Abstract
Ovarian granulosa cells (GC) play an important role in the growth and development of the follicle in the process known as folliculogenesis. In the present review, we focus on the recent developments in prohibitin (PHB) research in relation to GC physiological functions. PHB is a member of highly conserved eukaryotic protein family containing the repressor of estrogen activity (REA)/stomatin/prohibitin/flotillin/HflK/C (SPFH) domain [also known as the PHB domain] found in divergent species from prokaryotes to eukaryotes. PHB is ubiquitously expressed either in circulating free form or is present in multiple cellular compartments including mitochondria, nucleus and plasma membrane. In mitochondria, PHB is anchored to the mitochondrial inner membrane (IMM), and form complexes with the ATPases Associated with diverse cellular Activities (m-AAA) proteases. PHB continuously shuttles between the mitochondria, cytosol and nucleus. In the nucleus, PHB interacts with various transcription factors and modulate transcriptional activity directly or through interactions with chromatin remodeling proteins. Multiple functions have been attributed to the mitochondrial and nuclear prohibitin complexes such as cellular differentiation, anti-proliferation, morphogenesis and maintaining the functional integrity of the mitochondria. However, to date, the regulation of PHB expression patterns and GC physiological functions are not completely understood.
Keywords: Prohibitin (PHB), granulosa cells (GCs), differentiation, survival, mitochondria
INTRODUCTION
The ovarian granulosa cell (GC) is perhaps one of the most well studied endocrine cell. Ovarian GC plays an important role in the growth and development of the follicle during folliculogenesis. The unique structure and function of GCs create a unique microenvironment for oocyte growth and maturation resulting from their biochemical activity and close anatomical relationship (Amsterdam et al. 1989). Ovarian follicles have been shown to be the basic functional unit of the ovary and consist of an outer layer of theca cells which encircle inner layers of GC. These GCs are surrounding the oocyte and connected to each other and to the oocyte by development of gap junctions (Mora et al., 2012). In response to cyclic pituitary gonadotrophin (LH, FSH) secretion, the various follicular compartments interact in a highly integrated manner to secrete the sex steroids (estrogens and progestogens) that are required to produce a fertilizable ovum. Ovulation occurs in one of the two ovaries during a given cycle in mammalian species. Although pituitary gonadotrophins (LH, FSH) are the major regulators of follicular development (Richards & Midgley, 1976) and the blood concentrations of these hormones perfusing the two ovaries are identical, not all follicles are responsive to pituitary gonadotrophins during a given cycle. Only a limited number of the ‘selected’ follicles ovulate during the life span of the females, while most of the follicles become atretic. After ovulation, the GC undergo profound changes in their hormonal responsiveness and their capacity to produce steroids. These luteinized GC constitute the major component of the corpora lutea and are the main source of ovarian progestogens.
To understand the basis for the disparate maturation of ovarian follicles and the luteinization of maturing GC, functional and morphological correlates of intra-ovarian changes in different hormonal environments are required. In the present review, we are summarizing some of the recent developments in the PHB research in relation to GC physiology. Prohibitin (PHB) is a multifunctional protein associated with many cellular processes such as cell cycle, proliferation, apoptosis, senescence, cellular immortalization, adipogenesis and differentiation (Chowdhury et al., 2012). In the ovary, PHB is widely expressed and its expressions are age and follicular stage regulated (Thompson et al., 2004). Our group has made significant contributions by analyzing the patterns of PHB expression, distribution and the functional roles it plays in relation to GC physiology.
Role of granulosa cells in folliculogenesis
The development of ovarian follicles begins during fetal life with the transformation of primordial germ cells into oocytes enclosed in structures called follicles (Hirshfield, 1991; McGee and Hsueh, 2000). During fetal life, primordial germ cells (PGCs) migrate to the future gonad, undergo mitosis and give rise to oogonia (Gondos et al., 1971). The oogonia are then transformed into oocytes as they enter the first meiotic division. Primordial follicles are formed perinatally as the oocytes arrested in prophase of the first meiotic division become enveloped by a single layer of flattened GC that are surrounded by a basement membrane (Fritz and Speroff, 2011; Uyar et al., 2013). The follicular GC are completely separated from blood vessels and other cell types by the basement membrane lining of the follicle.
GCs are the somatic cells of the ovary which are closely associated with the developing oocyte. GC differentiate from either the coelomic epithelium or mesenchymal precursors, however, the embryological origin is still disputed. During puberty, through an unknown selection mechanism, individual primordial follicles are recruited from this resting pool to undergo growth and differentiation (McGee, 2000). During this process, flattened GC which surround the oocyte become cuboidal in shape, and support the formation of the primary follicle (Amsterdam et al., 1992; Gougeon and Chainy, 1987). At this stage GC proliferate and form multiple layers of somatic cells that surround the oocyte, resulting in the formation of a secondary follicle. This is followed by the formation of small, fluid-filled cavities within the follicle that coalesce to form the early antral (or tertiary) follicle (Soyal et al., 2000). In the absence of gonadotropin stimulation, these follicles become atretic and disappear from the ovary. However, once puberty begins, pituitary follicle stimulating hormone (FSH) stimulates further follicular growth with expression of follicle-stimulating hormone receptor (FSHR) in GC (McGee, 2000). Under the influence of FSH, the antrum continues to enlarge, resulting in the formation of a preovulatory (antral or late antral) follicle.
In the antral/preovulatory follicle formation of the antral cavity segregates the distribution of GC and creates four different GC layers: the outermost layer is the membrana granulosa, the inner most layer is the periantral, the intermediate layer is the cumulus oophorus, and the layer juxtaposed to the oocyte is the corona radiata (inner layer of cumulus oophorus) (Erickson and Shimasaki, 2000). These four layers are functionally heterogeneous, secrete different molecules and express various hormone receptors (Nguyen et al., 2012). The cumulus cells (surrounding the oocyte) are specialized GC which are distinct from the mural GC that line the antrum (Guzeloglu-Kayisli et al., 2012). At the time of ovulation, the cumulus oophorus loses its connections with the outer parietal GC so that the oocyte, enclosed in a large, expanded cumulus mass, is extruded in to the Fallopian tube in order to accomplish fertilization.
Ovarian cyclic events are highly specialized both in the structure and function of the GC. Thus, GC in the intact follicle possess marked morphological and biochemical heterogeneity among the different layers of cells, with respect to their location close to the oocyte or close to the basement membrane separating GC and theca cells (Amsterdan and Rotmenshe, 1987). This heterogeneity includes differential expression of LH receptors (Amsterdam et al., 1975), and the concentration of steroidogenic enzymes that are higher in the outer GC layers adjacent to the basement membrane (Zoller et al., 1978, 1979). Differentiated GC increases in volume and show morphometric changes in the mitochondria, nucleus, endoplasmic reticulum (ER) and Golgi complex with an increase in volume of lipid droplets, lysosomes and smooth ER. These changes are accompanied by the organelles associated with the enzymes necessary for steroid synthesis and the subsequent increase in progesterone secretion (Hsueh et al. 1984).
The oocyte within the preovulatory follicle will remain arrested in prophase I until after the LH surge, which precedes ovulation (Guzeloglu-Kayisli et al., 2012). During this period the cumulus cells undergo “cumulus expansion”, a process that requires cumulus cells to produce hyaluronic acid that is deposited into the extracellular space and further stabilized by secreted proteins (Eppig, 1991; Richards et al., 2002). This newly formed extracellular matrix binds the oocyte and cumulus cells together (Eppig, 1991; Richards et al., 2002). In the meantime, the oocyte resumes meiotic division and begins the process of maturation. The culmination of these process results in the formation of a mature cumulus-oocyte complex (COC) containing an oocyte arrested at the metaphase of the second meiotic division (MII) and ready for ovulation and subsequent fertilization (Guzeloglu-Kayisli et al., 2012).
