Abstract
Alkali burns to the cornea are among the most devastating injuries to the eye. The purpose of this study was to evaluate the effects of dexamethasone (Dex) or doxycycline (Doxy) on protease activity and corneal complications in a combined model (CM) of alkali burn and dry eye. C57BL/6 mice were subjected to the CM for 2 or 5 days (D). Mice were topically treated either with Dex (0.1%), Dox (0.025%) or vehicle QID and observed daily for appearance of corneal perforation. Quantitative real time PCR was performed to measure expression of inflammation cytokines and matrix metalloproteinases (MMP) in whole cornea lysates. No perforations were observed in the Dex-treated corneas. All wounds in Doxy-treated corneas were closed 2D post-injury, and they had significantly lower corneal opacity scores at days 4 and 5 post-injury compared to BSS treatment. Dex-treated corneas had the lowest corneal opacity scores. Dex treatment significantly decreased expression of IL-1β, IL-6, MMPs -1, -9, -13, and TIMP-1 after 2 days but increased levels of MMP-8, while Doxy treatment significantly decreased IL-1β, IL-6, MMP-8, and -9, compared to vehicle. Decreased MMP -1, -9 and -13 immunoreactivity and gelatinolytic activity were seen in corneas treated with Doxy and Dex compared to vehicle. Increased neutrophil infiltration and myeloperoxidase activity was noted in the vehicle group compared to Dex 2 days post-injury. These findings demonstrate that early initiation of anti-inflammatory therapy is very efficacious in preserving corneal clarity and facilitating wound healing, while modulating MMP production and suppressing neutrophil infiltration.
Keywords: alkali injury, corticoid, dexamethasone, doxycycline, dry eye, neutrophils, MMPs, ocular perforation
I. Introduction
Chemical/thermal injuries to the eye are potentially blinding conditions. Therapeutic strategies for ocular surface alkali injuries have been directed toward promoting epithelial healing and suppressing inflammation and tissue destruction, during the acute phase.1 Treatment of the chronic phases, including the anatomical sequelae, sometimes require a multidisciplinary approach and, very often, surgical procedures such as amniotic membrane, keratolimbal stem cell, or cornea transplantation.2 Although these approaches have slightly improved visual outcomes, visual outcomes from these injuries still poor, in large part due to inadequate control of inflammatory and proteolytic components of the wound healing response. Among the treatments proposed, several inhibitors of MMPs and inflammation have been used with some success.3–5
Previous studies have demonstrated a marked anticollagenolytic effect for the tetracycline family antibiotics in rat, rabbit, and human corneas, both in vivo and in vitro.6–9 Doxycycline (Doxy) was discovered in the early 1960s as a semisynthetic long-acting tetracycline antibiotic that inhibits ribosomes in a wide variety of bacteria. It has also proven to be effective in treating common ocular surface/cornea diseases such as acne rosacea, recurrent corneal erosions, and sterile corneal ulceration.10 Subantimicrobial doses have been reported to be effective for treating chronic meibomian gland dysfunction11 and are approved adjunctive treatment for adult periodontitis.12,13 Doxy, administered by subconjunctival injections or drops, successfully prevented neutrophil infiltration and promoted healing in corneas subjected to alkali burn from half-mustard in an animal model.14,15
It has been suggested that early systemic and topical administration of corticosteroids after alkali injury may be of benefit by decreasing inflammation, scarring, and neovascularization, improving visual outcomes and reducing the need for transplantation.16–18 Early (1970s) reports of corticosteroid use showing decreased efficacy in deep alkali burns in rabbits may have limited its use as a therapeutic option.16,19
Dry eye is an unrecognized comorbidity that can markedly worsen the outcome of ocular surface chemical burns by increasing the risk of sterile corneal ulceration. We have previously reported that combining alkali burn with dry eye leads to delayed wound healing, greater corneal opacity, greater production and activity of MMPs, and increased neutrophil infiltration.20 We hypothesize that early anti-inflammatory therapy can modulate the deleterious effects of alkali burn associated with dry eye. The purpose of this study was to investigate the efficacy of the corticosteroid dexamethasone (Dex) and Doxy, a broad MMP inhibitor, in controlling inflammation and MMP production in a combined model of alkali-burn associated with dry eye.
II. Materials and Methods
A. Animals
Female C57BL/6 mice aged 6–8 weeks were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and subjected to alkali burn associated with dry eye as previously described.20 Animals were euthanized after 2 or 5 days unless otherwise noted. At least 28 animals without corneal perforations were used per topical treatment group (Balanced Salt Solution [BSS] Doxy, Dex) and per time point: 6 for histology, 15 for real-time PCR, 4 for myeloperoxidase assay, and 3 for gelatinase zymography. Corneal opacity and wound closure rate were evaluated in twelve live mice that were subsequently used for either histology or PCR. Twenty-two naïve control animals were used. The contralateral eyes served as an untreated control (UT). Appearance of corneal perforation was a criterion for early euthanasia.
This research protocol was approved by the Baylor College of Medicine Center for Comparative Medicine, and it conformed to the standards in the ARVO Statement for the use of animals in Ophthalmic and Vision Research.
B. Alkali Burn Associated with Dry Eye Mouse Model
After systemic anesthesia with isoflurane using a vaporizer (SomnoSuite, Kent Scientific, Torrington, Connecticut), a unilateral alkali burn (AB) was created on the right eye of 6–8 week old C57BL/6 mice. This was achieved by placing one 2.0 mm diameter filter paper disc that had been presoaked with 1N NaOH on the central cornea for 10 seconds, followed by extensive rinsing with balanced salt solution (Alcon, Fort Worth, TX, USA), as previously described.20,21 Precautions were taken to avoid damage to the peripheral cornea, conjunctiva, and lids. AB was created at day 0 and animals were sacrificed after 2 or 5 days. After anesthesic recovery, mice were subjected to environmentally-induced dry eye to create a combined model (CM) of alkali burn and dry eye.
Desiccating stress (DS) was induced in female C57BL/6 mice aged 6–8 weeks by sterile subcutaneous injection of 0.5 mg/1 mL scopolamine hydrobromide (Sigma-Aldrich, St. Louis, MO) QID into alternating flanks and exposure to a drafty low humidity (<30% relative humidity) environment as previously described.22 Mice received no antibiotic eyedrops.
C. Treatment Regimen
1. Short-term Dosing and Short-term Combined Model
Mice subjected to the combined model for 2 and 5 days were topically treated either with 2 μL sodium phosphate dexamethasone (Dex, 0.1%, Spectrum Laboratory, Gardena, CA), 2 μL of freshly prepared doxycycline USP (Doxy, 0.025%,Sigma-Aldrich, St. Louis, MO) or vehicle (balanced salt solution, BSS, Alcon, Fort Worth, TX) QID and compared to naïve control corneas.
2. Extended Dosing after Short-term Combined Model
To evaluate if prolonged anti-inflammatory therapy promoted corneal melting, a distinct group of animals (n=six/group) were subjected to the combined model and dosed with either BSS, Doxy or Dex QID for 5 days. On the 6th day, mice were placed in a vivarium room with normal environment (relative humidity >40–60%) and topical treatment continued for another 16 days, for a total of 21 days of topical regimen.
D. Clinical Findings
1. Ocular Perforation and Opacity Score
Eyes of all treatment groups were examined daily under a microscope (SMZ 1500, Nikon, Melville, New York) for presence of corneal perforation. Once corneal perforation was observed, mice were euthanized. The number of corneal perforations occurring each day was recorded and survival curves were calculated using Graph Pad Prism 6.0 software (GraphPad Software Incorporation, San Diego, CA). Mice with corneal perforations were not used any further studies.
Corneal opacity was graded by two masked observers in images taken with a color digital camera DS-Fi1 (Melville, New York) as previously described.23 Corneal opacity was scored using a scale of 0–4 (grade 0=completely clear, grade 1=slightly hazy, iris and pupils easily visible, grade 2= slightly opaque, iris and pupils still detectable, grade 3=opaque, pupils hardly detectable, and grade 4=completely opaque with no view of the pupils).
