Abstract
Rnd3, also known as RhoE, belongs to the Rnd subclass of the Rho family of small GTP-binding proteins. Rnd proteins are unique due to their inability to switch from a GTP-bound to GDP-bound conformation. Even though studies of the biological function of Rnd3 are far from being concluded, information is available regarding its expression pattern, cellular localization, and its activity, which can be altered depending on the conditions. The compiled data from these studies implies that Rnd3 may not be a traditional small GTPase. The basic role of Rnd3 is to report as an endogenous antagonist of RhoA signaling-mediated actin cytoskeleton dynamics, which specifically contributes to cell migration and neuron polarity. In addition, Rnd3 also plays a critical role in arresting cell cycle distribution, inhibiting cell growth, and inducing apoptosis and differentiation. Increasing data have shown that aberrant Rnd3 expression may be the leading cause of some systemic diseases; particularly highlighted in apoptotic cardiomyopathy, developmental arrhythmogenesis and heart failure, hydrocephalus, as well as tumor metastasis and chemotherapy resistance. Therefore, a better understanding of the function of Rnd3 under different physiological and pathological conditions, through the use of suitable models, would provide a novel insight into the origin and treatment of multiple human diseases.
Keywords: Rnd3/RhoE, small GTPase, system physiology, cardiovascular diseases, nervous system disorders, tumorigenesis, chemotherapy resistance
Introduction
Small guanosine triphosphatases (GTPases) are a family of hydrolase enzymes that can bind and hydrolyze guanosine triphosphate (GTP) (86). They are a certain type of G-proteins that are homologous to the alpha subunit of heterotrimeric G-proteins. Three classes of regulators are found to regulate GTPase protein activity: guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs), and guanine nucleotide dissociation inhibitors (GDIs). GEFs facilitate the exchange of GDP with GTP which activates GTPases. GAPs and GDIs, however, inhibit the activity of GTPases. GAPs hydrolyze GTP into guanosine diphosphate (GDP); while GDIs prevent interaction between GTPases and GEFs (14, 17, 84). The switch between the active GTP-bound and inactive GDP-bound form is termed as GTP-GDP cycling and is the main mechanism of small GTPase activity modulation (22).
The most well-known GTPase family members are the Ras GTPases and are therefore sometimes called the Ras superfamily. Ras homologous (Rho) family GTPases consist of over 150 varieties in mammals and are part of the Ras superfamily, which includes the Ras, Rho, Rab, Ran, and Arf families (139). As for the Rho family, it includes Rnd1, Rnd2, Rnd3/RhoE, RhoH/TTF, RhoF/Rif, RhoBTB1, RhoBTB2, Miro-1, Miro-2, and RhoD (108). Most of the Rho GTPases are functionally changed from the GTP-bound to the GDP-bound state. The Rnd proteins (Rnd1, Rnd2, Rnd3/RhoE, RhoH, RhoBTB1, and RhoBTB2) belong to a distinct branch of Rho GTPases and show a relatively late evolutional history (117). Rnd3/RhoE was first cloned by Forster and co-workers in the year 1996. It showed a unique characteristic in that it lacks GTPase activity, similar to Rnd1 and Rnd2 (38), which means that Rnd3 has a distinct manner in which to regulate its activity. Some stimuli including genomic stress and growth signals mediate its expression at both the mRNA and protein levels. Also, post-transcriptional modifications such as phosphorylation and prenylation can change its cellular location as well as its stability, which in turn regulates its activity.
In earlier studies, the function of Rnd3 was found to associate with RhoA/Rho-associated coiled-coil kinase 1 (ROCK1) signaling, but not RhoA/ROCK2, in the mediation of actin cytoskeletal dynamics (54). Overexpression of Rnd3 in Bac1.2F5 macrophages attenuated RhoA/ROCK1-mediated actin cytoskeletal assembly. The cell morphology was altered into a “round” shape, and displayed a decrease in stress fibers and an extension of filopodia and pseudopodia (54). Morphological changes such as these help maintain cell migration and cellular polarity in certain cell types.
Besides its ROCK1 inhibitory role, recent studies revealed several new functions of Rnd3, which strongly suggests a diversity of regulatory mechanisms that might be independent of RhoA/ROCK1 signaling (78, 103, 136, 146, 147). These discoveries provide a foundation for the understanding of this small G protein from a different perspective and adds complexity to Rnd3-mediated biological regulations. Moreover, the expression levels of Rnd3 have been suggested to be associated with human diseases and abnormal animal phenotypes; indicating its important role in the pathogenesis of human diseases. In this article, we review the physiological and pathological functions of Rnd3 from a systematic organ point of view paralleled with summaries of its molecular signaling pathways and mechanisms, as well as associated diseases. It has become clear that a more in depth understanding of the molecular regulations and tissue-specific functions of Rnd3 will lead to novel therapeutic targets for the treatment and diagnosis of human diseases.
Physiological Function of Rnd3
Actin Cytoskeletal Dynamics
The eukaryotic cytoskeleton refers to a protein fiber network mainly consisting of microtubules, microfilaments, and intermediate filaments in cells (13). Actin microfilaments are the thinnest fibers of the cytoskeleton that directly affect the cell shape, motility, cytokinesis, and phagocytosis. Rho GTPases have diverse effects on the organization of the actin filament system. Rho, Rac, and Cdc42 are three well-known members involved in regulating the organization of the actin cytoskeleton (3, 54). It has been documented that Rho regulates the formation of actin stress fibers, whereas Rac regulates lamellipodium formation, and Cdc42 regulates filopodium formation in Swiss 3T3 fibroblast cells (71, 96, 110, 111).
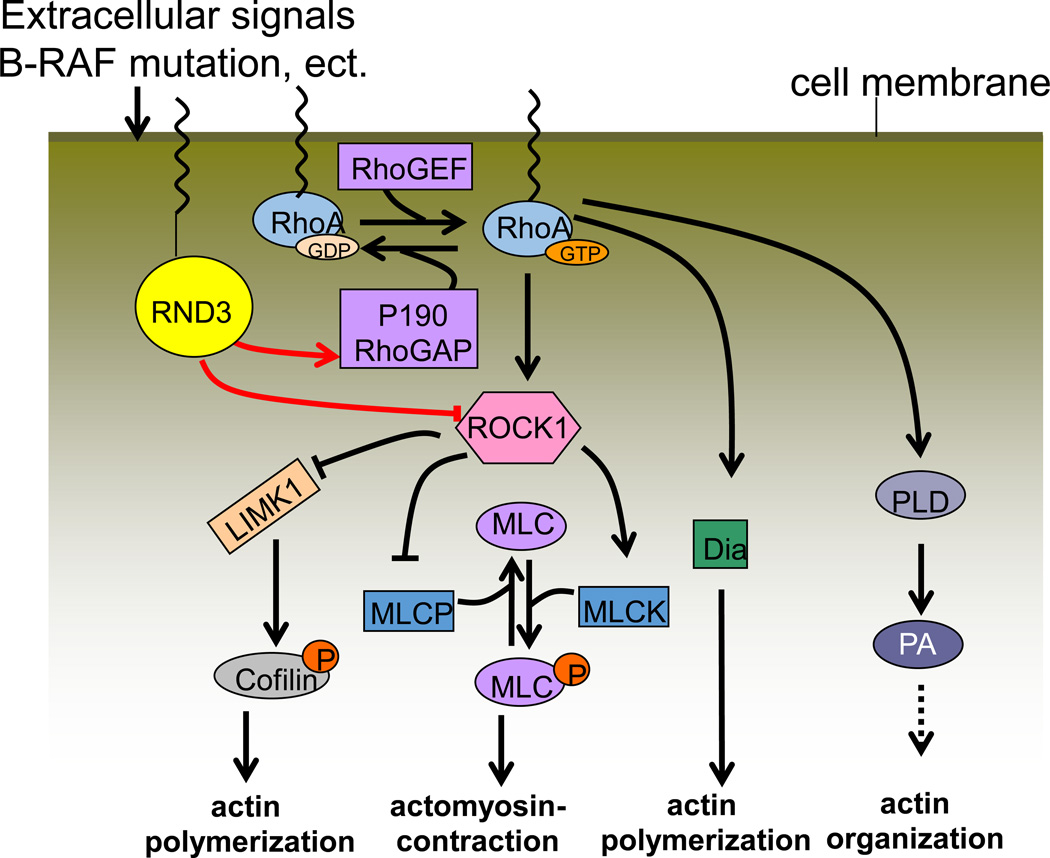
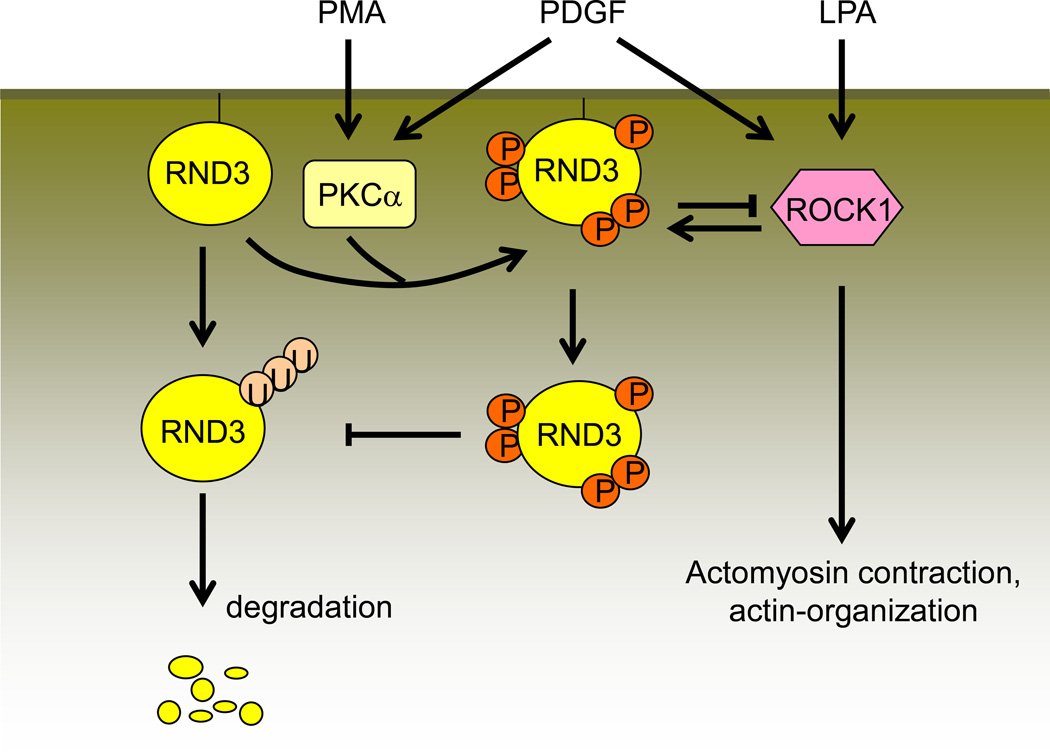
As an atypical member of the Rho GTPase family, Rnd3 is involved in mediating actin cytoskeletal rearrangement by inhibiting RhoA effector ROCK1 (Figure 1). Rnd3 can induce stress fiber disassembly by competitively binding to ROCK I and inhibiting it from phosphorylating downstream targets. Guasch and colleagues first revealed this by using Madin-Darby canine kidney (MDCK) epithelial cells and Bac1.2F5 macrophages (54). MDCK cells are comprised of stress fibers associated with integrin-containing adhesion complexes. Microinjection of Rnd3 into these cells induced the complete disappearance of stress fibers and caused the aggregation of actin filaments within the cytoplasm. However, Rnd3-induced actin reorganization led to the formation of extensions resembling filopodia and pseudopodia in macrophages, which do not contain stress fibers or associated local adhesions (33, 54, 109). Furthermore, Rnd3 also induced a transient decrease but subsequently stimulated a strong increase in stress fibers in different types of endothelial cells (49).
Figure 1. Rnd3 mediates actin cytoskeletal dynamics.
The switching of the GDP-bound to the GTP-bound RhoA form is a basic mechanism that mediates RhoA/ROCK1 signaling. Rnd3 promotes p190RhoGAP to hydrolyze the active GTP-bound form into the resting GDP-bound RhoA. Additionally, Rnd3 directly binds with ROCK1 and suppresses RhoA/ROCK1 signaling. MLC: myosin light chain; Dia: diaphanous; PLD: phospholipase D; PA: phosphatidic acid.
However, even in tumor cells, the function of Rnd3 may vary depending on the type of cells fabricating the tumor. In mammary epithelial tumor cells, RND3 regulates the assembly of the apical junction complex as well as tight junction sealing by mediating the co-localization of actin and inducing the localization of the adherent junction, protein beta-catenin, and the tight junction protein ZO-1 to sites of cell-cell contact (119). In BRAF mutant melanoma cells, RND3 mediates actin cytoskeletal rearrangement and is tightly associated with signal-mediated changes in cofilin phosphorylation. Depletion of RND3 elevates cofilin phosphorylation, stress fiber formation, and reduces cell invasion; indicating that RND3 is also a key contributor to oncogene-mediated reorganization of the actin cytoskeleton and focal adhesions (67). Recently, Georgess et al further revealed that the RND3/ROCK/Cofilin pathway is required for the promotion of podosome dynamics and patterning in osteoclast-mediated bone resorption (44). Taken together, RND3 mediates actin cytoskeletal rearrangement in a cell-context dependent manner.
Cell Polarity in Nervous System Development
Neurons and glial cells are the main components of the central nervous system (CNS). The polarity of neurons is particularly vital as neurons undergo complex morphological rearrangements to assemble into neuronal circuits and propagate signals. Spatiotemporal regulation of axons and dendrites is essential to maintain the polarity of neurons during development. During this process, the rearrangement of the actin cytoskeleton and microtubules plays a critical role in the initial establishment of polarity (126, 140).
It has been reported that Rnd3 is crucial for maintaining the polarity of neurons and glial cells in multiple aspects (74, 75, 102, 103, 105). In vivo results from a study showed that Rnd3 is required for the migration of cortical neurons. Deficiency of Rnd3 resulted in accumulation of F-actin in neuronal processes, and disruption in the migration of bipolar neurons (75). Disturbance of Rnd3 expression was also shown to lead to defects in proneural transcription factor Ascl1-driven cortical neuron distribution (103), which ensures integration of neurogenesis with neuronal migration and proper development of the cerebral cortex. Additional studies demonstrated that RND3 is involved in the synaptogenesis of hippocampal neurons (74). RND3-null hippocampal neurons exhibited a decrease in the total length and number of neurites, a reduction in axon outgrowth, and a delay in the process of neuronal polarization; probably due to a disruption in the RhoA/ROCK/LIMK/Cofilin signaling pathway (105) (Figure 2). In radial glial cells, RND3 was revealed to regulate interkinetic nuclear migration, control the orientation of the mitotic spindles, and maintain the adherent junctions by inhibiting actin filament polymerization (102).