The cellular physiological coordination between theca cells, the GC and the oocyte in the late follicular development (preantral and preovulatory follicles) occurs through the gap junctions. At an early stage of development of the follicle, contact between GC is characterized by adherence junctions, desmosomes, and small gap junctions even in primary and primordial follicles (Albertini and Anderson, 1974; Mitchell and Burghardt, 1986). However, during follicular growth there is a shift in the nature of these cell-cell contacts; while adherence junctions and desmosomes are down-regulated, gap junctions increase in number and size (Albertini and Anderson, 1974; Amsterdam et al., 1976). The complex bidirectional communication with the oocyte includes providing essential nutrients for oocyte development and accumulation of oocyte secreted metabolites (Eppig 1979, 1982, 1991, 1994, 1996, 2001; Eppig et al., 1996, 1997, 2005; Su et al., 2009). Moreover, the natural role of the GC includes hormonal activity with production of estradiol during follicular growth and secretion of progesterone after ovulation (Yong et al., 1992). Termination of communication between the oocyte and processes of the corona radiata cell layer transversing the zona pellucida serve as a major trigger for the resumption of oocyte meiotic division and follicle rupture, which is associated with massive internalization of gap junctions during follicular rupture (Amsterdam et al., 1976; Lindner et al., 1977; Larsen et al., 1986, 1987; Racowskv et al., 1989). Re-establishment of communication between corpus luteum cells occurs at an early stage of luteinization (Larsen et al., 1988). All these changes that occur in intercellular communication require rapid modulation in the organization of the junctional elements during folliculogenesis, ovulation and luteinization.
In summary, the major functions of GC include the production of sex steroids, various peptides required for folliculogenesis and ovulation, and follicular fluid.
The Prohibitin (PHB/PHB1)
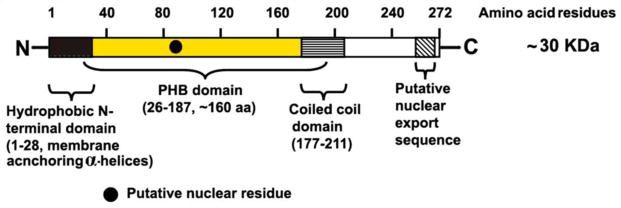
Prohibitin (PHB/PHB1) is a member of a highly evolutionary conserved protein family which includes repressor of estrogen activity (REA/PHB2), stomatins, HflKC, flotillins, HIR proteins and plant defense proteins (Nadiampalli et al., 2000; Nijtmans et al., 2002). PHB was first identified and PHB cDNA isolated by differential hybridization to RNA from normal versus regenerating rat liver (McClung et al., 1989). Subsequently, it was demonstrated that the microinjection of PHB mRNA into normal human diploid fibroblasts attenuated the DNA synthesis or led to growth inhibition, while suppression of PHB expression by anti-sense oligonucleotide stimulated proliferation (Liu et al., 1994; Nuell et al., 1991). However, once the cells enter S-phase, PHB-mediated growth arrest by blocking G1/S phase of cell cycle (antiproliferative effects) was no longer observed (Ikonen et al., 1995; McClung et al., 1995; Roskams et al., 1993). These studies suggested that PHB is an inhibitor of cellular proliferation, hence the name prohibitin. Later, it was shown that the PHB-attributed anti-proliferative effect is mediated by the 3′-untranslated region of the PHB mRNA rather than the coding region (Manjeshwar et al., 2003). In human, the PHB gene (hPHB) spans ~11 kb with 7 exons and mapped to chromosome 17q21 (Sato et al., 1992). The first exon and a small portion of the second exon comprise the 5′ untranslated region, whereas the largest exon, exon 7 contains ~700 bp of 3′ untranslated RNA. Several transcripts of the PHB gene with varying lengths of 3′ untranslated region have been identified (Jupe et al., 1996). The longer transcripts are present at higher levels in proliferating tissues and cells (Choongkittaworn et al., 1993). The abundance of PHB mRNA is inversely related to markers of cellular proliferation in different cells and tissues (Hussain-Hakimjee et al., 2006; Jupe et al., 1995; Lumpkin et al., 1995; Miyamoto et al., 2001; Tanno et al., 1997). Comparative genomic alignment studies have shown that the human and rat PHB genes are similar except for intron 2 and 3, which is ~1 kb larger in the rat gene (Altus et al., 1995). The hPHB gene encodes ~30 kDa protein, also known as B-cell receptor associated protein-32 (BAP32) gene (Figure 1).
Figure 1.
Schematic domain representation of human prohibitin (hPHB/hPHB1) protein. N, amino terminal; C, carboxylic terminal.
The human PHB2 (REA/hPHB2) (Montano et al., 1999), also referred as prohibitone (Lamers and Bacher, 1997)/B-cell receptor associated protein-37 (BAP37) (Terashima et al., 1994) gene (PHB2) is located on chromosome 12p13 (Ansari-Lari et al., 1997). This gene has 10 exons, with smaller introns than PHB and spans ~5.3 kb. The REA/PHB2/BAP37 gene encodes a protein of ~37 kDa. In eukaryotes PHB and PHB2 have highly conserved PHB domains. The PHB protein is 54% homologous with PHB2 (Chowdhury et al., 2012; Gamble et al., 2007) and has a single amino acid difference between rodents and humans (Altus et al., 1995). Orthologues of the PHB gene have been identified in several organisms including bacteria (de Monbrison and Picot, 2002; Narsimhan et al., 1997), plants (Snedden and Fromm, 1997; Takahashi et al., 2003), Trypanosoma brucei (Welburn and Murphy, 1998), Saccharomyces cerevisiae (yeast) (Kirchman et al., 2003; Tatsuta et al., 2005), and Drosophila (Eveleth et al., 1986).
Expression and cellular localization of prohibitin (PHB) during folliculogenesis
There are distinct differences in PHB levels that have been observed during mammalian folliculogenesis. Immunolocalization of PHB in rat ovarian sections have shown that PHB is expressed differentially in GCs, theca interstitial cells and the oocyte (Thompson et al., 2001, 2004). As follicles develop toward early or large antral stages, the theca-interstitial cells show more intense PHB expressions, whereas, a gradient expression of PHB was observed in the GC of early and large antral follicles (Thompson et al., 2004). Additionally, in porcine oocytes, zygotes, and blastocysts immunolocalized PHB shows a differential expression pattern (Thompson et al., 2004). In contrast to these published studies on PHB gene expression, only few studies have been performed that were designed to analyze the expression and distribution of pattern of REA/PHB2 in GCs of human or other mammalian or non-mammalian species (Coates et al., 2001; our unpublished data). In lower vertebrates (rainbow trout, Oncoryncus mykiss), REA/PHB2 mRNA abundance was shown to negatively correlate with the developmental potential of the egg (Bonnet et al., 2007).
Subcellular fractionation of rat ovarian GC followed by one and two-dimensional Western blot analyses showed that PHB is present as two major isoforms (Chowdhury et al., 2007; Thompson et al., 1997, 1999, 2004; our unpublished data). In the mitochondrial fraction, two major PHB 30-kDa protein spots are detected with isoelectric points of 5.6 and 5.8, whereas only one 30-kDa protein spot with an isoelectric point of 5.8 is observed in the nuclear fraction. Interestingly, no detectable spots are identified in the cytosolic fraction. Furthermore, when the respective protein fractions of mitochondria and nucleus are incubated with alkaline phosphatase, the acidic isoform (isoelectric point 5.6) completely disappears. These results suggest that PHB within the mitochondrial fraction is phosphorylated. Bio-informatics/computational analyses of PHB sequence structure have shown that there are several potential phosphorylation sites. The sequence of PHB contains 272 residues, 35 of which (>10%) are serine, threonine or tyrosine. It is probable that multiple sites within the PHB protein are phosphorylated concurrently. However, the functional significance of these phosphorylation sites and the identity of the kinases involved have not been well studied except our recent studies (Chowdhury et al., 2015).