2. Measurement of Corneal Epithelial Defect
Corneal epithelial healing was assessed daily by instilling 1 μL of 0.1% liquid sodium fluorescein onto the ocular surface. Corneas were rinsed with phosphate-buffered saline and photographed with a stereoscopic zoom microscope (SMZ 1500; Nikon, Melville, NY) under fluorescence excitation at 470 nm (DS-Qi1Mc, Nikon, Melville, NY). Corneal epithelial defects were graded in digital images by two masked observers in a categorical manner (present/absent) to generate a survival curve. Biological replicate scores were transferred to an excel database where the results were analyzed.
E. RNA Isolation and Quantitative PCR
Five whole corneas (including stroma) per group at 2 and 5 days post-injury were excised, minced and total RNA was extracted using a Qiagen MicroPlus RNeasy isolation® Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions, quantified by a NanoDrop® ND-2000 Spectrophotometer (Thermo Scientific, Wilmington, DE) and stored at −80°C. First-strand cDNA was synthesized with random hexamers by M-MuLV reverse transcription (Ready-To-Go You-Prime First-Strand Beads; GE Healthcare, Inc., Arlington Heights, NJ), as previously described.22 There were a total of three independent experiments.
Real-time polymerase chain reaction (PCR) was performed with specific Taqman MGB probes (Applied Biosystems, Inc., Foster City, CA) and PCR master mix (Taqman Gene Expression Master Mix), in a commercial thermocycling system (StepOnePlus™ Real-Time PCR System, Applied Biosystems), according to the manufacturer’s recommendations. Quantitative real time PCR was performed using gene expression assay primers and MGB probes specific for murine targets described in Table 1. The hypoxanthine guanine phosphoribosyl transferase (HPRT-1) gene was used as an endogenous reference for each reaction to correct for differences in the amount of total RNA added. The results of quantitative PCR were analyzed by the comparative CT method where target change = 2−ΔΔCT. The results were normalized by the CT value of HPRT-1 and the relative mRNA level in the untreated group was used as the calibrator.
Table 1.
Oligonucleotide primers used for real-time PCR.
| Gene Name | Symbol | Assay ID* |
|---|---|---|
| Interleukin 1 beta | IL1-β | Mm00434228 |
| Interleukin 6 | IL-6 | Mm99999064 |
| Chemokine (C-X-C motif) ligand 1 | CXCL1 | Mm04207460 |
| Matrix metalloproteinase 1 | MMP-1 | Mm00473493 |
| Matrix metalloproteinase 2 | MMP-2 | Mm00439506 |
| Matrix metalloproteinase 3 | MMP-3 | Mm00440295 |
| Matrix metalloproteinase 8 | MMP-8 | Mm00439509 |
| Matrix metalloproteinase 9 | MMP-9 | Mm00442991 |
| Matrix metalloproteinase 13 | MMP-13 | Mm00439491 |
| Tissue inhibitor of metalloproteinase-1 | TIMP1 | Mm00441818 |
| Tissue inhibitor of metalloproteinase-2 | TIMP2 | Mm00441825 |
| Tissue inhibitor of metalloproteinase-3 | TIMP3 | Mm00441826 |
| Tissue inhibitor of metalloproteinase-4 | TIMP4 | Mm00446568 |
| Hypoxanthine guanine phosphoribosyl transferase 1 | HPRT-1 | Mm00446968 |
Identification number from Life Technologies (www.lifetechnologies.com).
F. Immunofluorescence Microscopy
A total of six mice per experimental group at 2 and 5 days post-injury were euthanized. Eyes and adnexae were surgically excised, embedded in optimal cutting temperature compound (VWR, Suwanee, GA), and flash frozen in liquid nitrogen. Sagittal 8 μm tissue sections were cut with a cryostat (HM 500; Micron, Waldorf, Germany) and placed on glass slides that were stored at −80°C.
Immunofluorescent staining was performed in frozen tissue sections with rabbit polyclonal antibody anti-MMP-1 (1:50, NBP1-77209; Novus Biologicals), anti-MMP-9 (1:100; Santa Cruz Biotechnology, Dallas, TX), anti-IL-1β (1:100, Upstate-Millipore Corp., Bedford, MA), anti-neutrophil-gelatinase associated lipocalin (1:50, ab63929, Abcam, Cambridge, MA), goat anti-MMP-13 and goat anti-integrin β4 (1:100 dilution, sc-123630; 1:50 dilution, sc-6628, respectively, Santa Cruz Biotechnology, Dallas, TX, USA). Secondary goat-anti rabbit or donkey anti-goat Alexa-Fluor 488 conjugated IgG antibodies were used, as previously described.24 The images were captured and photographed by a Nikon fluorescence microscope (Eclipse E400 equipped with a DS–F1 digital camera; Nikon, Melville, NY).
G. Gelatin Zymography
The relative amount of MMP-9 in whole cornea lysates was measured by gelatin zymography, using a previously reported method.9,25 Whole cornea lysates prepared for MMP-9 activity (20 μg/sample, n=3/group/time point) were fractionated in an 8% polyacrylamide gel containing gelatin (0.5 mg/mL). The gels were soaked in 0.25% Triton X-100 for 30 minutes at room temperature, and incubated in a digestion buffer containing 5 mmol/L phenylmethyl sulfonyl fluoride at 37°C overnight. They were stained with 0.25% Coomassie brilliant blue R-250 in 40% methanol for 2 hours, and destained overnight in 10% acetic acid. Gelatinolytic activities appeared as clear bands of digested gelatin against a dark blue background of stained gelatin.
H. In Situ Zymography
In situ zymography was performed to localize the gelatinase activity in corneal cryosections using a previous reported method.25 Sections were thawed and incubated overnight with reaction buffer, 0.05 M Tris-HCl, 0.15 M NaCl, 5 mM CaCl2, and 0.2 mM NaN3, pH 7.6, containing 40 μg/mL FITC-labeled DQ gelatin, which was available in a gelatinase/collagenase assay kit (EnzChek, Molecular Probes, Eugene, OR, USA). As a negative control, 50 μM 1,10-phenanthroline, a metalloproteinase inhibitor, was added to the reaction buffer before applying the FITC-labeled DQ gelatin to frozen sections. Proteolysis of the FITC-labeled DQ gelatin substrate yields cleaved gelatin-FITC peptides that are fluorescent. The localization of fluorescence indicates the sites of net gelatinolytic activity. After incubation, the sections were washed three times with PBS for 5 minutes, an anti-fade Gel/Mount (Fisher, Atlanta, GA), and a 22×50 mm cover slip was applied. Areas of gelatinolytic activity of MMPs were viewed with a Nikon fluorescence microscope (Eclipse E400 equipped with a DS–F1 digital camera; Nikon, Melville, NY).
I. Immunohistochemistry
Immunohistochemistry was performed to detect neutrophils using rat anti-Gr-1 antibody (Ly6G; 1:250, clone 1A8, BD Pharmingen). Cryosections were stained with the primary antibody and appropriate biotinylated secondary antibody (1:100 biotin goat α-rat, BD Pharmingen, San Diego, CA) using a Vectastain Elite ABC kit and Nova Red reagents (Vector, Burlingame, CA). Six sections from each experimental group were examined and photographed with microscope equipped with a digital camera (Eclipse E400 with a DS-Qi1Mc; Nikon, Melville, NY, USA). The numbers of Gr-1 positive (+) cells were counted in cornea sections from each animal at 20X magnification and results averaged and expressed as the number of positive cells per cornea.
J. Myeloperoxidase Assay
Myeloperoxidase (MPO) activity was measured using a myeloperoxidase colorimetric activity assay kit as described by the manufacturer (Sigma-Aldrich, CA). Briefly, whole cornea lysates from experimental groups (n=4/group) were homogenized in MPO assay buffer and the homogenate was centrifuged at 14000 ×g for 20 min at 4°C. Total protein concentration was measured by the BCA protein assay as previously described.25
A 50 μg/sample was mixed with MPO assay buffer and MPO substrate, incubated at room temperature for 2 hours, and then mixed with tetramethylbenzidine probe. Fluorescence was measured at 412 nm using a Tecan Infinite (M200, Durham, NC) plate reader equipped with Magellan V6.55 software. Biologic replicate samples were averaged. Results are presented as mean ± SEM milliunits.
