Abstract
Selenium (Se) is an essential micronutrient that can be found at toxic concentrations in surface waters contaminated by runoff from agriculture and coal mining. Zebrafish (Danio rerio) embryos were exposed to aqueous Se in the form of selenate, selenite, and L-selenomethionine (SeMet) in an attempt to determine if oxidative stress plays a role in selenium embryo toxicity. Selenate and selenite exposure did not induce embryo deformities (lordosis and craniofacial malformation). L-selenomethionine, however, induced significantly higher deformity rates at 100 μg/L compared to controls. SeMet exposure induced a dose-dependent increase in the catalytic subunit of glutamate-cysteine ligase (gclc) and reached an 11.7-fold increase at 100 μg/L. SeMet exposure also reduced concentrations of TGSH, RGSH, and the TGSH:GSSG ratio. Pretreatment with 100 μM N-acetylcysteine (NAC) significantly reduced deformities in the zebrafish embryos secondarily treated with 400 μg/L SeMet from approximately 50% to 10% as well as rescued all three of the significant glutathione level differences seen with SeMet alone. Selenite exposure induced a 6.6-fold increase in expression of the glutathione-S-transferase pi class 2 (gstp2) gene, which is involved in xenobiotic transformation and possibly oxidative stress. These results suggest that aqueous exposure to SeMet can induce significant embryonic teratogenesis in zebrafish that are at least partially attributed to oxidative stress.
Introduction
Selenium (Se) is an essential micronutrient necessary for proper function of important antioxidant enzymes including glutathione peroxidases (Eisler 2000). However, Se has a narrow margin between essentiality and toxicity; doses of over 3 mg Se/kg dw, about 7 to 30 times the nutritional concentrations can be toxic in teleosts (Hilton et al. 1980, Janz et al. 2010). For example, rainbow trout require at least 3.5 μg/kg/d dietary inorganic Se but 4.6 mg/kg dw dietary Se as seleno-L-methionine caused significant decreases in body weight and fork length in larval rainbow trout (Hilton et al. 1980, Vidal et al. 2005). Oviparous vertebrates including fish, birds, amphibians, and reptiles are particularly susceptible to Se toxicity because Se is readily transferred from mother to embryo through vitellogenin and other egg yolk proteins (Kroll et al. 1991, Unrine et al. 2006, Bergeron et al. 2010). There are several hypothesized mechanisms of Se toxicity, including sulfur substitution, immune system disruption, and oxidative stress (reviewed by Janz et al. 2010). Recently, oxidative stress has received more attention as a possible mechanism for the teratogenicity associated with Se exposure (Spallholz 1997, Hoffman et al. 1998, Palace et al. 2004, Franson et al. 2007).
Selenium can be found in several different inorganic and organic forms that exhibit various toxicities to fish. Inorganic forms of Se, including selenate or selenite, are more commonly found in surface waters contaminated by Se, such as those effected by agricultural runoff in the San Joaquin valley, CA or mountain top removal coal mining in Appalachia (Presser et al. 1987, Lindberg et al. 2011). Some forms of Se, including diselenides and selenite, are active prooxidants and may combine with glutathione to generate superoxide radicals, indicating that Se toxicity may be a result of oxidative stress (Spallholz 1997). Selenite exposure in rainbow trout (Oncorhynchus mykiss) hepatocytes caused oxidative stress-induced cytotoxicity as measured by increased intracellular reactive oxygen species (ROS) formation, decreased reduced to oxidized glutathione ratio (GSH:GSSG), and increased lipid peroxidation (Misra et al. 2009).
Inorganic Se such as selenite can be biotransformed by algae and bacteria into organic forms including selenomethionine (SeMet), a selenoamino acid, which is considered to be one of the more toxic forms of Se generally (Maher et al. 2010). Extrapolated concentrations of various Se compounds sufficient to cause 5% mortality after a 10-day aqueous exposure revealed that SeMet was more toxic to newly hatched zebrafish compared to selenite and selenate (Niimi et al. 1976). Similar to selenite, oxidative stress was induced in rainbow trout hepatocytes as suggested by increased lipid peroxidation and decreased GSH:GSSG ratios (Misra et al. 2012). Decreased GSH: GSSG ratios were also observed in juvenile rainbow trout exposed to dietary SeMet (Schlenk et al. 2003) and in rainbow trout exposed to aqueous selenite, indicating oxidative stress, although aqueous exposure did not cause changes to levels of lipid peroxidation (Miller et al. 2007). There is evidence that SeMet may be transformed by rainbow trout embryos into methylselenol, which redox cycles with glutathione to produce the superoxide anion radical (Spallholz 1997, Palace et al. 2004, Spallholz et al. 2004). Additional in vitro work provided supporting evidence of SeMet conversion to alternative forms (e.g. methylselenol) capable of producing superoxide via methioninase (Miki et al. 2001, Wang et al. 2002). There is a need, however, for further data exploring the possibility of SeMet-induced oxidative stress in whole organisms.
The production of ROS by selenium species and other xenobiotics can induce the expression of several antioxidant genes (reviewed by Di Giulio and Meyer 2008). For example, ROS have been demonstrated to regulate the expression of glutamate cysteine ligase catalytic subunit (gclc), glutathione-S-transferases (GST), glutathione peroxidase 1 (gpx1), and manganese superoxide dismutase (MnSOD, sod2) (Di Giulio et al. 2008). Gclc is part of a heterodimeric enzyme involved in the synthesis of the tripeptide glutathione (GSH), an important non-enzymatic antioxidant (Franklin et al. 2009). GST pi class 2 (Gstp2) is a cytosolic enzyme important in the phase II detoxification of a variety of xenobiotic chemicals through conjugation with reduced glutathione (Schlenk et al. 2008). Gpx1 is an important antioxidant enzyme found in the cytosol and mitochondrial matrix that contains a selenocysteine moiety in its active site and is capable of reducing excess hydrogen peroxide to water and lipid hydroperoxides to their corresponding alcohols (Jones et al. 1981, Hussain et al. 2004). Sod2 is a mitochondrial antioxidant enzyme that can dismutate superoxide to hydrogen peroxide and water (Oberley et al. 1988). An increased expression of these genes can indicate responses to elevated cellular ROS production.
Although many antioxidant genes contain a regulatory region called the antioxidant response element (ARE) that is responsive to nuclear factor E2-related factor 2 (Nrf2), it is currently unknown if piscine gclc, gpx1, sod2, or gstp2 contain ARE regions. However, there are many examples of homologs to these genes in different species containing ARE-like sequences. Such sequences are found in human, rat, mouse, and zebrafish gstp (Ikeda et al. 2002, Ikeda et al. 2004, Suzuki et al. 2005). Human GCLC can be induced via an ARE sequence (Wild et al. 2000) and human GPX2 and GPX3 contain an ARE (Bierl et al. 2004). Mouse Sod2 also contains an ARE sequence (Jones et al. 1995). It is reasonable to assume that zebrafish gclc, gpx1, sod2, and gstp2 all include at least an ARE-like sequence that is responsive to oxidative stress.
