Abstract
The corneal stroma contains a population of mesenchymal cells subjacent to the limbal basement membrane with characteristics of adult stem cells. These ‘niche cells’ support limbal epithelial stem cell viability. In culture by themselves, the niche cells display a phenotype typical of mesenchymal stem cells. These stromal stem cells exhibit a potential to differentiate to multiple cell types, including keratocytes, thus providing an abundant source of these rare cells for experimental and bioengineering applications. Stromal stem cells have also shown the ability to remodel pathological stromal tissue, suppressing inflammation and restoring transparency. Because stromal stem cells can be obtained by biopsy, they offer a potential for autologous stem cell treatment for stromal opacities. This review provides an overview of the status of work on this interesting cell population.
Keywords: bioengineering, cell-based therapy, cornea, limbus, stem cells, stroma
I. Introduction
A. Corneal Stroma: Cells and Matrix
The physical strength and optical properties of the cornea derive mainly from the stroma, a tough connective tissue composed of a combination of specialized extracellular matrix (ECM) components organized with an elegant ultrastructure that provides both strength and transparency to this unique tissue. The stroma is populated and maintained by keratocytes, neural crest-derived mesenchymal cells, occupying about 3% of the stromal volume. After birth, the number of dividing keratocytes decreases, and in adult mammals, keratocytes have withdrawn from the cell cycle and become quiescent.1–5 Thus, unlike the self-renewing corneal epithelium, homeostasis of the stroma does not rely on the presence of an active population of stem cells.
B. Corneal Scarring
Scarring of the corneal stroma can occur in response to surgery, trauma, or infection. Corneal scars are long-lasting and disrupt vision for millions of people worldwide.6 Currently, surgical replacement of the stroma is the primary approach to restoration of vision in scarred corneas. The cells responsible for scar deposition are fibroblastic cells derived from stromal keratocytes.7 Upon wounding, keratocytes proximal to the site undergo apoptosis, and keratocytes distal to the wound become motile, mitotically active fibroblasts.2 Expression of α-smooth muscle actin has become a marker for cells involved in fibrotic ECM deposition.7,8 Secretion of fibrotic components is stable for months after healing in rabbit cornea.9 In humans, corneal scars can remain for decades.10
Damage to the corneal epithelium that does not involve the corneal stroma and retains some of the limbal stem cells can heal without scarring.3 Such epithelial wounds cause keratocyte apoptosis in the anterior stroma, and keratocytes peripheral to the injury migrate into the region and replicate. After epithelial debridement, mouse corneal stromal cells regain expression of stromal matrix components within 12 weeks after wounding.11 Keratocytes derived from the recipient have been identified in human donor keratoplasty tissue, indicating a potential for human keratocytes to repopulate and maintain stromal tissue.12 Such repopulation, however, is slow, sometimes requiring decades. It is clear from these studies that keratocytes do not conform to the classic definition of ‘terminal differentiation,’ and at least some cells in the stroma maintain the capability of replication, migration, and regeneration of transparent stromal tissue.
II. Stem Cells in the Stroma
A. Progenitor Potential of Stromal Cells
In vitro expansion of adult keratocytes typically leads to transformation to cells with a fibroblastic morphology, which produce a scar-like ECM rather than the specialized ECM required for corneal transparency.13 This fibroblastic transformation was considered irreversible, but recently it has become apparent that early-passage stromal cells maintain some potential to re-express differentiated keratocyte characteristics.14 However, the ability to differentiate to keratocytes after mitotic expansion is not equally distributed in the stromal cell population. About 3% of freshly isolated adult bovine stromal cells were found to grow clonally.15 These cells did not show keratocyte morphology or gene expression, rather expressed a number of genes typical of mesenchymal stem cells. When these cloned cells were shifted to a reducedmitogen culture medium, the clonal cells developed dendritic morphology and upregulated expression of keratan sulfate, keratocan, and ALDH3A1, all products highly expressed by differentiated keratocytes. The potential for keratocyte differentiation was maintained through greater than 50 population doublings, indicating that a progenitor phenotype was a stable property of these cells. These stromal progenitor cells exhibited normal karyotype and reached replicative senescence after 70–80 population doublings, demonstrating that they represent a population of non-transformed, adult diploid cells. As these cells differentiate to keratocytes, mRNA for several gene products present in embryonic neural and/or neural crest cells was markedly downregulated. The downregulated genes involve several associated with early ocular development including Six2, Six3, Notch1, and PAX6.15 These results demonstrate that corneal stromal cells are heterogeneous in their potential for self-renewal and include a small population of stem-like cells.
B. Mesenchymal Stem Cells in Human Corneal Stroma
Small populations of adult stem cells can be identified in many non-epithelial tissues. These cells, generically termed mesenchymal stem cells (MSCs), do not typically participate in the normal homeostasis of tissues in which they are located. MSCs share several key properties, including clonal growth, multipotent differentiation, and self-renewal, and have also been found to efflux fluorescent dyes, reducing the fluorescence of the MSCs, and thus allowing their identification by flow cytometry as a ‘side population.’