K. Statistical Analysis
Two-way analysis of variance (ANOVA) with Bonferroni post hoc testing was used for statistical comparisons of gene expression. P≤0.05 was considered statistical significant. These tests were performed using GraphPad Prism 6.0 software (GraphPad Incorporation, San Diego, CA).
III. Results
A. Clinical Findings after Anti-Inflammatory Therapy in Alkali Burn Associated with Dry Eye
1. Short-term Regimen
Corneal scarring and opacification are sight-threatening sequelae of severe chemical burns. Here we subjected mice to unilateral alkali burn and concomitant desiccating stress and topically treated them with either Doxy or Dex and compared the effects to BSS-treated corneas (Figure 1A). Representative images for corneal opacity and wound closure are shown on Figure 1B and C. The dex-treated corneas had the lowest corneal opacity scores among all treatment groups (Figure 1B, D).
Figure 1.
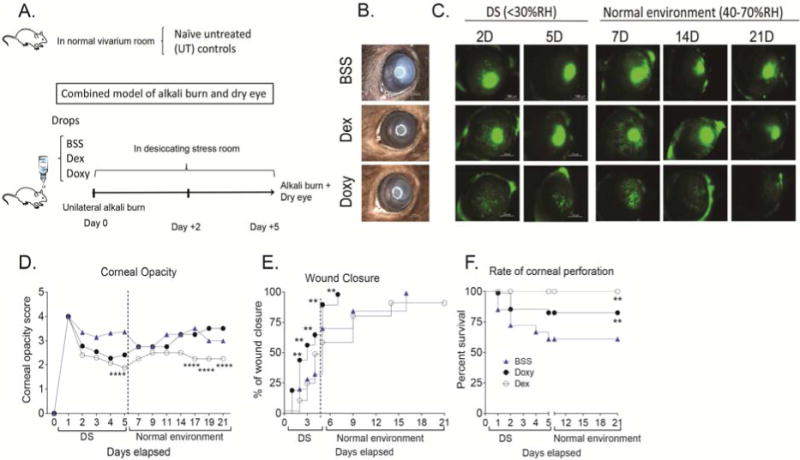
Anti-inflammatory therapy improves clinical parameters in a combined model (CM) of alkali burn and dry eye.
A. Schematic of experimental design of the combined model of alkali burn and dry eye. A unilateral alkali burn (AB) was created on the right cornea as described in materials and methods. Mice were then subjected to desiccating stress and topically treated with either balanced salt solution (BSS), dexamethasone (Dex) or doxycycline (Doxy). Control mice were kept in a normal vivarium room (untreated animals, UT).
B. Bright field digital images of alkali burn and dry eye combined model treated with either balanced salt solution (BSS), dexamethasone (Dex) or doxycycline (Doxy) 5 days (5D) post-injury. Scale bar=1000 μm.
C. Representative digital images of corneas post-injury stained with 0.1% sodium fluorescein after creation of alkali burn lesion and induction of dry eye topically treated with BSS, Dex or Doxy for 5 days and then topically treated for 16 days in normal environment. Scale bar=1000 μm.
D. Corneal opacity score in CM corneas topically treated with BSS, Dex, or Doxy. Asterisks show significant p value compared to vehicle.
E. Survival rate of wound closure in CM corneas topically treated with BSS, Dex, or Doxy.
F. Rate of ocular perforation in eyes subjected to CM topically treated with BSS, Dex, or Doxy.
*p<.05, **p<.01; ***p<.001, ****p<.0001.
BSS=corneas subjected to alkali burn and dry eye and treated topically with balanced salt solution, Dex=corneas subjected to alkali burn and dry eye and treated topically with dexamethasone, Doxy=corneas subjected to alkali burn and dry eye and treated topically with doxycycline, D=days.
Doxy-treated corneas healed significantly faster (p<.001) than BSS-treated corneas; with 100% wound closure 2 days post-injury (Figure 1E). In the Dex group, 60% of corneas had areas of epithelial defect, while 75% of BSS-treated corneas had epithelial defects 5 days post-injury.
BSS- and Doxy-treated corneas subjected to the combined model showed corneal perforations as early as day 1 after the initial insult; however, more BSS than Dox-treated corneas perforated (27 vs. 14%, p=.03, Figure 1E). No corneal perforations were observed in Dex-treated corneas for the duration of the experiment.
2. Extended Anti-inflammatory Therapy for up to 21 Days
Conflicting results of prolonged use of glucorticoids promoting corneal perforation after alkali burn in animal models have been reported.16,26–28 To investigate if prolonged anti-inflammatory treatment in mice after this combined model would also lead to corneal perforation, we subjected a separate group of mice to alkali burn and dry eye for 5 days, and subsequently placed them in a normal environment (RH 40–70%) for 16 days. Mice were continuously treated with either BSS, Doxy or Dex for a total of 21 days and corneal opacity, wound closure, and rate of corneal perforation were evaluated. We observed that if BSS- or Doxy-treated corneas did not perforate in the first 5 days, they did not perforate at all (Figure 1F), indicating that this is the critical period for corneal ulceration leading to perforation. Dex-treated corneas did not perforate even after continuous treatment for up to 21 days, indicating that prolonged therapy for up to 21 days does not promote corneal melting.
Doxy-treated corneas showed the fastest wound healing, but there was no difference in corneal opacity scores compared to vehicle-treated eyes (Figure 1B,D). Treatment with Dex showed incomplete wound closure similar to BSS controls even after 21 days; however, corneal opacity scores were significantly lower in Dex-treated corneas (Figure 1D).
B. Production of Inflammatory Cytokines and MMPS after Anti-Inflammatory Therapy in Alkali Burn Associated with Dry Eye
Our previous results showed that combining alkali burn and dry eye led to significantly increased levels of IL- and MMPs-1, -3, -8, -9, and -13 transcripts in the cornea compared to alkali burn alone.20 We investigated the efficacy of anti-inflammatory therapy with Doxy or Dex at 2 and 5 days post-injury by evaluating the expression of MMPs by real-time PCR, immunostaining, and zymography. Corneas subjected to alkali burn and dry eye and topically treated with BSS showed a significant increase in IL-6, MMPs -1, -3, -8, -9, -13 and TIMP-1 compared to naïve corneas. MMPs-1, -3, -8, -9,-13 and TIMP-1 transcripts peaked at 2 days and decreased by 5 days, while IL-6 continued to increase up to day 5 (Figure 2). No changes in MMP-2, TIMP-2, TIMP-3 or TIMP-4 were observed (data not shown).
Figure 2.
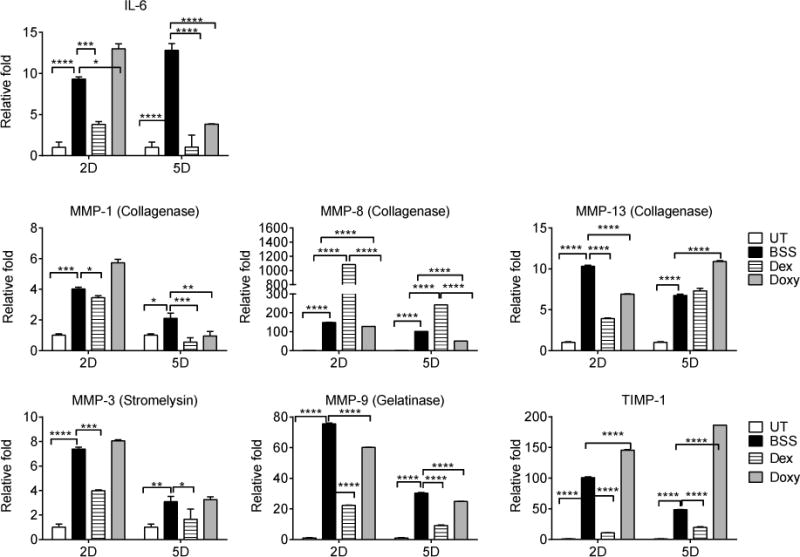
Inflammatory cytokines and MMPs decrease after anti-inflammatory therapy in a combined model (CM) of alkali burn and dry eye. Relative fold expression of IL-6, collagenases (MMPs -1, -8 and 13), the stromelysin MMP-3, the gelatinase MMP-9 and TIMP-1 in whole corneas subjected to a combined model of alkali burn and dry eye topically treated with BSS, Dex, or Doxy. Bar graphs show means ± SEM of one representative experiment with four samples per group/time point (experiment was repeated three times with similar results). UT=untreated cornea, BSS=corneas subjected to alkali burn and dry eye and treated topically with balanced salt solution, Dex=corneas subjected to alkali burn and dry eye and treated topically with dexamethasone, Doxy=corneas subjected to alkali burn and dry eye and treated topically with doxycycline, D=days.