The purpose of this study was to investigate the role of oxidative stress in Se-induced zebrafish (Danio rerio) embryo deformities. Zebrafish are highly valuable to toxicological research because of their rapid development, high fecundity, and fully sequenced genome, which provides the opportunity to conduct analysis on changes in gene expression that can provide insight into the mechanism behind Se embryo toxicity. We attempted to induce deformities in zebrafish embryos using aqueous exposures of several Se compounds, including selenate, selenite, and L-selenomethionine. N-acetylcysteine (NAC) was used in an attempt to rescue Se-induced deformities. N-acetylcysteine is an antioxidant shown to increase reduced glutathione (GSH) and scavenge oxidant species (Moldeus et al. 1986). Treatment with NAC has previously been demonstrated to increase GSH in rabbit hearts and increase expression of GSSG-reductase in rat liver and lung cells, indicating that it can increase GSH regeneration (Deflora et al. 1985, Ceconi et al. 1988). Concentrations of variously oxidized glutathione types were measured in zebrafish embryos exposed to Se with and without pre-treatment with NAC. Finally, gene expression analysis was performed on several genes involved in the oxidative stress response. Our results suggest that oxidative stress may be at least part of the mechanism of SeMet-induced deformities in zebrafish embryos.
Materials and methods
Chemicals
Sodium selenite and L-selenomethionine were purchased from Acros Organics (Thermo Fisher Scientific, Fair Law, NJ). Sodium selenate was purchased from Alfa Aesar (Ward Hill, MA). N-acetylcysteine was purchased from Sigma-Aldrich, LLC. (St. Louis, MO).
Zebrafish
EkkWill wildtype zebrafish (EkkWill Waterlife Resources, Ruskin, FL) were maintained in an Aquatic Habitats system (Aquatic Habitats, Apopka, FL) at 28°C on a 14:10 light:dark cycle in 60 mg/L salinity water (Instant Ocean, Foster & Smith, Rhinelander, WI). The adult zebrafish were fed a diet of newly hatched brine shrimp, Ziegler's Adult Zebrafish food (Aquatic Habitats), and Cyclop-eeze (Argent Chemical Laboratories, WA). Adults were placed in breeding chambers overnight and allowed to breed in the morning. Embryos were collected from the breeding chambers and screened for viability and cell stage. Healthy embryos that successfully reached the 8–32 cell stage (e.g. within approximately 2 hours of fertilization) were selected to use in dosing experiments. Embryos were rinsed with 30% Danieau media before treatment. All fish husbandry and experimental procedures were approved by the Duke University Institutional Animal Care and Use Committee via protocol A184-13-07.
Aqueous selenium zebrafish embryo exposures
All dosing solutions were prepared in 30% Danieau media and were vortexed immediately before dosing to ensure homogenous mixture and oxygen saturation. Zebrafish embryos were treated with control, 1, 50, 100, or 400 μg/L SeMet. In a separate dosing experiment 8–32 cell stage embryos were exposed to control, 30, 45, or 60 μg/L selenate (Na2SeO4) or selenite (Na2SeO3). Doses of selenate and selenite were chosen to reflect concentrations found in aquatic ecosystems (e.g. Lemly 2002, Lindberg et al. 2011). Selenium exposures were conducted in triplicate for each treatment condition using 6-well plastic plates. Zebrafish embryos (12–30 per well) were treated with 4 mL of dosing media and stored in an incubator at 28°C on a 14:10 light:dark cycle until analysis. The same number of embryos were used for each treatment (e.g., 12 in a negative control well and 12 in a Se-treated well) but this number varied between experiments (e.g., in one experimental replicate 12 embryos were used in each well and in another experimental replicate 30 embryos were used in each well) due to differences in embryo availability. Although the density of embryos could affect oxygen concentration and result in hypoxia-induced deformity (Küster and Altenberger 2008; Strecker et al. 2011), no increased mortality or deformity was observed in control wells in any experimental replicate, indicating that oxygen was sufficient.
Embryos were screened for toxicity at 48 hours post fertilization (hpf) using a Nikon SMZ 1500 microscope equipped with a Nikon Sight DS-Fi1 camera and NIS elements F4.00.06 imaging software (Nikon, Melville, NY, USA). Dead embryos were removed from wells. Embryos were counted as “deformed” if they had malformations of the spine (lordosis, kyphosis), pericardial edema, eye malformations, and/or craniofacial deformities.
Quantitative real-time PCR
Twelve to 30 zebrafish embryos exposed to selenate, selenite, or L-selenomethionine as previously described were manually dechorionated at 48 hpf using mild suction from a small transfer pipette and stored at −80°C until processing. Embryos were homongenized and mRNA was isolated from samples using RNA-Bee/chloroform extraction (Tel-test, Inc, Friendswood, TX) or a Direct-zol RNA MiniPrep kit (Zymo Research, Irvine, CA). mRNA quality and concentration were verified using a NanoDrop ND-100 (NanoDrop Technologies, Wilmington, DE). cDNA was synthesized using an Omniscript RT kit (Qiagen, Valencia, CA). Quantitative real-time PCR using SYBR Green Lightcycler Master Mix (Life Technologies, Carlsbad, CA) was conducted on an ABI 7300 quantitative real-time PCR machine. A thermal cycle of 10 minutes at 95°C, followed by 35 cycles of 15 seconds at 95°C, 1 minute at 60°C, and finally a dissociation curve. Samples were run in duplicate.
Primers for gpx1, gclc, gstp2, actb, and sod2 were published previously (Malek et al. 2004, Grimes et al. 2008, Van Tiem et al. 2011) and primer sequences and GenBank ID numbers are shown in Table 1. Quantitative real-time PCR data were analyzed on the ABI PRISM 7300 Sequence Detection System, Version 1.1 (Applied Biosystems, Inc., Foster City, CA). Average fold induction of mRNA was determined using the ddCT method (Livak et al. 2001) by comparing the target genes’ threshold cycles (CT) to those of the reference genes actb for the selenate/selenite exposures or eif1b for the SeMet exposures.
Table 1.