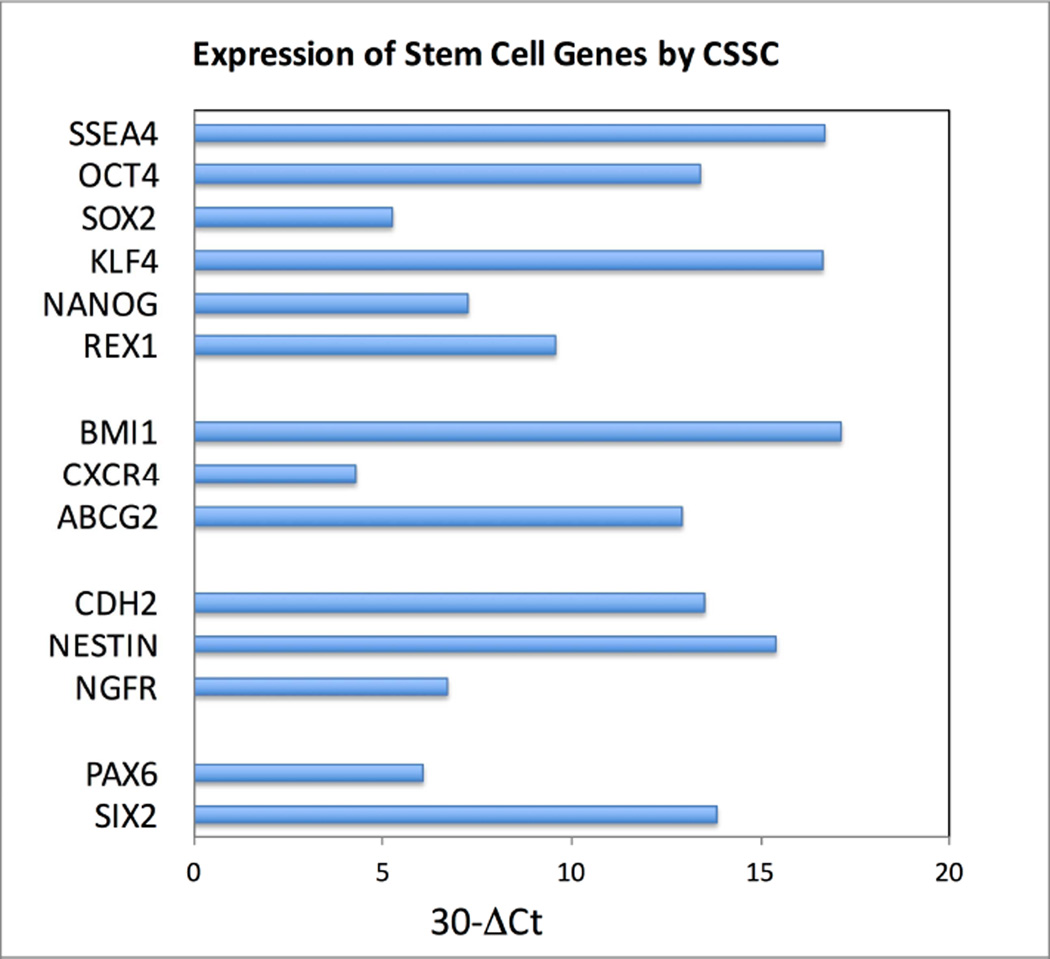
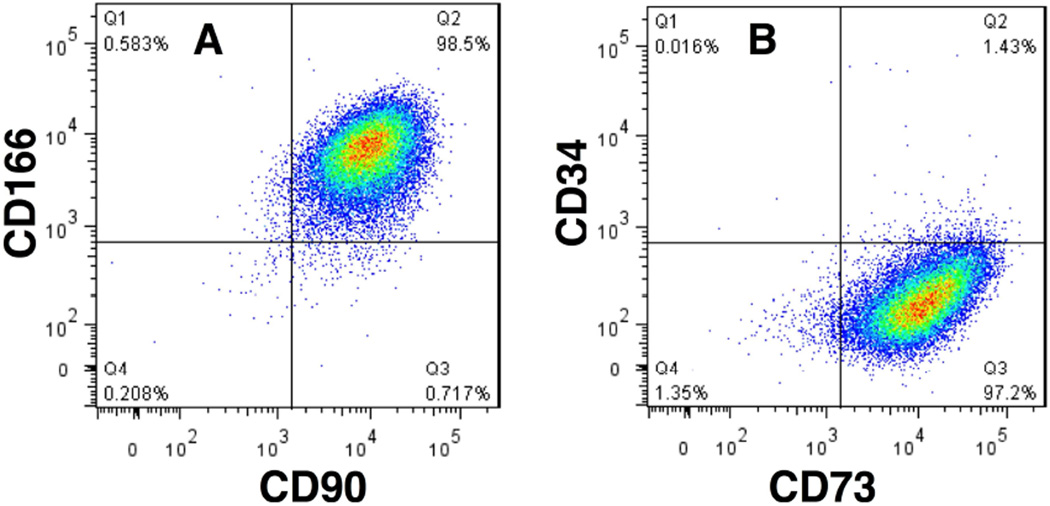
Side populations have been detected in many mammalian tissues and are used to isolate adult stem cells.16 Stem cells from human stroma were initially identified as such a side population, effluxing the dye Hoechst 33342.17 These cells (termed corneal stromal stem cells [CSSCs]) were present in early passage cells from human stroma at frequencies <1%. Dye efflux was blocked by verapamil, an inhibitor of the ABC cassette membrane transporter proteins responsible for the side population phenotype. FACS-isolated side population cells could be expanded clonally, with clones showing properties of MSCs. CSSC could be expanded through 100 cumulative population doublings.17 Gene array analysis identified a panel of genes highly expressed in CSSC that were weakly expressed in keratocytes, and vice versa. The CSSC-specific genes included MSC genes ABCG2, BMI1, CXCR4, as well as genes present in early corneal development PAX6 and Six2. Additionally, genes associated with neural development NGFR, NESTIN, and CDH2, as well as expression of genes associated with pluripotent cells, SSEA4, SOX2, REX1, NANOG, KLF4, OCT4 were observed (Figure 1).18 During expansion, cell surface proteins considered as markers of MSC, CD166, CD90, and CD73, were expressed on most CSSCs, whereas hematopoietic progenitor cell antigen CD34 was not (Figure 2).
Figure 1.
Expression of stem cell genes by CSSCs. CSSCs from digests of human limbal stroma were expanded in a low serum-culture medium that maintains their stem cell phenotype, and the relative expression of mRNA from genes identified in stem cells was determined by RT-PCR as described by Basu et al.31 The first group represents genes expressed in pluripotent cells (SSEA4, OCT4, SOX2, KLF4, NANOG, REX1). The second group represents genes expressed by MSC (BMI1, CXCR4, ABCG2). The third group represents markers of neural stem and progenitor cells (NESTIN, CDH2, NGFR). The final group (PAX6, SIX6) are expressed by ocular precursors in embryonic development. Delta Ct in the horizontal axis estimates the difference in abundance between the mRNA in question and 18S RNA as a power of 2.
Figure 2.