*p<.05; **p<.01; ***p<.001, ****p<.0001.
Doxy treatment significantly decreased MMPs -8, -9, -13 two days post-injury, while Dex treatment significantly decreased IL-6, MMPs -1, -3, -9, -13 and TIMP-1 but significantly increased MMP-8 RNA transcripts (up to a 1000 fold). Doxy treatment showed greater suppression of IL-6, and MMPs -8, -9, and MMP-13 five days after the initial injury than BSS (Figure 2).
The immunoreactivity of corneas to collagenases (MMPs -1, -13) and MMP-9 was evaluated by immunostaining (Figure 3). Minimal levels of MMP-1, -9 and -13 were present in the control corneas. Increased reactivity against these MMPs in the corneal epithelium was seen in BSS-treated corneas. Both Doxy- and Dex-treated corneas had decreased immunoreactivity of corneal epithelium to MMP-1, MMP-9 and MMP-13 compared to BSS treatment (Figure 3A).
Figure 3.
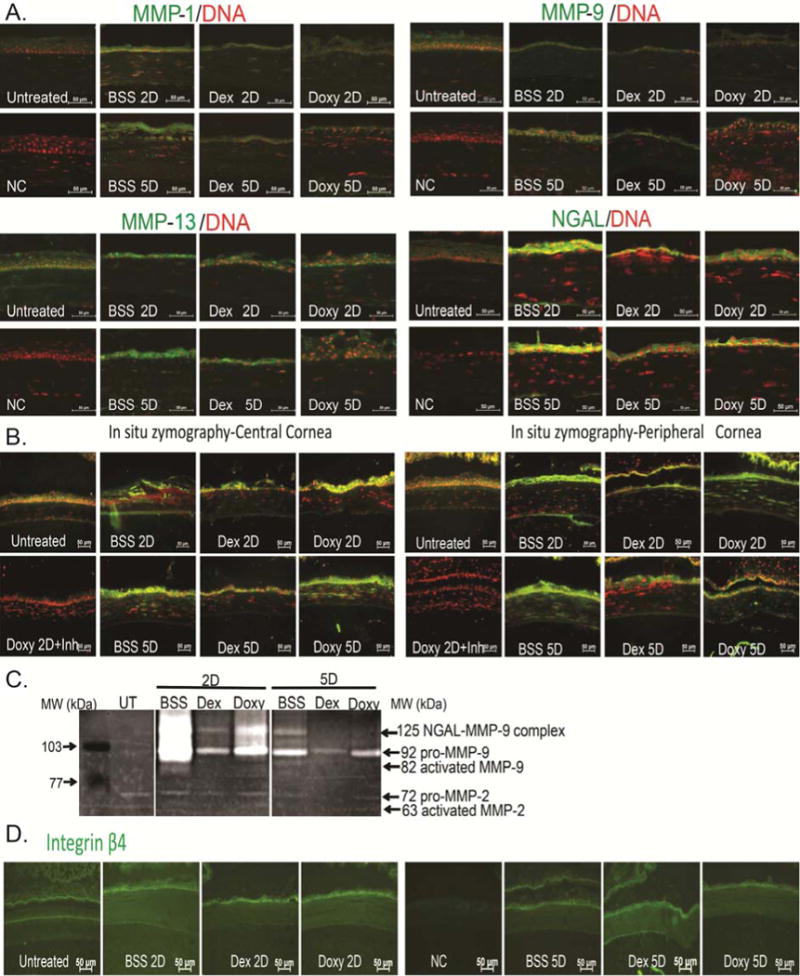
Anti-inflammatory therapy decreases MMP protein expression and gelatinolytic activity.
A. Representative merged digital images of cornea cryosections immunostained for MMP-1, MMP-9, MMP-13 and NGAL (all in green) with propidium iodide nuclei counterstaining (red) subjected to a combined model of alkali burn and dry eye topically treated with BSS, Dex, or Doxy for 2 or 5 days (2D or 5D, respectively). Scale bar=50 μm.
B. Representative merged digital images of in situ zymography of central and peripheral cornea (green) with propidium iodide nuclei counterstaining (red) in all treatment groups. Doxy 2D was incubated with inhibitor (inh) provided in the kit and used as negative control. Scale bar=50μm.
C. Representative gelatin zymogram showing MMP-9 and NGAL-MMP-9 bands in whole corneal lysates in the treatment groups.
D. Representative digital images of cornea cryosections immunostained for Integrin β4 (in green) in all treatment groups. Nuclear counterstaining was omitted to facilitate visualization of immunofluorescent staining. Scale bar=50 μm.
UT=untreated cornea, BSS=corneas subjected to alkali burn and dry eye and treated topically with balanced salt solution, Dex=corneas subjected to alkali burn and dry eye and treated topically with dexamethasone, Doxy=corneas subjected to alkali burn and dry eye and treated topically with doxycycline, D=days, NGAL=Neutrophil-gelatinase associated lipocalin, NC=negative control.
We performed in situ zymography to visualize areas in the cornea with the greatest protease activity. In situ zymography showed that central corneas and infiltrating stromal cells in the BSS group had higher collagenase and gelatinolytic activity than peripheral corneas from the same group. Both Dex and Doxy treatment decreased gelatinolytic activity compared to the BSS group and this was the greatest in in the Dex-treated corneas (Figure 3B).
MMP-9 activity was evaluated by gelatin zymography, which showed increased amounts of both pro and active MMP-9 bands in the BSS group compared to control corneas (day 2 > day 5). Dex and Doxy groups showed no activated MMP-9 bands and had reduced pro-MMP-9 bands, in both day 2 and 5 post-injury samples. Negligible amounts of MMP-2 were present in all groups (Figure 3C).
It is known that the epithelial basement membrane is quite resistant to alkali exposure and it is subsequently degraded by MMPs produced by the regenerating epithelium.29 To investigate this, we performed immunofluorescent staining for integrin β4, a component of the basement membrane in eyes that had no perforation. We observed that there was no disruption of the basement membrane in any of the treatment groups (Figure 3D).
C. Effects of Dexamethasone in NGAL Formation
Neutrophil-gelatinase associated lipocalin (NGAL), also known as Lipocalin 2, is involved in cell growth, migration, and differentiation and has anti-microbial properties.30–32 NGAL exists as a monomer (25kDa), a homodimer (46kDa) or as a heterodimer (125kDa).33 We investigated the expression of NGAL in the combined model by immunostaining frozen sections. While barely detected in naïve corneas, increased immunoreactivity to NGAL was observed in the epithelium of BSS-treated corneas. Treatment with Dex or Doxy decreased NGAL immunoreactivity (Figure 3A). We also observed that a 125-kDa band in our zymography gels, corresponding to the NGAL-MMP-9 complex, was markedly decreased in Dex-treated corneas compared to BSS 2 days after injury. Doxy had a moderate effect decreasing NGAL-MMP-9 complex (Figure 3C).