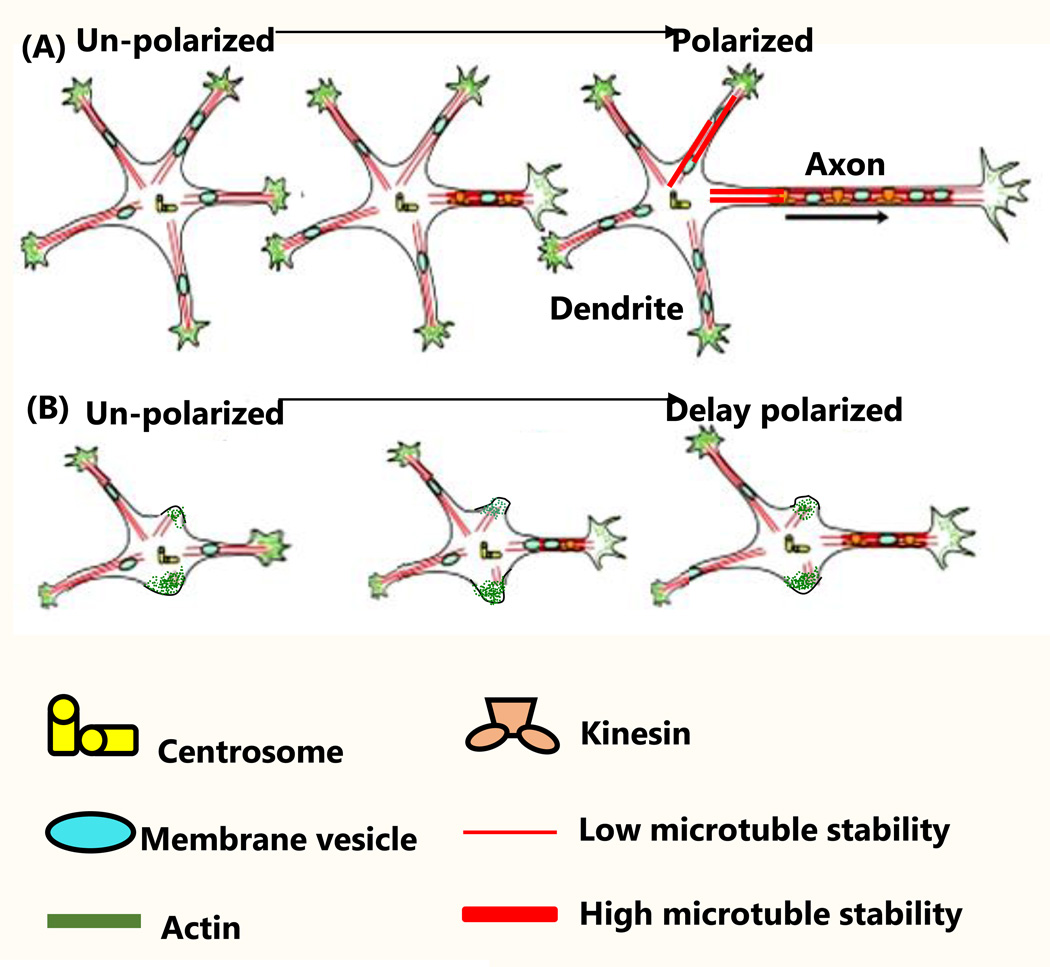
Figure 2. Rnd3 mediates neuron polarization.
(A) In Rnd3 WT neurons, intracellular stimuli induced dendrite and axon outgrowth with actin rearrangement (126). (B) In Rnd3-null neurons, the intracellular stimuli induced cell polarization but was delayed. This was evidenced by a reduction in the number of dendrites and shortened lengths of the axons. Figure 2A was adapted from ref. (140).
Given the fact that Rnd3 may affect neuronal polarity through actin-dependent (actin cytoskeletal arrangement) and/or actin-independent manners (e.g. inhibition of cyclin D1) (102), a deeper understating of Rnd3-mediated neurogenesis would provide important insights into the treatment and diagnosis of nervous system diseases.
Cell Apoptosis and Survival
P53 plays a critical role in mediating cell survival and apoptosis during a genotoxic stress response. It has been found that Rnd3 is a downstream target of p53, which favors cell survival through the inhibition of ROCK1-mediated apoptosis (100, 156). Ongusaha and co-workers showed that Rnd3 was transcriptionally induced in response to various DNA damaging agents, resulting in the disassembly of actin stress fibers. Knockdown of Rnd3 not only prevented the disassembly of the actin cytoskeleton, but also promoted apoptosis (100). However, it should be noted that inhibition of ROCK activity does not protect against apoptosis in all cell types, implying that additional factors likely contribute to the ultimate outcome (29).
An early study from our laboratory proposed that an increase in caspase-3 cleaves the 160 kDa native ROCK1 protein into a 130 kDa ROCK1 fragment. Upregulation of cleaved ROCK1 in heart subspecies provokes cardiomyocyte apoptosis and therefore contributes to the development of human heart failure (20). By using the Rnd3 gene depletion mouse model, our lab further revealed that genetic deletion of Rnd3 results in the hyperactivation of ROCK1 signaling in mouse hearts, which leads to apoptotic cardiomyopathy and heart failure (147). We went even further and generated the mutant mouse line, Rnd3+/−Rock−/−. The results showed that Rnd3+/−Rock−/− mice only partially rescued the cardiac apoptosis after transverse aortic constriction (TAC) surgery when compared to the Rnd3+/− mice; indicating the existence of a ROCK1-independent signaling pathway participating in cardiac apoptosis in the Rnd3-depleted hearts.
Previously, Boswell et al proposed that in UVB-irradiated human keratinocyte cells the upregulation of RND3 repressed apoptosis independent of the status of p53 and ROCK1 (16); suggesting p53- and/or ROCK1-independent mechanisms exist in cells other than cardiomyocytes involving Rnd3-mediated cell apoptosis. An alternative mechanism could be anoikis-induced apoptosis, which can be induced by integrin-mediated cell adhesion signaling, therefore activating Rnd3 expression-caused cell detachment (24).
Further investigations have revealed that Rnd3 participates in apoptosis in a cell type-dependent and microenvironment-dependent manner (10, 76, 107, 120, 151). In multiple human cancer cells, including U87 glioblastoma cells, T84 colon carcinoma cells, and A375 malignant melanoma cells, forced expression of RND3 induces apoptosis which may be through the inactivation of retinoblastoma protein (Rb) (107). Bektic et al showed that forced expression of RND3 in DU-145 RND3-negative prostate cancer cells promoted apoptosis by inducing caspase-3 (10). Additionally, other apoptosis-associated molecules/signaling pathways such as BAX (76, 151), BCL-2 (151), plakoglobin (120), and PTEN/PI3K/p-Akt (151) have been reported to be associated with RND3 abnormalities (Figure 3). However, the involvement of Rnd3 in apoptosis remains largely unknown.
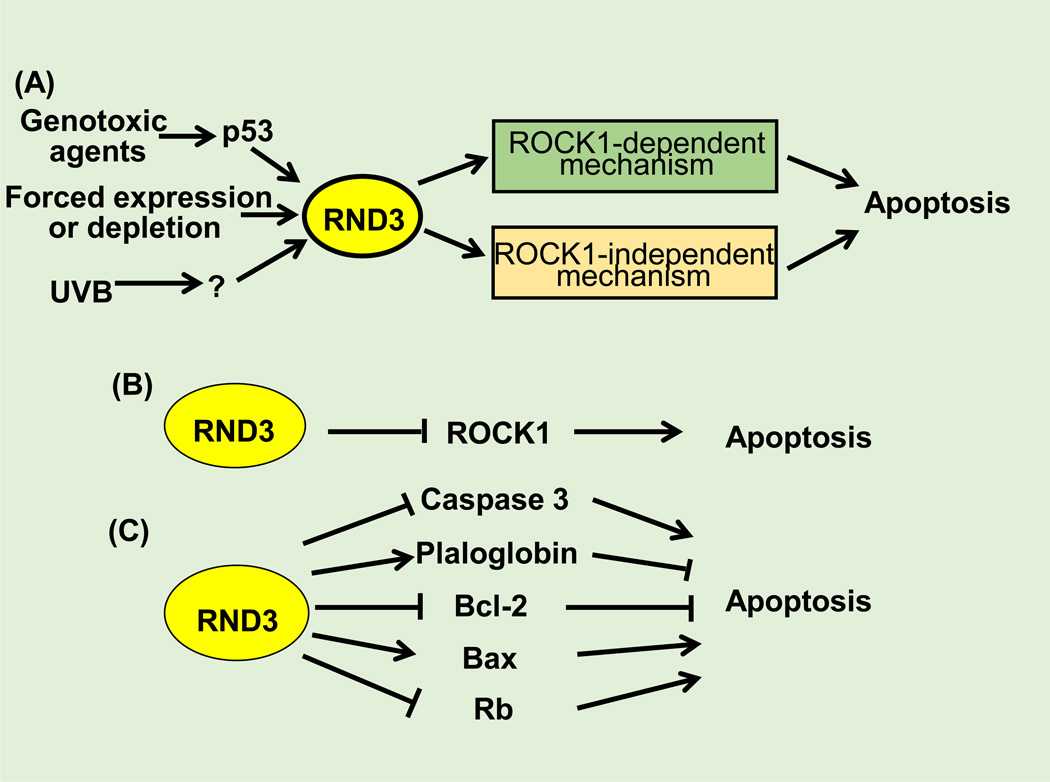
Figure 3. Rnd3 mediates apoptosis.
(A) Rnd3 is involved in apoptosis through ROCK1-dependent and –independent manners. (B) The ROCK1-dependent mechanism refers to that Rnd3 attenuates apoptosis through direct inhibition of ROCK1 activation. (C) The ROCK1-independent mechanism means that Rnd3 is involved in apoptosis through mediation of caspase3, BAX, Rb, plaloglobin, and BCL-2.
Cell Cycle Distribution
Cell cycle progression is the series of events that take place in a cell leading to its division and duplication; eventually resulting in two daughter cells. This process is strictly regulated by cyclins and cyclin-dependent kinases (CDKs) (95). Cyclin D is one of the major cyclins produced in terms of its functional importance. It binds with four Cdks: Cdk2, 4, 5, and 6. An early study showed that induction of Rnd3 expression in 3T3 fibroblast cells led to G1 stage accumulation. Furthermore, quiescent Rnd3-expressing cells failed to progress through the G1/S transition when they were stimulated by serum to enter the cell cycle. Mechanistically, Rnd3-induced cell cycle arrest is due to the inhibition of cyclin D1 or p21cip1 expression in a ROCK and RhoA inhibition-independent pathway (136). However, Rnd3 does not bind to the cyclin D1 promoter and affect its expression at the transcriptional level but mediates cyclin D1 expression at the post-transcriptional level. Interestingly, overexpression of cyclin D1 alone is not sufficient to overcome the Rnd3-induced cell cycle arrest and other molecules might also be participating in this regulation (136). In DU-145 cells, a prostate cancer cell line, the upregulation of RND3 inhibited the expression of Cdc2 and cyclin B1. Consequently, the cell cycle was arrested at the G2/M phase (10). Taken together, Rnd3 may affect the cell cycle at multiple stages as well as multiple involved factors (Figure 4).
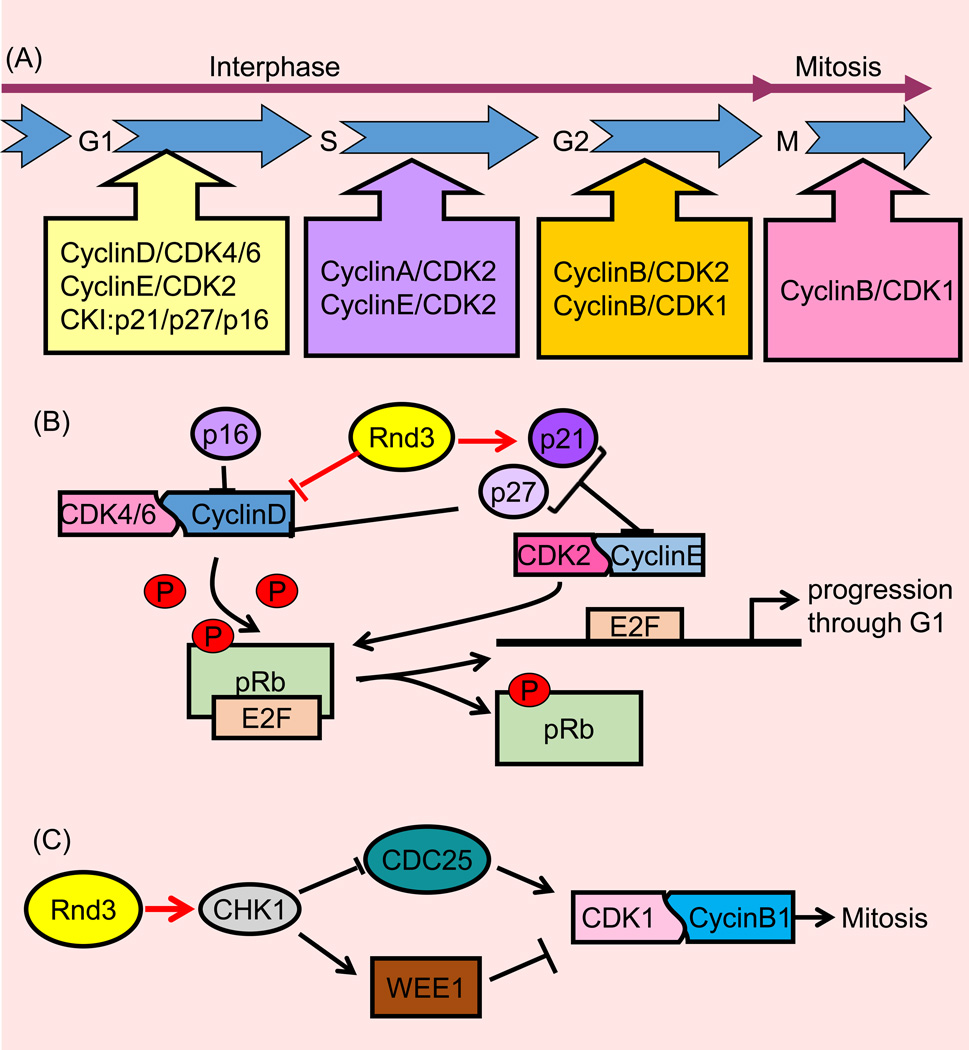
Figure 4. Rnd3 mediates the cell cycle.
(A) The cell cycle includes the transition from the G1 to S phase followed by the G2 to M phase transition. Upon stimulation, the cell enters the cell cycle going from the G0 to G1 stage. The appropriate stimuli lead to cell cycle completion; resulting in the production of two daughter cells. In each stage, special cyclins bind with cyclin kinase (CDK) to drive the progression of the cell cycle. This progression is inhibited by cyclin kinase inhibitor (CDI). M stage refers to mitosis in which two daughter cells are produced, the preceding stages can be referred to as interphase. (B) Rnd3 arrests the cell cycle at the G1 stage. Upon receiving a pro-mitotic extracellular signal, cyclin D binds with CDK4 and CDK6. As a result, low levels of phosphorylated pRb protein are phosphorylated into a hyperphosphorylated condition. Next, the hyperphosphorylated pRb release the nuclear transcription factor E2F from the E2F/DP1/Rb complex. The free E2F drives downstream gene expression including cyclin E, cyclin A, DNA polymerase, thymidine kinase, etc. The increasing cyclin E binds with CDK2 to further phosphorylate pRb and push the cell cycle from the G1 to S phase. In this stage, Rnd3 attenuates cyclin D and p21 expression and arrests the cell cycle at the G1 stage. (C) In the G2/M stage, Rnd3 attenuates CDK1 and cyclin B expression and therefore arrests the cell cycle at the G2/M stage. CHK1: checkpoint kinase 1; WEE1: a kind of mitosis inhibitor kinase; CDC25: cell division cycle 25, a dual-specificity phosphatase.
Cell Differentiation
During the cell differentiation process, an upregulation of Rnd3 expression was observed in various types of models. Guo et al (55) reported that there was a 9.4-fold increase in the RND3 mRNA expression levels in the well-differentiated type 3 lobules (Lob 3) of parous women’s breast tissues when compared with those of the undifferentiated type 1 lobules (Lob 1) of nulliparous women’s breast tissues. These findings indicate that differential RND3 expression occurs during normal human breast lobular differentiation. The differentiation and fusion of skeletal myoblast cells is a critical process during the formation into multinucleated myotubes. Fortier et al (37) revealed that enhanced Rnd3 expression, with a subsequent inhibition of RhoA/ROCK1 activity was crucial for myoblast fusion. Mei et al demonstrated that the disruption in Rho signaling, through the introduction of Rnd3 and a reduction in ROCK2, RhoA, and RhoGAP, was required for G protein subunit [Gα(z)]-induced myogenic differentiation in mouse myoblast C2C12 cells (91). In an in vitro model of villous cytotrophoblast fusion, Collett et al treated BeWo choriocarcinoma cells with a cell permeable cyclic AMP analogue, dibutyryl cyclic AMP (dbcAMP). As a result, there was a strong upregulation of Rnd3 24h after dbcAMP treatment, coinciding with the observation of the onset of cell fusion (28). Liebig with colleagues (77) also observed that RND3 protein levels were specifically and transiently upregulated upon basal keratinocyte differentiation, while RND3 depletion induced hyperproliferation and delayed initiation of keratinocyte differentiation. However, contrary to these reports, Rnd3 was found to be downregulated in cultured liver sinusoidal endothelium (LSEC) and was accompanied by an increase in stress fibers when compared to freshly isolated LSEC, suggesting a microenvironment-dependent differentiation program in LSEC (45). In addition, Rnd3 was also downregulated in rat intestinal epithelial IEC-6 cells during differentiation (88). So far, the mechanisms of cell differentiation involving Rnd3 are not clear since both positive and negative aspects of Rnd3 expression have been revealed; which implies a complicated role of Rnd3 in cell differentiation. Stringent regulation of Rnd3 expression spatiotemporally, such as through the use of the CRISPR/Cas9 system (32, 69), or in specific stem cell models might provide a novel insight into the underlying relationship between Rnd3 and cell differentiation.
