi: 10.1007/s00586-012-2483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tonack M, Hitzig SL, Craven BC, Campbel KA. Predicting life satisfaction after spinal cord injury in a Canadian sample. Spinal Cord. 2008;46:380–5. doi: 10.1038/sj.sc.3102088. [DOI] [PubMed] [Google Scholar]
- 9.Veerbeek JM, Kwakkel G, van Wegen EE, Ket JC, Heymans MW. Early prediction of outcome of activities of daily living after stroke a systematic review. Stroke. 2011;42:1482–8. doi: 10.1161/STROKEAHA.110.604090. [DOI] [PubMed] [Google Scholar]
- 10.Soh SE, Morris ME, McGinley JL. Determinants of health-related quality of life in Parkinson’s disease: a systematic review. Parkinsonism Relat Disord. 2011;17:1–9. doi: 10.1016/j.parkreldis.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Kwakkel G, Wagenaar RC, Kollen BJ, Lankhorst GJ. Predicting disability in stroke--a critical review of the literature. Age Ageing. 1996;25:479–89. doi: 10.1093/ageing/25.6.479. [DOI] [PubMed] [Google Scholar]
- 12.Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66:271–3. [PubMed] [Google Scholar]
- 13.Fairbank JC, Pynsent PB. The Oswestry disability index. Spine. 2000;25:2940–53. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 14.Benzel EC, Lancon J, Kesterson L, Hadden T. Cervical laminectomy and dentate ligament section for cervical spondylotic myelopathy. J Spinal Disord. 1991;4:286–95. doi: 10.1097/00002517-199109000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Lee SI, Ghasemzadeh H, Mortazavi BJ, Sarrafzadeh M. A pervasive assessment of motor function: a lightweight grip strength tracking system. IEEE J Biomed Health Inform. 2013;17:1023–30. doi: 10.1109/JBHI.2013.2262833. [DOI] [PubMed] [Google Scholar]
- 16.Getachew R, Lee SI, Yew A, Kimball J, Lu DS, Garst JH, Ghalehsari N, Paak BH, Razaghy M, Espinal M, Ostowari A, Ghavamrezaii A, Pourtaheri S, Sarrafzadeh M, Lu DC. Utilization of a novel digital measurement tool for quantitative assessment of upper extremity motor dexterity: a controlled pilot study. J Neuroeng Rehabil. 2014;11:121. doi: 10.1186/1743-0003-11-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellazzi R, Zupan B. Predictive data mining in clinical medicine: current issues and guidelines. Int J Med Inform. 2008;77:81–97. doi: 10.1016/j.ijmedinf.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Furey TS, Cristianini N, Duffy N, Bednarski DW. Support vector machine classification and validation of cancer tissue samples using microarray expression data. Bioinformatics. 2000;16:906–14. doi: 10.1093/bioinformatics/16.10.906. [DOI] [PubMed] [Google Scholar]
- 19.Moro FD, Abate A, Lanckriet GR, Arandjelovic G, Gasparella P, Bassi P, Mancini M, Pagano F. A novel approach for accurate prediction of spontaneous passage of ureteral stones: support vector machines. Kidney Int. 2006;69:157–60. doi: 10.1038/sj.ki.5000010. [DOI] [PubMed] [Google Scholar]
- 20.Smola AJ, Schölkopf B. A tutorial on support vector regression. Statistics and computing. 2003;14:199–222. [Google Scholar]
- 21.Yu JS, Ongarello S, Fiedler R, Chen XW, Toffolo G, Cobelli C, Trajanoski Z. Ovarian cancer identification based on dimensionality reduction for high-throughput mass spectrometry data. Bioinformatics. 2005;21:2200–9. doi: 10.1093/bioinformatics/bti370. [DOI] [PubMed] [Google Scholar]
- 22.Yu W, Liu T, Valdez R, Gwinn M. Application of support vector machine modeling for prediction of common diseases: the case of diabetes and pre-diabetes. BMC Med Inform Decis Mak. 2010;10:16. doi: 10.1186/1472-6947-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nirala AP, Husain M, Vatsal DK. A retrospective study of multiple interbody grafting and long segment strut grafting following multilevel anterior cervical decompression. Br J Neurosurg. 2004;18:227–32. doi: 10.1080/02688690410001732643. [DOI] [PubMed] [Google Scholar]