D. Effects of Anti-inflammatory Therapy on Neutrophil Infiltration
Neutrophils are the first inflammatory cells to infiltrate the site of injury. We have previously reported that combining alkali burn with dry eye significantly increases neutrophil infiltration in wounded corneas.20 We observed increased immunoreactivity of MMP-8 in the corneal epithelium, stroma, and endothelium after 2 and 5 days in the combined injury model (Figure 4A). In addition, we investigated the expression of the cytokine IL-1β and the chemokine CXCL1 since they have been implicated in the migration of neutrophils.34,35 Dex treatment in the combined model significantly decreased the early peak of IL-1β and CXCL1 two days after initial injury, as well as at day 5. A significantly decreased expression of IL-1β and CXCL1 by day 5 was observed in the Doxy-treated group compared to BSS (Figure 4B,C).
Figure 4.
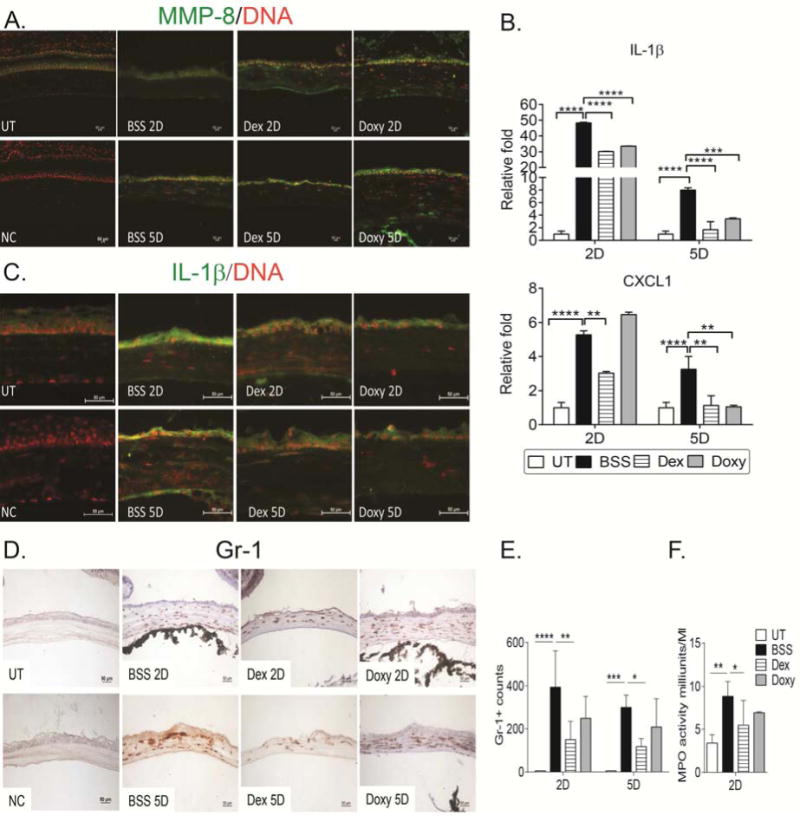
Anti-inflammatory therapy decreases neutrophil infiltration.
A. Representative merged digital images of corneal cryosections immunostained for MMP-8 (in green) with propidium iodide nuclei counterstaining (red) in corneas subjected to the combined model topically treated with BSS, Dex or Doxy. NC= negative control. Scale bar=100μm.
B. Relative fold expression of IL-1β and CXCL1 in whole corneas subjected to alkali burn and dry eye topically treated with BSS, Dex, or Doxy. Bar graphs show means ± SEM of one representative experiment with five samples per group/time point (experiment was repeated three times with similar results). UT=untreated cornea.
*p<.05; **p<.01; ***p<.001, ****p<.0001.
C. Representative merged digital images of central cornea cryosections immunostained for IL-1β (in green) with propidium iodide nuclei counterstaining (red) in corneas subjected to combined model topically treated with BSS, Dex, or Doxy. NC=negative control. Scale bar=50μm.
D. Representative images of Gr-1+ cells (red) of central cornea cryosections from animals subjected to a combined model of alkali burn and dry eye topically treated with BSS, Dex or Doxy used to generate the bar graph showing counts in D.
E. Bar graphs (mean±SEM) of Gr-1+ cell counts in experimental groups.
F. Myeloperoxidase (MPO) activity in whole corneas lysates from animals subjected to a combined model of alkali burn and dry eye topically treated with BSS, Dex or Doxy 2 days after injury (mean±SEM).
*p<.05; ***p<.001; ****p<.0001.
UT=untreated cornea, BSS=corneas subjected to alkali burn and dry eye and treated topically with balanced salt solution, Dex=corneas subjected to alkali burn and dry eye and treated topically with dexamethasone, Doxy=corneas subjected to alkali burn and dry eye and treated topically with doxycycline, D=days. NC=negative control
To investigate if topical treatment would decrease neutrophil infiltration, we performed immunohistochemistry for the neutrophil marker Gr-1 in cornea cryosections, and investigated myeloperoxidase (MPO) activity in whole corneal lysates. In naïve corneas, a few resident neutrophils were found at the limbal area (data not shown). A significant influx of Gr-1+ cells was observed in BSS-treated corneas 2 and 5 days post-injury; the infiltration was not restricted to the limbal area, but extended to the central cornea (Figure 4D). Dex-treated corneas had a significant decrease in Gr-1+ cell counts compared to BSS corneas at days 2 and 5 post-injury (Figure 4D, 4E).
MPO activity has been used as a parameter of neutrophil infiltration.36 We measured MPO activity in corneal lysates in the treatment groups 2 days after injury. Significantly higher MPO activity was seen in BSS-treated corneas compared to naïve controls, and significantly lower MPO activity was present in the Dex group, confirming the IHC results (Figure 4F).
IV. Discussion
It is well recognized that alkali burns to the cornea induce a severe inflammation and MMP production.16,26 We have previously reported that inhibition of tear production and exposure to a desiccating environment markedly worsened corneal inflammation and matrix degradation, leading to perforation in nearly 30% of corneas acutely subjected to alkali burn.20 Here we report that although topical therapy with broad anti-inflammatory agents (doxycycline and dexamethasone) decreased corneal perforations, production and activation of MMPs, and neutrophil infiltration, these two prototype drugs have differential activity. Differential effects observed with dexamethasone treatment included: 1) greater potency decreasing MMPs and inflammatory cytokines; 2) significant increase of MMP-8 transcripts; 3) decrease in expression of TIMP1; 4) greater reduction in NGAL-MMP-9 complex formation; and 5) decreased neutrophil infiltration. The combination of these factors resulted in less corneal opacification in dexamethasone-treated corneas; however, faster wound healing was observed in doxycycline group.