Studies in various mammalian cells have demonstrated that PHB forms heterodimeric large molecular weight (~1 MDa) ring complexes with REA and present in multiple copies (Choongkittaworn et al., 1993; Hussain-Hakimjee et al., 2006; Jupe et al., 1995, 1996; Sato et al., 1992) at the mitochondrial inner membrane (IMM) (Bach et al., 2002; Eveleth and Marsh, 1986; McClung et al., 1989). These multimeric PHB complexes act as chaperone/holdase binding and stabilizing subunits of the respiratory chain complex proteins in the mitochondria (Nijtmans et al., 2000; Steglich et al., 1999). Recent studies have also suggested a scaffolding role for PHB complexes to target lipid rafts (Merkwirth and Langer, 2009; Merkwirth et al., 2008). Furthermore, there is also compelling evidence that both PHB and REA are localized in the nucleus and are able to modulate transcriptional activity by interacting with various transcription factors, either directly or through interactions with chromatin remodeling proteins (Matsuyama et al., 1997). Moreover, PHB and REA are translocated from the cytoplasm/mitochondria to the nucleus or vice-versa in different cells including GC of ovarian follicles undergoing atresia, and these proteins are markedly visible in early and large antral follicle cells (Thompson et al., 2004, Chowdhury et al., unpublished work). In support of these observations, amino-acid alignments and bioinformatic studies have demonstrated that both PHB and REA have a putative nuclear localization signal, that consist of hydrophobic stretches found at the amino terminal end to anchor the membrane. In addition, a coiled coil (CC) structure present at the C-terminal end constitutes a putative signal peptide (Chowdhury et al., 2012, 2014; Joshi et al., 2003; Rastogi et al., 2006; Winter et al., 2007). The CC-region at the carboxy terminal end is crucial for the assembly of prohibitin complexes and is exposed to the intermembrane space. The PHB domain is characteristic of the SPFH-family of membrane proteins (Winter et al., 2007).
Prohibitin (PHB) roles in follicular growth, differentiation and maturation
Ovarian follicular development is tightly regulated by crosstalk between cell death and survival signals, which include both endocrine and intra-ovarian regulators such as growth factors, cytokines, gonadal steroids etc. Ultimately, they regulate follicular ovulation or atresia by controlling follicular cell proliferation, growth, differentiation or apoptosis. The initial stages of folliculogenesis are independent of gonadotropic hormones, whereas antral follicles become responsive to and then dependent on FSH. Here we highlight some important physiological functions of PHB in GC during folliculogenesis (Figure 2).
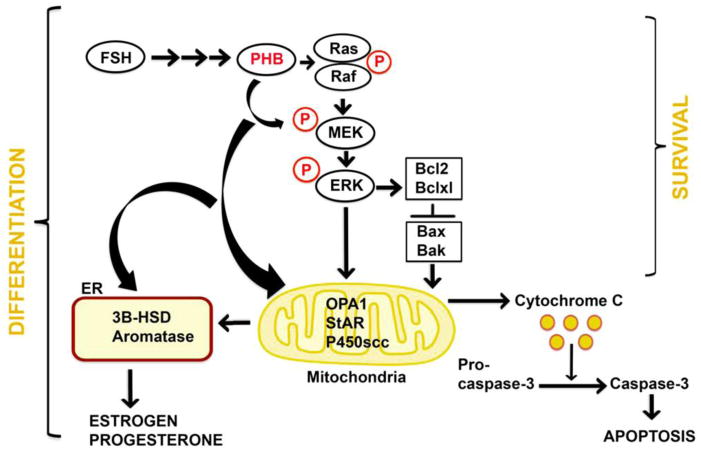
Figure 2.
Schematic representation of involvement of the prohibitin (PHB/PHB1) in granulosa cell (GCs) survival and differentiation functions. The schematic diagram showing the supportive role played by PHB/pPHB through Ras-Raf-MEK-ERK pathway in FSH induced differentiation and survival role in GCs. FSH induces up-regulation of PHB/pPHB, OPA1 (optic atrophy 1 (autosomal dominant)), steroidogenic acute regulatory protein (StAR), p450 cholesterol side-chain cleavage enzyme (p450scc), hydroxysteroid dehydrogenase-3β (HSD-3β), and aromatase (cyp19/cyp11a) leading to stimulation of synthesis and secretions of estrogen (E2) and progesterone (P4). Moreover, PHB acting through phospho-PHB (pPHB) mediate activation of pERK expression resulting in enhancement of the Bcl/Bcl-xL pathway and inhibition of Bax-Bak pathway. These events directly inhibit the release of cytochrome c from the inter-mitochondrial space resulting in inhibition of the downstream activation of cleaved caspase 3. ER-Endoplasmic reticulum, FSH-Follicle stimulating hormone, P-phosphate, Bcl2-B-cell CLL/lymphoma 2, Bclxl-B-cell lymphoma-extra large, Bax-BCL2-Associated X Protein, Bak-Bcl-2 homologous antagonist/killer, Ras-Rat sarcoma protein, Raf-Rapidly Accelerated Fibrosarcoma, MEK-Mitogen-activated protein kinase, ERK-Extracellular signal regulated kinase.
a. Gonadotropins-dependent expression of PHB in support of granulosa cells differentiation
At present, our understanding of the hormonal dependent expression of PHB in GC is limited. However, studies from our laboratory have demonstrated that in immature female rats treated with equine chorionic gonadotropin (eCG) or eCG with normal (preimmune) rabbit serum (NRS) followed by PHB immunostaining of ovaries showed high expression and rapid turnover of PHB in the ovarian follicles and GC, whereas the theca-interstitial cells within the preantral follicle showed low expression of PHB. This increased expression of PHB corresponded with follicular growth, and decreased after the ovulatory luteinizing hormone (LH) surge and during follicular atresia (Thompson et al., 2004). Moreover, one and two-dimensional Western blot analyses further confirmed that gonadotropin stimulated over-expression of PHB levels was associated with an elevation of the more acidic isoform (phosphorylated form) of PHB in GC (Thompson et al., 1997, 2004). In addition, we demonstrated that treatment of immature rats with pregnant mare serum gonadotropin (PMSG) followed by human chorionic gonadotropin (hCG) treatment induced transient expression of PHB genes in ovary, GC or thecal layers (Jo et al., 2004; Chowdhury et al., unpublished data). The mRNA expression levels of the PHB increased initially with PMSG treatment followed by a decrease or no change. In contrast, hCG treatment maintained the mRNA expression of PHB genes (Jo et al., 2004). However, treatment of immature rats with PMSG alone enhanced differential expression of the PHB proteins in the ovary, GCs and thecal layers (Chowdhury et al., unpublished data).
The entire differentiation paradigm induced by gonadotropin (FSH) receptor activation in GC in the intact animal can be duplicated in primary GC cultured in presence of FSH on an extracellular matrix in a serum-free environment that mimic the avascular milieu within the follicle (Hsueh et al., 1984). Addition of FSH in presence of testosterone (T) to GC under these culture conditions initiates a differentiation program which is completed within 48–72h. Taking advantage of these “physiological” culture conditions and using a proteomic approach we analyzed the pattern of intracellular PHB expression during ongoing differentiation of rat GC from preantral and early antral follicles (Chowdhury et al., 2015; Thompson et al., 1997). These studies demonstrated that increased PHB expression occurred during the transitional stages of rat ovarian follicular differentiation. Recent studies using pre-antral GCs isolated from diethylstilbestrol (DES)-treated rats and antral GC isolated from equine chorionic gonadotropin (eCG)-treated rats have shown that PHB is regulated by FSH in a follicular stage-dependent manner in vitro and that the role of PHB in the regulation of steroidogenesis is dependent on the differentiation status of GC (Wang et al., 2013a,b).
Furthermore, PHB is phosphorylated in the presence of FSH+T in GC, since we observed a shift in the mobility of the protein toward the basic region in 2-D Western blot analysis upon phosphatase treatment, indicating that PHB is phosphorylated (Chowdhury et al., 2015). This phosphorylation was not inhibited by LY294002 and H89 suggesting that PHB is not a substrate for PKB/AKT or PKA under these experimental conditions. Moreover, phosphorylation was inhibited by PD98059 and SB203580 suggesting that PHB is a substrate for MEK1 and p38MAPK during GC differentiation (Chowdhury et al., 2015).