Primers used for zebrafish (Danio rerio) quantitative real time polymerase chain reaction assay
| Gene | GenBank ID | Direction | Sequence (5’-3’) |
|---|---|---|---|
| actb | AF057040 | Forward | AAG ATC AAG ATC ATT GCT CCC |
| Reverse | CCA GAC TCA TCG TAC TCC T | ||
| eif1b | NM_199588 | Forward | GCC TTC AAG AAG AAA TTT GCC |
| Reverse | CCG TGG ACT TTG AGC TG | ||
| gstp2 | AB194128 | Forward | TCT GGA CTC TTT CCC GTC TCT CAA |
| Reverse | ATT CAC TGT TTG CCG TTG CCG | ||
| sod2 | Y12236 | Forward | CTA GCC CGC TGA CAT TAC ATC |
| Reverse | GAG CGG AAG ATT GAG GAT TG | ||
| gpx1 | AW232474 | Forward | AGA TGT CAT TCC TGC ACA CG |
| Reverse | AAG GAG AAG CTT CCT CAG CC | ||
| gclc | NM_199277 | Forward | AAG TGG ATG AGG GAG TTT GTT GCC |
| Reverse | CTT GTG GAG CAG GTC GTA GTT GAT |
Antioxidant rescue
Zebrafish embryos were collected and screened as described previously. The 100 μM dose of NAC was chosen based on preliminary studies as a high dose that was non-lethal and did not induce deformities in zebrafish embryos (unpublished data). Five to 20 embryos per replicate were pretreated in 5 mL of either 100 μM NAC or control in 65 mm diameter glass petri dishes using the same number of embryos for control and treated plates within an experiment. Embryos were incubated at 28°C overnight for an NAC exposure period of approximately 24 hours, after which they were thoroughly rinsed with 30% Danieau before being transferred into clean petri dishes with 5 mL of either 400 μg/L SeMet or control media (30% Danieau), to create the following treatments: control, 100 μM NAC, 400 μg/L SeMet, and 100 μM NAC+ 400 μg/L SeMet. The exposure dose of 400 μg/L SeMet was chosen because it caused a significant amount of deformities without mortality while the 100 μM NAC dose was chosen as a non-lethal concentration that was effective at rescuing deformities. Embryos were screened at 72 hpf for toxicity as described previously after the embryos incubated for 48 hours in the SeMet treatments. Each treatment group was replicated three times and the entire dosing experiment was repeated four times.
Glutathione levels
Variously oxidized glutathione types including total glutathione (TGSH), reduced glutathione (RGSH) and oxidized glutathione (GSSG) were measured spectrophotometrically as described by Hermes-Lima and Storey (1996) and Massarsky et al. (2013). Zebrafish embryos in groups of approximately 25 embryos were dosed as above for the NAC rescue experiment and flash frozen at 72 hpf. Groups were then homogenized by probe sonication in 5% sulfosalicylic acid, centrifuged at 5000 g for 5 min at 4°C and the supernatant split to measure TGSH and GSSG. TGSH was measured at 412 nm over 10 minutes following the addition of 5,5-dithiobis-2-nitrobenzoic acid (DTNB), NADPH, and glutathione reductase. GSSG was similarly measured after 1.5 hr incubation with 2-vinylpyridine and NaOH and was measured against a GSSG standard curve. Reduced glutathione was calculated from the equation of RGSH = TGSH – 2GSSG. All glutathione levels are presented as pmol of glutathione per larva. The ratio of total or reduced to oxidized glutathione (or vice versa) is often used as measure of oxidative stress. The ratio of TGSH:GSSG is presented here with lower ratios indicative of more oxidative conditions.
Statistics
The proportion of deformed embryos in each treatment was calculated using the number of embryos left alive and deformed in each well. All results are reported as mean ± standard error. Statistical analysis was performed using SigmaPlot 12.5 (Systat Software Inc., San Jose, CA) and GraphPad Prism 6 (La Jolla, CA). Selenate, selenite, and SeMet exposure mortality and deformity data (as expressed in proportion dead or deformed) was analyzed using a Kruskal-Wallis one-way analysis of variance (ANOVA) by ranks followed by a Dunn's multiple comparisons test. A two-way ANOVA was performed on the mortality and deformity values and one-way ANOVAs were performed within each glutathione type for the antioxidant rescue experiments (α = 0.05).
A four parameter nonlinear dose-response analysis without constraints was used in GraphPad Prism 6 to determine the EC50 for the SeMet exposures. Fold changes in gene expression ddCT values were compared using an unpaired t-test.
Results
Selenium induced deformities in zebrafish embryos
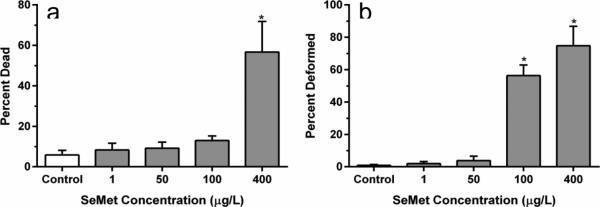
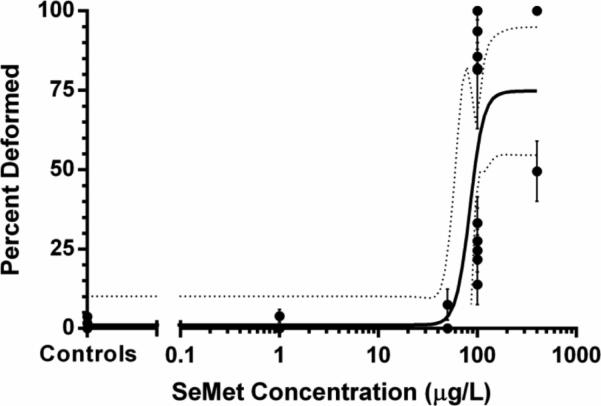
Mortality was significantly different between treatment groups in the SeMet dosed embryos (H = 19.384, p < 0.001) with 100 and 400 μg/L SeMet exposures resulting in significantly higher mortality compared to controls (p < 0.05, Figure 1A). Zebrafish embryos exposed to the highest two doses of SeMet also exhibited significantly increased incidences of lordosis and craniofacial deformities (Figures 1B, 2A-C, H = 19.384, p < 0.001). For example, at 100 μg/L and 400 μg/L SeMet, 56 ± 7% and 74 ± 5% of embryos exhibited deformities, respectively, which were significantly higher than the deformity rate of 0.9 ± 0.5% in controls (p < 0.05, Figure 1B). An EC50 value of 84 μg/L (95% confidence interval of 57–123 μg/L) was calculated for SeMet for zebrafish embryo deformities (Figure 3) based on the fitted equation:
Fig. 1.