MSC-surface markers on CSSCs. CSSCs, passage 3, were stained for cell surface antigens and analyzed by flow cytometry. Horizontal and vertical lines show the maximum fluorescence of cells stained with non-specific isotope control antibodies using procedures described by Du et al.17 A. 98% of CSSCs stained for both CD90 (Thy-1) and CD166 (ALCAM). B. 97% of CSSCs stained for CD73 (NT5E), but <2% were positive for the hematopoietic stem cell marker CD34.
The presence of MSCs in the stromas of other species was demonstrated at about the same time as that of human CSSC. Stem cells from stroma of both mouse and rabbit could be expanded clonally in attachment-free cultures as floating spheroids.19–21 Re-plating of the spheres on plastic substratum led to expression of keratocan as well as neural specific proteins, such as ß-III tubulin, and of alpha smooth muscle actin. These results support the idea that all mammalian corneas contain a population of multipotent MSCs.
As with most MSC populations, rabbit, mouse, and human CSSCs exhibit a broad differentiation potential. CSSCs cultured in chondrogenic media underwent upregulation of mRNA and protein for cartilage ECM molecules, including collagen II, aggrecan and collagen oligomatrix protein (COMP).17 Under these conditions, the CSSCs deposited ECM staining with toluidine blue, a characteristic specific to cartilage. Similarly, when cultured in a neural induction culture medium, CSSCs showed upregulation of glial fibrillary acidic protein (GFAP) and neurofilament protein. Differentiation to distinct cellular lineages demonstrates multipotency of CSSCs, a key identifier of stem cells. Importantly, when CSSCs were placed in a serum-free medium supplemented with insulin and ascorbate, they upregulated expression of keratocyte-specific markers ALDH3A1, CXADR, PTDGS, and PDK4.17 These results are significant with regard to potential therapeutic use of these cells. Expansion of keratocytes in culture results in fibroblastic transformation and loss of keratocyte phenotype. Expanding cultures of stem cells and then differentiating them to keratocytes could provide cells useful for both bioengineering and for cell-based therapeutic applications.
C. Tissue Localization of Stromal Stem Cells
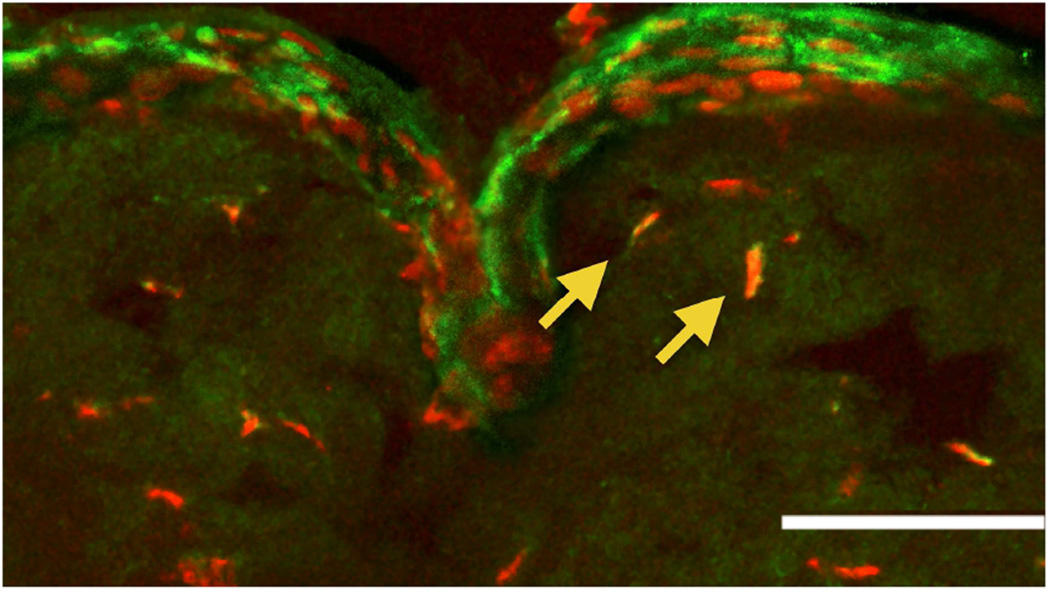
Stromal stem cells were originally observed in the transitional zone between cornea and sclera known as the limbus, based on immunostaining for ABCG2 and PAX6 proteins.17 The stained cells localized to anterior stroma immediately subjacent to the epithelial basement membrane, in regions where the basement membrane has ripples and folds (Figure 3). These anatomical features, termed the palisades of Vogt, provide the niche for limbal epithelial stem cells (LESCs).22 The presence of MSCs in the limbal stroma was subsequently confirmed in a number of independent studies. Optically, a population of highly reflective cells was observed in the anterior limbal stroma of living patients using in vivo laser scanning confocal microscopy.23 Immunostaining of corneal sections showed these cells to express CD90 and CD105, markers typical of MSCs.
Figure 3.
Limbal localization of stromal stem cells. Cells in the anterior stroma of human cornea were detected which express both PAX6 (red) and ABCG2 (green). (Arrows) Most of these cells were near the folded region of the epithelial basement membrane known as the palisades of Vogt. The white bar represents 50 µm. (Reprinted with permission from Du Y et al.17)
Polisetty et al observed that shallow biopsies of limbal tissue, carried out for the purpose of isolating LESCs, contained mesenchymal cells with properties similar to those of bone marrow MSCs.24 These cells exhibited clonal growth, multipotent differentiation, and expression of an array of cell-surface markers similar to that of bone marrow-MSC and distinct from LESC markers. Studies by Tseng and colleagues have shown that digestion of limbal tissue with collagenase isolates clumps of limbal epithelial cells in association with mesenchymal stromal cells.25–27 The stromal cells in these aggregates exhibited a number of stem cell properties and associated with LESCs via interaction between CXCR4 and SDF-1, a receptor/ligand pair that is active in localization of hematopoetic stem cells to their niche in bone marrow.28
The association between LESCs and stromal cells was further explored by Higa et al, who described cellular processes of N-cadherin-expressing epithelial cells passing through the epithelial basement membrane and making contact with stromal mesenchymal cells, which stained for aquaporin-1 and vimentin.29 These results support the idea of direct cell-cell contacts between the LESCs and anterior stromal cells. Such contacts were confirmed in an elegant anatomical study using serial block-face scanning electron microscopy (SBFSE) to create a three-dimensional reconstruction of the LESC niche.30 In these images, Dziasko et al showed long processes of stromal cells extending through fenestrations in the epithelial basement membrane, making contact with basal epithelial cells. Identity of the LESC-associated anterior stromal cells as CSSCs was confirmed by Basu et al, who isolated the epithelial-mesenchymal cell aggregates from mock biopsies with collagenase treatment and then compared the mesenchymal cells from these isolates to CSSC cells in terms of their clonal growth, expression of stem cell genes, formation of spheres, and ability to differentiate to functional keratocytes.31 This study showed that anterior limbal mesenchymal ‘niche’ cells could not be distinguished in vitro from the previously characterized CSSCs.31
In summary, data from multiple studies are consistent in support of a model that a population of multipotent MSCs is present in stroma of human and other mammalian corneas, and these cells are localized in the limbal palisades immediately subjacent to the epithelial basement membrane, where they maintain a close association with LESCs.