Our study showed that corneas treated with doxycycline healed faster than controls, had a smaller number of corneal perforations, decreased levels of MMP-9, MMP-13, TIMP-1, IL-1β and CXCL1 (at day 5) gene transcripts, decreased immunoreactivity of MMPs-1, -9 and 13 and levels of gelatinases in the cornea. Doxycycline has been extensively used in vitro and in vivo to decrease MMPs after inflammatory stimuli.7,10,25,37,38 Oral doxycycline is an FDA-approved drug to treat periodontitis,39 another disease in which MMPs are involved. We have previously reported that doxycycline decreases levels of MMP-9 mRNA transcripts and prevents DS-induced increase in inflammatory cytokines IL-1 and TNF-α.25 The inhibitory effect of doxycycline on MMP-9 was also confirmed on osmotically stressed human corneal epithelia cells.40 Doxycycline functions as a noncompetitive inhibitor of MMPs by interacting with the zinc or calcium atoms within the structural center of these enzymes that is required for their stability.41 Chelation of these metal ions by doxycycline could be responsible for the decreased collagenase.6
Dexamethasone is a potent prototype corticosteroid. Topical dexamethasone treatment of corneas subjected to alkali burn and dry eye had profound anti-MMP and anti-inflammatory effects compared to its vehicle, while it increased MMP-8 expression by 1000-fold. MMP-8 is rarely detected in corneal epithelium,7 but it has been reported to show increased immunoreactivity in the context of inflammation.42,43
MMP-8, MMP-9 and lipocalin-2 are some of the many proteins stored in peroxidase negative granules in neutrophils.44 MMP-8 facilitates neutrophil infiltration in many models, including LPS stimulation of the cornea.45–47 However, MMP-8-deficient mice showed delayed healing of cutaneous skin wounds, which was accompanied by increased inflammation and expression of MMP-1, MMP-13 and MMP-9.48 We explored the role of MMP-8 during dexamethasone treatment in alkali-burned dry eye corneas using a combination of gene knockout (KO) and pharmacological inhibition. Our recent results showed that topical dexamethasone treatment lost its efficacy in controlling inflammation and MMP expression in MMP-8KO mice subjected to alkali-burn associated with dry eye, suggesting that some of dexamethasone effects are mediated through MMP-8 (de Paiva, et al, IOVS 2015,56, E-Abstract 5617 and manuscript submitted)
Dexamethasone treatment paradoxically decreased TIMP-1, while doxycycline treatment did not. TIMP-1 binds MMP-9, while TIMP-2 binds MMP-2 and MMP-14. Once recognized as negative regulators of MMPs, TIMPs are now recognized as bi-functional regulators.49 TIMP-2 exogenous application stimulated wound closure,50 while overexpression of TIMP-1 in the epidermis of a transgenic mouse decreased re-epithelization following a cutaneous wound.
We observed an increase in NGAL immunoreactivity in the corneal epithelium of alkali-burned and dry eye. A 125-kDa noted in zymograms was markedly inhibited by dexamethasone and moderately by doxycycline, compared to BSS vehicle. This 125kDa band corresponds to the NGAL-MMP-9 complex.30,32 NGAL binds covalently to MMP-9 and this complex has been reported to protect MMP-9 from degradation and preserve MMP-9 activity in a concentration-dependent manner.30,33 Levels of serum NGAL-MMP-9 complex were increased in patients with active ulcerative colitis, correlated with neutrophil infiltration and it has been proposed as a surrogate marker of mucosal healing.51 NGAL immunoreactivity increased in the colonic epithelium in inflammatory and neoplastic, colorectal diseases.52 Increased levels of NGAL-MMP-9 have been reported in tears upon awakening53 and observed in tears of Sjögren syndrome patients.54 Therefore, the presence of NGAL-MMP-9 complex in BSS-treated corneas may indicate a mechanism to preserve MMP-9 activity. Our findings indicate that dexamethasone directly decreases MMP-9 synthesis and decreases its activity by suppressing NGAL.
Our results showed that topical steroid therapy greatly decreases inflammatory cytokines (IL-1β and IL-6), chemokine (CXCL1), and expression and activity of MMPs. It is interesting to note that dexamethasone was more effective in inhibiting these mediators than doxycycline. Dexamethasone greatly preserved corneal clarity, decreased neutrophil infiltration and expression of the neutrophil chemoattractant, CXCL1. Our results are in agreement with thouse of Saud and colleagues, who showed an improvement in corneal opacity with no corneal perforations in rabbits subjected to alkali burn and subconjunctival injections of the corticosteroid triamcinolone.27 Several enzymes and proteases, including collagenases, are stored within the cytoplasmic granules of neutrophils. Experiments showed that depletion of neutrophils dramatically improved the fate of alkali burn injuries.19,55 Doxycycline and dexamethasone have been shown to decrease neutrophil migration.6,19,56,57
Davis and associates showed that treatment of human alkali burns with topical steroids for longer than 10 days after the initial injury was safe and did not induce corneal melting.58 Among the anti-inflammatory treatments after alkali burn, the use of a topical corticosteroid is by far the most controversial, specifically its use beyond the 6th day post-injury. While several animal models (rabbits and, more recently, mice) have been used to study alkali burns, there is no consensus regarding the strength of alkali used to induce the burn (0.5N, 1N, 2N of NaOH), mode of application (pre-wet discs vs. topical instillation of alkali as eyedrops) or the duration of the contact (in case of pre-wetted discs, 10 seconds to 2 minutes) and volume of irrigation agents after the injury (from “brief irrigation” to “copious”, to “2 liters”). The diversity in models makes generalization regarding steroid-induced corneal perforations difficult. Donshik and colleagues reported that treatment with dexamethasone sodium phosphate was just partially efficacious in preventing deep corneal ulcers and descemetoceles following alkali injury in rabbits, but they used a strong (2N) sodium hydroxide-embedded filter paper and very brief rinsing.16 It has also been demonstrated that steroid treatment retarded corneal reepithelization, 0.1% dexamethasone was able to control inflammation without inducing corneal perforation in a milder model in rabbits that used 0.1N NaOH filter discs to create the injury.59
When evaluating corneal wounds, it is important to take into consideration the type of corneal injury. Thermal burns treated early with either medroxyprogesterone or prednisolone showed a significant reduction on deep corneal ulcers in albino rabbits compared to vehicle, but not if treatment was started 6 days post-injury.60 The species itself must also be considered. It seems that alkali burns in rabbits will evolve to perforation more frequently than in mice. We have not observed corneal perforations after alkali burns in mice unless these mice are subjected to a desiccating stress and cholinergic blockade.20 In our experiment with prolonged dosing of dexamethasone (up to 21 days), we did not observe any corneal perforations.
Another consideration when evaluating corneal wounds is the size of the wound. In our preliminary results, we observed that large corneal burns (>3 mm) induced greater production of MMPs than smaller burns (2 mm; data not shown). Thus, it is possible that efficacy of anti-inflammatory agents will also vary according to the size of the initial lesion.
Alkali burns of the cornea are potentially sight-threatening, as alkali can penetrate rapidly into the eye and cause intense inflammation. There is a consensus that the prognosis of an alkali burn depends on the area of the insult, the penetration of the agent, and early treatment controlling host response and inflammation. We did not find any basement membrane disruption after combining alkali injury and dry eye.
The visual outcomes from alkali injuries are poor, largely because of inadequate control of inflammatory and proteolytic components of the wound healing response, leading to corneal perforation, corneal opacification, and corneal neovascularization. Prospective clinical trials evaluating efficacy of anti-inflammatory therapy in alkali burn are urgently needed. Our study showed that early initiation anti-inflammatory therapy is critical in controlling acute host response. A recent retrospective study using an algorithmic approach showed significant improvement in outcomes and visual acuity in mild ocular alkali burns compared to eyes treated 2 years prior to the implementation of the algorithm. This algorithm included frequent use of topical corticosteroids, oral doxycycline, and oral vitamin C, along with amniotic membrane grafting if needed.61
V. Conclusions
Taken together, our results show that the stroma ulceration following alkali burn is not simply the passive breakdown of alkali-denatured collagen and proteoglycans; it is a complex process involving interactions between different cell types, regulation of collagenases, cytokines and chemokines. Early topical anti-inflammatory therapy appears to inhibit sight-threatening corneal ulceration and perforation in our mouse model, and these findings support the use of these anti-inflammatory agents in the treatment of human cornea alkali burns.
Acknowledgments
We thank Mahira Zaheer and Kevin Tesareski for their expert technical assistance.
Supported by W81XWH-12-1-0616 (CSDP), NIH Training Grant T32 AI053831 (FB), NEI/NIH Core Grant EY-002520, RPB Research to Prevent Blindness, The Oshman Foundation, William Stamps Farish Fund, and The Hamill Foundation.
Abbreviations
- AB
Alkali burn
- BID
Bis in die i.e., twice a day
- BSS
Balanced salt solution
- CM
Combined model (AB+DS)
- D
Days
- Dex
Dexamethasone
- Doxy
Doxycycline
- DS
Desiccating stress
- QID
Quater in die i.e., 4 times a day
- M-MuLV
Moloney murine leukemia virus
- MMP
Matrix metalloproteinase
- MPO
Myeloperoxidase
- UT
Untreated
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no commercial or proprietary interest in any concept or product described in this article. The funding agencies had no role in the design of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Presented in part as abstracts at the annual meeting of the Association for Research in Vision and Ophthalmology 2010, 2013, and 2014.