Other Physiological Functions
A report showed that Rnd3 plays a role in glucose uptake in adipocytes (26), indicating Rnd3 may be involved in metabolism.
Pathological Functions of Rnd3
Cardiovascular Disorders
Cardiovascular system disorders, including ischemic heart diseases, currently account for the majority of morbidity and mortality worldwide (43, 101); implying a great need for further investigation in this field. In a previous study, primary information from genetic association analysis showed that UTR-3 RND3 SNP (rs115015150) is a risk factor for preeclampsia and the development of maternal cardiovascular disease later in life (61). Results of one microarray analysis showed that the upregulation of Rnd3 is involved in urocortins (UCNs)-mediated cardioprotection against myocardial ischemia/reperfusion (I/R) injury in rats (8). Furthermore, another study based on microarray screening indicated that Rnd3 mRNA was significantly downregulated in failing human hearts (GDS651/212724_at/RND3 in NCBI GEO profiles). These early reports have proposed the possible association of RND3 with the progression of cardiovascular diseases. However, these studies fail to provide complete investigations and direct evidence.
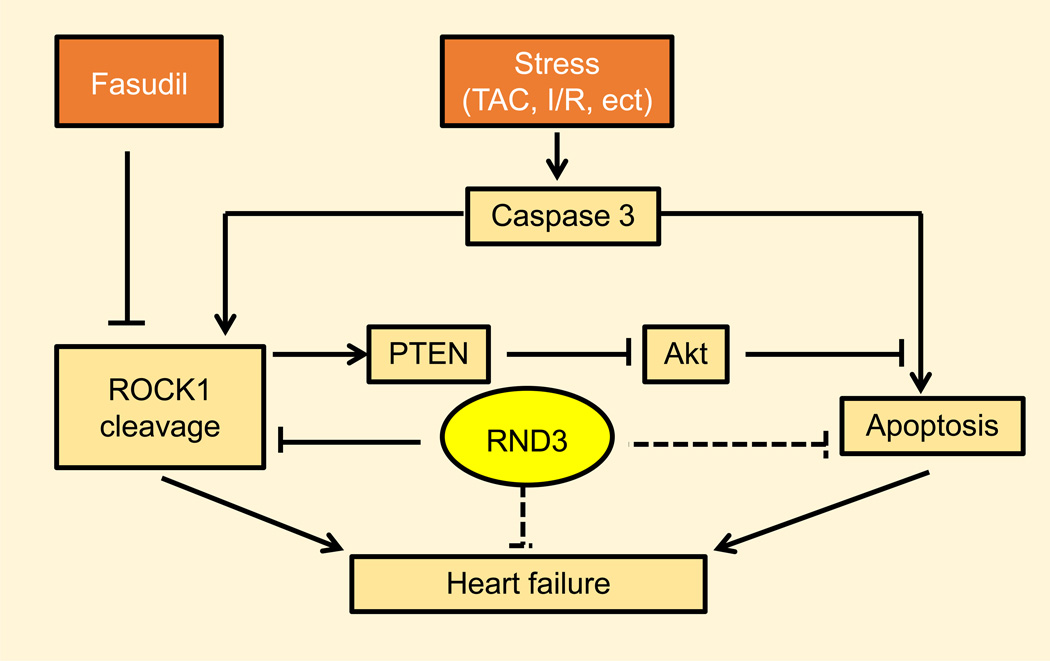
The relationship between Rnd3 deficiency and cardiovascular disease wasn’t revealed until recently. Our laboratory has directly linked decreases in Rnd3 to apoptotic cardiomyopathy, which may be a reason for the development of pressure overload-induced heart failure (147). We found that when the physiological conditions are not significantly altered there are no drastic morphological differences between the Rnd3+/+ (Rnd3 WT) and Rnd3+/− (Rnd3 Heterozygous) mice (Figure 5). The Rnd3+/− mice are normal in size and fertility, but the Rnd3-null mice suffer high embryonic lethality after E11.5 (Table 1). Through the use of these Rnd3 gene-trap mice, however, we found that Rnd3+/− haploinsufficient mice are susceptible to TAC-induced hemodynamic stress and develop apoptotic cardiomyopathy with heart failure at a later stage (147). Mechanistically, we determined that the insufficiency of Rnd3 may be partly associated with the enhanced activation of ROCK1 signaling in the pressure-overloaded hearts since there was increased caspase-3 activity; which was consistent with an earlier report from our laboratory (20). However, application of ROCK1 inhibitor, Fasudil, or knockout of ROCK1 in Rnd3 haploinsufficient mice only partly rescued the heart functions. We concluded that there must be a ROCK1-independent mechanism leading to the Rnd3 deficiency-induced heart failure (147) . Thus, our investigation first proposed the relationship between Rnd3 deficiency and apoptotic cardiomyopathy in animal hearts in both a ROCK1-dependent and -independent manner (Figure 6).
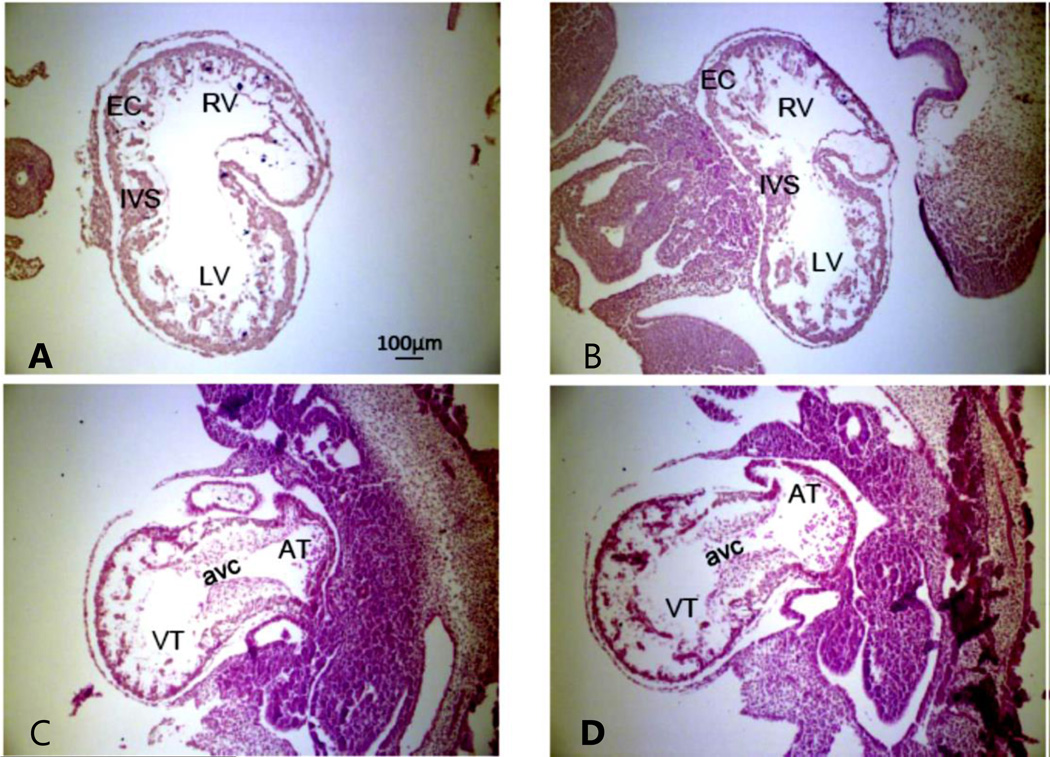
Figure 5. E10.5 embryos with H&E staining show no obvious morphological defects in the Rnd3 null heart.
Rnd3 WT E10.5 mouse heart coronal (A) and sagittal (C) sections, Rnd3−/− E10.5 mouse heart coronal (B) and sagittal (D) sections. EC: endocardial cushion; RV: right ventricle; LV: left ventricle; OFT: outflow tract; IVS: interventricular septum; VT: ventricular; AT: atrium; AVC: AV canal. Objective lens: 10X. This figure was published as Figure S1 in ref. (145) and republished with press authorization.
Table 1.
Rnd3−/− mice were lethal around E11.0
| Developmental stage |
Total embryos |
Wild-type | Heterozygote | Homozygote | P value |
|---|---|---|---|---|---|
| E9.5 | 128 | 32(25.0%) | 64(50.0%) | 32(25.0%) | 1.000 |
| E10.5 | 169 | 45(26.6%) | 75(44.4%) | 49(29.0%) | 0.313 |
| E11.0 | 238 | 53(22.3%) | 138(58.0%) | 47(19.7%) | 0.041 |
| E11.5 | 160 | 42(26.3%) | 98(61.2%) | 20(12.5%) | <0.001 |
| E12.5 | 154 | 48(31.2%) | 94(61.0%) | 12(7.8%) | <0.001 |
| postnatal | 900 | 282(31.3%) | 610(67.8%) | 8(0.9%) | <0.001 |
Chi-square analysis was performed for mouse genotyping data based on Mendelian ratios. This table was published as Table S1 in ref. (145) and republished with press authorization.
Figure 6. Rnd3 involvement in overpressure-induced cardiomyocyte apoptosis and heart failure.
After TAC surgery, full length 160 kDa ROCK1 was cleaved into 130 kDa pieces. This cleaved ROCK1 promotes cardiomyocyte apoptosis and therefore leads to heart failure. Fasudil, a chemical inhibitor of ROCK1, attenuates TAC-promoted apoptosis and heart failure. Rnd3, the endogenous ROCK1 inhibitor, may directly attenuate ROCK1-induced apoptosis and may also inhibit apoptosis through a ROCK1-indendent manner, however, it remains unknown. For more details, please refer to our published articles; ref. (20) and (147).
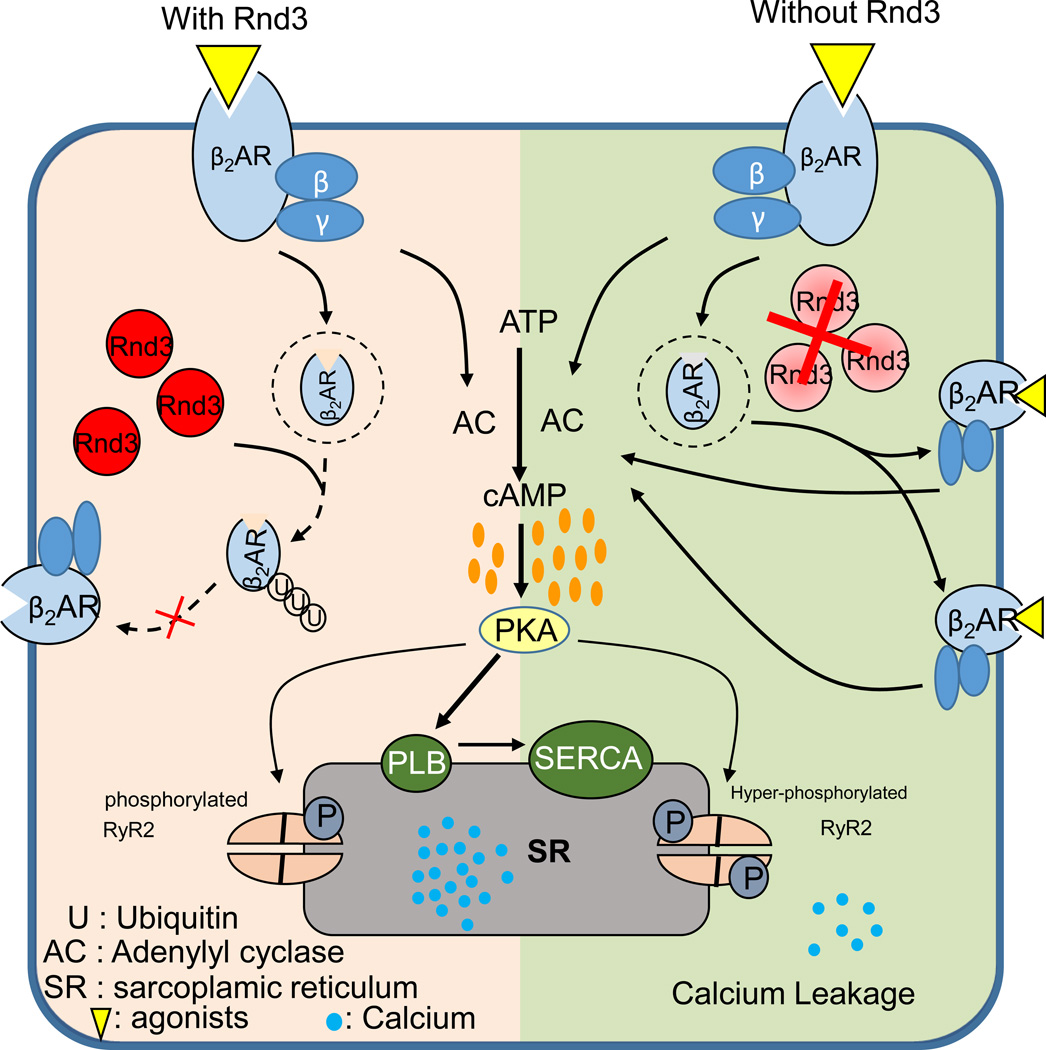
Besides induced apoptotic cardiomyopathy in adult mice, Rnd3 deficiency may also result in a cardiovascular disorder at the embryonic stage. Our previous observation showed high embryonic lethality around E11.5 in Rnd3-null mice (Table 1). In further investigation, we found that Rnd3−/− embryonic mouse hearts suffered from fetal arrhythmias. Mechanistically, lack of Rnd3 leads to attenuated β2AR ubiquitination, thus the accumulating β2AR protein promotes PKA signaling, which in turn results in dysfunction of the ryanodine receptor 2 (RyR2) calcium release channels and fetal arrhythmias as seen in the phenotype (Figure 7) (145).
Figure 7. Proposed model outlining the molecular mechanism of the Rnd3-deficiency-mediated calcium dysregulation.
Downregulation of Rnd3 attenuated β2-adrenergic receptor (β2AR) lysosomal targeting and ubiquitination, which in turn resulted in elevated β2AR protein levels and led to hyperactivation of protein kinase A (PKA) signaling. The PKA activation destabilized ryanodine receptor type 2 (RyR2) Ca2+ release channels, contributing to calcium leakage. This figure was published in ref. (145) and republished with the press authorization.
Taken together, through Rnd3 deficient mice, we found the indispensable role of Rnd3 in cardiac protection at both the embryonic and adult stage. Subsequently, spatiotemporal deletion of Rnd3 in mice will provide an interesting future endeavor in order to investigate the precise functions of Rnd3 in the mouse heart.