D. Function of Corneal Stromal Stem Cells In Vivo
A number of studies contribute evidence that, in vivo, CSSCs provide a biological support system for maintenance of the active population of LESCs in the limbal niche in which both cell types reside.25,27,32–38 In vitro, limbal fibroblasts supported expansion of LESCs better than 3T3 cells32 or scleral fibroblasts,33 and this ability was enhanced by expansion of the CSSCs in culture conditions that maintain the stem-like phenotype of the CSSCs.27 When CSSCs and LESCs were co-isolated in clusters using collagenase digestion, the LESCs expanded more rapidly and formed more holoclones, than without the niche cells.34 The co-isolated cells were found to be associated via the interaction of SDF-1 and its receptor CXCR4, a connection that reduced differentiation of LESCs.26 Disruption of the CXCR4/SDF-1 axis using AMD3100 reduced sphere and holoclone formation by LESCs.26 In co-cultures of limbal epithelial cells and limbal stromal cells, IL6 secretion was found to activate STAT3 signaling in the epithelial cell population, and this signaling pathway was proposed as an active component in the niche function of stromal cells.39.
These studies and the anatomical proximity of these two cell populations provide convincing evidence that a primary role for CSSCs in vivo is homeostatic maintenance of the LESC population in the limbal niche. The potential for CSSCs to mediate immune responses and to initiate stromal regeneration (discussed below) suggest other potential in vivo roles, but currently little evidence documents those activity in vivo.
E. Embryonic Origins of Corneal Stromal Stem Cells In Vivo
Corneal epithelium is an ectodermal derivative, but stromal and endothelial cells are derived from neural crest. Expression of PAX6, as well as Six2, Six3, and Notch1, by CSSCs suggests a neural crest lineage for these CSSCs.15 Other origins are possible, however. Bone marrow-derived cells are typically present in the corneal stroma, and some authors have proposed a bone marrow origin for all MSCs.40 Another hypothesis proposes that adult MSCs derive from perivascular cells (pericytes) in each tissue.41 The plasticity of stem cells makes it difficult to test this hypothesis in human CSSCs. Mice, however, have a population of multipotent progenitor cells in the stromal limbus analogous to the human CSSCs. As with human CSSCs, the mouse stromal stem cells do not express CD45, suggesting that they are not bone marrow-derived cells.21 In lethally irradiated mice rescued by transplantation of bone marrow cells expressing enhanced green fluorescent protein (EGFP), fluorescent cells were present in the stoma, but clonal spheres formed by the stromal progenitor cells did not contain bone marrow-derived cells, as indicated by the absence of green cells.21 Additionally, spheres from transgenic mice with P0-Cre/Floxed-stop-EGFP as well as Wnt1-Cre/Floxed-stop-EGFP were EGFP-positive , indicating an ocular and neural crest embryonic lineage of the stromal progenitor cells.21 These results were confirmed by the expression of the embryonic neural crest markers Twist, Snail, Slug, and Sox9 in these spheres. The similar character of CSSC and mouse stromal progenitor cells supports the idea that stromal stem cells are derivatives of ocular neural crest and are not derived from pericytes or other bone marrow progenitor cells.
F. Differentiation to Keratocytes
When cultured in low-mitogen, ascorbate-containing media, CSSCs express an array of genes characteristic of keratocytes.17 When the cells are removed from substratum and cultured as a pellet, a more complete keratocyte gene expression pattern was observed and significant amounts of ECM were deposited, some with tracts of aligned collagen fibrils, similar to that seen in stroma in vivo.18 When cultured on substratum of parallel aligned polymeric nanofibers, CSSCs produced layers of highly parallel collagen fibers with packing and fibril diameter close to that of human stromal lamellae.42 Additional studies demonstrated that a stroma-like organization of the tissue by CSSCs could also be elicited by silk or polycarbonate substrata with parallel grooves to align the cells, demonstrating a role for topographical cues in guiding organization of tissue.43,44 The presence of TGF-β3 and FGF2 in combination resulted in more abundant and stroma-like organization of the ECM produced by CSSCs.45
CSSCs also adopt keratocyte phenotype and function in vivo. When injected into mouse corneal stroma, human CSSCs expressed keratocyte mRNA and protein, depositing human corneal matrix components.46 These injected cells remained viable for months, apparently having permanently become quiescent keratocytes. These results suggest that keratocytes represent a default lineage for the CSSC; the implication of this is that some aspect of the limbal microenvironment maintains the stem/progenitor character of CSSCs in vivo, but, if they enter the stroma the CSSCs spontaneously, differentiate to keratocytes. The proximity of CSSCs and LESCs in vivo suggests the possibility that each of these populations provides symbiotic support for maintenance of the stem cell phenotype of the other. Beyond these observations, however, little is known about participation of CSSCs in normal stromal homeostasis or in tissue healing and/or regeneration in response to trauma or pathology.