Moreover, we identified the PHB phosphorylation site under these culture conditions as tyrosine 249 (Y249), threonine 258 (T258) and tyrosine 259 (T259) (Chowdhury et al., 2015; Rikova et al., 2007). Most interestingly, Y249 in PHB is located within the Raf-binding domain of PHB, residues 243–275 (Rajalingam et al., 2005). PHB/Raf interactions have been reported to be essential for the activation of the pathway (Rajalingam et al., 2005). We have observed that depletion of PHB had a negative impact on FSH-induced phosphorylation of ERK1/2 without changes in its protein expression levels. These studies suggest that PHB is phosphorylated during GCs differentiation, and we have identified Y249, T258 and T259 as a main phosphorylation sites in PHB in response to MEK1 activation by FSH+T showing that the ERK1/2 phosphorylation events occuring in response to FSH+T is PHB-dependent in GC. These findings indicate that there is a mutual hierarchical relationship between PHB and the Ras-Raf-MEK1-ERK1/2 pathway. PHB plays an indispensable role in the activation of the Raf-MEK-ERK pathway through Ras (Rajalingam et al., 2005). The activation of c-Raf by Ras requires a direct interaction of c-Raf with PHB. Moreover, c-Raf kinase fails to interact with the active Ras induced by epidermal growth factor (EGF) in the absence of PHB. These studies strongly support the notion that PHB transcription, translation and phosphorylation are hormonally dependent in GC. Hormone dependent expression of PHB has been demonstrated in other non-reproductive tissues (Dixit et al., 2004).
b. Roles of prohibitin (PHB) in granulosa cell survival through Ras-Raf-MEK-Erk pathway
From birth, only a small portion of the primordial follicles that are present in the mammalian ovary will reach the ovulatory stage, the rest will succumb to follicular atresia. Follicular atresia is mediated by apoptosis, which is initially starts in the GCs layer (Boone et al., 1997; Palumbo et al., 1994), followed by apoptosis of the theca cells (Tilly et al., 1991). Ovarian follicular atresia is induced by both the extrinsic (death receptor) and intrinsic (mitochondrial) pathway in GC (Jiang et al., 2003). The intrinsic pathway is activated within the cell, and is characterized by the permeabilization of the outer mitochondrial membrane (OMM) resulting in the release of pro-apoptotic factors such as cytochrome c, Smac, Omi into the cytosol and loss of mitochondrial function (Jin et al., 2005). Caspase-3 is the most characterized effector caspase involved in this process, and its activation leads to the final stages of cellular death. Published studies have confirmed that Caspase-3 content is very high in the atretic preovulatory follicular GC (Boone et al., 1997; Matikainen et al., 2001). In addition, follicles isolated from caspase-3 null ovaries do not show GC apoptosis in response to serum starvation, suggesting the critical role of caspase-3 in GC apoptosis (Matikainen et al., 2001).
The actions of PHB in GC are dependent on the stage of differentiation (Chowdhury et al., 2007; Wang et al., 2013a,b). Our studies have shown that infection of undifferentiated GC from preantral follicles with a PHB adenoviral construct resulted in over-expression of PHB that markedly attenuated ceramide-, staurosporine-, campothecine- and serum withdrawl-induced apoptosis via the intrinsic apoptotic pathway (Chowdhury et al., 2007, 2011, 2013, 2014; Wang et al., 2013a,b; our unpublished data). Further, these studies have confirmed that over-expression of PHB maintains the mitochondrial transmembrane potential by inhibiting cytochrome c release and activation of caspase-3 (Chowdhury et al., 2007, 2011, 2013).
In addition, studies performed by our group have also shown that over-expression of PHB in undifferentiated GCs isolated from preantral follicle delayed apoptosis by enhancing the transcription and translation of anti-apoptotic genes (Bcl2, Bclxl) in STS-treated GC (Chowdhury et al., 2006, 2013). The apoptosis-preventing effect of Bcl2 and Bclxl are counteracted by the expression of proapoptotic proteins Bax and Bak (Chowdhury et al., 2013). These studies suggest that an imbalance of the Bax and Bak versus Bcl2 and Bclxl ratios tilts the scales toward cell death and sensitizes cells to a wide variety of cell death stimuli. However, ectopic PHB over-expression in GC prevented triggering by apoptotic stimuli, thereby supporting the role of the Bax/Bcl2, Bax/Bclxl, Bak/Bcl2, and Bak/Bclxl as key checkpoint rheostat (Chowdhury et al., 2013). Anti-apoptotic (Bcl-2, Bcl-xL) or pro-apoptotic (Bax, Bak) Bcl2 proteins are the prototype of a large family of proteins (more than 30 members), which share a high degree of homology although they exert different functions in the apoptotic pathway (Chowdhury and Bhat, 2010). In healthy cells, Bax and Bak are mainly located in the cytosol as monomers with a minor pool loosely attached to mitochondria. Apoptotic stimuli induces structural changes in Bax and Bak proteins which oligomerize in the OMM to result in MOMP formation and the release of inter-membrane space proteins including cytochrome c. In contrast, the anti-apoptotic Bcl2 and Bclxl proteins are able to neutralize pro-apoptotic proteins and thus inhibit Bax/Bak activity and MOMP formation. Molecular studies performed in our laboratory that were designed to elucidate the mechanisms involved in this regulatory process suggest that upon induction of apoptosis by STS, mitochondrial PHB through phosphorylated PHB (phospho-PHB, pPHB) mediate activation of pMEK–pERK expression. This result in enhancement of the Bcl/Bcl-xL pathway and inhibition of Bax-Bak inhibiting the release of cytochrome c from the inter-mitochondrial space and inhibiting of the downstream activation of cleaved caspase 3 (Chowdhury et al., 2013). Collectively, these studies suggest that PHB is likely to be a key factor in GC survival pathway acting through the Bcl family of proteins.
Strong published experimental evidence in support of the relationship between PHB phosphorylation and activation of the Ras-Raf-MEK-Erk pathway in cellular survival has been provided by our laboratory and other research groups. PHB/Raf interactions have previously been reported to be essential for activation of the Ras-Raf-MEK1-ERK1/2 pathway (Rajalingam et al., 2005). In support of this observation, our laboratory has consistently observed that depletion of PHB had a negative impact on STS-induced phosphorylation of ERK1/2 without changes in its protein expression levels. These studies show that, in addition to PHB being required for MEK1 activity, PHB is also a potential target of MEK1, suggesting that a possible regulatory loop is activated during GC differentiation and survival that is mediated by PHB and affects the Ras-Raf-MEK1-ERK1/2 pathway. These findings indicate that there is a mutual hierarchical relationship between PHB and the Ras-Raf-MEK1-ERK1/2 pathway (Chowdhury et al., 2013, 2014; our unpublished work) and that PHB plays an indispensable role in the activation of the Ras-Raf-MEK-ERK pathway (Rajalingam et al., 2005). Similar studies have shown in other cells/cell lines (Chowdhury et al., 2014 and references therein). Taken together, these studies indicate that PHB act as a survival factor by influencing the regulation of anti-apoptotic gene expression in ovarian GC.
In addition, our laboratory has shown that the prohibitins (PHB and REA) are required for morphogenesis of mitochondrial cristae, as revealed by an ultrastructural analysis of mitochondria in PHB-deficient mouse embryonic fibroblasts (MEFs) (Chowdhury et al., 2013; Merkwirth et al., 2009). This experimental observation suggest that the disrupted cristae morphology might facilitate the release of cytochrome c from the intracristal space, thereby explaining the increased sensitivity of prohibitin-deficient MEFs or GC to apoptotic stimuli. Published studies have shown that this occurs in other cell lines and animal models (Antral-Sanz et al., 2003; Berger and Yaffe, 1998; Kasashima et al., 2006; Osman et al., 2009). The aberrant mitochondrial morphology observed in the absence of the prohibitins can be explained by an altered processing of OPA1 (Merkwirth et al., 2008), a large dynamin-like GTPase found in the mitochondrial intermembrane space (IMS) that regulates both mitochondrial fusion and cristae morphogenesis (Hoppins et al., 2007). Since the prohibitins are required for normal OPA1 processing this explains the mitochondrial morphology defects observed in Phb2−/− cells. Normal mitochondrial fusion depends on expression of both long and short OPA1 isoforms (Ishihara et al., 2006; Song et al., 2007). Indeed, ectopic expression of a non-cleavable long OPA1 isoform was able to restore the tubular mitochondrial network, cristae morphogenesis and apoptotic resistance in Phb2−/− MEFs (Merkwirth et al., 2008). Similar results are found when PHB was ectopically expressed in sturosporine (STS) induced GC (Chowdhury et al., 2013, 2014). These experiments confirm that OPA1 processing is the key process regulated by the prohibitins in MEFs. However, the exact mechanisms by which prohibitins affect OPA1 processing remains to be determined.
c. Conditional knock-down effects of prohibitin (PHB) in granulosa cells
A wide range of functional roles for PHB and its orthologue members have been described in invertebrates. Studies have shown that PHB null yeast strains have reduced lifespan. The PHB orthologues in Drosophila, Cc, is essential for normal development and differentiation during larvae to pupae metamorphosis (Eveleth and Marsh, 1986). By using RNA-interference technology it has ben shown that PHB is essential during embryonic development, somatic and germline differentiation in the larval gonad in Caenorhabditis elegans (Antral-Sanz et al., 2003). Further, the genetic deletion of PHB in mice is lethal before embryonic day 6.5 (our unpublished data), implying that these proteins may play a critical role in the early stages of development in vertebrates. However, heterozygous mice show no appreciable defects in fertility (our unpublished work). Moreover, our unpublished detailed analysis of GC-conditional knockout mice studies suggest that PHB is essential for folliculogenesis (Chowdhury et al., unpublished work).