Nervous System
The expression profiles of Rnd3 in the CNS were examined in mouse postnatal development (7), and showed that Rnd3 protein expression is detected in all regions of the adult brain and spinal cord, with the highest levels in the olfactory bulb and cortex. Rnd3 protein expressions were significantly higher in all of the regions of the CNS during the first 2–3 weeks of postnatal development, and gradually decreased to lower levels as development approached adulthood. The Rnd3-null mice displayed obvious nervous system disorders, including lack of the common peroneal nerve, reduction in the number of spinal motor neurons, and delay of neuromuscular maturation; and therefore resulted in a series of abnormal neuromuscular symptoms (92). More evidence has shown that Rnd3-deficiency affects the development of calbindin-expressing cells in the olfactory bulb (6). Recent findings in our group revealed that Rnd3 gene function insufficiency is involved in the development of hydrocephalus (78). Hydrocephalus is a neurological disorder characterized by an excessive accumulation of cerebrospinal fluid; the mechanisms involved in hydrocephalus remain largely unknown (150). We used the Rnd3-null mouse (Rnd3−/−) model to address this disorder. We found that Rnd3-null mice developed severely dilated third ventricles, however, the fourth ventricles remained normal. The phenotype was also accompanied by narrowed or disconnected aqueducts. Mechanistically, we discovered that the insufficiency of Rnd3 led to Notch1 signaling activation-induced hyperplasia in ependymal cells (Figure 8). Our report is the first investigation that directly linked Rnd3-deficency to Notch1 hyperactivation (78), which was further confirmed by other groups (129). Gene expression profiling in brain subependymal giant cell astrocytomas (SEGAs) showed that Rnd3 was one of the mammalian targets of rapamycin (mTOR) effector genes, which could represent a novel therapeutic approach for treatment of SEGAs (132). Another study showed that ethanol-driven inflammatory response in the brain causes upregulation of Rnd3 and participates in actin cytoskeletal disorganization through decreases in both RhoA and Rac. The inflammatory response is elicited through the activation of the IRAK/ERK/NF-kB pathway and COX-2 expression (53). Finally, the differential expression profile of Rnd3 in the brains of MDMA and cocaine-treated mice showed alterations in cell cytoskeletons. Given the unique characteristics of gene expression, Rnd3 might also be a good candidate for research of drug abuse (89). Collectively, aberrant Rnd3 expression in a variety of nervous system disorders provides a potential therapeutic target for the associated diseases.
Figure 8. A proposed model outlining the molecular mechanism of Rnd3 deficiency-mediated hydrocephalus development.
(A) Upon activation, Notch receptors on ependymal cell membranes are cleaved into NICD, an intracellular isoform, which is translocated into the nucleus. In the nucleus, Rnd3 controls NICD protein accessibility to MAML and CSL through physical interaction with NICD. In the absence of Rnd3, Notch signaling is significantly enhanced due to the extra amount of NICD available for MAML and CSL to form transcriptomes; which facilitates ependymal cell proliferation resulting in aqueductal stenosis and hydrocephalus. (B) Illustrations of aqueductal stenosis formation. Depletion of Rnd3 promotes ependymal cell proliferation through an enhanced Notch signaling mechanism. The overgrowth of ependymal cells leads to the formation of multiple ependymal cell layers, inward cellular folding, and eventually luminal stenosis or closure. This figure was published as Figure 7 in ref. (78) and republished with press authorization.
Tumor
Rnd3 Expression Profile
To this date, most of the publications about the biofunction of Rnd3 focus on tumors. There are two different viewpoints pertaining to the function of Rnd3 in tumor biology. The first one is that Rnd3 is deemed as a tumor suppressor gene, elucidated by the evidence that loss of Rnd3 contributes to tumorigenesis or clinical progression. On the other hand, a small portion of investigations indicate that Rnd3 might be an oncogene. Overexpression of Rnd3 promotes the progression of tumors. Evidence focused on hepatocellular carcinoma (HCC) (52, 81, 83, 142), breast cancer (142, 155, 156), squamous cell carcinoma (151), mesenchymal tumor (12), prostate cancer (10, 90), colorectal carcinoma (82, 156), and lung cancer (155, 156) strongly support the rationale that Rnd3 may act as a tumor suppressor gene. Our lab recently revealed that RND3 is substantially downregulated in glioblastoma multiform (GBM), and this downregulation is negatively associated with the clinical progression and prognosis of patients (manuscript submitted). However, results from non-small cell lung cancer (30, 148), colorectal carcinoma (153), pancreatic cancer (50), and gastric carcinoma (35) studies showed that high RND3 expression positively correlated with tumor progression. However, there is some controversy over the results from lung cancer and colorectal carcinoma observations. This discrepancy indicates a diversified and cell-type specific Rnd3 function in cancer cells.
Tumor Migration and Metastasis
Early reports revealed that Rnd3 mediates actin cytoskeletal organization and cell adhesion which helps cell migration (54). Increasing data indicates that aberrant Rnd3 expression contributes to cancer cell migration and metastasis. Using DNA microarray technology, Trojan and colleagues screened that RND3 is related to the high metastatic potential of prostate cancer cells (131). Rnd3 mediates nerve growth factor (NGF)-induced neurite outgrowth in rat pheochromocytoma PC12 cells through inhibition of RhoA/ROCK1 signaling (128). On the molecular level, Pinner et al found that RND3 might compete with 3-phosphoinositide-dependent protein kinase-1 (PDK1) to bind with ROCK1 in A375 melanoma cells; affecting its ability to drive cortical acto-myosin contraction (106). In addition, mutant BRAF-promoted RND3 expression in highly invasive human melanoma cell lines, WM115 and WM793, and was strongly associated with an elevated invasive behavior in a 3D dermal-like environment (65); which further strengthens the notion that RND3 is required for melanoma cell metastasis. However, a report also indicated that suppression of BRAF in WM793 cells with a BRAF inhibitor led to the attenuation of RND3 expression and eventually resulted in enhanced cell migration in residual cells (66); implying a discrepancy with one of the previous studies (65). In contrast to the observations in melanoma cells, depression of RND3 promotes the invasion and metastasis of immortalized fibroblast cell line, Cen3tel (12). This relation is also extended to hepatocellular carcinomas (51, 52, 142). However, high RND3 expression in gastric cancer (35) and colorectal cancer (153) promotes metastasis and invasion in cancer cells.
The epithelial-mesenchymal transition (EMT) is a process by which epithelial cells lose their cell polarity and cell-cell adhesion, and gain migratory and invasive properties to become mesenchymal-like cells. EMT is a key element that drives epithelia-derived cancerous cell metastasis, which has been well addressed in many laboratories (40, 60, 104, 127). Zhou et al first observed that RND3 expression led to the upregulation of vimentin and the downregulation of E-cadherin in gastric cancer cells, thereby promoting cell migration (152). This evidence infers that aberrant RND3 expression is linked to the EMT process. However, contradictory to the gastric cancer cell results, Grise et al observed that attenuated RND3 expression in hepatocyte carcinoma led to the downregulation of E-cadherin, therefore promoting cell migration (52). Thus, aberrant Rnd3 expression may mediate cancer cell metastasis partially through the EMT process; though the molecular mechanism needs further investigation in various types of cancer.
The contradiction in relationships of Rnd3 expression in various types of tumor cells and the clinical progression is not yet clear. Maybe the background genetic mutation status such as p53 and BRF in different tumor cells bypass the Rnd3-inhibited cell cycle progression.
Chemotherapy Resistance
Rnd3 correlates to drug resistance in tumor cells. Overexpression of RND3 promotes the multi-drug resistance phenotype of SGC790I gastric cancer cells by post-transcriptionally decreasing BAX expression (76). Downregulation of RND3 is related to chemotherapy resistance in cisplatin (DDP) resistant human gastric carcinoma SGC7901/DDP cells (21, 124) and DDP-resistant nasopharyngeal carcinoma cell line, CNE2/DDP (79). Enhanced RND3 expression also plays a role in the chemotherapy resistance of lung cancer cells, A549/DDP (141). DDP is the first member of a class of platinum-containing anti-cancer drugs. Initial platinum responsiveness is high but the majority of cancer patients will eventually relapse with cisplatin-resistance disease. Mechanisms of cisplatin resistance have been proposed including changes in cellular uptake and efflux of the drug, increased detoxification of the drug, inhibition of apoptosis, and increased DNA repair (125). These reports proposed the role of RND3 in the DDP-resistance of tumor cells. RND3, along with other mTOR signaling genes (MRAS, PRKAA2, PLD1), have been found to be differentially expressed in paclitaxel-treated hypopharynx cancer cells (144). Apart from the function of Rnd3 in apoptosis-mediation, its associated mechanisms of chemotherapy resistance remain unknown.
Reproductive System
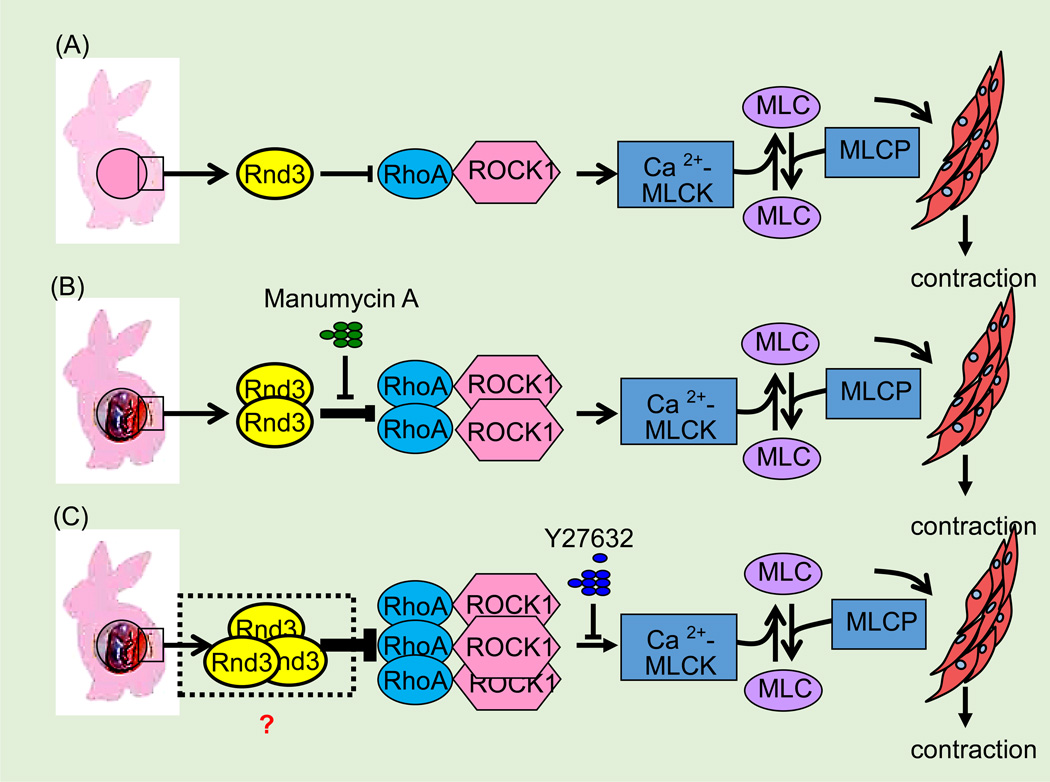
Accumulating data have shown that Rnd3 may have a function in the myometrium of the uterus during pregnancy. In rabbit and rat animal models, increasing Rnd3 expression was observed in myometrium samples from pregnant females compared to samples from non-pregnant females; this upregulation of Rnd3 correlated with an inhibition of ROCK-mediated Ca2+ sensitization during pregnancy (19, 64) (Figure 9). As for human myometria (73), the expression and function of RND3 may be similar to that of the animals (19, 64). Rnd3 might represent a potential target for the development of new treatments for pre-term labor complications.
Figure 9. Rnd3 antagonizes RhoA/ROCK1 signaling during various stages of pregnancy.
(A) In the non-pregnant rabbit uterus, constitutively expressed Rnd3 antagonizes RhoA/ROCK1 signaling which maintains the smooth muscle cells of the uterus in the quiescent state. (B) During the mid-pregnancy stage (15 days) in the rabbit uterus, upregulated Rnd3 antagonizes enhanced RhoA/ROCK1 signaling and maintains the calcium-dependent increase in Ca2+ MLCK and tension at a higher level (Ca2+ sensitization). This effect can be recovered by treatment of muscle strips from mid-pregnancy myometrium with farnesyl-transferase inhibitor, Manumycin A. (C) During late pregnancy (31 days), upregulation of RhoA and Rho kinase expression is associated with an increase in Ca2+ sensitivity of contractile proteins that is inhibited by the Rho kinase inhibitor, Y-27632. MLC: myosin light chain; Ca2+-MLCK: Ca2+-calmodulin-activated myosin light chain kinase; MCLP: myosin light chain phosphatase.
Endometriosis is a common gynecological condition, though its etiology remains unclear. Molecular epidemiological data indicate that a dysregulation of RND3 occurs in autologous ectopic and eutopic endometria; implying that Rnd3 is overtly associated with endometriosis (57). Subsequently, genetic analysis of a case-control cohort using a genome-wide association study (GWAS) revealed that the SNP of RND3-RBM43 on 2q23.3 was identified to be in association with endometriosis (1). Therefore, Rnd3 may represent a potential diagnostic marker or therapeutic target for endometriosis.
Other reports have also shown that Rnd3 is a significant gene that is involved in the pre-ovulatory LH surge-impacted follicular theca and granulosa transcriptomes (25). In addition, RND3 is downregulated in estradiol-treated prostatic stromal cells (11).
Other Disorders
The bacteria Staphylococcus aureus produces the toxic-shock inducing molecule, enterotoxin B (SEB), which is a common cause of food poisoning. Downregulated Rnd3 differentially expressed transcripts (DETs) were detected in SEB-exposed renal proximal tubule epithelial cells (RPTEC); which coincided with an increase in RhoA mRNA and actin stress fiber formation (59). Although the function of Rnd3 is unknown in response to treatment, the changes in Rho family protein expression imply involvement in enterotoxic shock with marked acute vasodilation, leading to severe hypotension.
Irritable bowel syndrome (IBS) is a common functional gut disorder characterized by abdominal pain, discomfort, diarrhea, and/or constipation related to gut motility disorder. In an IBS study, the female hormone estrogen was found to be related to the development of IBS symptoms by modulating gut motility and sensitivity to mechanical distention. Rat ileal circular smooth muscle tissues were treated with 17β-estradiol in order to mimic IBS symptoms. Induced RhoA/ROCK signaling led to the phosphorylation of CPI-17, a phosphorylation-dependent inhibitor of myosin phosphatase, ultimately leading to increased Ca2+ sensitization and smooth muscle contractions. Upregulation of Rnd3, Rnd2, and RhoG have been observed to counteract the effects of RhoA. It is suggested that Rnd proteins, including Rnd3, can inhibit the RhoA mediated Ca2+ sensitization of contractile mechanisms, which are mediated by CPI-17 phosphorylation in ileal smooth muscle tissues (122).
RND3 has also been reported to affect the migration of bronchial smooth muscle cells (BSMCs) in asthma-related airway remodeling (72). If bronchial epithelial cells are exposed to isophorone diisocyanate (IPDI), an aliphatic diisocyanate used to manufacture polyurethane plastics, or other low molecular weight chemicals occupational adult asthma can occur, resulting in increased BSMC proliferation and migration through the activation of FAK, Src, ERK1/2, and AKT. However, as a downstream target, RND3 has only been found to be responsible for BSMC migration, not proliferation (72). The regulatory role of RND3 in BSMC migration provides a new critical function of Rnds involving airway remodeling.
Rnd3 Regulation
Transcriptional Regulation
Since Rnds have no GTPase and keep a state of constitutive activation (41), transcriptional regulation is a basic mechanism for regulating the level of Rnd3 activation. Accumulating data also show that Rnd3 can be induced at transcriptional levels by various stimuli (115, 117). The stimuli mediating Rnd3 expression is included in Table 2. Notably, it is known that hypoxia inducible factor-1α (HIF-1α) directly binds to the Rnd3 gene promoter and drives its expression. HIF-1α is found to be the most specific and key transcription factor that mediates cellular hypoxia response, so far (99). HIF-1α is essential in pathological phenomena such as hypoxia, ischemia, and inflammatory injury; which mediate the cellular response to hypoxia through a series of transcriptional regulations for hypoxic response gene expression (137). An early report from Zhou et al validated that HIF-1α binds to a hypoxia-responsive element (HRE) in the RND3 promoter and directly mediates RND3 expression in gastric cancer cells (152).
Table 2.