G. Immune Modulation by Corneal Stromal Stem Cells
There is a considerable body of evidence documenting the immune-modulatory properties of MSCs, including the fact that these cells have therapeutic effectiveness in immune-incompatible individuals in clinical trials.47–50 Similar to other MSCs, CSSCs expanded in culture are able to alter aspects of the cellular immune response. Injection of human CSSCs into mouse corneal stroma in vivo resulted in no xenogeneic T-cell-mediated immune rejection of these cells.46 Injection of human corneal fibroblasts, on the other hand, produced a marked increase in CD45+ cells after 1 week. Immunostaining of the injected tissue at 2 weeks showed CD3+ T-cells associated with the injected human fibroblasts; however, no T-cells were detected in corneas injected with CSSCs.46 The fibroblast-injected eyes exhibited visible haze, increasing after 2 weeks, but the CSSC-injected eyes remained clear. Finally, chimeric mice rescued with EGFP-bone marrow cells exhibited only a transient influx of green cells after CSSC injection.46 Conversely, injected human corneal fibroblasts elicited a strong influx of green cells into the cornea, lasting more than 10 days. Inhibition of T-cell proliferation by limbal stromal MSCs has also been confirmed by in vitro experiments with cells cultured from both human and rabbit corneas.36 These data all support an immunomodulatory function for CSSCs. Such properties may enhance the effectiveness and stability of CSSCs in allogeneic cell-based or tissue engineered therapeutic applications.
H. Corneal Stromal Stem Cells and Corneal Regeneration
Corneal scarring involves long-term alterations in the matrix structure of stromal ECM, including changes in collagen molecular type, fibril diameter, and spacing.51 The deletion of lumican, a major stromal proteoglycan, in mice results in a scar-like phenotype with hazy corneas due to increases in the size and organization of stromal collagen fibrils.51 When CSSCs were injected into the stromas of lumican knockout mice, human lumican accumulated in the cornea, indicating that the CSSCs were depositing human ECM.46 Additionally, the large heterogeneous collagen fibrils that characterize lumican knockout ECM were no longer observed, and the architecture of the tissue became indistinguishable from that of wild-type mice. Corneal thickness and transparency were similar to that of normal corneas. A similar effect was observed if stem cells from human umbilical cord were used.52 This kind of complete restructuring of existing connective tissue can take place only by removal of existing ECM structures and deposition of new native tissue. To be able to accomplish this process, it would appear that the CSSCs are capable of mediating the remodeling and regeneration of stromal ECM.
The regenerative properties of CSSCs were further demonstrated in a model of mouse corneal wound healing. Wounds were generated by debridement of epithelium and anterior stromal tissue with a rotating burr, resulting in deposition of opaque scar tissue, accompanied by loss of stromal lamellar organization, large and disorganized collagen fibrils, and deposition of collagen type III, hyaluronan, and fibronectin.53 When CSSCs were layered on the surface of the cornea in fibrin gel at the time of wounding, the debrided stromal tissue was replaced with ECM containing normal collagen organization and fibril diameter. Fibrotic ECM components were absent and corneal transparency was normal.31 In this model, human ECM components were detected close to the CSSCs at the anterior surface of the stroma, but much of the wound region was replaced with mouse tissue. These results support the concept that the CSSCs are not directly replacing the debrided stromal tissue, but rather that they influence the tissue deposited by mouse cells during wound healing in a paracrine fashion.
Regenerative responses to paracrine stimulation by MSCs have been documented in other organs, including lung, liver, kidney, and heart. The mechanisms by which these effects are mediated have been proposed to involve cytokines, chemokines, exosomes, and modulation of the immune response by the stem cells.54–58 Considering the close association of infiltrating immune cells (particularly T-cells) with corneal scarring,59–67 and the documented effect on Tcell infiltration by CSSCs in damaged mouse cornea (described above), it seems likely that corneal regeneration by CSSCs involves their ability to alter cellular immune response in the healing tissue.
III. Therapeutic Applications of Corneal Stromal Stem Cells
Although most studies of human CSSCs have been carried out with cadaveric tissue bank material, limbal biopsies are being actively carried out in order to isolate autologous LESCs for restoration of the this population to eyes with limbal stem cell deficiency.22,24,68,69 Mesenchymal cells present in this biopsy tissue have been shown to be fully potent CSSCs, capable of generating stromal tissue in vitro and of preventing corneal scarring in mice.31 This recent discovery opens the door to treatment of stromal scarring with autologous cells. Corneas with anterior scarring that contain a competent endothelium are often treated with use of a partial thickness anterior lamellar keratoplasty. The efficacy of this process is similar to that of penetrating keratoplasty, but it is considered safer.70 Unfortunately, many of these grafts are lost within 3–5 years.71
Clinical trials are already underway with an acellular collagen as partial thickness lamellar grafts.72,73 The ability of CSSCs to produce multilayer stromal tissue makes them an excellent candidate for this application. Such grafts would represent a cellularized alternative to the cross-linked collagen, and thus would not need to undergo extensive remodeling. Since the CSSCs represent a pure, non-immune, autologous cell population, the potential for rejection should be minimal in this application. Development of such stromal replacement constructs with CSSCs is therefore a high priority in the therapeutic application of CSSCs.
In addition to utility in bioengineering stromal tissue, CSSCs may have the potential to provide direct cell-based therapy for corneal scarring. Application in human patients of the procedure developed for mice, in which cell are layered on the cornea in fibrin gel, after epithelial debridement, would leave the corneal essentially intact, reducing loss of strength and potential for infection of sutures and glaucoma that can be associated with keratoplasty. Applications to initiate clinical trials using this approach are in process (personal communication, V. Sangwan, S. Basu 2015); thus, we may soon understand whether the regenerative nature of CSSCs can be applied directly to therapy for human corneal scarring.
IV. Summary and Conclusions
Corneal stromal stem cells are a population of neural crest-derived mesenchymal stem cells localized in the limbal stroma, subjacent to the epithelial basement membrane. Multiple studies show that the CSSCs associate with and support the function of LESCs. In vitro, CSSCs maintain an enhanced potential to become functional keratocytes after multiple rounds of expansion compared to the majority of the cells in the stroma. CSSCs in vitro can produce collective tissue similar in composition and structure to that of human stroma and thus present an excellent potential for use in corneal bioengineering applications. Their ability to regenerate normal stromal tissue in a mouse scar model and their immunomodulatory properties present an exciting prospect for use of CSSCs in direct cell-based therapy for human corneal scarring.