Percentage of 48 hpf zebrafish embryos dead (a) and deformed (b) after treatment with L-selenomethionine (SeMet). Error bars are standard error and asterisks represent significant differences from controls (P<0.05)
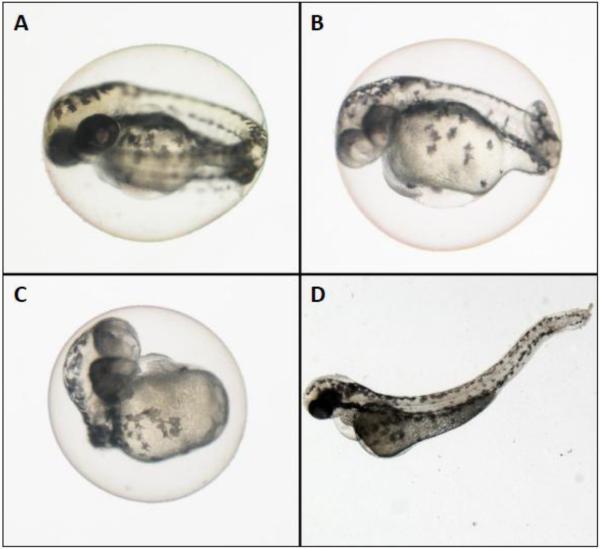
Fig. 2.
Zebrafish (Danio rerio) embryos A. control (48 hpf) B. 100 μg/L L-selenomethionine (48 hpf) exposed embryo with craniofacial and tail deformities with pericardial edema C. 100 μg/L L-selenomethionine (48 hpf) exposed embryo with significant deformity D. 30 μg/L selenite (72 hpf) exposed embryo with lordosis and pericardial edema
Fig. 3.
Dose response curve for deformities induced in zebrafish embryos by L-selenomethionine exposure. Dotted lines represent the 95% confidence interval. Calculated EC50 = 84 μg/L
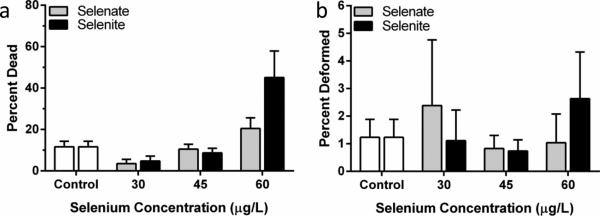
Mortality differed significantly between selenate and selenite treatments (Kruskal-Wallis, H = 18.898, p = 0.004) although further analysis with a post-hoc test using Dunn's Method showed no significant differences between Se treated embryos compared to controls (Figure 4A). Craniofacial and skeletal deformities were occasionally identified in zebrafish embryos exposed to aqueous selenate (maximum average 2.4 ± 2%) and selenite (maximum average 2.6 ± 2%, Figure 2D). However, the frequencies of these deformities were not significantly different from those of the controls (1.2 ± 0.7%) at any doses (p = 0.316, Figure 4B).
Fig. 4.
Percentage of 48 hpf zebrafish embryos dead (a) and deformed (b) after treatment with various concentrations of selenate and selenite. Error bars represent standard error.
While we observed no detectable effects of selenite or selenate on embryonic zebrafish development, SeMet exposure induced a high frequency of severe deformities. Since the detrimental effects of SeMet were not observed with NAC-pretreated embryos, we attributed at least some portion of the observed toxicity to oxidative stress. Aqueous exposures to either selenate or selenite did not lead to an increase in craniofacial and skeletal deformities relative to controls. In contrast, aqueous SeMet exposure induced a significant number of deformities in zebrafish embryos with a calculated EC50 concentration of 84 μg/L. A dose-dependent increase in deformities was observed with increasing doses of SeMet. Deformities observed in embryos dosed with 100 or 400 μg/L SeMet were often severe, and included a total lack of head development and/or significant truncation of the spine and tail.
Gene expression
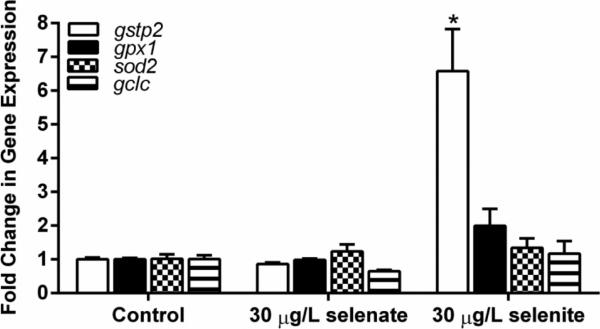
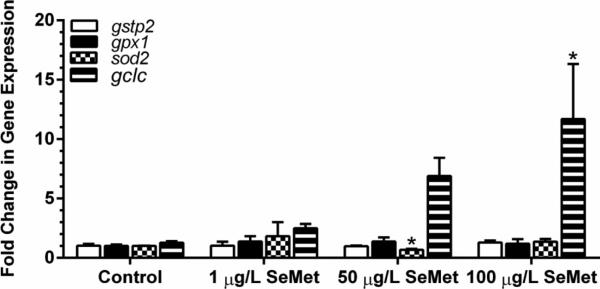
Gene expression analyses of oxidative stress responsive genes in embryos exposed to 30 μg/L aqueous selenite showed a 7 ± 1 fold increase in expression of gstp2 compared to controls. sod2, gpx1, and gclc expression was not increased in selenite exposed embryos (Figure 5). Embryos exposed to 30 μg/L aqueous selenate did not show changes in expression for any of the genes analyzed. sod2 showed a small but significant decrease (0.67 ± 0.09-fold) in expression in zebrafish embryos exposed to 50 μg/L SeMet (Figure 6, p < 0.05). The expression of gclc showed a clear increasing dose-response with exposure to SeMet, resulting in a significant 11.7 ± 4.6-fold increase (P < 0.05) at 100 μg/L SeMet.
Fig. 5.
Fold change in gene expression for embryos treated with control, selenate, or selenite aqueous exposures. Asterisk indicates significance (p ≤ 0.05) from respective control. Error bars represent standard error
Fig. 6.
Fold change in gene expression in embryos treated with control or L-selenomethionine (SeMet) aqueous exposures. Asterisk indicates significance (p ≤ 0.05) from respective control. Error bars represent standard error
Antioxidant rescue with N-acetylcysteine
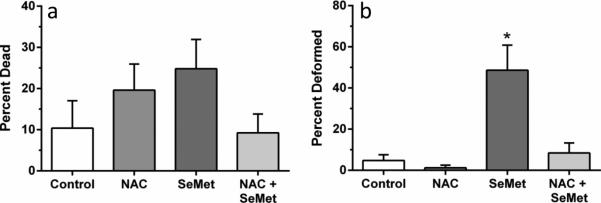
No significant differences in mortality were observed between treatment groups (p = 0.389, Figure 7A). Significant differences in the proportion of deformities were observed between embryos exposed to NAC in combination with SeMet versus embryos treated with SeMet alone (p < 0.001, Figure 7B). Pretreatment with NAC was protective against SeMet induced embryo deformities; embryos pretreated with NAC showed a frequency of deformity that was indistinguishable from controls while embryos without the pretreatment exposed to SeMet alone showed an average deformity frequency of almost 50% compared to approximately 10% with NAC pretreatment. No significant difference in deformities was observed between control embryos and NAC only treated embryos. Deformities in the co-exposed NAC + SeMet treatment groups were frequently mild (slight lordosis or craniofacial deformity, small pericardial edema) while the SeMet alone exposed embryos showed deformities ranging from mild to severe (lack of trunk/tail development, significant craniofacial deformity and pericardia edema).