Factors that mediate Rnd3 expression and activation
| Inducer | Function | References |
|---|---|---|
| UVB | Upregulates Rnd3 expression | (15, 93) |
| Chemotherapeutic agents |
Upregulates Rnd3 expression | (123) |
| TGF-β1 signaling | Downregulates Rnd3 expression | (130) |
| Mechanical stress | Upregulates Rnd3 expression | (31) |
| Hypoxia | Upregulates Rnd3 expression | (152) |
| Cyclic AMP | Upregulates Rnd3 expression | (28) |
| Eccentric exercise | Upregulates Rnd3 expression | (85) |
| Urocortin hormones | Upregulates Rnd3 expression | (8) |
| 17beta-estradiol | Upregulates Rnd3 expression | (122) |
| Staphylococcal enterotoxin B |
Downregulates Rnd3 expression | (59) |
| Ethanol | Upregulates Rnd3 expression | (53) |
| PDGFRβ signaling | Upregulates Rnd3 expression | (5) |
| MDMA and cocaine | Upregulates Rnd3 expression | (89) |
| PKC signaling | Translocation and phosphorylation | (87) |
| Zymogen serine protease factor VII |
Upregulates Rnd3 expression | (18) |
| NF-κB2 | Upregulates Rnd3 | (94) |
| Trichostatin A | Upregulates Rnd3 expression | (23) |
| Ucocorticoid | Downregulates Rnd3 activity | (118) |
| Thyrotropin | Downregulates Rnd3 expression | (134) |
Also in gastric cancer cells, it has been reported that histone deacetylation at the epigenetic level might regulate RND3 expression (23). Treatment of gastric cancer cells with trichostatin A, to inhibit histone deacetylation, enhances the activity of the RND3 promoter. While treatment with 5-Aza-2’-deoxycytidine, to inhibit DNA methylation, affects neither the RND3 promoter activity nor RND3 expression levels (23). However, the transcriptional regulation of Rnd3 at the epigenetic level should be tested in more types of cell lines.
Post-Transcriptional Regulation
Phosphorylation and Farnesylation
Post-translational modifications such as phosphorylation and prenylation are additional critical regulation methods involving Rnd3 activity, as well as subcellular localization. ROCK1 and PKC are two crucial factors for Rnd3 phosphorylation (87, 114). It was documented that the amino acid residues out of the core GTP-binding domain near the N-terminal and C-terminal in Rnd3 are crucial for its phosphorylation (115). The phosphorylated Rnd3 is localized in the cytosol while the unphosphorylated Rnd3 remains isolated to the cellular membrane. Rnd3 protein stability is also increased after phosphorylation (114). Interestingly, the crystal structure of Rnd3 provides the structural basis for its multi-site phosphorylation (36, 68). There is a negative feedback loop between Rnd3 and ROCK1. On one hand, ROCK1 promotes Rnd3 phosphorylation and stabilizes Rnd3; on the other hand, ROCK1-phosphorylated Rnd3 enhances Rnd3 regulation of ROCK1 function (113). Rnd3 can be inactivated through the ubiquitin/proteasomal degradation mechanism (80). However, the phosphorylated Rnd3 is resistant to degradation, and its activity is improved (114) (Figure 10).
Figure 10. Rnd3 phosphorylation and degradation.
Unphosphorylated Rnd3 binds to the cell membrane and is degraded through the ubiquitin/proteasome system. PMA-activated PKCα and LPA-activated ROCK1 promote the phosphorylation of Rnd3. The phosphorylation of Rnd3 enhances its stability and causes its translocation into the cytoplasm where it is resistant to ubiquitin/proteasome system degradation. The phosphorylated Rnd3 also binds with ROCK1 and therefore attenuates ROCK1 functions. PMA: phorbol 12-myristate-13-acetate; PDGF: platelet-derived growth factor; LPA: lysophosphatidic acid (117).
Characteristic differences of small GTPases are due to specific changes in several amino acids, except for the highly conserved region responsible for GTP hydrolyzation (38). Rnd3 can be farnesylated through the addition of a 15-carbon isoprenoid, known as a farnesyl group. This farnesyl group is added to proteins that bear a CaaX motif, a four amino acid sequence at the carboxyl-terminus of the protein. Rnd3 is farnesylated in vivo, which is required for association with the plasma membrane and with an unidentified cellular structure that may play a role in cell adhesion (38). Additionally, farnesylation of Rnd3 leads to subcellular translocalization of the protein, which indicates that it may be involved in multiple biophysical and biochemical interactions of multiple signaling pathways.
MicroRNA
MicroRNAs (miRNAs) are endogenous non-coding small RNAs at approximately 22-bp in length, that mediate gene expression at the post-transcriptional level by binding complementarily to the 3’ untranslated region (UTR) of target messenger RNAs (mRNAs) (2, 9). Most of the identified miRNAs inhibit gene expression, while a small portion of miRNAs upregulate gene expression (133). MiRNAs exhibit a tight association with Rnd3 expression. Xiao et al confirmed that miR-200b interacts with the 3’ UTR of RND3 by conducting luciferase assays in HeLa cells, and thereby directly manipulated RND3 expression at the mRNA level. As a consequence, cyclin D1, a downstream target of RND3, was increased along with S-phase entry (143). The association between miR-200b and RND3 could be extended to colorectal carcinoma (39) and human Tenon’s capsule fibroblasts (HTFs) (130). Using the informatics and qRT-PCR approach, Hurteau et al identified that Rnd3 may also be a target of miR-200c (58). In colorectal carcinoma, miR-17 also directly targets RND3 in vitro and promotes cell proliferation, tumor growth, and cell cycle progression (82). In addition, miR-503 transcriptionally mediates RND3 expression, and affects its cisplatin sensitivity in cisplatin-resistant lung cancer cells, A549/DDP (141). Given this, Rnd3 is thought to act mainly as a tumor suppressor gene. The miRNA-attenuated RND3 expression may be one of the reasons for RND3 downregulation in many types of human tumor tissues.
Interaction of Rnd3 with Other Molecules/Signaling
RhoA/ROCK1
ROCK1 and ROCK2 are downstream effectors of the small GTPase RhoA, however, Rnd3 only inhibits RhoA/ROCK1 signaling, not RhoA/ROCK2 (112, 113). The similarity in Rnd3 and RhoA sequences suggests that Rnd3 competes with RhoA for interaction with ROCK1 (112). Interestingly, Rnd3 binds to the amino-terminal region of ROCK1, encompassing the kinase domain at a site distant from the carboxyl-terminal RhoA-binding site. Rnd3 binding inhibits ROCK1 from phosphorylating its downstream target, myosin light chain phosphatase (MLCP); therefore increasing the activity of the phosphatase to dephosphorylate myosin II, resulting in reduced actomyosin contractility. Differing from previous studies, Chun et al indicated that RND3 can also bind to the kinase domain of ROCK1 and inhibit the catalytic activity of ROCK1. The effect of inhibition was notably increased in obese type 2 diabetic patients, accounting for defective ROCK1 activity (27).
Apart from directly binding to the N-terminal kinase domain of ROCK1 (42), Rnd3 also antagonizes RhoA/ROCK1 signaling through other effectors. P190RhoGap and Syx are two main effectors that are involved in Rnd3-mediated RhoA/ROCK1 signaling activation (48, 138).
p190RhoGAP
GTPase-activating proteins (GAP) function as a mediator to hydrolyze GTP to GDP. GAPs inhibit intrinsic GTPase activity by converting the GTPase from the active form (GTP-bound) to the inactive form (GDP-bound). Among the near 70 different GAPs in the human genome, p190RhoGap is the most highly and ubiquitously expressed regulatory protein of the family (22). It was shown that Rnd3 protein binds to p190RhoGAP and increases its GAP activity towards GDP-bound RhoA, and reduces the RhoA-ROCK1 activity as a result (48, 138). Further details revealed that a KERRA (Lys-Glu-Arg-Arg-Ala) sequence of amino acids in the N-terminus of Rnd3 determined its binding and activation to p190RhoGAP (98). C2C12 myoblast cells in the absence of Rnd3 exhibited defective p190RhoGAP activation and RhoA inhibition during myoblast fusion (37).
Syx
Similar to p190RhoGAP, synectin-binding RhoA exchange factor (Syx) is another effector of Rnd3. Using mass spectrometry, Syx was identified as a new Rnd3 target (48). Like p190RhoGAP, Syx binds to Rnd3 utilizing a region similar to the classic Raf1 Ras-binding domain (RBD). Functionally, it was found that inhibition of Syx by RNAi in zebrafish at the one-cell stage resulted in embryos with a shortened anterior-posterior body axis. In addition, an Rnd3-binding defective mutant of Syx1B, containing a mutation in the RBD, was even more potent in rescuing the embryonic defects than the wild-type Syx1B; indicating that Rnd3 negatively regulates Syx activity in vivo. Furthermore, the binding of Syx leads to improvement in Rnd3 stability (47).
Notch1 Intracellular Domain (N1-ICD)
Notch signaling pathway consists of the Notch receptor, ligand, DNA binding proteins, and effector molecules; all of which are highly conserved in evolution and exist widely in both vertebrates and invertebrates. Notch signaling pathways are activated through the interaction between adjacent cells, which contributes to the regulation of cell, tissue, and organ differentiation and development (70). An early report on somite segmentation and differentiation in Xenopus embryos proposed the correlation of Notch signaling to Rnd3 expression (46). Recently, our study directly confirmed that Rnd3 is a negative regulator of Notch signaling, in which Rnd3 mediates Notch1 intracellular domain (N1-ICD) protein degradation through ubiquitination rather than transcriptional regulation (78) (Figure 8). This relationship was further tested in different cancer cells by different groups, including ours, given the solid function of Notch signaling in tumorigenesis (129). We successfully tested this proposal in GBM and further confirmed that depletion of Rnd3 promotes Notch1 signaling activation through the mechanism of RND3 physically interacting with NICD, CSL, and MAML1. The Notch transcriptional complex factors promote NICD ubiquitination and facilitate the degradation of these cofactor proteins by facilitating the binding of NICD to E3 ligase FBW7 (manuscript submitted). Dissimilarly, a report on skin epithelia-derived squamous cell carcinomas revealed that RND3 controls a key step in Notch1 signaling activation by positively mediating NICD (157). Because the biofunction of Notch signaling is cell-context dependent, it seems that the relationship between Rnd3 and Notch signaling needs further investigation.
β2-Adrenergic Receptor (β2-AR)
The β2-AR is a beta-adrenergic receptor within the cellular membrane that reacts with adrenaline as a hormone or neurotransmitter. After binding with its effectors, the class C L-type calcium channel CaV1.2, the receptor-channel complex is coupled to the Gs G protein, which activates adenylyl cyclase. This activation catalyzes the formation of cyclic adenosine monophosphate (cAMP) which then activates protein kinase A; thus producing a wide range of biological effects, including an increase in the http://en.wikipedia.org/wiki/Heart_ventriclecontractility and automaticity of cardiac muscle and the heart rate in the sinoatrial node. Recently, our study revealed for the first time that Rnd3 is involved in β2-AR ubiquitination (145). As a result, the insufficiency of Rnd3 leads to attenuated β2-AR ubiquitination and in turn results in the hyperactivation of PKA signaling; which eventually leads to fatal arrhythmias due to the high intracellular Ca2+ levels caused by PKA-destabilized ryanodine receptor type 2 (RyR2) channels (Figure 7).
PlexinB2
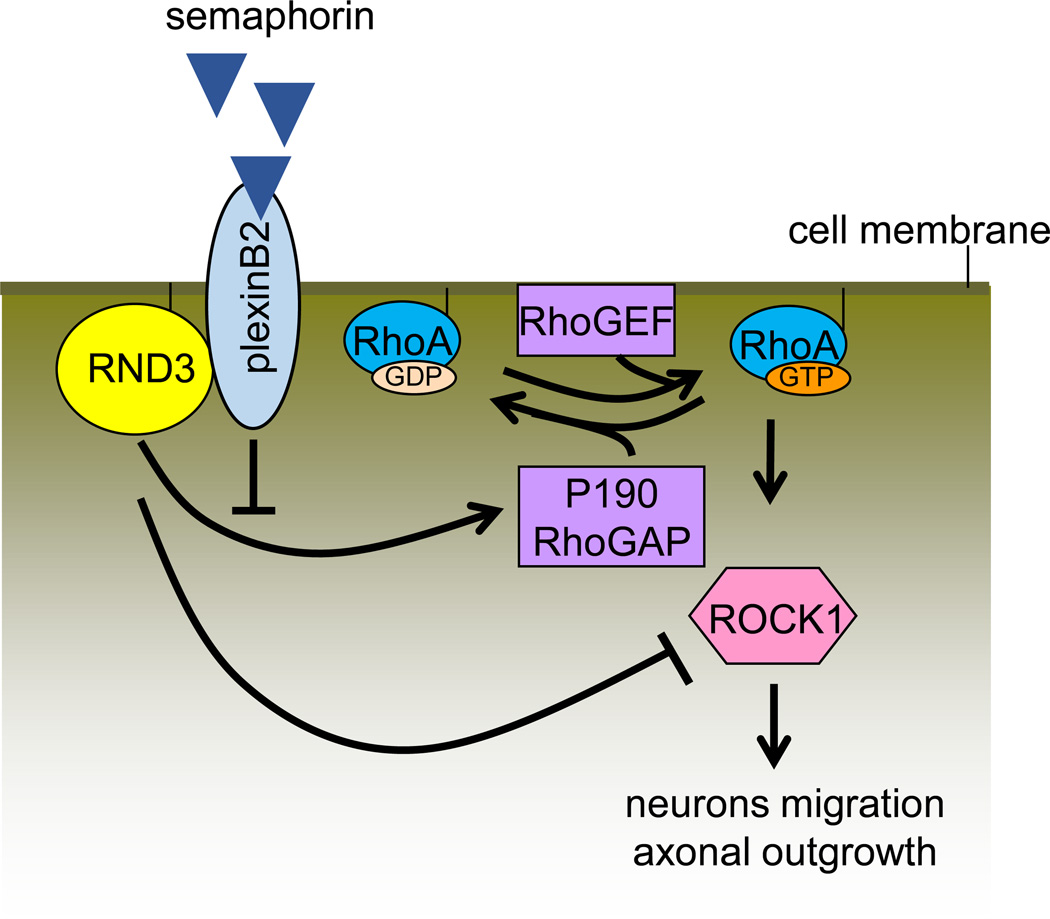
Plexins are cell surface receptors for semaphorin molecules; their interaction governs cell adhesion and migration in a variety of tissues (56). Early reports have shown that the binding of Semaphorin 4D (Sema4D) receptor PlexinB1 with Rnd1 mediates growth cone collapse of hippocampal neurons (97). Recent studies confirmed that PlexinB2 receptor interacts physically and functionally with Rnd3, and therefore stimulates RhoA activity in migrating cortical neurons (4). Mechanistically, PlexinB2 competes with p190RhoGAP for binding to Rnd3, blocking the Rnd3-mediated inhibition of RhoA and also recruiting RhoGEFs to directly stimulate RhoA activity (Figure 12).
Socius
Utilizing yeast two-hybrid screening and Rnd1 as bait, a protein known as Socius was identified to be the effector of Rnd GTPase (63). Although the biological function of Socius remains largely unknown, it has been demonstrated that it directly binds to Rnd1, Rnd2, and Rnd3 through its COOH-terminal region and participates in the Rnd GTPase-induced signal transduction pathways; leading to the reorganization of the actin cytoskeleton.
Other Molecules
Some other molecules including Skp2, 14-3-3 protein, Nanog, Plakoglobin, Foxd3, 4E-BP1, AES, and PDK1 are reported to associate with Rnd3 and mediate it at a post-transcriptional level in terms of expression, localization, and activation (Table 3).
Table 3.