CONCLUSIONS
At present our understanding of the complex biology of the PHB in GC physiology is limited and our current functional studies remain a work in progress. In this review, we have provided some of the experimental evidence supporting a conserved role for the PHB in GCs physiology. However, the individual PHB or REA proteins are likely to have functionally different effects compared to the PHB/REA heterodimers. This is particularly relevant because recent evidence suggests that the nuclear prohibitins may have overlapping functions in modulating gene expression, particularly steroid hormone mediated gene expression. Post-translational modifications such as phosphorylation or ubiquitination that are important in modulating of the various functional roles of the respective prohibitins at very early stages in GC physiology. The detailed conditional knock-out experiments currently underway in our laboratory will most likely to be important for determining the in vivo physiological significance of the various interactions of the PHB in relation to its respective functions during folliculogenesis.
Acknowledgments
This study was supported in part by National Institutes of Health Grants 1RO1HD057235, HD41749, 1SC3GM113751, U54-CA118638 and G12-RR03034. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Grant #C06 RR18386 from NIH/NCRR.
Footnotes
Disclosure statement: The authors have nothing to disclose.
References
- Albertini DF, Anderson E. The appearance and structure of intercellular connections during the ontogeny of the rabbit ovarian follicle with particular reference to gap junctions. J Cell Biol. 1974;63(1):234–50. doi: 10.1083/jcb.63.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altus MS, Wood CM, Stewart DA, Roskams AJ, Friedman V, Henderson T, Owens GA, Danner DB, Jupe ER, Dell’Orco RT, Keith McClung J. Regions of evolutionary conservation between the rat and human prohibitin-encoding genes. Gene. 1995;158:291–294. doi: 10.1016/0378-1119(95)00164-2. [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Josephs R, Lieberman ME, Lindner HR. Organization of intramembrane particles in freeze-cleaved gap junctions of rat graafian rollicles: optical-diffraction analysis. J Cell Sci. 1976;21(1):93–105. doi: 10.1242/jcs.21.1.93. [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Koch Y, Lieberman ME, Lindner HR. Distribution of binding sites for human chorionic gonadotropin in the preovulatory follicle of the rat. J Cell Biol. 1975;67(3):894–900. doi: 10.1083/jcb.67.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A, Plehn-Dujowich D, Suh BS. Structure-function relationships during differentiation of normal and oncogene-transformed granulosa cells. Biol Reprod. 1992;46(4):513–22. doi: 10.1095/biolreprod46.4.513. [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Rotmensch S, Ben-Ze’ev A. Coordinated regulation of morphological and biochemical differentiation in a steroidogenic cell: the granulosa cell model. Trends Biochem Sci. 1989;14(9):377–82. doi: 10.1016/0968-0004(89)90012-1. [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Rotmensch S. Structure-function relationships during granulosa cell differentiation. Endocr Rev. 1987;8(3):309–37. doi: 10.1210/edrv-8-3-309. [DOI] [PubMed] [Google Scholar]
- Ansari-Lari MA, Shen Y, Muzny DM, Lee W, Gibbs RA. Large-scale sequencing in human chromosome 12p13: experimental and computational gene structure determination. Genome Res. 1997;7:268–280. doi: 10.1101/gr.7.3.268. [DOI] [PubMed] [Google Scholar]
- Artal-Sanz M, Tsang WY, Willems EM, Grivell LA, Lemire BD, van der Spek H, Nijtmans LG. The mitochondrial prohibitin complex is essential for embryonic viability and germline function in Caenorhabditis elegans. J Biol Chem. 2003;278:32091–32099. doi: 10.1074/jbc.M304877200. [DOI] [PubMed] [Google Scholar]
- Back JW, Sanz MA, De Jong L, De Koning LJ, Nijtmans LG, De Koster CG, Grivell LA, Van Der Spek H, Muijsers AO. A structure for the yeast prohibitin complex: Structure prediction and evidence from chemical crosslinking and mass spectrometry. Protein Sci. 2002;11(10):2471–2478. doi: 10.1110/ps.0212602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger KH, Yaffe MP. Prohibitin family members interact genetically with mitochondrial inheritance components in Saccharomyces cerevisiae. Mol Cell Biol. 1998;18:4043–4052. doi: 10.1128/mcb.18.7.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet E, Fostier A, Bobe J. Microarray-based analysis of fish egg quality after natural or controlled ovulation. BMC Genomics. 2007;8:55. doi: 10.1186/1471-2164-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone DL, Carnegie JA, Rippstein PU, Tsang BK. Induction of apoptosis in equine chorionic gonadotropin (eCG)-primed rat ovaries by anti-eCG antibody. Biol Reprod. 1997;57:420–427. doi: 10.1095/biolreprod57.2.420. [DOI] [PubMed] [Google Scholar]
- Choongkittaworn NM, Kim KH, Danner DB, Griswold MD. Expression of prohibitin in rat seminiferous epithelium. Biol Reprod. 1993;49:300–310. doi: 10.1095/biolreprod49.2.300. [DOI] [PubMed] [Google Scholar]
- Chowdhury I, Bhat GK. Mitochondria—In cellular life and death, Chapter 5. In: Svensson OL, editor. In mitochondria structure, functions and dysfunctions. USA: NOVA Publishers; 2010. pp. 247–376. [Google Scholar]
- Chowdhury I, Branch A, Olatinwo M, Thomas K, Matthews R, Thompson WE. Prohibitin (PHB) acts as a potent survival factor against ceramide induced apoptosis in rat granulosa cells. Life Sci. 2011;89:295–303. doi: 10.1016/j.lfs.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury I, Garcia-Barrio M, Harp D, Thomas K, Matthews R, Thompson WE. The emerging roles of prohibitins in folliculogenesis. Front Biosci (Elite Ed) 2012;4:690–699. doi: 10.2741/410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury I, Tharakan B, Bhat GK. Caspases—An update. Comp Biochem Physiol B Biochem Mol Biol. 2008;151:10–27. doi: 10.1016/j.cbpb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Chowdhury I, Tharakan B, Bhat GK. Current concepts in apoptosis: The physiological suicide program revisited. Cell Mol Biol Lett. 2006;11:506–525. doi: 10.2478/s11658-006-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury I, Thompson WE, Thomas K. Prohibitins role in cellular survival through Ras-Raf-MEK-ERK pathway. J Cell Physiol. 2014;229(8):998–1004. doi: 10.1002/jcp.24531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury I, Thompson WE, Welch C, Thomas K, Matthews R. Prohibitin (PHB) inhibits apoptosis in rat granulosa cells (GCs) through the extracellular signal-regulated kinase 1/2 (ERK1/2) and the Bcl family of proteins. Apoptosis. 2013;18:1513–1525. doi: 10.1007/s10495-013-0901-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury I, Xu W, Stiles JK, Zeleznik A, Yao X, Matthews R, Thomas K, Thompson WE. Apoptosis of rat granulosa cells after staurosporine and serum withdrawal is suppressed by adenovirus-directed overexpression of prohibitin. Endocrinology. 2007;148:206–217. doi: 10.1210/en.2006-0187. [DOI] [PubMed] [Google Scholar]