References
- 1.Reim M, Redbrake C, Schrage N. Chemical and thermal injuries of the eyes. Surgical and medical treatment based on clinical and pathophysiological findings. Arch Soc Esp Oftalmol. 2001;76:79–124. [PubMed] [Google Scholar]
- 2.Luengo GF, Lavigne V, Gatto S, et al. Advances in corneal stem-cell transplantation in rabbits with severe ocular alkali burns. J Cataract Refract Surg. 2007;33:1958–65. doi: 10.1016/j.jcrs.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Fini ME, Cui TY, Mouldovan A, et al. An inhibitor of the matrix metalloproteinase synthesized by rabbit corneal epithelium. Invest Ophthalmol Vis Sci. 1991;32:2997–3001. [PubMed] [Google Scholar]
- 4.Kato T, Saika S, Ohnishi Y. Effects of the matrix metalloproteinase inhibitor GM6001 on the destruction and alteration of epithelial basement membrane during the healing of post-alkali burn in rabbit cornea. Jpn J Ophthalmol. 2006;50:90–5. doi: 10.1007/s10384-005-0287-8. [DOI] [PubMed] [Google Scholar]
- 5.Sosne G, Szliter EA, Barrett R, et al. Thymosin beta 4 promotes corneal wound healing and decreases inflammation in vivo following alkali injury. Exp Eye Res. 2002;74:293–9. doi: 10.1006/exer.2001.1125. [DOI] [PubMed] [Google Scholar]
- 6.Seedor JA, Perry HD, McNamara TF, et al. Systemic tetracycline treatment of alkali-induced corneal ulceration in rabbits. Arch Ophthalmol. 1987;105:268–71. doi: 10.1001/archopht.1987.01060020122043. [DOI] [PubMed] [Google Scholar]
- 7.Li DQ. Regulated expression of collagenases MMP-1, -8, and -13 and stromelysins MMP-3, -10, and -11 by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:2928–36. doi: 10.1167/iovs.02-0874. [DOI] [PubMed] [Google Scholar]
- 8.Li DQ, Chen Z, Song XJ, et al. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:4302–11. doi: 10.1167/iovs.04-0299. [DOI] [PubMed] [Google Scholar]
- 9.Li DQ, Lokeshwar BL, Solomon A, et al. Regulation of MMP-9 production by human corneal epithelial cells. Exp Eye Res. 2001;73:449–59. doi: 10.1006/exer.2001.1054. [DOI] [PubMed] [Google Scholar]
- 10.Dursun D, Kim MC, Solomon A, Pflugfelder SC. Treatment of recalcitrant recurrent corneal erosions with inhibitors of matrix metalloproteinase-9, doxycycline and corticosteroids. Am J Ophthalmol. 2001;132:8–13. doi: 10.1016/s0002-9394(01)00913-8. [DOI] [PubMed] [Google Scholar]
- 11.Yoo SE, Lee DC, Chang MH. The effect of low-dose doxycycline therapy in chronic meibomian gland dysfunction. Korean J Ophthalmol. 2005;19:258–63. doi: 10.3341/kjo.2005.19.4.258. [DOI] [PubMed] [Google Scholar]
- 12.Caton JG, Ciancio SG, Blieden TM, et al. Treatment with subantimicrobial dose doxycycline improves the efficacy of scaling and root planing in patients with adult periodontitis. J Periodontol. 2000;71:521–32. doi: 10.1902/jop.2000.71.4.521. [DOI] [PubMed] [Google Scholar]
- 13.Akpek EK, Merchant A, Pinar V, Foster CS. Ocular rosacea: patient characteristics and follow-up. Ophthalmology. 1997;104:1863–7. [PubMed] [Google Scholar]
- 14.Lee EJ, Scott GD, Rosembaum J, Planck SR. Inhibition of neutrophil migration in the injured murine cornea by Mmp-2/9 blockade. Invest Ophthalmol Vis Sci. 2008;49 E-Abstract 2388. [Google Scholar]
- 15.Gordon MK, Desantis A, Deshmukh M, et al. Doxycycline hydrogels as a potential therapy for ocular vesicant injury. J Ocul Pharmacol Ther. 2010;26:407–19. doi: 10.1089/jop.2010.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donshik PC, Berman MB, Dohlman CH, et al. Effect of topical corticosteroids on ulceration in alkali-burned corneas. Arch Ophthalmol. 1978;96:2117–20. doi: 10.1001/archopht.1978.03910060497024. [DOI] [PubMed] [Google Scholar]
- 17.Brodovsky SC, McCarty CA, Snibson G, et al. Management of alkali burns: an 11-year retrospective review. Ophthalmology. 2000;107:1829–35. doi: 10.1016/s0161-6420(00)00289-x. [DOI] [PubMed] [Google Scholar]
- 18.Mattax JB, McCulley JP. Corneal surgery following alkali burns. Int Ophthalmol Clin. 1988;28:76–82. doi: 10.1097/00004397-198802810-00011. [DOI] [PubMed] [Google Scholar]
- 19.Kenyon KR, Berman M, Rose J, Gage J. Prevention of stromal ulceration in the alkali-burned rabbit cornea by glued-on contact lens. Evidence for the role of polymorphonuclear leukocytes in collagen degradation. Invest Ophthalmol Vis Sci. 1979;18:570–87. [PubMed] [Google Scholar]
- 20.Bian F, Pelegrino FS, Pflugfelder SC, et al. Desiccating stress-induced MMP production and activity worsens wound healing in alkali-burned corneas. Invest Ophthalmol Vis Sci. 2015;56:4908–18. doi: 10.1167/iovs.15-16631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi H, Igarashi T, Fujimoto C, et al. Immunohistochemical observation of amniotic membrane patching on a corneal alkali burn in vivo. Jpn J Ophthalmol. 2007;51:3–9. doi: 10.1007/s10384-006-0389-y. [DOI] [PubMed] [Google Scholar]
- 22.de Paiva CS, Chotikavanich S, Pangelinan SB, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2:243–53. doi: 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoeruek E, Ziemssen F, Henke-Fahle S, et al. Safety, penetration and efficacy of topically applied bevacizumab: evaluation of eyedrops in corneal neovascularization after chemical burn. Acta Ophthalmol. 2008;86:322–8. doi: 10.1111/j.1600-0420.2007.01049.x. [DOI] [PubMed] [Google Scholar]
- 24.de Paiva CS, Pangelinan SB, Chang E, et al. Essential role for c-Jun N-terminal kinase 2 in corneal epithelial response to desiccating stress. Arch Ophthalmol. 2009;127:1625–31. doi: 10.1001/archophthalmol.2009.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83:526–35. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Brown SI, Akiya S, Weller CA. Prevention of the ulcers of the alkali-burned cornea. Preliminary studies with collagenase inhibitors. Arch Ophthalmol. 1969;82:95–7. doi: 10.1001/archopht.1969.00990020097023. [DOI] [PubMed] [Google Scholar]
- 27.Saud EE, Moraes HV, Jr, Marculino LG, et al. Clinical and histopathological outcomes of subconjunctival triamcinolone injection for the treatment of acute ocular alkali burn in rabbits. Cornea. 2012;31:181–7. doi: 10.1097/ICO.0b013e318221ce99. [DOI] [PubMed] [Google Scholar]
- 28.Newsome NA, Gross J. Prevention by medroxyprogesterone of perforation in the alkali-burned rabbit cornea: inhibition of collagenolytic activity. Invest Ophthalmol Vis Sci. 1977;16:21–31. [PubMed] [Google Scholar]
- 29.Pal-Ghosh S, Pajoohesh-Ganji A, Tadvalkar G, Stepp MA. Removal of the basement membrane enhances corneal wound healing. Exp Eye Res. 2011;93:927–36. doi: 10.1016/j.exer.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kubben FJ, Sier CF, Hawinkels LJ, et al. Clinical evidence for a protective role of lipocalin-2 against MMP-9 autodegradation and the impact for gastric cancer. Eur J Cancer. 2007;43:1869–76. doi: 10.1016/j.ejca.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–32. [PubMed] [Google Scholar]
- 32.Gupta K, Shukla M, Cowland JB, et al. Neutrophil gelatinase-associated lipocalin is expressed in osteoarthritis and forms a complex with matrix metalloproteinase 9. Arthritis Rheum. 2007;56:3326–35. doi: 10.1002/art.22879. [DOI] [PubMed] [Google Scholar]