Factors of Rnd3 interaction and function
| Rnd3 partner | Function | References |
|---|---|---|
| p190RhoGAP | Downregulates RhoA | (38, 138) |
| Syx | Increases Rnd3 stability | (47, 48) |
| ROCK1 | Phosphorylation, location, and stability | (114) |
| Socius | Loss of stress fibers | (63) |
| PlexinB1 | --- | (34) |
| PlexinB2 | Competes with p190RhoGAP for binding to Rnd3 | (4) |
| Notch1-ICD | Decreases N1-ICD ubiquitination | (78) |
| Mediates N1-ICD nuclear translocation | (157) | |
| β2-AR | Promotes β2-AR ubiquitination | (145) |
| HIF-1α | Upregulates Rnd3, promotes cancer metastasis |
(152) |
| Skp2 | Mediates proteasomal degradation of Rnd3 |
(80) |
| 14-3-3 | Mediates Rnd3 translocation | (116) |
| Nanog | Cell migration | (154) |
| Plakoglobin | Cell apoptosis | (120) |
| Foxd3 | Downregulates Rnd3 expression | (62) |
| 4E–BP1 | Cell proliferation | (135) |
| AES | Mediates Rnd3 expression | (142) |
| PDK1 | Competes with Rnd3 for binding to ROCK1 |
(106) |
Conclusions and Prospective
Until now many physiological roles of Rnd3 have been demonstrated. The study of these basic functions help us to understand the multiple functions of the Rnd3 gene. As the endogenous antagonist of RhoA signaling, Rnd3 alters actin cytoskeletal rearrangement; regulating cell morphology, cell migration, neuron polarity, and smooth muscle cell contraction. On the other hand, Rnd3 affects the cell cycle mainly through the inhibition of cyclinD1, therefore suppressing cell proliferation. Rnd3 is also a mediator in cell apoptosis and survival. These molecular mechanisms need to be thoroughly elucidated through the use of Rnd3 overexpression and Rnd3 depletion cell models. The genome-editing strategy, such as the CRISPR/Cas9 system, (121, 149) would be helpful in the knockdown of Rnd3 for the creation of heterozygous or homozygous phenotypes in animal models. We have established Rnd3-null cell lines using the CRISPR/Cas9 technology in order to better understand the biofunction of Rnd3 in vitro. Recent studies have been released in which Rnd3 was shown to be involved in cell differentiation. However, more studies should be extended using various types of stem cells, because Rnd3 defects lead to disorders in cortex neuron development (7, 103) and myoblast cell differentiation (37).
Aberrant Rnd3 expression contributes to the development of various systemic disorders. The contribution of Rnd3 to pathological conditions may partly be in a ROCK1-dependent manner; which was mostly demonstrated by in vitro cell culture studies and tissue screening assays. However, through the establishment of the Rnd3 gene knockout mouse model, our laboratory found that aberrant Rnd3 expression plays a critical role in some systematic diseases through a ROCK1-independent mechanism (78, 145, 147). Therefore, using a conditional Rnd3 knockout mouse would be a powerful tool for research of environmental stress-induced diseases such as heart failure, asthma, intestinal motility dysfunction, and so on. The main functions and mechanisms that Rnd3 contributes to in various physiological and pathological conditions are shown in Table 4.
Table 4.
Aberrant Rnd3 expression functions through ROCK1-dependent and –independent manners
| ROCK1-dependent mechanism | Physiological and pathological conditions |
|---|---|
| Cell polarity | CNS development, cell migration |
| Cell contraction | Pregnancy and habitual abortion, asthma |
| Apoptosis | Heart failure |
| ROCK1-independent mechanism | Physiological and pathological conditions |
| Cell proliferation (Notch1) | Hydrocephalus, tumor |
| Ca 2+ leakage (β2AR/PKA) | Arrhythmias |
| Apoptosis (BAX, BCL-2, Rb, etc.) | Heart failure, tumor |
| Chemoresistance (BAX) | Tumor |
| Cell cycle arrest (cyclin D, cyclinB1, p21) Cell differentiation (Notch1, etc.) EMT |
Tumor Myoblast infusion, somite formation Tumor metastasis |
| - | Endometriosis |
EMT: Epithelial-mesenchymal transformation.
Rnd3 overexpression is observed in some types of tumors while downregulation has been reported in other types. Future investigations of the involved molecular mechanisms are necessary to address this discrepancy. Taking this into account, the polymorphism of Rnd3 SNP and Rnd3 mutations should be considered. Interestingly, Rnd3 also contributes to the chemotherapeutic resistance of cancer; so future investigations should focus on targeting Rnd3 in order to expand our knowledge of available cancer treatments.
So far, reports have shown that Rnd3 expression is mediated upon environmental stress and some cytokine signaling. The molecular mechanisms involving Rnd3 expression and its effectors need more attention (e.g. the interaction between Rnd3 and Notch1-ICD, HIF-1α, and NFκB).
Figure 11. PlexinB2 and Rnd3 competitively bind to p190RhoGAP.
Rnd3 promotes p190RhoGAP activity and therefore promotes hydrolyzation of GTP to GDP, which in turn attenuates RhoA/ROCK1 signaling. As a receptor of semaphoring, PlexinB2 competitively binds to p190RhoGAP and inhibits p190RhoGAP activity; subsequently driving neuron migration and axonal outgrowth.
Acknowledgments
This work was supported by the National Natural Science Foundation of China 81170121 (WJ), the Technology Innovation Project of Department of Education of Guangdong Province 2013KJCX0088 (WJ); the China Scholarship Council (X. Yue); the American Heart Association Postdoctoral Fellowship 13POST17260043 (X.Yang.); the American Heart Association Grant-in-Aid 0855030F, the NIH-NHLBI R01HL102314, R01HL123593, R21HL094844, and K02HL098956 (JC).
References
- 1.Albertsen HM, Chettier R, Farrington P, Ward K. Genome-wide association study link novel loci to endometriosis. PLoS One. 2013;8:e58257. doi: 10.1371/journal.pone.0058257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 3.Aspenstrom P, Fransson A, Saras J. Rho GTPases have diverse effects on the organization of the actin filament system. Biochem J. 2004;377:327–337. doi: 10.1042/BJ20031041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azzarelli R, Pacary E, Garg R, Garcez P, van den Berg D, Riou P, Ridley AJ, Friedel RH, Parsons M, Guillemot F. An antagonistic interaction between PlexinB2 and Rnd3 controls RhoA activity and cortical neuron migration. Nat Commun. 2014;5:3405. doi: 10.1038/ncomms4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ball SG, Shuttleworth CA, Kielty CM. Platelet-derived growth factor receptor-alpha is a key determinant of smooth muscle alpha-actin filaments in bone marrow-derived mesenchymal stem cells. Int J Biochem Cell Biol. 2007;39:379–391. doi: 10.1016/j.biocel.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Ballester-Lurbe B, Gonzalez-Granero S, Mocholi E, Poch E, Garcia-Manzanares M, Dierssen M, Perez-Roger I, Garcia-Verdugo JM, Guasch RM, Terrado J. RhoE deficiency alters postnatal subventricular zone development and the number of calbindin-expressing neurons in the olfactory bulb of mouse. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0846-1. [DOI] [PubMed] [Google Scholar]
- 7.Ballester-Lurbe B, Poch E, Mocholi E, Guasch RM, Perez-Roger I, Terrado J. RhoE is spatiotemporally regulated in the postnatal mouse CNS. Neuroscience. 2009;163:586–593. doi: 10.1016/j.neuroscience.2009.06.062. [DOI] [PubMed] [Google Scholar]
- 8.Barry SP, Lawrence KM, McCormick J, Soond SM, Hubank M, Eaton S, Sivarajah A, Scarabelli TM, Knight RA, Thiemermann C, Latchman DS, Townsend PA, Stephanou A. New targets of urocortin-mediated cardioprotection. J Mol Endocrinol. 2010;45:69–85. doi: 10.1677/JME-09-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 10.Bektic J, Pfeil K, Berger AP, Ramoner R, Pelzer A, Schafer G, Kofler K, Bartsch G, Klocker H. Small G-protein RhoE is underexpressed in prostate cancer and induces cell cycle arrest and apoptosis. Prostate. 2005;64:332–340. doi: 10.1002/pros.20243. [DOI] [PubMed] [Google Scholar]
- 11.Bektic J, Wrulich OA, Dobler G, Kofler K, Ueberall F, Culig Z, Bartsch G, Klocker H. Identification of genes involved in estrogenic action in the human prostate using microarray analysis. Genomics. 2004;83:34–44. doi: 10.1016/s0888-7543(03)00184-8. [DOI] [PubMed] [Google Scholar]
- 12.Belgiovine C, Frapolli R, Bonezzi K, Chiodi I, Favero F, Mello-Grand M, Dei Tos AP, Giulotto E, Taraboletti G, D’Incalci M, Mondello C. Reduced expression of the ROCK inhibitor Rnd3 is associated with increased invasiveness and metastatic potential in mesenchymal tumor cells. PLoS One. 2010;5:e14154. doi: 10.1371/journal.pone.0014154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernal SD, Stahel RA. Cytoskeleton-associated proteins: their role as cellular integrators in the neoplastic process. Crit Rev Oncol Hematol. 1985;3:191–204. doi: 10.1016/s1040-8428(85)80026-3. [DOI] [PubMed] [Google Scholar]
- 14.Bokoch GM, Der CJ. Emerging concepts in the Ras superfamily of GTP-binding proteins. FASEB J. 1993;7:750–759. doi: 10.1096/fasebj.7.9.8330683. [DOI] [PubMed] [Google Scholar]
- 15.Boswell SA, Ongusaha P, Lee SW. The role of RhoE in the UVB response of human keratinocytes. Journal of Investigative Dermatology. 2006;126:137–137. [Google Scholar]
- 16.Boswell SA, Ongusaha PP, Nghiem P, Lee SW. The protective role of a small GTPase RhoE against UVB-induced DNA damage in keratinocytes. J Biol Chem. 2007;282:4850–4858. doi: 10.1074/jbc.M610532200. [DOI] [PubMed] [Google Scholar]
- 17.Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990;348:125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- 18.Camerer E, Gjernes E, Wiiger M, Pringle S, Prydz H. Binding of factor VIIa to tissue factor on keratinocytes induces gene expression. The Journal of biological chemistry. 2000;275:6580–6585. doi: 10.1074/jbc.275.9.6580. [DOI] [PubMed] [Google Scholar]
- 19.Cario-Toumaniantz C, Reillaudoux G, Sauzeau V, Heutte F, Vaillant N, Finet M, Chardin P, Loirand G, Pacaud P. Modulation of RhoA-Rho kinase-mediated Ca2+ sensitization of rabbit myometrium during pregnancy - role of Rnd3. J Physiol. 2003;552:403–413. doi: 10.1113/jphysiol.2003.047738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang J, Xie M, Shah VR, Schneider MD, Entman ML, Wei L, Schwartz RJ. Activation of Rho-associated coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proc Natl Acad Sci U S A. 2006;103:14495–14500. doi: 10.1073/pnas.0601911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang L, Guo F, Wang Y, Lv Y, Huo B, Wang L, Liu W. MicroRNA-200c regulates the sensitivity of chemotherapy of gastric cancer SGC7901/DDP cells by directly targeting RhoE. Pathol Oncol Res. 2014;20:93–98. doi: 10.1007/s12253-013-9664-7. [DOI] [PubMed] [Google Scholar]
- 22.Chardin P. Function and regulation of Rnd proteins. Nat Rev Mol Cell Biol. 2006;7:54–62. doi: 10.1038/nrm1788. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Zhou H, Li Q, Qiu M, Li Z, Tang Q, Liu M, Zhu Y, Huang J, Lang N, Liu Z, Deng Y, Zhang S, Bi F. Epigenetic modification of RhoE expression in gastric cancer cells. Oncol Rep. 2011;25:173–180. [PubMed] [Google Scholar]
- 24.Chiarugi P, Giannoni E. Anoikis: a necessary death program for anchorage-dependent cells. Biochem Pharmacol. 2008;76:1352–1364. doi: 10.1016/j.bcp.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 25.Christenson LK, Gunewardena S, Hong X, Spitschak M, Baufeld A, Vanselow J. Research resource: preovulatory LH surge effects on follicular theca and granulosa transcriptomes. Mol Endocrinol. 2013;27:1153–1171. doi: 10.1210/me.2013-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chun KH, Araki K, Ongusaha P, Lee DH, Lee S, Kim YB. RhoE GTPase stimulates glucose uptake through a PI 3-kinase dependent mechanism in 3T3-L1 Adipocytes. Diabetes. 2008;57:A358–A358. [Google Scholar]
- 27.Chun KH, Choi KD, Lee DH, Jung Y, Henry RR, Ciaraldi TP, Kim YB. In vivo activation of ROCK1 by insulin is impaired in skeletal muscle of humans with type 2 diabetes. American journal of physiology Endocrinology and metabolism. 2011;300:E536–E542. doi: 10.1152/ajpendo.00538.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collett GP, Goh XF, Linton EA, Redman CW, Sargent IL. RhoE is regulated by cyclic AMP and promotes fusion of human BeWo choriocarcinoma cells. PLoS One. 2012;7:e30453. doi: 10.1371/journal.pone.0030453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Croft DR, Olson MF. Transcriptional regulation of Rho GTPase signaling. Transcription. 2011;2:211–215. doi: 10.4161/trns.2.5.16904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuiyan Z, Jie H, Fang Z, Kezhi Z, Junting W, Susheng S, Xiaoli F, Ning L, Xinhua M, Zhaoli C, Kang S, Bin Q, Baozhong L, Sheng C, Meihua X, Jie H. Overexpression of RhoE in Non-small Cell Lung Cancer (NSCLC) is associated with smoking and correlates with DNA copy number changes. Cancer Biol Ther. 2007;6:335–342. doi: 10.4161/cbt.6.3.3663. [DOI] [PubMed] [Google Scholar]
- 31.de Araujo RM, Oba Y, Kuroda S, Tanaka E, Moriyama K. RhoE regulates actin cytoskeleton organization in human periodontal ligament cells under mechanical stress. Arch Oral Biol. 2014;59:187–192. doi: 10.1016/j.archoralbio.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- 33.Dowrick PG, Prescott AR, Warn RM. Scatter factor affects major changes in the cytoskeletal organization of epithelial cells. Cytokine. 1991;3:299–310. doi: 10.1016/1043-4666(91)90498-3. [DOI] [PubMed] [Google Scholar]
- 34.Fansa EK, Dvorsky R, Zhang SC, Fiegen D, Ahmadian MR. Interaction characteristics of Plexin-B1 with Rho family proteins. Biochem Biophys Res Commun. 2013;434:785–790. doi: 10.1016/j.bbrc.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Feng B, Li K, Zhong H, Ren G, Wang H, Shang Y, Bai M, Liang J, Wang X, Fan D. RhoE promotes metastasis in gastric cancer through a mechanism dependent on enhanced expression of CXCR4. PLoS One. 2013;8:e81709. doi: 10.1371/journal.pone.0081709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fiegen D, Blumenstein L, Stege P, Vetter IR, Ahmadian MR. Crystal structure of Rnd3/RhoE: functional implications. FEBS Lett. 2002;525:100–104. doi: 10.1016/s0014-5793(02)03094-6. [DOI] [PubMed] [Google Scholar]
- 37.Fortier M, Comunale F, Kucharczak J, Blangy A, Charrasse S, Gauthier-Rouviere C. RhoE controls myoblast alignment prior fusion through RhoA and ROCK. Cell Death Differ. 2008;15:1221–1231. doi: 10.1038/cdd.2008.34. [DOI] [PubMed] [Google Scholar]