Fig. 7.
Percentage of 72 hpf zebrafish embryos dead (a) and deformed (b) after treatment with 100 uM N-acetylcysteine (NAC) and/or 400 μg/L L-selenomethionine (SeMet). Error bars are standard error. Asterisk represents significant difference from all other treatments (p<0.001)
Glutathione
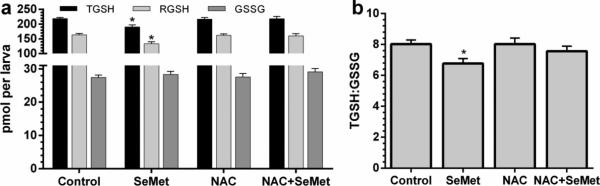
Treatment of zebrafish embryos with 400 μg/L SeMet resulted in a significant decrease in TGSH, RGSH, and TGSH:GSSG ratio compared to all other treatments (all p < 0.05), but no significant difference in GSSG level (Figure 8). These decreases in glutathione levels were rescued by the pretreatment with NAC as no significant differences were observed between NAC+SeMet treatment and controls.
Fig. 8.
Glutathione levels in zebrafish embryos at 72 hpf (a) including total glutathione (TGSH), reduced glutathione (RGSH), and oxidized glutathione (GSSG). The ratio of TGSH:GSSG is plotted in (b). Error bars are standard error. Asterisks represent significant difference from all other treatments within glutathione type
Discussion
In order to explore the role of oxidative stress in zebrafish embryo selenium deformities, we performed toxicity assays, measured oxidative stress gene expression and glutathione levels, and performed rescue experiments with the antioxidant NAC. We showed significantly elevated rates of deformities for organic SeMet but not inorganic selenate or selenite. SeMet exposure also resulted in significantly increased expression of gclc at 100 μg/L and significantly reduced concentrations of TGSH, RGSH, and the TGSH:GSSG ratio. Pretreatment of embryos with the antioxidant NAC before SeMet exposure rescued embryos from deformities as well as rescued concentrations of TGSH and, RGSH, and the TGSH:GSSG ratio. These results support the theory that oxidative stress plays a role in the deformities caused by exposure to the organic selenium species, selenomethionine.
A clear difference was observed between the teratogenicity of organic and inorganic compounds. Significant rates of deformities were observed in zebrafish embryos exposed to aqueous SeMet but not selenate or selenite. SeMet exposure induced significantly higher rates of deformities than control at 100 and 400 μg/L and resulted in a calculated deformity EC50 of 84 μg/L, or approximately 34 μg/L Se by weight. At 48 hpf exposure to 400 μg/L SeMet resulted in 56% mortality and 74% of those remaining alive being severely deformed with craniofacial abnormalities, pronounced spinal curvature, and/or extreme pericardial edema. Alternatively, inorganic selenate and selenite induced average deformity rates only up to 2.6% at 60 μg/L selenite and no treatments were significantly different than controls. Concentrations of inorganic Se higher than 60 μg/L were tested in preliminary range finding experiments (data not shown), but high rates of mortality at 48 hpf left only a few embryos to score for deformities, and so higher concentrations were not used.
The expressions of four genes involved with the oxidative stress response and xenobiotic transformation were measured: gclc, gpx1, sod2, and gstp2. Selenite exposure significantly increased the expression of gstp2 6.6-fold over control embryos. Although it is not currently known whether zebrafish gstp2 contains an antioxidant response element, the closely homologous zebrafish gstp1 does contain an ARE-like sequence that is regulated by Nrf2 (Suzuki et al. 2005), suggesting that gstp2 expression may be sensitive to changes in redox status. The expression of other oxidative stress response genes did not show significant changes compared to controls for aqueous selenate or selenite. SeMet exposure elicited a small but significant decrease in expression of sod2 in embryos exposed to 50 μg/L SeMet. Limited previous data exist for changes in sod2 expression in fish exposed to Se. Expression of sod2 in macrophages and rat hepatocytes varied with selenite exposure depending on the tissue type and species tested (Shilo et al. 2004, Shilo et al. 2008).
Expression of gclc in zebrafish embryos exposed to 100 μg/L SeMet was significantly increased 11.7-fold in zebrafish embryos. gclc expression has previously been demonstrated to increase in zebrafish embryos upon exposure to the pro-oxidant tert-butylhydroperoxide, the aryl hydrocarbon receptor agonist β-napthaflavone, and fullerene C60 (Timme-Laragy et al. 2009, Usenko et al. 2009). Changes in gclc expression have been shown to parallel changes in the GCL protein, which is important in the biosynthesis of glutathione (Franklin et al. 2009; Krzywanski et al. 2004).
Total glutathione and reduced glutathione were measured and the ratio of TGSH:GSSG calculated as an index of oxidative status of the embryo (Di Giulio and Meyer 2008). Zebrafish embryos exposed to 400 μg/L SeMet had significantly lower concentrations of total and reduced glutathione as well as a significantly lower ratio of TGSH:GSSG compared to controls (Figure 8). Oxidized glutathione (GSSG), however, was not significantly altered by SeMet treatment. Selenomethionine may be oxidized to selenoxide as seen in Japanese medaka (Oryzias latipes) embryos, which resulted in a depletion of glutathione following aqueous exposure (Lavado et al. 2012). Our results resemble those of mallard ducks (Anas platyrhynchos) fed selenomethionine that showed a dose-dependent increase in the hepatic ratio of GSSG:GSH (equivalent to a decrease in GSH:GSSG) and an increase in hydroperoxides that cause lipid peroxidation (Heinz et al. 1988, Hoffman et al. 1998). Misra et al (2012) saw the same dose-dependent trend towards a more oxidative cellular environment (lower GSH:GSSG) in isolated rainbow trout (Oncorhynchus mykiss) hepatocytes. The reduction of TGSH with SeMet exposure in zebrafish embryos could be leading to the significant increase in gclc expression. Glutamate cysteine ligase is the rate limiting enzyme in GSH biosynthesis and expression of its component parts (i.e. gclc) is at least partially controlled by GSH concentration (Franklin et al. 2009; Krzywanski et al. 2004).