- 33.Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem. 2001;276:37258–65. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]
- 34.De Filippo K, Dudeck A, Hasenberg M, et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121:4930–7. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- 35.Oliveira SH, Canetti C, Ribeiro RA, Cunha FQ. Neutrophil migration induced by IL-1beta depends upon LTB4 released by macrophages and upon TNF-alpha and IL-1beta released by mast cells. Inflammation. 2008;31:36–46. doi: 10.1007/s10753-007-9047-x. [DOI] [PubMed] [Google Scholar]
- 36.Paterson CA, Williams RN, Parker A. Characteristics of polymorphonuclear leukocyte infiltration into alkali burned eye and the influence of sodium citrate. Exp Eye Res. 1984;39:701–8. doi: 10.1016/0014-4835(84)90069-1. [DOI] [PubMed] [Google Scholar]
- 37.Boyle JR, McDermott E, Crowther M, et al. Doxycycline inhibits elastin degradation and reduces metalloproteinase activity in a model of aneurysmal disease. J Vasc Surg. 1998;27:354–61. doi: 10.1016/s0741-5214(98)70367-2. [DOI] [PubMed] [Google Scholar]
- 38.Gilbertson-Beadling S, Powers EA, Stamp-Cole M, et al. The tetracycline analogs minocycline and doxycycline inhibit angiogenesis in vitro by a non-metalloproteinase-dependent mechanism. Cancer Chemother Pharmacol. 1995;36:418–24. doi: 10.1007/BF00686191. [Published erratum appears in Cancer Chemother Pharmacol 1995;37(1–2):194.] [DOI] [PubMed] [Google Scholar]
- 39.Gu Y, Walker C, Ryan ME, et al. Non-antibacterial tetracycline formulations: clinical applications in dentistry and medicine. J Oral Microbiol. 2012;4 doi: 10.3402/jom.v4i0.19227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pflugfelder SC, Farley W, Luo L, et al. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am J Pathol. 2005;166:61–71. doi: 10.1016/S0002-9440(10)62232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samtani S, Amaral J, Campos MM, et al. Doxycycline-mediated inhibition of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2009;50:5098–106. doi: 10.1167/iovs.08-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Brien TP, Li QJ, Sauerburger F, et al. The role of matrix metalloproteinases in ulcerative keratolysis associated with perioperative diclofenac use. Ophthalmology. 2001;108:656–9. doi: 10.1016/s0161-6420(00)00590-x. [DOI] [PubMed] [Google Scholar]
- 43.Reviglio VE, Rana TS, Li QJ, Ashraf MF, et al. Effects of topical nonsteroidal antiinflammatory drugs on the expression of matrix metalloproteinases in the cornea. J Cataract Refract Surg. 2003;29:989–97. doi: 10.1016/s0886-3350(02)01737-6. [DOI] [PubMed] [Google Scholar]
- 44.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5:1317–27. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Quintero PA, Knolle MD, Cala LF, et al. Matrix metalloproteinase-8 inactivates macrophage inflammatory protein-1 alpha to reduce acute lung inflammation and injury in mice. J Immunol. 2010;184:1575–88. doi: 10.4049/jimmunol.0900290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin M, Jackson P, Tester AM, et al. Matrix metalloproteinase-8 facilitates neutrophil migration through the corneal stromal matrix by collagen degradation and production of the chemotactic peptide Pro-Gly-Pro. Am J Pathol. 2008;173:144–53. doi: 10.2353/ajpath.2008.080081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lenglet S, Mach F, Montecucco F. Role of matrix metalloproteinase-8 in atherosclerosis. Mediators Inflamm. 2013;2013:659282. doi: 10.1155/2013/659282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gutierrez-Fernandez A, Inada M, Balbin M, et al. Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8) FASEB J. 2007;21:2580–91. doi: 10.1096/fj.06-7860com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordon GM, Austin JS, Sklar AL, et al. Comprehensive gene expression profiling and functional analysis of matrix metalloproteinases and TIMPs, and identification of ADAM-10 gene expression, in a corneal model of epithelial resurfacing. J Cell Physiol. 2011;226:1461–70. doi: 10.1002/jcp.22306. [DOI] [PubMed] [Google Scholar]
- 50.Terasaki K, Kanzaki T, Aoki T, et al. Effects of recombinant human tissue inhibitor of metalloproteinases-2 (rh-TIMP-2) on migration of epidermal keratinocytes in vitro and wound healing in vivo. J Dermatol. 2003;30:165–72. doi: 10.1111/j.1346-8138.2003.tb00367.x. [DOI] [PubMed] [Google Scholar]
- 51.de Bruyn M, Arijs I, Wollants WJ, et al. Neutrophil gelatinase B-associated lipocalin and matrix metalloproteinase-9 complex as a surrogate serum marker of mucosal healing in ulcerative colitis. Inflamm Bowel Dis. 2014;20:1198–207. doi: 10.1097/MIB.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 52.Nielsen BS, Borregaard N, Bundgaard JR, et al. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut. 1996;38:414–20. doi: 10.1136/gut.38.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Markoulli M, Papas E, Cole N, Holden BA. The diurnal variation of matrix metalloproteinase-9 and its associated factors in human tears. Invest Ophthalmol Vis Sci. 2012;53:1479–84. doi: 10.1167/iovs.11-8365. [DOI] [PubMed] [Google Scholar]
- 54.Solomon A, Dursun D, Liu Z, et al. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–92. [PubMed] [Google Scholar]
- 55.Foster CS, Zelt RP, Mai-Phan T, Kenyon KR. Immunosuppression and selective inflammatory cell depletion. Studies on a guinea pig model of corneal ulceration after ocular alkali burning. Arch Ophthalmol. 1982;100:1820–4. doi: 10.1001/archopht.1982.01030040800019. [DOI] [PubMed] [Google Scholar]
- 56.Martin RR, Warr GA, Couch RB, et al. Effects of tetracycline on leukotaxis. J Infect Dis. 1974;129:110–6. doi: 10.1093/infdis/129.2.110. [DOI] [PubMed] [Google Scholar]
- 57.Zentay Z, Sharaf M, Qadir M, et al. Mechanism for dexamethasone inhibition of neutrophil migration upon exposure to lipopolysaccharide in vitro: role of neutrophil interleukin-8 release. Pediatr Res. 1999;46:406–10. doi: 10.1203/00006450-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 58.Davis AR, Ali QK, Aclimandos WA, Hunter PA. Topical steroid use in the treatment of ocular alkali burns. Br J Ophthalmol. 1997;81:732–4. doi: 10.1136/bjo.81.9.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chung JH, Kang YG, Kim HJ. Effect of 0.1% dexamethasone on epithelial healing in experimental corneal alkali wounds: morphological changes during the repair process. Graefes Arch Clin Exper Ophthalmol. 1998;236:537–45. doi: 10.1007/s004170050118. [DOI] [PubMed] [Google Scholar]
- 60.Phillips K, Arffa R, Cintron C, et al. Effects of prednisolone and medroxyprogesterone on corneal wound healing, ulceration, and neovascularization. Arch Ophthalmol. 1983;101:640–3. doi: 10.1001/archopht.1983.01040010640024. [DOI] [PubMed] [Google Scholar]
- 61.Al-Moujahed A, Chodosh J. Outcomes of an algorithmic approach to treating mild ocular alkali burns. JAMA Ophthalmol. 2015;133:1214–6. doi: 10.1001/jamaophthalmol.2015.2302. [DOI] [PubMed] [Google Scholar]