- 38.Foster R, Hu KQ, Lu Y, Nolan KM, Thissen J, Settleman J. Identification of a novel human Rho protein with unusual properties: GTPase deficiency and in vivo farnesylation. Mol Cell Biol. 1996;16:2689–2699. doi: 10.1128/mcb.16.6.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu Y, Liu X, Zhou N, Du L, Sun Y, Zhang X, Ge Y. MicroRNA-200b stimulates tumour growth in TGFBR2-null colorectal cancers by negatively regulating p27/kip1. J Cell Physiol. 2014;229:772–782. doi: 10.1002/jcp.24497. [DOI] [PubMed] [Google Scholar]
- 40.Gao D, Vahdat LT, Wong S, Chang JC, Mittal V. Microenvironmental regulation of epithelial-mesenchymal transitions in cancer. Cancer Res. 2012;72:4883–4889. doi: 10.1158/0008-5472.CAN-12-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garavini H, Riento K, Phelan JP, McAlister MS, Ridley AJ, Keep NH. Crystal structure of the core domain of RhoE/Rnd3: a constitutively activated small G protein. Biochemistry. 2002;41:6303–6310. doi: 10.1021/bi025651h. [DOI] [PubMed] [Google Scholar]
- 42.Garg R, Riento K, Keep N, Morris JD, Ridley AJ. N-terminus-mediated dimerization of ROCK-I is required for RhoE binding and actin reorganization. Biochem J. 2008;411:407–414. doi: 10.1042/BJ20071342. [DOI] [PubMed] [Google Scholar]
- 43.Gaziano T, Reddy KS, Paccaud F, Horton S, Chaturvedi V. Cardiovascular Disease. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, editors. Disease Control Priorities in Developing Countries. Washington (DC): World Bank; 2006. [Google Scholar]
- 44.Georgess D, Mazzorana M, Terrado J, Delprat C, Chamot C, Guasch RM, Perez-Roger I, Jurdic P, Machuca-Gayet I. Comparative transcriptomics reveals RhoE as a novel regulator of actin dynamics in bone-resorbing osteoclasts. Mol Biol Cell. 2014;25:380–396. doi: 10.1091/mbc.E13-07-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geraud C, Schledzewski K, Demory A, Klein D, Kaus M, Peyre F, Sticht C, Evdokimov K, Lu S, Schmieder A, Goerdt S. Liver sinusoidal endothelium: a microenvironment-dependent differentiation program in rat including the novel junctional protein liver endothelial differentiation-associated protein-1. Hepatology. 2010;52:313–326. doi: 10.1002/hep.23618. [DOI] [PubMed] [Google Scholar]
- 46.Goda T, Takagi C, Ueno N. Xenopus Rnd1 and Rnd3 GTP-binding proteins are expressed under the control of segmentation clock and required for somite formation. Dev Dyn. 2009;238:2867–2876. doi: 10.1002/dvdy.22099. [DOI] [PubMed] [Google Scholar]
- 47.Goh LL, Manser E. The GTPase-deficient Rnd proteins are stabilized by their effectors. J Biol Chem. 2012;287:31311–31320. doi: 10.1074/jbc.M111.327056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goh LL, Manser E. The RhoA GEF Syx is a target of Rnd3 and regulated via a Raf1-like ubiquitin-related domain. PLoS One. 2010;5:e12409. doi: 10.1371/journal.pone.0012409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gottesbuhren U, Garg R, Riou P, McColl B, Brayson D, Ridley AJ. Rnd3 induces stress fibres in endothelial cells through RhoB. Biol Open. 2013;2:210–216. doi: 10.1242/bio.20123574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gress TM, Muller-Pillasch F, Geng M, Zimmerhackl F, Zehetner G, Friess H, Buchler M, Adler G, Lehrach H. A pancreatic cancer-specific expression profile. Oncogene. 1996;13:1819–1830. [PubMed] [Google Scholar]
- 51.Grise F, Bidaud-Meynard A, Dugot-Senant N, Rullier A, Bioulac-Sage P, Zucman-Rossi J, Rosenbaum J, Moreau V. Under-expression of Rnd3/RhoE in hepatocellular carcinoma: implication in tumoral hepatocyte invasion. Bulletin Du Cancer. 2010;97:S29–S30. [Google Scholar]
- 52.Grise F, Sena S, Bidaud-Meynard A, Baud J, Hiriart JB, Makki K, Dugot-Senant N, Staedel C, Bioulac-Sage P, Zucman-Rossi J, Rosenbaum J, Moreau V. Rnd3/RhoE Is down-regulated in hepatocellular carcinoma and controls cellular invasion. Hepatology. 2012;55:1766–1775. doi: 10.1002/hep.25568. [DOI] [PubMed] [Google Scholar]
- 53.Guasch RM, Blanco AM, Perez-Arago A, Minambres R, Talens-Visconti R, Peris B, Guerri C. RhoE participates in the stimulation of the inflammatory response induced by ethanol in astrocytes. Exp Cell Res. 2007;313:3779–3788. doi: 10.1016/j.yexcr.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 54.Guasch RM, Scambler P, Jones GE, Ridley AJ. RhoE regulates actin cytoskeleton organization and cell migration. Mol Cell Biol. 1998;18:4761–4771. doi: 10.1128/mcb.18.8.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo S, Russo IH, Russo J. Difference in gene expression profile in breast epithelial cells from women with different reproductive history. Int J Oncol. 2003;23:933–941. [PubMed] [Google Scholar]
- 56.Hota PK, Buck M. Plexin structures are coming: opportunities for multilevel investigations of semaphorin guidance receptors, their cell signaling mechanisms, and functions. Cell Mol Life Sci. 2012;69:3765–3805. doi: 10.1007/s00018-012-1019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu WP, Tay SK, Zhao Y. Endometriosis-specific genes identified by real-time reverse transcription-polymerase chain reaction expression profiling of endometriosis versus autologous uterine endometrium. J Clin Endocrinol Metab. 2006;91:228–238. doi: 10.1210/jc.2004-1594. [DOI] [PubMed] [Google Scholar]
- 58.Hurteau GJ, Spivack SD, Brock GJ. Potential mRNA Degradation Targets of hsa-miR-200c,identified using informatics and qRT-PCR. Cell Cycle. 2006;5:1951–1956. doi: 10.4161/cc.5.17.3133. [DOI] [PubMed] [Google Scholar]
- 59.Ionin B, Hammamieh R, Shupp JW, Das R, Pontzer CH, Jett M. Staphylococcal enterotoxin B causes differential expression of Rnd3 and RhoA in renal proximal tubule epithelial cells while inducing actin stress fiber assembly and apoptosis. Microb Pathog. 2008;45:303–309. doi: 10.1016/j.micpath.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 60.Jiang H, Gao M, Shen Z, Luo B, Li R, Jiang X, Ding R, Ha Y, Wang Z, Jie W. Blocking PI3K/Akt signaling attenuates metastasis of nasopharyngeal carcinoma cells through induction of mesenchymal-epithelial reverting transition. Oncol Rep. 2014;32:559–566. doi: 10.3892/or.2014.3220. [DOI] [PubMed] [Google Scholar]
- 61.Johnson MP, Brennecke SP, East CE, Dyer TD, Roten LT, Proffitt JM, Melton PE, Fenstad MH, Aalto-Viljakainen T, Makikallio K, Heinonen S, Kajantie E, Kere J, Laivuori H, Group FS, Austgulen R, Blangero J, Moses EK. Genetic dissection of the pre-eclampsia susceptibility locus on chromosome 2q22 reveals shared novel risk factors for cardiovascular disease. Mol Hum Reprod. 2013;19:423–437. doi: 10.1093/molehr/gat011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Katiyar P, Aplin AE. FOXD3 regulates migration properties and Rnd3 expression in melanoma cells. Mol Cancer Res. 2011;9:545–552. doi: 10.1158/1541-7786.MCR-10-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Katoh H, Harada A, Mori K, Negishi M. Socius is a novel Rnd GTPase-interacting protein involved in disassembly of actin stress fibers. Molecular and cellular biology. 2002;22:2952–2964. doi: 10.1128/MCB.22.9.2952-2964.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim YS, Hori M, Yasuda K, Ozaki H. Differences in the gestational pattern of mRNA expression of the Rnd family in rat and human myometria. Comp Biochem Physiol A Mol Integr Physiol. 2005;142:410–415. doi: 10.1016/j.cbpa.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 65.Klein RM, Aplin AE. Rnd3 regulation of the actin cytoskeleton promotes melanoma migration and invasive outgrowth in three dimensions. Cancer Res. 2009;69:2224–2233. doi: 10.1158/0008-5472.CAN-08-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klein RM, Higgins PJ. A switch in RND3-RHOA signaling is critical for melanoma cell invasion following mutant-BRAF inhibition. Mol Cancer. 2011;10:114. doi: 10.1186/1476-4598-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klein RM, Spofford LS, Abel EV, Ortiz A, Aplin AE. B-RAF regulation of Rnd3 participates in actin cytoskeletal and focal adhesion organization. Mol Biol Cell. 2008;19:498–508. doi: 10.1091/mbc.E07-09-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Komander D, Garg R, Wan PT, Ridley AJ, Barford D. Mechanism of multi-site phosphorylation from a ROCK-I:RhoE complex structure. Embo J. 2008;27:3175–3185. doi: 10.1038/emboj.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Konermann S, Brigham MD, Trevino AE, Joung J, Abudayyeh OO, Barcena C, Hsu PD, Habib N, Gootenberg JS, Nishimasu H, Nureki O, Zhang F. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2014 doi: 10.1038/nature14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Molecular and cellular biology. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuo PL, Huang MS, Huang SK, Ni WC, Hung JY, Ko YC, Hung CH, Tsai YM, Duh TH, Hsu YL. Signalling pathway of isophorone diisocyanate-responsive interleukin-8 in airway smooth muscle cells. Eur Respir J. 2011;37:1226–1236. doi: 10.1183/09031936.00192109. [DOI] [PubMed] [Google Scholar]
- 73.Lartey J, Gampel A, Pawade J, Mellor H, Bernal AL. Expression of RND proteins in human myometrium. Biol Reprod. 2006;75:452–461. doi: 10.1095/biolreprod.105.049130. [DOI] [PubMed] [Google Scholar]
- 74.Lesiak A, Pelz C, Ando H, Zhu M, Davare M, Lambert TJ, Hansen KF, Obrietan K, Appleyard SM, Impey S, Wayman GA. A genome-wide screen of CREB occupancy identifies the RhoA inhibitors Par6C and Rnd3 as regulators of BDNF-induced synaptogenesis. PLoS One. 2013;8:e64658. doi: 10.1371/journal.pone.0064658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li J, Anton ES. Rnd-ing up RhoA activity to link neurogenesis with steps in neuronal migration. Dev Cell. 2011;20:409–410. doi: 10.1016/j.devcel.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 76.Li K, Lu Y, Liang J, Luo G, Ren G, Wang X, Fan D. RhoE enhances multidrug resistance of gastric cancer cells by suppressing Bax. Biochem Biophys Res Commun. 2009;379:212–216. doi: 10.1016/j.bbrc.2008.12.044. [DOI] [PubMed] [Google Scholar]
- 77.Liebig T, Erasmus J, Kalaji R, Davies D, Loirand G, Ridley A, Braga VM. RhoE Is required for keratinocyte differentiation and stratification. Mol Biol Cell. 2009;20:452–463. doi: 10.1091/mbc.E07-11-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin X, Liu B, Yang X, Yue X, Diao L, Wang J, Chang J. Genetic deletion of Rnd3 results in aqueductal stenosis leading to hydrocephalus through up-regulation of Notch signaling. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:8236–8241. doi: 10.1073/pnas.1219995110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu X, Chen L, Feng B, Liu G. Reversing effect of sorcin in the drug resistance of human nasopharyngeal carcinoma. Anat Rec (Hoboken) 2014;297:215–221. doi: 10.1002/ar.22832. [DOI] [PubMed] [Google Scholar]
- 80.Lonjedo M, Poch E, Mocholi E, Hernandez-Sanchez M, Ivorra C, Franke TF, Guasch RM, Perez-Roger I. The Rho family member RhoE interacts with Skp2 and is degraded at the proteasome during cell cycle progression. J Biol Chem. 2013;288:30872–30882. doi: 10.1074/jbc.M113.511105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luo H, Dong Z, Zou J, Zeng Q, Wu D, Liu L. Down-regulation of RhoE is associated with progression and poor prognosis in hepatocellular carcinoma. Journal of surgical oncology. 2012;105:699–704. doi: 10.1002/jso.23019. [DOI] [PubMed] [Google Scholar]
- 82.Luo H, Zou J, Dong Z, Zeng Q, Wu D, Liu L. Up-regulated miR-17 promotes cell proliferation, tumour growth and cell cycle progression by targeting the RND3 tumour suppressor gene in colorectal carcinoma. Biochem J. 2012;442:311–321. doi: 10.1042/BJ20111517. [DOI] [PubMed] [Google Scholar]
- 83.Ma W, Wong CCL, Tung EKK, Wong CM, Ng IOL. RhoE is frequently down-regulated in hepatocellular carcinoma (HCC) and suppresses HCC invasion through antagonizing the Rho/Rho-Kinase/Myosin phosphatase target pathway. Hepatology. 2013;57:152–161. doi: 10.1002/hep.25987. [DOI] [PubMed] [Google Scholar]
- 84.Macara IG. The ras superfamily of molecular switches. Cell Signal. 1991;3:179–187. doi: 10.1016/0898-6568(91)90043-t. [DOI] [PubMed] [Google Scholar]
- 85.MacNeil LG, Melov S, Hubbard AE, Baker SK, Tarnopolsky MA. Eccentric exercise activates novel transcriptional regulation of hypertrophic signaling pathways not affected by hormone changes. PLoS One. 2010;5:e10695. doi: 10.1371/journal.pone.0010695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Madaule P, Axel R. A novel ras-related gene family. Cell. 1985;41:31–40. doi: 10.1016/0092-8674(85)90058-3. [DOI] [PubMed] [Google Scholar]
- 87.Madigan JP, Bodemann BO, Brady DC, Dewar BJ, Keller PJ, Leitges M, Philips MR, Ridley AJ, Der CJ, Cox AD. Regulation of Rnd3 localization and function by protein kinase C alpha-mediated phosphorylation. Biochem J. 2009;424:153–161. doi: 10.1042/BJ20082377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maria Vieites J, Torre Rde L, Ramirez Mdel C, Torres MI, Sanchez-Pozo A, Gil A, Suarez A. Exogenous nucleosides accelerate differentiation of rat intestinal epithelial cells. Br J Nutr. 2008;99:732–738. doi: 10.1017/S0007114507837457. [DOI] [PubMed] [Google Scholar]
- 89.Marie-Claire C, Salzmann J, David A, Courtin C, Canestrelli C, Noble F. Rnd family genes are differentially regulated by 3,4-methylenedioxymethamphetamine and cocaine acute treatment in mice brain. Brain Res. 2007;1134:12–17. doi: 10.1016/j.brainres.2006.11.065. [DOI] [PubMed] [Google Scholar]
- 90.Matheu G, Benejam JM, Villalonga P. Immunohistochemical expression of RhoE in benign prostate glands and prostatic adenocarcinoma. Virchows Archiv. 2007;451:408–409. [Google Scholar]
- 91.Mei H, Ho MK, Yung LY, Wu Z, Ip NY, Wong YH. Expression of Galpha(z) in C2C12 cells restrains myogenic differentiation. Cell Signal. 2011;23:389–397. doi: 10.1016/j.cellsig.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 92.Mocholi E, Ballester-Lurbe B, Arque G, Poch E, Peris B, Guerri C, Dierssen M, Guasch RM, Terrado J, Perez-Roger I. RhoE Deficiency Produces Postnatal Lethality, Profound Motor Deficits and Neurodevelopmental Delay in Mice. PLoS One. 2011;6:e19236. doi: 10.1371/journal.pone.0019236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murakami T, Fujimoto M, Ohtsuki M, Nakagawa H. Expression profiling of cancer-related genes in human keratinocytes following non-lethal ultraviolet B irradiation. J Dermatol Sci. 2001;27:121–129. doi: 10.1016/s0923-1811(01)00124-4. [DOI] [PubMed] [Google Scholar]
- 94.Nadiminty N, Dutt S, Tepper C, Gao AC. Microarray analysis reveals potential target genes of NF-kappaB2/p52 in LNCaP prostate cancer cells. Prostate. 2010;70:276–287. doi: 10.1002/pros.21062. [DOI] [PubMed] [Google Scholar]
- 95.Nigg EA. Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. Bioessays. 1995;17:471–480. doi: 10.1002/bies.950170603. [DOI] [PubMed] [Google Scholar]