We have shown selenomethionine to be teratogenic to zebrafish embryos, significantly increase gclc expression, and significantly alter glutathione levels to reflect a more oxidized cellular environment. We used 24 hour N-acetylcysteine pretreatment in an attempt to rescue SeMet's teratogenicity and glutathione impact. NAC pretreatment rescued zebrafish embryos from deformities seen with SeMet alone (Figure 7b) and qualitatively decreased the severity of those deformities (data not shown). NAC pretreatment also rescued the TGSH, RGSH, and the ratio of TGSH:GSSG to control levels. Pretreatment with NAC alone did not result in any significant difference, contrary to other reports of increased GSH (i.e. Timme-Laragy et al. 2009). These results indicate that NAC may have prevented oxidative stress in SeMet-exposed embryos by scavenging free radicals, although additional experiments should be done to demonstrate the production of free radicals in SeMet-exposed embryos. The rescue of these SeMet-induced glutathione deficiencies by NAC pretreatment further supports the idea that oxidative stress is a major mechanism of Se toxicity.
There is considerable previous research demonstrating that Se exposure can cause significant developmental defects in fish embryos (Teh et al. 2004, Holm et al. 2005, Muscatello et al. 2006), and that oxidative stress may be an important mechanism behind this teratogenicity. The evidence for the role of oxidative stress in Se-induced embryo toxicity, however, remains conflicted. Kupsco et al. (2014) found no evidence of lipid peroxidation in Japanese medaka exposed to aqueous SeMet and hypersalinity, and they suggested that other mechanisms such as activation of the unfolded protein response may play a role in the 100% deformity rate observed in the medaka embryos. Vidal et al. 2005 found that dietary SeMet exposure in larval rainbow trout did not cause changes in lipid peroxidation or GSH:GSSG ratios, which did not support the oxidative stress hypothesis. Those data, in addition to the results presented here, suggest that Se-induced embryo toxicity may be caused by multiple mechanisms possibly including substitution-based protein malfunction, oxidative stress, unfolded protein response, and immune dysfunction (reviewed by Janz et al. 2010). Our NAC rescue of SeMet-induced toxicity supports the hypothesis that that aqueous exposure to SeMet causes oxidative stress in zebrafish embryos, resulting in developmental defects not seen with exposure to selenate or selenite. Further work should be done to measure free radical production from SeMet exposure to support the oxidative stress hypothesis.
Acknowledgements
We would like to thank Drs. D. Hinton, A. Massarsky and A. Bone, T. Lindberg, J. Brandt, and M. Chernick for assistance and advice. This work was made possible by a grant to The Duke University Nicholas School of the Environment from the Foundation for the Carolinas, as well as the National Institute of Environmental Health Science grant T32ES07031 to support the Duke Integrated Toxicology and Environmental Health Program.
References
- Bergeron CM, Bodinof CM, Unrine JM, Hopkins WA. Bioaccumulation and maternal transfer of mercury and selenium in amphibians. Environ Toxicol Chem. 2010;29:989–997. doi: 10.1002/etc.125. doi:Doi 10.1002/Etc.125. [DOI] [PubMed] [Google Scholar]
- Bierl C, Voetsch B, Jin RC, Handy DE, Loscalzo J. Determinants of human plasma glutathione peroxidase (GPx-3) expression. J Biol Chem. 2004;279:26839–26845. doi: 10.1074/jbc.M401907200. doi:DOI 10.1074/jbc.M401907200. [DOI] [PubMed] [Google Scholar]
- Ceconi C, Curello S, Cargnoni A, Ferrari R, Albertini A, Visioli O. The role of glutathione status in the protection against ischemic and reperfusion damage - effects of N-acetyl cysteine. J Molec and Cell Cardiol. 1988;20:5–13. doi: 10.1016/s0022-2828(88)80174-3. doi:Doi 10.1016/S0022-2828(88)80174-3. [DOI] [PubMed] [Google Scholar]
- Deflora S, Bennicelli C, Camoirano A, et al. In vivo effects of N-acetylcysteine on glutathione metabolism and on the biotransformation of carcinogenic and or mutagenic compounds. Carcinogenesis. 1985;6:1735–1745. doi: 10.1093/carcin/6.12.1735. [DOI] [PubMed] [Google Scholar]
- Di Giulio RT, Meyer JN. Reactive oxygen species and oxidative stress. In: Di Giulio RT, Hinton DE, editors. The Toxicology of Fishes. CRC Press; Boca Raton: 2008. pp. 273–324. [Google Scholar]
- Eisler R. Handbook of chemical risk assessment: Health hazards to humans, plants, and animals. Boca Raton: 2000. [Google Scholar]
- Franklin CC, Backos DS, Mohar I, White CC, Forman HJ, Kavanagh TJ. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol Aspects Med. 2009;30:86–98. doi: 10.1016/j.mam.2008.08.009. doi:DOI 10.1016/j.mam.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franson JC, Hoffman DJ, Wells-Berlin A, et al. Effects of dietary selenium on tissue concentrations, pathology, oxidative stress, and immune function in common eiders (Somateria mollissima). J Toxicol Environ Health A. 2007;70:861–874. doi: 10.1080/15287390701212760. doi:Doi 10.1080/15287390701212760. [DOI] [PubMed] [Google Scholar]
- Grimes AC, Erwin KN, Stadt HA, Hunter GL, Gefroh HA, Tsai HJ, Kirby ML. PCB126 exposure disrupts zebrafish ventricular and branchial but not early neural crest development. Toxicol Sciences. 2008;106:193–205. doi: 10.1093/toxsci/kfn154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CC. p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res. 2004;64:2350–2356. doi: 10.1158/0008-5472.can-2287-2. [DOI] [PubMed] [Google Scholar]
- Heinz GH, Hoffman DJ, Gold LG. Toxicity of organic and inorganic selenium to mallard ducklings. Arch Environ Contam Toxicol. 1988;17:561–568. doi: 10.1007/BF01055823. [DOI] [PubMed] [Google Scholar]
- Hermes-Lima M, Storey KB. Relationship between anoxia exposure and antioxidant status inthe frog Rana pipiens. Am J Physiol. 1996;217:918–925. doi: 10.1152/ajpregu.1996.271.4.R918. [DOI] [PubMed] [Google Scholar]