Acknowledgments
Sources of support: This work was supported by NIH grant EY016415 (to J.L.F.), NIH Core grant P30-EY08098, and grants from Louis J. Fox Center for Vision Restoration (Pittsburgh), and an unrestricted grant from Research to Prevent Blindness, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no commercial or proprietary interest in any concept or product discussed in this article.
References
- 1.Szentmáry N, Nagy ZZ, Resch M, et al. Proliferation and apoptosis in the corneal stroma in longterm follow-up after photorefractive keratectomy. Pathol Res Pract. 2005;201:399–404. doi: 10.1016/j.prp.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Wilson SE. Analysis of the keratocyte apoptosis, keratocyte proliferation, and myofibroblast transformation responses after photorefractive keratectomy and laser in situ keratomileusis. Trans Am Ophthalmol Soc. 2002;100:411–433. [PMC free article] [PubMed] [Google Scholar]
- 3.Zieske JD, Guimaraes SR, Hutcheon AE. Kinetics of keratocyte proliferation in response to epithelial debridement. Exp Eye Res. 2001;72:33–39. doi: 10.1006/exer.2000.0926. [DOI] [PubMed] [Google Scholar]
- 4.Jester JV, Lee YG, Huang J, et al. Postnatal corneal transparency, keratocyte cell cycle exit and expression of ALDH1A1. Invest Ophthalmol Vis Sci. 2007;48:4061–4069. doi: 10.1167/iovs.07-0431. [DOI] [PubMed] [Google Scholar]
- 5.Zieske JD. Corneal development associated with eyelid opening. Int J Dev Biol. 2004;48:903–911. doi: 10.1387/ijdb.041860jz. [DOI] [PubMed] [Google Scholar]
- 6.Johnson GJ. Vision 2020: The right to sight: report on the Sixth General Assembly of the International Agency for the Prevention of Blindness (IAPB) Community Eye Health. 1999;12:59–60. [PMC free article] [PubMed] [Google Scholar]
- 7.Funderburgh JL, Mann MM, Funderburgh ML. Keratocyte phenotype mediates proteoglycan structure: a role for fibroblasts in corneal fibrosis. J Biol Chem. 2003;278:45629–45637. doi: 10.1074/jbc.M303292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999;18:529–551. doi: 10.1016/s1350-9462(98)00033-0. [DOI] [PubMed] [Google Scholar]
- 9.Cintron C, Kublin CL. Regeneration of corneal tissue. Dev Biol. 1977;61:346–357. doi: 10.1016/0012-1606(77)90304-9. [DOI] [PubMed] [Google Scholar]
- 10.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 11.Carlson EC, Wang IJ, Liu CY, et al. Altered KSPG expression by keratocytes following corneal injury. Mol Vis. 2003;9:615–623. [PubMed] [Google Scholar]
- 12.Wollensak G, Green WR. Analysis of sex-mismatched human corneal transplants by fluorescence in situ hybridization of the sex-chromosomes. Exp Eye Res. 1999;68:341–346. doi: 10.1006/exer.1998.0611. [DOI] [PubMed] [Google Scholar]
- 13.Long CJ, Roth MR, Tasheva ES, et al. Fibroblast growth factor-2 promotes keratan sulfate proteoglycan expression by keratocytes in vitro. J Biol Chem. 2000;275:13918–13923. doi: 10.1074/jbc.275.18.13918. [DOI] [PubMed] [Google Scholar]
- 14.Ren R, Hutcheon AE, Guo XQ, et al. Human primary corneal fibroblasts synthesize and deposit proteoglycans in long-term 3-D cultures. Dev Dyn. 2008;237:2705–2715. doi: 10.1002/dvdy.21606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funderburgh ML, Du Y, Mann MM, et al. PAX6 expression identifies progenitor cells for corneal keratocytes. FASEB J. 2005;19:1371–1373. doi: 10.1096/fj.04-2770fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golebiewska A, Brons NH, Bjerkvig R, Niclou SP. Critical appraisal of the side population assay in stem cell and cancer stem cell research. Cell Stem Cell. 2011;8:136–147. doi: 10.1016/j.stem.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Du Y, Funderburgh ML, Mann MM, et al. Multipotent stem cells in human corneal stroma. Stem Cells. 2005;23:1266–1275. doi: 10.1634/stemcells.2004-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du Y, Sundarraj N, Funderburgh ML, et al. Secretion and organization of a cornea-like tissue in vitro by stem cells from human corneal stroma. Invest Ophthalmol Vis Sci. 2007;48:5038–5045. doi: 10.1167/iovs.07-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amano S, Yamagami S, Mimura T, Uchida S, Yokoo S. Corneal stromal and endothelial cell precursors. Cornea. 2006;25:S73–S77. doi: 10.1097/01.ico.0000247218.10672.7e. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida S, Shimmura S, Shimazaki J, et al. Serum-free spheroid culture of mouse corneal keratocytes. Invest Ophthalmol Vis Sci. 2005;46:1653–1658. doi: 10.1167/iovs.04-1405. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida S, Shimmura S, Nagoshi N, et al. Isolation of multipotent neural crest-derived stem cells from the adult mouse cornea. Stem Cells. 2006;24:2714–2722. doi: 10.1634/stemcells.2006-0156. [DOI] [PubMed] [Google Scholar]
- 22.Shortt AJ, Secker GA, Munro PM, et al. Characterization of the limbal epithelial stem cell niche: novel imaging techniques permit in vivo observation and targeted biopsy of limbal epithelial stem cells. Stem Cells. 2007;25:1402–1409. doi: 10.1634/stemcells.2006-0580. [DOI] [PubMed] [Google Scholar]
- 23.Mathews S, Chidambaram JD, Lanjewar S, et al. In vivo confocal microscopic analysis of normal human anterior limbal stroma. Cornea. 2015;34:464–470. doi: 10.1097/ICO.0000000000000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polisetty N, Fatima A, Madhira SL, et al. Mesenchymal cells from limbal stroma of human eye. Mol Vis. 2008;14:431–442. [PMC free article] [PubMed] [Google Scholar]