- 96.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 97.Oinuma I, Ishikawa Y, Katoh H, Negishi M. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science. 2004;305:862–865. doi: 10.1126/science.1097545. [DOI] [PubMed] [Google Scholar]
- 98.Oinuma I, Kawada K, Tsukagoshi K, Negishi M. Rnd1 and Rnd3 targeting to lipid raft is required for p190 RhoGAP activation. Mol Biol Cell. 2012;23:1593–1604. doi: 10.1091/mbc.E11-11-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ong SG, Hausenloy DJ. Hypoxia-inducible factor as a therapeutic target for cardioprotection. Pharmacol Ther. 2012;136:69–81. doi: 10.1016/j.pharmthera.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 100.Ongusaha PP, Kim HG, Boswell SA, Ridley AJ, Der CJ, Dotto GP, Kim YB, Aaronson SA, Lee SW. RhoE is a pro-survival p53 target gene that inhibits ROCK I-mediated apoptosis in response to genotoxic stress. Curr Biol. 2006;16:2466–2472. doi: 10.1016/j.cub.2006.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 101.Organization WH. The top 10 causes of death. http://whoint/mediacentre/factsheets/fs310/en/
- 102.Pacary E, Azzarelli R, Guillemot F. Rnd3 coordinates early steps of cortical neurogenesis through actin-dependent and -independent mechanisms. Nat Commun. 2013;4:1635. doi: 10.1038/ncomms2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pacary E, Heng J, Azzarelli R, Riou P, Castro D, Lebel-Potter M, Parras C, Bell DM, Ridley AJ, Parsons M, Guillemot F. Proneural transcription factors regulate different steps of cortical neuron migration through Rnd-mediated inhibition of RhoA signaling. Neuron. 2011;69:1069–1084. doi: 10.1016/j.neuron.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peng Z, Wang CX, Fang EH, Wang GB, Tong Q. Role of epithelial-mesenchymal transition in gastric cancer initiation and progression. World J Gastroenterol. 2014;20:5403–5410. doi: 10.3748/wjg.v20.i18.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peris B, Gonzalez-Granero S, Ballester-Lurbe B, Garcia-Verdugo JM, Perez-Roger I, Guerri C, Terrado J, Guasch RM. Neuronal polarization is impaired in mice lacking RhoE expression. J Neurochem. 2012;121:903–914. doi: 10.1111/j.1471-4159.2012.07733.x. [DOI] [PubMed] [Google Scholar]
- 106.Pinner S, Sahai E. PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE. Nat Cell Biol. 2008;10:127–137. doi: 10.1038/ncb1675. [DOI] [PubMed] [Google Scholar]
- 107.Poch E, Minambres R, Mocholi E, Ivorra C, Perez-Arago A, Guerri C, Perez-Roger I, Guasch RM. RhoE interferes with Rb inactivation and regulates the proliferation and survival of the U87 human glioblastoma cell line. Exp Cell Res. 2007;313:719–731. doi: 10.1016/j.yexcr.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 108.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 109.Ridley AJ, Comoglio PM, Hall A. Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Molecular and cellular biology. 1995;15:1110–1122. doi: 10.1128/mcb.15.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 111.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 112.Riento K, Guasch RM, Garg R, Jin B, Ridley AJ. RhoE binds to ROCK I and inhibits downstream signaling. Mol Cell Biol. 2003;23:4219–4229. doi: 10.1128/MCB.23.12.4219-4229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Riento K, Ridley AJ. Inhibition of ROCK by RhoE. In: Balch WE, Der CJ, Hall A, editors. Methods in Enzymology, Vol 406, Regulators and Effectors of Small Gtpases: Rho Family. 2006. pp. 533–541. [DOI] [PubMed] [Google Scholar]
- 114.Riento K, Totty N, Villalonga P, Garg R, Guasch R, Ridley AJ. RhoE function is regulated by ROCK I-mediated phosphorylation. Embo J. 2005;24:1170–1180. doi: 10.1038/sj.emboj.7600612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Riento K, Villalonga P, Garg R, Ridley A. Function and regulation of RhoE. Biochem Soc Trans. 2005;33:649–651. doi: 10.1042/BST0330649. [DOI] [PubMed] [Google Scholar]
- 116.Riou P, Kjaer S, Garg R, Purkiss A, George R, Cain RJ, Bineva G, Reymond N, McColl B, Thompson AJ, O’Reilly N, McDonald NQ, Parker PJ, Ridley AJ. 14-3-3 proteins interact with a hybrid prenyl-phosphorylation motif to inhibit g proteins. Cell. 2013;153:640–653. doi: 10.1016/j.cell.2013.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Riou P, Villalonga P, Ridley AJ. Rnd proteins: multifunctional regulators of the cytoskeleton and cell cycle progression. Bioessays. 2010;32:986–992. doi: 10.1002/bies.201000060. [DOI] [PubMed] [Google Scholar]
- 118.Rubenstein NM, Callahan JA, Lo DH, Firestone GL. Selective glucocorticoid control of Rho kinase isoforms regulate cell-cell interactions. Biochem Biophys Res Commun. 2007;354:603–607. doi: 10.1016/j.bbrc.2007.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rubenstein NM, Chan JF, Kim JY, Hansen SH, Firestone GL. Rnd3/RhoE induces tight junction formation in mammary epithelial tumor cells. Exp Cell Res. 2005;305:74–82. doi: 10.1016/j.yexcr.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 120.Ryan KR, Lock FE, Heath JK, Hotchin NA. Plakoglobin-dependent regulation of keratinocyte apoptosis by Rnd3. J Cell Sci. 2012;125:3202–3209. doi: 10.1242/jcs.101931. [DOI] [PubMed] [Google Scholar]
- 121.Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelsen TS, Heckl D, Ebert BL, Root DE, Doench JG, Zhang F. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–87. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shimomura A, Ohama T, Hori M, Ozaki H. 17 beta-Estradiol Induces Gastrointestinal Motility Disorder by Decreasing CPI-17 Phosphorylation Via Changes in Rho-Family G-Protein Rnd Expression in Small Intestine. Journal of Veterinary Medical Science. 2009;71:1591–1597. doi: 10.1292/jvms.001591. [DOI] [PubMed] [Google Scholar]
- 123.Shurin GV, Tourkova IL, Shurin MR. Low-dose chemotherapeutic agents regulate small Rho GTPase activity in dendritic cells. J Immunother. 2008;31:491–499. doi: 10.1097/CJI.0b013e318176fae4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Song W, Jiang R, Zhao CM. Role of integrin-linked kinase in multi-drug resistance of human gastric carcinoma SGC7901/DDP cells. Asian Pac J Cancer Prev. 2012;13:5619–5625. doi: 10.7314/apjcp.2012.13.11.5619. [DOI] [PubMed] [Google Scholar]
- 125.Stordal B, Davey M. Understanding cisplatin resistance using cellular models. IUBMB Life. 2007;59:696–699. doi: 10.1080/15216540701636287. [DOI] [PubMed] [Google Scholar]
- 126.Tahirovic S, Bradke F. Neuronal polarity. Cold Spring Harb Perspect Biol. 2009;1:a001644. doi: 10.1101/cshperspect.a001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Talbot LJ, Bhattacharya SD, Kuo PC. Epithelial-mesenchymal transition, the tumor microenvironment, and metastatic behavior of epithelial malignancies. Int J Biochem Mol Biol. 2012;3:117–136. [PMC free article] [PubMed] [Google Scholar]
- 128.Talens-Visconti R, Peris B, Guerri C, Guasch RM. RhoE stimulates neurite-like outgrowth in PC12 cells through inhibition of the RhoA/ROCK-I signalling. J Neurochem. 2010;112:1074–1087. doi: 10.1111/j.1471-4159.2009.06526.x. [DOI] [PubMed] [Google Scholar]
- 129.Tang Y, Hu C, Yang H, Cao L, Li Y, Deng P, Huang L. Rnd3 regulates lung cancer cell proliferation through notch signaling. PLoS One. 2014;9:e111897. doi: 10.1371/journal.pone.0111897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tong J, Fu Y, Xu X, Fan S, Sun H, Liang Y, Xu K, Yuan Z, Ge Y. TGF-beta1 stimulates human Tenon’s capsule fibroblast proliferation by miR-200b and its targeting of p27/kip1 and RND3. Invest Ophthalmol Vis Sci. 2014;55:2747–2756. doi: 10.1167/iovs.13-13422. [DOI] [PubMed] [Google Scholar]
- 131.Trojan L, Schaaf A, Steidler A, Haak M, Thalmann G, Knoll T, Gretz N, Alken P, Michel MS. Identification of metastasis-associated genes in prostate cancer by genetic profiling of human prostate cancer cell lines. Anticancer Res. 2005;25:183–191. [PubMed] [Google Scholar]
- 132.Tyburczy ME, Kotulska K, Pokarowski P, Mieczkowski J, Kucharska J, Grajkowska W, Roszkowski M, Jozwiak S, Kaminska B. Novel proteins regulated by mTOR in subependymal giant cell astrocytomas of patients with tuberous sclerosis complex and new therapeutic implications. Am J Pathol. 2010;176:1878–1890. doi: 10.2353/ajpath.2010.090950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Valinezhad Orang A, Safaralizadeh R, Kazemzadeh-Bavili M. Mechanisms of miRNA-Mediated Gene Regulation from Common Downregulation to mRNA-Specific Upregulation. Int J Genomics. 2014;2014:970607. doi: 10.1155/2014/970607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vandeput F, Zabeau M, Maenhaut C. Identification of differentially expressed genes in thyrotropin stimulated dog thyroid cells by the cDNA-AFLP technique. Mol Cell Endocrinol. 2005;243:58–65. doi: 10.1016/j.mce.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 135.Villalonga P, Fernandez de Mattos S, Ridley AJ. RhoE inhibits 4E–BP1 phosphorylation and eIF4E function impairing cap-dependent translation. J Biol Chem. 2009;284:35287–35296. doi: 10.1074/jbc.M109.050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Villalonga P, Guasch RM, Riento K, Ridley AJ. RhoE inhibits cell cycle progression and Ras-induced transformation. Mol Cell Biol. 2004;24:7829–7840. doi: 10.1128/MCB.24.18.7829-7840.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Walmsley SR, Cadwallader KA, Chilvers ER. The role of HIF-1alpha in myeloid cell inflammation. Trends Immunol. 2005;26:434–439. doi: 10.1016/j.it.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 138.Wennerberg K, Forget MA, Ellerbroek SM, Arthur WT, Burridge K, Settleman J, Der CJ, Hansen SH. Rnd proteins function as RhoA antagonists by activating p190 RhoGAP. Curr Biol. 2003;13:1106–1115. doi: 10.1016/s0960-9822(03)00418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–846. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- 140.Witte H, Bradke F. The role of the cytoskeleton during neuronal polarization. Curr Opin Neurobiol. 2008;18:479–487. doi: 10.1016/j.conb.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 141.Wu Y, Guo L, Liu J, Liu R, Liu M, Chen J. [The reversing and molecular mechanisms of miR-503 on the drug-resistance to cisplatin in A549/DDP cells] Zhongguo Fei Ai Za Zhi. 2014;17:1–7. doi: 10.3779/j.issn.1009-3419.2014.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xia H, Li M, Chen L, Leng W, Yuan D, Pang X, Chen L, Li R, Tang Q, Bi F. Suppression of RND3 activity by AES downregulation promotes cancer cell proliferation and invasion. Int J Mol Med. 2013;31:1081–1086. doi: 10.3892/ijmm.2013.1321. [DOI] [PubMed] [Google Scholar]
- 143.Xia W, Li J, Chen L, Huang B, Li S, Yang G, Ding H, Wang F, Liu N, Zhao Q, Fang T, Song T, Wang T, Shao N. MicroRNA-200b regulates cyclin D1 expression and promotes S-phase entry by targeting RND3 in HeLa cells. Mol Cell Biochem. 2010;344:261–266. doi: 10.1007/s11010-010-0550-2. [DOI] [PubMed] [Google Scholar]
- 144.Xu CZ, Shi RJ, Chen D, Sun YY, Wu QW, Wang T, Wang PH. Potential biomarkers for paclitaxel sensitivity in hypopharynx cancer cell. Int J Clin Exp Pathol. 2013;6:2745–2756. [PMC free article] [PubMed] [Google Scholar]
- 145.Yang X, Wang T, Lin X, Yue X, Wang Q, Wang G, Fu Q, Ai X, Chiang DY, Miyake CY, Wehrens XH, Chang J. Genetic Deletion of Rnd3/RhoE Results in Mouse Heart Calcium Leakage Through Upregulation of Protein Kinase A Signaling. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.116.304940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang X, Wang T, Lin X, Yue X, Wang Q, Wang G, Fu Q, Ai X, Chiang DY, Miyake CY, Wehrens XH, Chang J. Genetic Deletion of Rnd3/RhoE Results in Mouse Heart Calcium Leakage Through Upregulation of Protein Kinase A Signaling. Circ Res. 2015;116:e1–e10. doi: 10.1161/CIRCRESAHA.116.304940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yue X, Yang X, Lin X, Yang T, Yi X, Dai Y, Guo J, Li T, Shi J, Wei L, Fan GC, Chen C, Chang J. Rnd3 haploinsufficient mice are predisposed to hemodynamic stress and develop apoptotic cardiomyopathy with heart failure. Cell Death Dis. 2014;5:e1284. doi: 10.1038/cddis.2014.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang C, Zhou F, Li N, Shi S, Feng X, Chen Z, Hang J, Qiu B, Li B, Chang S, Wan J, Shao K, Xing X, Tan X, Wang Z, Xiong M, He J. Overexpression of RhoE has a prognostic value in non-small cell lung cancer. Ann Surg Oncol. 2007;14:2628–2635. doi: 10.1245/s10434-007-9457-x. [DOI] [PubMed] [Google Scholar]
- 149.Zhang F, Wen Y, Guo X. CRISPR/Cas9 for genome editing: progress, implications and challenges. Hum Mol Genet. 2014;23:R40–R46. doi: 10.1093/hmg/ddu125. [DOI] [PubMed] [Google Scholar]
- 150.Zhang J, Williams MA, Rigamonti D. Genetics of human hydrocephalus. J Neurol. 2006;253:1255–1266. doi: 10.1007/s00415-006-0245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhao H, Yang J, Fan T, Li S, Ren X. RhoE functions as a tumor suppressor in esophageal squamous cell carcinoma and modulates the PTEN/PI3K/Akt signaling pathway. Tumour Biol. 2012;33:1363–1374. doi: 10.1007/s13277-012-0384-5. [DOI] [PubMed] [Google Scholar]
- 152.Zhou J, Li K, Gu Y, Feng B, Ren G, Zhang L, Wang Y, Nie Y, Fan D. Transcriptional up-regulation of RhoE by hypoxia-inducible factor (HIF)-1 promotes epithelial to mesenchymal transition of gastric cancer cells during hypoxia. Biochem Biophys Res Commun. 2011;415:348–354. doi: 10.1016/j.bbrc.2011.10.065. [DOI] [PubMed] [Google Scholar]
- 153.Zhou J, Yang J, Li K, Mo P, Feng B, Wang X, Nie Y, Fan D. RhoE is associated with relapse and prognosis of patients with colorectal cancer. Ann Surg Oncol. 2013;20:175–182. doi: 10.1245/s10434-012-2472-6. [DOI] [PubMed] [Google Scholar]
- 154.Zhou Y, Li S, Huang Q, Xie L, Zhu X. Nanog suppresses cell migration by downregulating thymosin beta4 and Rnd3. J Mol Cell Biol. 2013;5:239–249. doi: 10.1093/jmcb/mjt002. [DOI] [PubMed] [Google Scholar]
- 155.Zhu Y, Xu F, Zhou J, Liu S, Huang J, Luo D, Qiu M, Jin B, Bi F. [Expression of RhoE in lung and breast cancer and its clinical significance] Zhongguo Fei Ai Za Zhi. 2008;11:85–89. doi: 10.3779/j.issn.1009-3419.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 156.Zhu Y, Zhou J, Xia H, Chen X, Qiu M, Huang J, Liu S, Tang Q, Lang N, Liu Z, Liu M, Zheng Y, Bi F. The Rho GTPase RhoE is a p53-regulated candidate tumor suppressor in cancer cells. Int J Oncol. 2014;44:896–904. doi: 10.3892/ijo.2014.2245. [DOI] [PubMed] [Google Scholar]
- 157.Zhu Z, Todorova K, Lee KK, Wang J, Kwon E, Kehayov I, Kim HG, Kolev V, Dotto GP, Lee SW, Mandinova A. Small GTPase RhoE/Rnd3 is a critical regulator of Notch1 signaling. Cancer Res. 2014;74:2082–2093. doi: 10.1158/0008-5472.CAN-12-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]