- Chowdhury I, Thomas K, Zeleznik A, Thompson WE. Prohibitin (PHB)/phospho-PHB plays a supportive role in the FSH signaling pathway in rat granulosa cell differentiation. 2015 doi: 10.1530/JME-15-0278. In communication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates PJ, Nenutil R, McGregor A, Picksley SM, Crouch DH, Hall PA, Wright EG. Mammalian prohibitin proteins respond to mitochondrial stress and decrease during cellular senescence. Exp Cell Res. 2001;265:262–273. doi: 10.1006/excr.2001.5166. [DOI] [PubMed] [Google Scholar]
- de Monbrison F, Picot S. Introducing antisense oligonucleotides into Pneumocystis carinii. J Microbiol Methods. 2002;50(2):211–213. doi: 10.1016/s0167-7012(02)00033-7. [DOI] [PubMed] [Google Scholar]
- Dixit VD, Sridaran R, Edmonsond MA, Taub D, Thompson WE. Gonadotropin releasing hormone attenuates pregnancy-associated thymic involution and modulates the expression of antiproliferative gene product prohibitin. Endocrinology. 2003;144:1496–1505. doi: 10.1210/en.2002-220955. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Maintenance of meiotic arrest and the induction of oocyte maturation in mouse oocyte-granulosa cell complexes developed in vitro from preantral follicles. Biol Reprod. 1991;45(6):824–30. doi: 10.1095/biolreprod45.6.824. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. FSH stimulates hyaluronic acid synthesis by oocyte-cumulus cell complexes from mouse preovulatory follicles. Nature. 1979;281(5731):483–4. doi: 10.1038/281483a0. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev. 1996;8(4):485–9. doi: 10.1071/rd9960485. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. The relationship between cumulus cell-oocyte coupling, oocyte meiotic maturation, and cumulus expansion. Dev Biol. 1982;89(1):268–72. doi: 10.1016/0012-1606(82)90314-1. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Further reflections on culture systems for the growth of oocytes in vitro. Hum Reprod. 1994;9(6):974–6. doi: 10.1093/oxfordjournals.humrep.a138669. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Pendola FL, Wigglesworth K, Pendola JK. Mouse oocytes regulate metabolic cooperativity between granulosa cells and oocytes: amino acid transport. Biol Reprod. 2005;73(2):351–7. doi: 10.1095/biolreprod.105.041798. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Intercommunication between mammalian oocytes and companion somatic cells. Bioessays. 1991;13:569–574. doi: 10.1002/bies.950131105. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001;122:829–838. doi: 10.1530/rep.0.1220829. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Chesnel F, Hirao Y, O’Brien MJ, Pendola FL, Watanabe S, Wigglesworth K. Oocyte control of granulosa cell development: how and why. Hum Reprod. 1997;12(suppl):127–132. [PubMed] [Google Scholar]
- Eppig JJ, O’Brien M, Wigglesworth K. Mammalian oocyte growth and development in vitro. Mol Reprod Dev. 1996;44:260–273. doi: 10.1002/(SICI)1098-2795(199606)44:2<260::AID-MRD17>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Erickson GF, Shimasaki S. The role of the oocyte in folliculogenesis. Trends Endocrinol Metab. 2000;11(5):193–8. doi: 10.1016/s1043-2760(00)00249-6. [DOI] [PubMed] [Google Scholar]
- Eveleth DD, Jr, Marsh JL. Sequence and expression of the Cc gene, a member of the dopa decarboxylase gene cluster of Drosophila: possible translational regulation. Nucleic Acid Res. 1986;14:6169–6183. doi: 10.1093/nar/14.15.6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz MA, Speroff L. Clinical gynecologic endocrinology and infertility. 8. Wolters Kluwer, Lippincott Williams & Wilkins; Philadelphia: 2011. [Google Scholar]
- Gamble SC, Chotai D, Odontiadis M, Dart DA, Brooke GN, Powell SM, Reebye V, Varela-Carver A, Kawano Y, Waxman J, Bevan CL. Prohibitin, a protein downregulated by androgens, represses androgen receptor activity. Oncogene. 2007;26(12):1757–1768. doi: 10.1038/sj.onc.1209967. [DOI] [PubMed] [Google Scholar]
- Gondos B, Bhiraleus P, Hobel CJ. Ultrastructural observations on germ cells in human fetal ovaries. Am J Obstet Gynecol. 1971;110(5):644–52. doi: 10.1016/0002-9378(71)90245-6. [DOI] [PubMed] [Google Scholar]
- Gougeon A, Chainy GB. Morphometric studies of small follicles in ovaries of women at different ages. J Reprod Fertil. 1987;81(2):433–42. doi: 10.1530/jrf.0.0810433. [DOI] [PubMed] [Google Scholar]
- Guzeloglu-Kayisli O, Lalioti MD, Aydiner F, Sasson I, Ilbay O, Sakkas D, Lowther KM, Mehlmann LM, Seli E. Embryonic poly(A)-binding protein (EPAB) is required for oocyte maturation and female fertility in mice. Biochem J. 2012;446(1):47–58. doi: 10.1042/BJ20120467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annu Rev Biochem. 2007;76:751–780. doi: 10.1146/annurev.biochem.76.071905.090048. [DOI] [PubMed] [Google Scholar]
- Hsueh AJ, Adashi EY, Jones PB, Welsh TH., Jr Hormonal regulation of the differentiation of cultured ovarian granulosa cells. Endocr Rev. 1984;5(1):76–127. doi: 10.1210/edrv-5-1-76. [DOI] [PubMed] [Google Scholar]
- Hussain-Hakimjee EA, Peng X, Mehta RR, Mehta RG. Growth inhibition of carcinogen-transformed MCF-12F breast epithelial cells and hormone-sensitive BT-474 breast cancer cells by 1alpha-hydroxyvitamin D5. Carcinogenesis. 2006;27:551–559. doi: 10.1093/carcin/bgi231. [DOI] [PubMed] [Google Scholar]
- Ikonen E, Fiedler K, Parton RG, Simons K. Prohibitin, an antiproliferative protein, is localized to mitochondria. FEBS Lett. 1995;358:273–277. doi: 10.1016/0014-5793(94)01444-6. [DOI] [PubMed] [Google Scholar]
- Ishihara N, Fujita Y, Oka T, Mihara K. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 2006;25(13):2966–2977. doi: 10.1038/sj.emboj.7601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JY, Cheung CK, Wang Y, Tsang BK. Regulation of cell death and cell survival gene expression during ovarian follicular development and atresia. Front Biosci. 2003;8:222–237. doi: 10.2741/949. [DOI] [PubMed] [Google Scholar]
- Jin Z, El-Deiry WS. Overview of cell deathsignaling pathways. Cancer Biol Ther. 2005;4:139–163. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- Jo M, Gieske MC, Payne CE, Wheeler-Price SE, Gieske JB, Ignatius IV, Curry TE, Jr, Ko CM. Development and Application of a Rat Ovarian Gene Expression Database. Endocrinology. 2004;145:5384–5396. doi: 10.1210/en.2004-0407. [DOI] [PubMed] [Google Scholar]
- Joshi B, Ko D, Ordonez-Ercan D, Chellappan SPA. putative coiled-coil domain of prohibitin is sufficient to repress E2F1-mediated transcription and induce apoptosis. Biochem Biophys Res Commun. 2003;312:459–466. doi: 10.1016/j.bbrc.2003.10.148. [DOI] [PubMed] [Google Scholar]
- Jupe ER, Liu XT, Kiehlbauch JL, McClung JK, Dell’Orco RT. The 3′ untranslated region of prohibitin and cellular immortalization. Exp Cell Res. 1996;224:128–135. doi: 10.1006/excr.1996.0120. [DOI] [PubMed] [Google Scholar]
- Jupe ER, Liu XT, Kiehlbauch JL, McClung JK, Dell’Orco RT. Prohibitin antiproliferative activity and lack of heterozygosity in immortalized cell lines. Exp Cell Res. 1995;218:577–580. doi: 10.1006/excr.1995.1194. [DOI] [PubMed] [Google Scholar]
- Kasashima K, Ohta E, Kagawa Y, Endo H. Mitochondrial Functions and Estrogen Receptor-dependent Nuclear Translocation of Pleiotropic Human Prohibitin. J Biol Chem. 2006;281:36401–36410. doi: 10.1074/jbc.M605260200. [DOI] [PubMed] [Google Scholar]