- 25.Xie H-T, Chen S-Y, Li G-G, Tseng SCG. Isolation and expansion of human limbal stromal niche cells. Invest Ophthalmol Vis Sci. 2012;53:279–286. doi: 10.1167/iovs.11-8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie H-T, Chen S-Y, Li G-G, Tseng SCG. Limbal epithelial stem/progenitor cells attract stromal niche cells by SDF-1/CXCR4 signaling to prevent differentiation. Stem Cells. 2011;29:1874–1885. doi: 10.1002/stem.743. [DOI] [PubMed] [Google Scholar]
- 27.Li G-G, Zhu Y-T, Xie H-T, Chen S-Y, Tseng SCG. Mesenchymal stem cells derived from human limbal niche cells. Invest Ophthalmol Vis Sci. 2012;53:5686–5697. doi: 10.1167/iovs.12-10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rankin SM. Chemokines and adult bone marrow stem cells. Immunol Lett. 2012;145:47–54. doi: 10.1016/j.imlet.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Higa K, Kato N, Yoshida S, et al. Aquaporin 1-positive stromal niche-like cells directly interact with N-cadherin-positive clusters in the basal limbal epithelium. Stem Cell Res. 2013;10:147–155. doi: 10.1016/j.scr.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Dziasko MA, Armer HE, Levis HJ, et al. Localisation of epithelial cells capable of holoclone formation in vitro and direct interaction with stromal cells in the native human limbal crypt. PLoS One. 2014;9:e94283. doi: 10.1371/journal.pone.0094283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basu S, Hertsenberg AJ, Funderburgh ML, et al. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci Transl Med. 2014;6:266ra172. doi: 10.1126/scitranslmed.3009644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Sun H, Li X, et al. Utilization of human limbal mesenchymal cells as feeder layers for human limbal stem cells cultured on amniotic membrane. J Tissue Eng Regen Med. 2010;4:38–44. doi: 10.1002/term.216. [DOI] [PubMed] [Google Scholar]
- 33.Ainscough SL, Linn ML, Barnard Z, et al. Effects of fibroblast origin and phenotype on the proliferative potential of limbal epithelial progenitor cells. Exp Eye Res. 2011;92:10–19. doi: 10.1016/j.exer.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Chen SY, Hayashida Y, Chen MY, et al. A new isolation method of human limbal progenitor cells by maintaining close association with their niche cells. Tissue Eng Part C Methods. 2011;17:537–548. doi: 10.1089/ten.tec.2010.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashmani K, Branch MJ, Sidney LE, et al. Characterization of corneal stromal stem cells with the potential for epithelial transdifferentiation. Stem Cell Res Ther. 2013;4:75. doi: 10.1186/scrt226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bray LJ, Heazlewood CF, Munster DJ, et al. Immunosuppressive properties of mesenchymal stromal cell cultures derived from the limbus of human and rabbit corneas. Cytotherapy. 2014;16:64–73. doi: 10.1016/j.jcyt.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Katikireddy KR, Dana R, Jurkunas UV. Differentiation potential of limbal fibroblasts and bone marrow mesenchymal stem cells to corneal epithelial cells. Stem Cells. 2014;32:717–729. doi: 10.1002/stem.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang M, Wang B, Wan P, et al. Roles of limbal microvascular net and limbal stroma in regulating maintenance of limbal epithelial stem cells. Cell Tissue Res. 2015;359:547–563. doi: 10.1007/s00441-014-2032-4. [DOI] [PubMed] [Google Scholar]
- 39.Notara M, Shortt AJ, Galatowicz G, et al. IL6 and the human limbal stem cell niche: a mediator of epithelial-stromal interaction. Stem Cell Res. 2010;5:188–200. doi: 10.1016/j.scr.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Sosnova M, Bradl M, Forrester JV. CD34+ corneal stromal cells are bone marrow-derived and express hemopoietic stem cell markers. Stem Cells. 2005;23:507–515. doi: 10.1634/stemcells.2004-0291. [DOI] [PubMed] [Google Scholar]
- 41.Corselli M, Chen CW, Crisan M, et al. Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol. 2010;30:1104–1109. doi: 10.1161/ATVBAHA.109.191643. [DOI] [PubMed] [Google Scholar]
- 42.Wu J, Du Y, Watkins SC, et al. The engineering of organized human corneal tissue through the spatial guidance of corneal stromal stem cells. Biomaterials. 2012;33:1343–1352. doi: 10.1016/j.biomaterials.2011.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karamichos D, Funderburgh ML, Hutcheon AEK, et al. A role for topographic cues in the organization of collagenous matrix by corneal fibroblasts and stem cells. PLoS One. 2014;9:e86260. doi: 10.1371/journal.pone.0086260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu J, Rnjak-Kovacina J, Du Y, Funderburgh ML, et al. Corneal stromal bioequivalents secreted on patterned silk substrates. Biomaterials. 2014;35:3744–3755. doi: 10.1016/j.biomaterials.2013.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu J, Du Y, Mann MM, et al. Bioengineering organized, multilamellar human corneal stromal tissue by growth factor supplementation on highly aligned synthetic substrates. Tissue Eng Part A. 2013;19:2063–2075. doi: 10.1089/ten.tea.2012.0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Du Y, Carlson EC, Funderburgh ML, et al. Stem cell therapy restores transparency to defective murine corneas. Stem Cells. 2009;27:1635–1642. doi: 10.1002/stem.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stagg J, Galipeau J. Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Curr Mol Med. 2013;13:856–867. doi: 10.2174/1566524011313050016. [DOI] [PubMed] [Google Scholar]
- 48.Atoui R, Chiu RC. Immune responses after mesenchymal stem cell implantation. Methods Mol Biol. 2013;1036:107–120. doi: 10.1007/978-1-62703-511-8_10. [DOI] [PubMed] [Google Scholar]
- 49.Fibbe WE, Bernardo ME. Control of immune responses by mesenchymal stromal cells. Rinsho Ketsueki. 2014;55:2190–2194. [PubMed] [Google Scholar]