- 24.Carol MP, Ducker TB. Cervical spondylitic myelopathies: surgical treatment. J Spinal Disord. 1988;1:59–65. [PubMed] [Google Scholar]
- 25.Toledano M, Bartleson JD. Cervical spondylotic myelopathy. Neurol Clin. 2013;31:287–305. doi: 10.1016/j.ncl.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Singh A, Tetreault L, Casey A, Laing R, Statham P, Fehlings MG. A summary of assessment tools for patients suffering from cervical spondylotic myelopathy: a systematic review on validity, reliability and responsiveness. Eur Spine J. 2013 doi: 10.1007/s00586-013-2935-x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Cook CE, Wilhelm M, Cook AE, Petrosino C, Isaacs R. Clinical tests for screening and diagnosis of cervical spine myelopathy: a systematic review. J Manipulative Physiol Ther. 2011;34:539–546. doi: 10.1016/j.jmpt.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Eismann EA, Lucky AW, Cornwall R. Hand function and quality of life in children with epidermolysis bullosa. Pediatr Dermatol. 2014;31:176–82. doi: 10.1111/pde.12262. [DOI] [PubMed] [Google Scholar]
- 29.Kutner NG, Zhang R, Butler AJ, Wolf SL, Alberts JL. Quality-of-life change associated with robotic-assisted therapy to improve hand motor function in patients with subacute stroke: a randomized clinical trial. Phys Ther. 2010;90:493–504. doi: 10.2522/ptj.20090160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doppman JL. The mechanism of ischemia in anteroposterior compression of the spinal cord. Invest Radiol. 1990;25:444–52. doi: 10.1097/00004424-199004000-00022. [DOI] [PubMed] [Google Scholar]
- 31.Tetreault LA, Nouri A, Singh A, Fawcett M, Fehlings MG. Predictors of outcome in patients with cervical spondylotic myelopathy undergoing surgical treatment: a survey of members from AOSpine International. World Neurosurg. 2014;81:623–33. doi: 10.1016/j.wneu.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 32.Bertalanffy H, Eggert HR. Clinical long-term results of anterior discectomy without fusion for treatment of cervical radiculopathy and myelopathy. A follow-up of 164 cases. Acta Neurochir (Wien) 1988;90:127–135. doi: 10.1007/BF01560567. [DOI] [PubMed] [Google Scholar]
- 33.Alafifi T, Kern R, Fehlings M. Clinical and MRI predictors of outcome after surgical intervention for cervical spondylotic myelopathy. J Neuroimaging. 2007;17:315–22. doi: 10.1111/j.1552-6569.2007.00119.x. [DOI] [PubMed] [Google Scholar]
- 34.Chiles BW, Leonard MA, Choudhri HF, Cooper PR. Cervical spondylotic myelopathy: patterns of neurological deficit and recovery after anterior cervical decompression. Neurosurgery. 1999;44:762–70. doi: 10.1097/00006123-199904000-00041. [DOI] [PubMed] [Google Scholar]
- 35.Tetreault LA, Kopjar B, Vaccaro A, Yoon ST, Arnold PM, Massicote EM, Fehlings MG. A clinical prediction model to determine outcomes in patients with cervical spondylotic myelopathy undergoing surgical treatment: data from the prospective, multi-center AOSpine North America study. J Bone Joint Surg Am. 2013;95:1659–66. doi: 10.2106/JBJS.L.01323. [DOI] [PubMed] [Google Scholar]
- 36.Fujiwara A, Kobayashi N, Saiki K, Kitagawa T, Tamai K, Saotome K. Association of the Japanese Orthopaedic Association score with the Oswestry Disability Index, Roland-Morris Disability Questionnaire, and short-form 36. Spine. 2003;28:1601–7. [PubMed] [Google Scholar]