- Kirchman PA, Miceli MV, West RL, Jiang JC, Kim S, Jazwinski SM. Prohibitins and Ras2 protein cooperate in the maintenance of mitochondrial function during yeast aging. Acta Biochim Pol. 2003;50(4):1039–56. [PubMed] [Google Scholar]
- Larsen WJ, Wert SE, Brunner GD. A dramatic loss of cumulus cell gap junctions is correlated with germinal vesicle breakdown in rat oocytes. Dev Biol. 1986;113:517–21. doi: 10.1016/0012-1606(86)90187-9. [DOI] [PubMed] [Google Scholar]
- Larsen WJ, Wert SE, Brunner GD. Differential modulation of rat follicle cell gap junction populations at ovulation. Dev Biol. 1987;122(1):61–71. doi: 10.1016/0012-1606(87)90332-0. [DOI] [PubMed] [Google Scholar]
- Lamers MC, Bacher S. Prohibitin and prohibitone, ubiquitous and abundant proteins that are reluctant to reveal their real identity. Int Arch Allergy Immunol. 1997;113:146–149. doi: 10.1159/000237530. [DOI] [PubMed] [Google Scholar]
- Liu XT, Stewart CA, King RL, Danner DA, Dell’Orco RT, McClung JK. Prohibitin expression during cellular senescence of human diploid fibroblasts. Biochem Biophys Res Commun. 1994;201:409–414. doi: 10.1006/bbrc.1994.1716. [DOI] [PubMed] [Google Scholar]
- Lumpkin CK, Jr, Moore TL, Tarpley MD, Taylor JM, Badger TM, McClung JK. Acute ethanol and selected growth suppressor transcripts in regenerating rat liver. Alcohol. 1995;12:357–362. doi: 10.1016/0741-8329(95)00018-m. [DOI] [PubMed] [Google Scholar]
- Manjeshwar S, Branam DE, Lerner MR, Brackett DJ, Jupe ER. Tumor suppression by the prohibitin gene 3′untranslated region RNA in human breast cancer. Cancer Res. 2003;63:5251–5256. [PubMed] [Google Scholar]
- Matikainen T, Perez GI, Zheng TS, Kluzak TR, Rueda BR, Flavell RA, Tilly JL. Caspase-3 Gene Knockout Defines Cell Lineage Specificity for Programmed Cell Death Signaling in the Ovary. Endocrinol. 2001;142:2468–2480. doi: 10.1210/endo.142.6.8078. [DOI] [PubMed] [Google Scholar]
- Matsuyama S, Kubo K, Ohashi F, Takamori Y. Partial cloning of prohibitin cDNA from canine, feline, bovine, equine, and rabbit liver mRNA by RT-PCR. J Vet Med Sci. 1997;59:201–203. doi: 10.1292/jvms.59.201. [DOI] [PubMed] [Google Scholar]
- McClung JK, Danner DB, Stewart DA, Smith JR, Schneider EL, Lumpkin CK, Dell’Orco RT, Nuell MJ. Isolation of a cDNA that hybrid selects antiproliferative mRNA from rat liver. Biochem Biophys Res Commun. 1989;164:1316–1322. doi: 10.1016/0006-291x(89)91813-5. [DOI] [PubMed] [Google Scholar]
- McClung JK, Jupe ER, Liu XT, Dell’Orco RT. Prohibitin: potential role in senescence, development, and tumor suppression. Exp Gerontol. 1995;30:99–124. doi: 10.1016/0531-5565(94)00069-7. [DOI] [PubMed] [Google Scholar]
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21(2):200–14. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- Merkwirth C, Langer T. Prohibitin function within mitochondria: essential roles for cell proliferation and cristae morphogenesis. Biochim Biophys Acta. 2009;1793(1):27–32. doi: 10.1016/j.bbamcr.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Merkwirth C, Dargazanli S, Tatsuta T, Geimer S, Löwer B, Wunderlich FT, von Kleist-Retzow JC, Waisman A, Westermann B, Langer T. Prohibitins control cell proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes Dev. 2008;22(4):476–88. doi: 10.1101/gad.460708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PA, Burghardt RC. The ontogeny of nexuses (gap junctions) in the ovary of the fetal mouse. Anat Rec. 1986;214(3):283–288. doi: 10.1002/ar.1092140307. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Qin J, Safer B. Detection of early gene expression changes during activation of human primary lymphocytes by in vitro synthesis of proteins from polysomeassociated mRNAs. Protein Sci. 2001;10:423–433. doi: 10.1110/ps.21301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano MM, Ekena K, Delage-Mourroux R, Chang W, Martini P, Katzenellenbogen BS. An estrogen receptor-selective coregulator that potentiates the effectiveness of antiestrogens and represses the activity of estrogens. Proc Natl Acad Sci USA. 1999;96:6947–6952. doi: 10.1073/pnas.96.12.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora JM1, Fenwick MA, Castle L, Baithun M, Ryder TA, Mobberley M, Carzaniga R, Franks S, Hardy K. Characterization and significance of adhesion and junction-related proteins in mouse ovarian follicles. Biol Reprod. 2012;86(5):153, 1–14. doi: 10.1095/biolreprod.111.096156. [DOI] [PubMed] [Google Scholar]
- Nadimpalli R, Yalpani N, Johal GS, Simmons CR. Prohibitins, stomatins and plant desease response genes compose a superfamily that controls cell proliferation, ion channels regulation and death. J Biol Chem. 2000;275:29579–29586. doi: 10.1074/jbc.M002339200. [DOI] [PubMed] [Google Scholar]
- Narasimhan S, Armstrong M, McClung JK, Richards FF, Spicer EK. Prohibitin, a putative negative control element present in Pneumocystis carinii. Infect Immun. 1997;65(12):5125–5130. doi: 10.1128/iai.65.12.5125-5130.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Lee S, Hatzirodos N, Hummitzsch K, Sullivan TR, Rodgers RJ, Irving-Rodgers HF. Spatial differences within the membrana granulosa in the expression of focimatrix and steroidogenic capacity. Mol Cell Endocrinol. 2012;363(1–2):62–73. doi: 10.1016/j.mce.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Nijtmans LG, Artal SM, Grivell LA, Coates PJ. The mitochondrial PHB complex: roles in mitochondrial respiratory complex assembly, aging and degenerative disease. CMLS, Cell Mol Life Sci. 2002;59:143–155. doi: 10.1007/s00018-002-8411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijtmans LG, de Jong L, Artal Sanz M, Coates PJ, Berden JA, Back JW, Muijsers AO, van der Spek H, Grivell LA. Prohibitins act as a membrane-bound chaperone for the stabilization of mitochondrial proteins. EMBO J. 2000;19:2444–2451. doi: 10.1093/emboj/19.11.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuell MJ, Stewart DA, Walker L, Friedman V, Wood CM, Owens GA, Smith JR, Schneider EL, Dell’ Orco R, Lumpkin CK, Danner DB, McClung Keith. Prohibitin, an evolutionarily conserved intracellular protein that blocks DNA synthesis in normal fibroblasts and HeLa cells. Mol Cell Biol. 1991;11:1372–81. doi: 10.1128/mcb.11.3.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman C, Merkwirth C, Langer T. Prohibitins and the functional compartmentalization of mitochondrial membranes. J Cell Sci. 2009;122(Pt 21):3823–3830. doi: 10.1242/jcs.037655. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Yeh J. In situ localization of apoptosis in the rat ovary during follicular atresia. Biol Reprod. 1994;51:888–895. doi: 10.1095/biolreprod51.5.888. [DOI] [PubMed] [Google Scholar]
- Racowsky C, Baldwin KV, Larabell CA, DeMarais AA, Kazilek CJ. Down-regulation of membrana granulosa cell gap junctions is correlated with irreversible commitment to resume meiosis in golden Syrian hamster oocytes. Eur J Cell Biol. 1989;49(2):244–51. [PubMed] [Google Scholar]
- Rajalingam K, Wunder C, Brinkmann V, Churin Y, Hekman M, Sievers C, Rapp UR, Rudel T. Prohibitin is required for Ras-induced Raf-MEK-ERK activation and epithelial cell migration. Nat Cell Biol. 2005;7:837–843. doi: 10.1038/ncb1283. [DOI] [PubMed] [Google Scholar]