- Hilton JW, Hodson PV, Slinger SJ. The requirement and toxicity of selenium in rainbow trout (Salmo gairdneri). J Nutr. 1980;110:2527–2535. doi: 10.1093/jn/110.12.2527. [DOI] [PubMed] [Google Scholar]
- Hoffman DJ, Heinz GH. Effects of mercury and selenium on glutathione metabolism and oxidative stress in Mallard ducks. Environ Toxicol Chem. 1998;17:161–166. [Google Scholar]
- Holm J, Palace V, Siwik P, et al. Developmental effects of bioaccumulated selenium in eggs and larvae of two salmonid species. Environ Toxicol Chem. 2005;24:2373–2381. doi: 10.1897/04-402r1.1. doi:Doi 10.1897/04-402r1.1. [DOI] [PubMed] [Google Scholar]
- Hussain SP, Amstad P, He PJ, et al. p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res. 2004;64:2350–2356. doi: 10.1158/0008-5472.can-2287-2. doi:Doi 10.1158/0008-5472.Can-2287-2. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Nishi S, Sakai M. Transcription factor Nrf2/MafK regulates rat placental glutathione S-transferase gene during hepatocarcinogenesis. Biochem J. 2004;380:515–521. doi: 10.1042/BJ20031948. doi:10.1042/BJ20031948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Serria MS, Kakizaki I, et al. Activation of mouse Pi-class glutathione S-transferase gene by Nrf2(NF-E2-related factor 2) and androgen. Biochem J. 2002;364:563–570. doi: 10.1042/BJ20011756. doi:10.1042/BJ20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz DM, DeForest DK, Brooks ML, et al. Selenium toxicity to aquatic oganisms. In: Chapman PM, Adams JB, Brooks ML, et al., editors. Ecological Assessment of Selenium in the Aquatic Environment. CRC Press; Boca Raton: 2010. pp. 141–232. [Google Scholar]
- Jones DP, Eklow L, Thor H, Orrenius S. Metabolism of hydrogen-peroxide in isolated hepatocytes - relative contributions of catalase and glutathione-peroxidase in decomposition of endogenously generated H2O2. Arch Biochem Biophys. 1981;210:505–516. doi: 10.1016/0003-9861(81)90215-0. doi:Doi 10.1016/0003-9861(81)90215-0. [DOI] [PubMed] [Google Scholar]
- Jones PL, Kucera G, Gordon H, Boss JM. Cloning and characterization of the murine manganous superoxide dismutase-encoding gene. Gene. 1995;153:155–161. doi: 10.1016/0378-1119(94)00666-g. doi:037811199400666G [pii] [DOI] [PubMed] [Google Scholar]
- Kleinow KM, Brooks AS. Selenium compounds in the fathead minnow (Pimephales promelas)–I. Uptake, distribution, and elimination of orally administered selenate, selenite and l-selenomethionine. Comp Biochem Phys C. 1986;83:61–69. doi: 10.1016/0742-8413(86)90013-7. [DOI] [PubMed] [Google Scholar]
- Kroll KJ, Doroshov SI. Williot P, editor. Vitellogenin: Potential vehicle for selenium bioaccumulation in oocytes of the white sturgeon (Acipenser transmontanus). Acipenser. CEMAGREF, Borgeaux. 1991:99–106. [Google Scholar]
- Krzywanski DM, Dickinson DA, Iles KE, Wigley AF, Franklin CC, Liu RM, Kavanagh TJ, Forman HJ. Variable regulation of glutamate cysteine ligase subunit proteins affects glutathione biosynthesis in response to oxidative stress. Arch Biochem Biophys. 2004;423:116–125. doi: 10.1016/j.abb.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Kupsco A, Schlenk D. Mechanisms of selenomethionine developmental toxicity and the impacts of combined hypersaline conditions on Japanese medaka (Oryzias latipes). Environ Sci Technol. 2014;48:7062–7068. doi: 10.1021/es5019948. doi:10.1021/es5019948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küster E, Altenburger R. Oxygen decline in biotesting of environmental samples–Is there a need for consideration in the acute zebrafish embryo test? Environ Toxicol. 2008;23:745–750. doi: 10.1002/tox.20377. doi:10.1002/tox.20377. [DOI] [PubMed] [Google Scholar]
- Lavado R, Shi DL, Schlenk D. Effects of salinity on the toxicity and biotransformation of L-selenomethionine in Japanese medaka (Oryzias latipes) embryos: Mechanisms of oxidative stress. Aquat Toxicol. 2012;108:18–22. doi: 10.1016/j.aquatox.2011.07.001. doi:DOI 10.1016/j.aquatox.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Lemly AD. Selenium Assessment in Aquatic Ecosystems: A Guide for hazard evaluation and water quality criteria. Springer; New York: 2002. [Google Scholar]
- Lindberg TT, Bernhardt ES, Bier R, et al. Cumulative impacts of mountaintop mining on an Appalachian watershed. Proc Natl Acad Sci U S A. 2011;108:20929–20934. doi: 10.1073/pnas.1112381108. doi:DOI 10.1073/pnas.1112381108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. doi:10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maher WA, Roach A, Doblin MA, et al. Environmental sources, speciation, and partitioning of selenium. In: Chapman PM, Adams WJ, Brooks ML, et al., editors. Ecological Assessment of Selenium in the Aquatic Environment. CRC Press; Boca Raton: 2010. pp. 47–92. [Google Scholar]
- Malek RL, Sajadi H, Abraham J, Grundy MA, Gerhard GS. The effects of temperature reduction on gene expression and oxidative stress in skeletal muscle from adult zebrafish. Comp Biochem Phys C. 2004;138:363–373. doi: 10.1016/j.cca.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Massarsky A, Dupuis L, Taylor J, Eisa-Beygi S, Strek L, Trudeau VL, Moon TW. Assessment of nanosilver toxicity during zebrafish (Danio rerio) development. Chemosphere. 2013;92:59–66. doi: 10.1016/j.chemosphere.2013.02.060. [DOI] [PubMed] [Google Scholar]
- Miki K, Xu M, Gupta A, Ba Y, Tan Y, Al-Refaie W, Bouvet M, Makuuchi M, Moossa AR, Hoffman RM. Methioninase cancer gene therapy with selenomethionine as suicide prodrug substrate. Cancer Res. 2001;61:6805–6810. [PubMed] [Google Scholar]
- Miller LL, Wang F, Palace VP, Hontela A. Effects of acute and subchronic exposures to waterborne selenite on the physiological stress response and oxidative stress indicators in juvenile rainbow trout. Aquat Toxicol. 2007;83:263–271. doi: 10.1016/j.aquatox.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Misra S, Hamilton C, Niyogi S. Induction of oxidative stress by selenomethionine in isolated hepatocytes of rainbow trout (Oncorhynchus mykiss). Toxicol In Vitro. 2012;26:621–629. doi: 10.1016/j.tiv.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Misra S, Niyogi S. Selenite causes cytotoxicity in rainbow trout (Oncorhynchus mykiss) hepatocytes by inducing oxidative stress. Toxicol In Vitro. 2009;23:1249–1258. doi: 10.1016/j.tiv.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Moldeus P, Cotgreave IA, Berggren M. Lung Protection by a Thiol-Containing Antioxidant - N-Acetylcysteine. Respiration. 1986;50:31–42. doi: 10.1159/000195086. [DOI] [PubMed] [Google Scholar]