- 50.Knaan-Shanzer S. Concise review: the immune status of mesenchymal stem cells and its relevance for therapeutic application. Stem Cells. 2014;32:603–608. doi: 10.1002/stem.1568. [DOI] [PubMed] [Google Scholar]
- 51.Hassell JR, Birk DE. The molecular basis of corneal transparency. Exp Eye Res. 2010;91:326–335. doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu H, Zhang J, Liu CY, et al. Cell therapy of congenital corneal diseases with umbilical mesenchymal stem cells: lumican null mice. PLoS One. 2010;5:e10707. doi: 10.1371/journal.pone.0010707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boote C, Du Y, Morgan S, et al. Quantitative assessment of ultrastructure and light scatter in mouse corneal debridement wounds. Invest Ophthalmol Vis Sci. 2012;53:2786–2795. doi: 10.1167/iovs.11-9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li J, Huang S, Wu Y, et al. Paracrine factors from mesenchymal stem cells: a proposed therapeutic tool for acute lung injury and acute respiratory distress syndrome. Int Wound J. 2014;11:114–121. doi: 10.1111/iwj.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu T, Liu Y, Wang B, Li G. The roles of mesenchymal stem cells in tissue repair and disease modification. Curr Stem Cell Res Ther. 2014;9:424–431. doi: 10.2174/1574888x09666140616125446. [DOI] [PubMed] [Google Scholar]
- 56.Berardis S, Dwisthi Sattwika P, Najimi M, Sokal EM. Use of mesenchymal stem cells to treat liver fibrosis: current situation and future prospects. World J Gastroenterol. 2015;21:742–758. doi: 10.3748/wjg.v21.i3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li N, Wang C, Jia L, Du J. Heart regeneration, stem cells, and cytokines. Regen Med Res. 2014;2:6. doi: 10.1186/2050-490X-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsuji K, Kitamura S. Trophic factors from tissue stem cells for renal regeneration. Stem Cells Int. 2015;2015:537204. doi: 10.1155/2015/537204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakamoto T, Ueno H, Sonoda K, et al. Blockade of TGF-beta by in vivo gene transfer of a soluble TGF-beta type II receptor in the muscle inhibits corneal opacification, edema and angiogenesis. Gene Ther. 2000;7:1915–1824. doi: 10.1038/sj.gt.3301320. [DOI] [PubMed] [Google Scholar]
- 60.Yamanaka O, Liu C-Y, Kao WW-Y. Fibrosis in the anterior segments of the eye. Endocr Metab Immune Disord Drug Targets. 2010;10:331–335. doi: 10.2174/1871530311006040331. [DOI] [PubMed] [Google Scholar]
- 61.Asano-Kato N, Toda I, Fukumoto T, et al. Detection of neutrophils in late-onset interface inflammation associated with flap injury after laser in situ keratomileusis. Cornea. 2004;23:306–310. doi: 10.1097/00003226-200404000-00016. [DOI] [PubMed] [Google Scholar]
- 62.Ueno M, Lyons BL, Burzenski LM, et al. Accelerated wound healing of alkali-burned corneas in MRL mice is associated with a reduced inflammatory signature. Invest Ophthalmol Vis Sci. 2005;46:4097–4106. doi: 10.1167/iovs.05-0548. [DOI] [PubMed] [Google Scholar]
- 63.Allen SJ, Mott KR, Ljubimov AV, Ghiasi H. Exacerbation of corneal scarring in HSV-1 gK-immunized mice correlates with elevation of CD8+CD25+ T cells in corneas of ocularly infected mice. Virology. 2010;399:11–22. doi: 10.1016/j.virol.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chan MF, Li J, Bertrand A, et al. Protective effects of matrix metalloproteinase-12 following corneal injury. J Cell Sci. 2013;126:3948–3960. doi: 10.1242/jcs.128033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matundan H, Mott KR, Ghiasi H. Role of CD8+ T cells and lymphoid dendritic cells in protection from ocular herpes simplex virus 1 challenge in immunized mice. J Virol. 2014;88:8016–8027. doi: 10.1128/JVI.00913-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu K, Harris DL, Yamaguchi T, et al. A dual role for corneal dendritic cells in herpes simplex keratitis: local suppression of corneal damage and promotion of systemic viral dissemination. PLoS One. 2015;10:e0137123. doi: 10.1371/journal.pone.0137123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Osorio Y, Cai S, Hofman FM, et al. Involvement of CD8+ T-cells in exacerbation of corneal scarring in mice. Curr Eye Res. 2004;29:145–151. doi: 10.1080/02713680490504632. [DOI] [PubMed] [Google Scholar]
- 68.Mariappan I, Maddileti S, Savy S, et al. In vitro culture and expansion of human limbal epithelial cells. Nat Protoc. 2010;5:1470–1479. doi: 10.1038/nprot.2010.115. [DOI] [PubMed] [Google Scholar]
- 69.Sangwan VS, Basu S, Vemuganti GK, et al. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: a 10-year study. Br J Ophthalmol. 2011;95:1525–1529. doi: 10.1136/bjophthalmol-2011-300352. [DOI] [PubMed] [Google Scholar]
- 70.Liu H, Chen Y, Wang P, et al. Efficacy and safety of deep anterior lamellar keratoplasty vs. penetrating keratoplasty for keratoconus: a meta-analysis. PLoS One. 2015;10:e0113332. doi: 10.1371/journal.pone.0113332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feizi S, Javadi MA, Javadi F, Jafarinasab MR. Deep anterior lamellar keratoplasty in keratoconic patients with versus without vernal keratoconjunctivitis. J Ophthalmic Vis Res. 2015;10:112–117. doi: 10.4103/2008-322X.163768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fagerholm P, Lagali NS, Merrett K, et al. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci Transl Med. 2010;2:46ra61. doi: 10.1126/scitranslmed.3001022. [DOI] [PubMed] [Google Scholar]
- 73.Buznyk O, Pasyechnikova N, Islam MM, et al. Bioengineered corneas grafted as alternatives to human corneas in three high-risk patients. Clin Transl Sci. 2015;8:558–562. doi: 10.1111/cts.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]