- Rastogi S, Joshi B, Dasgupta P, Morris M, Wright K, Chellappan S. Prohibitin Facilitates Cellular Senescence by Recruiting Specific Corepressors To Inhibit E2F Target Genes. J Biol Chem. 2006;26:4161–4171. doi: 10.1128/MCB.02142-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JS, Midgley AR., Jr Protein hormone action: a key to understanding ovarian follicular and luteal cell development. Biol Reprod. 1976;14(1):82–94. doi: 10.1095/biolreprod14.1.82. [DOI] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Ochsner S, Hsieh M, Doyle KH, Falender AE, Lo YK, Sharma SC. Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent Prog Horm Res. 2002;57:195–220. doi: 10.1210/rp.57.1.195. [DOI] [PubMed] [Google Scholar]
- Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, Hu Y, Tan Z, Stokes M, Sullivan L, Mitchell J, Wetzel R, Macneill J, Ren JM, Yuan J, Bakalarski CE, Villen J, Kornhauser JM, Smith B, Li D, Zhou X, Gygi SP, Gu TL, Polakiewicz RD, Rush J, Comb MJ. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131(6):1190–203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- Roskams AJ, Friedman V, Wood CM, Walker L, Owens GA, Stewart DA, Altus MS, Danner DB, Liu XT, McClung JK. Cell cycle activity and expression of prohibitin mRNA. J Cell Physiol. 1993;157:289–295. doi: 10.1002/jcp.1041570211. [DOI] [PubMed] [Google Scholar]
- Sato T, Saito H, Swensen J, Olifant A, Wood C, Danner D, Sakamoto T, Takita K, Kasumi F, Miki Y, Skolnick M, Nakamura Y. The human prohibitin gene located on chromosome 17q21 is mutated in sporadic breast cancer. Cancer Res. 1992;52:1643–1646. [PubMed] [Google Scholar]
- Snedden WA, Fromm H. Characterization of the plant homologue of prohibitin, a gene associated with antiproliferative activity in mammalian cells. Plant Mol Biol. 1997;33:753–756. doi: 10.1023/a:1005737026289. [DOI] [PubMed] [Google Scholar]
- Song Z, Chen H, Fiket M, Alexander C, Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol. 2007;178(5):749–755. doi: 10.1083/jcb.200704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyal SM, Amleh A, Dean J. FIGalpha, a germ cell-specific transcription factor required for ovarian follicle formation. Development. 2000;127(21):4645–54. doi: 10.1242/dev.127.21.4645. [DOI] [PubMed] [Google Scholar]
- Steglich G, Neupert W, Langer T. Prohibitins regulate membrane protein degradation by the m-AAA protease in mitochondria. Mol Cell Biol. 1999;19:3435–3442. doi: 10.1128/mcb.19.5.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Eppig JJ. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumuluscell metabolism. Semin Reprod Med. 2009;27(1):32–42. doi: 10.1055/s-0028-1108008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Kawasaki T, Wong HL, Suharsono U, Hirano H, Shimamoto K. Hyperphosphorylation of a mitochondrial protein, prohibitin, is induced by calyculin A in a rice lesion-mimic mutant cdr1. Plant Physiol. 2003;132(4):1861–9. doi: 10.1104/pp.103.021733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno S, Fukuda I, Saito Y, Ogawa K. Prohibitin expression is decreased in the regenerating liver but not in chemically induced hepatic tumors in rats. Jpn J Cancer Res. 1997;88:1155–1164. doi: 10.1111/j.1349-7006.1997.tb00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuta T, Model K, Langer T. Formation of membrane bound ring complexes by prohibitin in mitochondria. Mol Biol Cell. 2005;16:248–259. doi: 10.1091/mbc.E04-09-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima M, Kim KM, Adachi T, Nielsen PJ, Reth M, Kohler G, Lamers MC. The IgM antigen receptor of B lymphocytes is associated with prohibitin and a prohibitin related protein. EMBO J. 1994;13:3782–3792. doi: 10.1002/j.1460-2075.1994.tb06689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WE, Asselin E, Branch A, Stiles JK, Sutovsky P, Lai L, Im G-S, Prather RS, Isom SC, Rucker E, III, Tsang B. Regulation of prohibitin expression during follicular development and atresia in the mammalian ovary. Biol Reprod. 2004;71:282–290. doi: 10.1095/biolreprod.103.024125. [DOI] [PubMed] [Google Scholar]
- Thompson WE, Branch A, Whittaker JA, Lyn D, Zilberstein M, Mayo KE, Thomas K. Characterization of prohibitin in a newly established rat ovarian granulose cell line. Endocrinology. 2001;142:4076–4085. doi: 10.1210/endo.142.9.8354. [DOI] [PubMed] [Google Scholar]
- Thompson WE, Powell JM, Whittaker JA, Sridaran R, Thomas KH. Immunolocalization and expression of prohibitin, a mitochondrial associated protein within the rat ovaries. Anat Rec. 1999;256:40–48. doi: 10.1002/(SICI)1097-0185(19990901)256:1<40::AID-AR6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Thompson WE, Sanbuissho A, Lee GY, Anderson E. Steroidogenic acute regulatory (StAR) protein (p25) and prohibitin (p28) from cultured rat ovarian granulosa cells. J Reprod Fertil. 1997;109:337–348. doi: 10.1530/jrf.0.1090337. [DOI] [PubMed] [Google Scholar]
- Tilly JL, Kowalski KI, Johnson AL, Hsueh AJ. Involvement of apoptosis in ovarian follicular atresia and postovulatory regression. Endocrinology. 1991;129:2799–2801. doi: 10.1210/endo-129-5-2799. [DOI] [PubMed] [Google Scholar]
- Uyar A, Torrealday S, Seli E. Cumulus and granulosa cell markers of oocyte and embryo quality. Fertil Steril. 2013;99(4):979–97. doi: 10.1016/j.fertnstert.2013.01.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Leader A, Tsang BK. Follicular stage-dependent regulation of apoptosis and steroidogenesis by prohibitin in rat granulosa cells. J Ovarian Res. 2013;6(1):23. doi: 10.1186/1757-2215-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Leader A, Tsang BK. Inhibitory roles of prohibitin and chemerin in FSH-induced rat granulosa cell steroidogenesis. Endocrinology. 2013;154(2):956–67. doi: 10.1210/en.2012-1836. [DOI] [PubMed] [Google Scholar]
- Welburn SC, Murphy NB. Prohibitin and RACK homologues are up-regulated in trypanosomes induced to undergo apoptosis and in naturally occurring terminally differentiated forms. Cell Death Differ. 1998;5(7):615–22. doi: 10.1038/sj.cdd.4400393. [DOI] [PubMed] [Google Scholar]
- Winter A, Kämäräinen O, Hofmann A. Molecular modeling of prohibitin domains. Proteins. 2007;68(1):353–62. doi: 10.1002/prot.21355. [DOI] [PubMed] [Google Scholar]
- Yong EL, Baird DT, Yates R, Reichert LE, Jr, Hillier SG. Hormonal regulation of the growth and steroidogenic function of human granulosa cells. J Clin Endocrinol Metab. 1992;74(4):842–9. doi: 10.1210/jcem.74.4.1548349. [DOI] [PubMed] [Google Scholar]
- Zoller LC, Weisz J. Identification of cytochrome P-450, and its distribution in the membrana granulosa of the preovulatory follicle, using quantitative cytochemistry. Endocrinology. 1978;103(1):310–3. doi: 10.1210/endo-103-1-310. [DOI] [PubMed] [Google Scholar]
- Zoller LC, Weisz J. A quantitative cytochemical study of glucose-6-phosphate dehydrogenase and delta 5-3 beta-hydroxysteroid dehydrogenase activity in the membrana granulosa of the ovulable type of follicle of the rat. Histochemistry. 1979;62(2):125–35. doi: 10.1007/BF00493314. [DOI] [PubMed] [Google Scholar]