- Muscatello JR, Bennett PM, Himbeault KT, Belknap AM, Janz DM. Larval deformities associated with selenium accumulation in northern pike (Esox lucius) exposed to metal mining effluent. Environ Sci Technol. 2006;40:6506–6512. doi: 10.1021/es060661h. [DOI] [PubMed] [Google Scholar]
- Niimi AJ, LaHam QN. Relative toxicity of organic and inorganic compounds of selenium to newly hatched zebrafish (Brachydanio rerio). Can J Zool. 1976;54:501–509. doi: 10.1139/z76-056. [DOI] [PubMed] [Google Scholar]
- Oberley LW, Oberley TD. Role of antioxidant enzymes in cell Immortalization and transformation. Mol Cell Biochem. 1988;84:147–153. doi: 10.1007/BF00421049. doi:Doi 10.1007/Bf00421049. [DOI] [PubMed] [Google Scholar]
- Palace VP, Spallholz JE, Holm J, Wautier K, Evans RE, Baron CL. Metabolism of selenomethionine by rainbow trout (Oncorhynchus mykiss) embryos can generate oxidative stress. Ecotoxicol Environ Saf. 2004;58:17–21. doi: 10.1016/j.ecoenv.2003.08.019. doi:10.1016/j.ecoenv.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Palmer MA, Bernhardt ES, Schlesinger WH, Eshleman KN, Foufoula-Georgiou E, Hendryx MS, Lemly AD, Likens GE, Loucks OL, Power ME, White PS, Wilcock PR. Science and regulation. Mountaintop mining consequences. Science. 2010;327:148–149. doi: 10.1126/science.1180543. [DOI] [PubMed] [Google Scholar]
- Presser TS, Ohlendorf HM. Biogeochemical Cycling of Selenium in the San-Joaquin Valley, California, USA. J Environ Manage. 1987;11:805–821. doi:Doi 10.1007/Bf01867247. [Google Scholar]
- Schlenk D, Celander M, Gallagher EP, et al. Biotransformation in Fishes. In: Di Giulio R, Hinton DE, editors. The Toxicology of Fishes. CRC Press; Boca Raton: 2008. pp. 153–234. [Google Scholar]
- Schlenk D, Zubcov N, Zubcov E. Effects of salinity on the uptake, biotransformation, and toxicity of dietary seleno-L-methionine to rainbow trout. Toxicol Sciences. 2003;75:309–313. doi: 10.1093/toxsci/kfg184. [DOI] [PubMed] [Google Scholar]
- Shilo S, Aharoni-Simon M, Tirosh O. Selenium attenuates expression of MnSOD and Uncoupling Protein 2 in J774.2 macrophages: Molecular mechanism for its cell-death and antiinflammatory activity. Antioxid Redox Signal. 2004;7:276–286. doi: 10.1089/ars.2005.7.276. [DOI] [PubMed] [Google Scholar]
- Shilo S, Pardo M, Aharoni-Simon M, Glibter S, Tirosh O. Selenium supplementation increases liver MnSOD expression: Molecular mechanism for hepato-protection. J Inorg Biochem. 2008;102:110–118. doi: 10.1016/j.jinorgbio.2007.07.027. doi:DOI 10.1016/j.jinorgbio.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Spallholz JE. Free radical generation by selenium compounds and their prooxidant toxicity. Biomed Environ Sci. 1997;10:260–270. [PubMed] [Google Scholar]
- Strecker R, Seiler TB, Hollert H, Braunbeck T. Oxygen requirements of zebrafish (Danio rerio) embryos in embryos toxicity tests with environmental samples. Comp Biochem Phys C. 2011;153:318–327. doi: 10.1016/j.cbpc.2010.12.002. doi:10.1016/j.cbpc.2010. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Takagi Y, Osanai H, Li L, Takeuchi M, Katoh Y, Kobayashi M, Yamamoto M. Pi class glutathione S-transferase genes are regulated by Nrf 2 through an evolutionarily conserved regulatory element in zebrafish. Biochem J. 2005;388:65–73. doi: 10.1042/BJ20041860. doi:BJ20041860 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh SJ, Deng X, Deng DF, et al. Chronic effects of dietary selenium on juvenile Sacramento splittail (Pogonichthys macrolepidotus). Environ Sci Technol. 2004;38:6085–6093. doi: 10.1021/es049545+. doi:Doi 10.1021/Es049545+ [DOI] [PubMed] [Google Scholar]
- Timme-Laragy AR, Van Tiem LA, Linney EA, Di Giulio RT. Antioxidant responses and NRF2 in synergistic developmental toxicity of PAHs in zebrafish. Toxicol Sci. 2009;109:217–27. doi: 10.1093/toxsci/kfp038. doi:10.1093/toxsci/kfp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unrine JM, Jackson BP, Hopkins WA, Romanek C. Isolation and partial characterization of proteins involved in maternal transfer of selenium in the western fence lizard (Sceloporus occidentalis). Environ Toxicol Chem. 2006;25:1864–1867. doi: 10.1897/05-598r.1. doi:Doi 10.1897/05-598r.1. [DOI] [PubMed] [Google Scholar]
- Useko CY, Harper SL, Tanguay RL. Fullerene C60 exposure elicits an oxidative stress response in embryonic zebrafish. Toxicol Appl Pharmacol. 2009;229:44–55. doi: 10.1016/j.taap.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA, U. S. E. P. A. Draft Aquatic Life Water Quality Criteria for Selenium EPA-822-D-04-001. Washington, DC: 2004. [Google Scholar]
- Van Tiem LA, Di Giulio RT. AHR2 knockdown prevents PAH-mediated cardiac toxicity and XRE-and ARE-associated gene induction in zebrafish (Danio rerio). Toxicol Appl Pharm. 2011;254:280–287. doi: 10.1016/j.taap.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal D, Bay SM, Schlenk D. Effects of dietary selenomethionine on larval rainbow trout (Oncorhynchus mykiss). Arch Environ Contam Toxicol. 2005;49:71–75. doi: 10.1007/s00244-004-0160-7. [DOI] [PubMed] [Google Scholar]
- Wang Z, Jiang C, Lu J. Induction of caspase-mediated apoptosis and cell-cycle G1 arrest by selenium metabolite methylselenol. Mol Carcinog. 2002;34:113–20. doi: 10.1002/mc.10056. doi:10.1002/mc.10056. [DOI] [PubMed] [Google Scholar]
- Wild AC, Mulcahy RT. Regulation of gamma-glutamylcysteine synthetase subunit gene expression: Insights into transcriptional control of antioxidant defenses. Free Rad Res. 2000;32:281–301. doi: 10.1080/10715760000300291. doi:Doi 10.1080/10715760000300291. [DOI] [PubMed] [Google Scholar]