Abstract
Background
The purpose of this study was to investigate the prevalence of metabolic syndrome and its co-morbidities in patients with primary knee osteoarthritis and to assess if the severity of metabolic syndrome, and components, correlates with the severity of osteoarthritis symptoms.
Methods
A case controlled analysis of 70 patients with osteoarthritis compared to a control group of 81 patients. Each patient underwent clinical review including history, examination, and pathology tests. The case-group all had stage IV osteoarthritis as determined by radiographs and intra-operative assessment. In addition a visual analogue scale (VAS), Hospital for Special Surgery knee score (HSS), and Hamilton Depression scores were completed.
Results
The prevalence of hypertension, obesity, dyslipidemia and metabolic syndrome was significantly higher in the patients with osteoarthritis compared to the control group. There is a significant correlation between the degree of hypertension, the presence of dyslipidemia or hyperglycemia and the severity of osteoarthritis symptoms. Variables hypertension, low HDL-C levels, and the number of co-morbidities were all identified as risk factors for increased osteoarthritis symptoms.
Conclusions
There is a correlation between the number of metabolic disorders, the severity of hypertension and severity of osteoarthritis symptoms. Hypertension and decreased HDL-cholesterol were positive risk factors for increased osteoarthritis symptomatology.
Keywords: Hyperglycemia, hypertension, metabolic syndrome X, obesity, osteoarthritis
Introduction
The link between osteoarthritis and metabolic syndrome has previously been demonstrated across different cultures (1-3). It has been suggested that the core of both is a chronic low-grade systemic inflammation. This has led some authors to consider osteoarthritis to be part of a greater inflammatory metabolic syndrome (4-7).
It has long been thought that obesity contributes to primary osteoarthritis of the knee through static and dynamic loads on the cartilage, leading to chronic cartilage degeneration (8-10). However this does not explain the increased incidence in the non weight-bearing joints of obese patients (11,12). More than just mechanical stress, therefore, is responsible for causing an increased prevalence of osteoarthritis in the obese population and the role of metabolic disorders has been recognized (5).
The purpose of this study was to investigate the prevalence of metabolic syndrome and its comorbidities in patients with chronic grade IV primary knee osteoarthritis and to see if there was any correlation between the severity of metabolic syndrome and the severity of osteoarthritis symptoms.
Methods
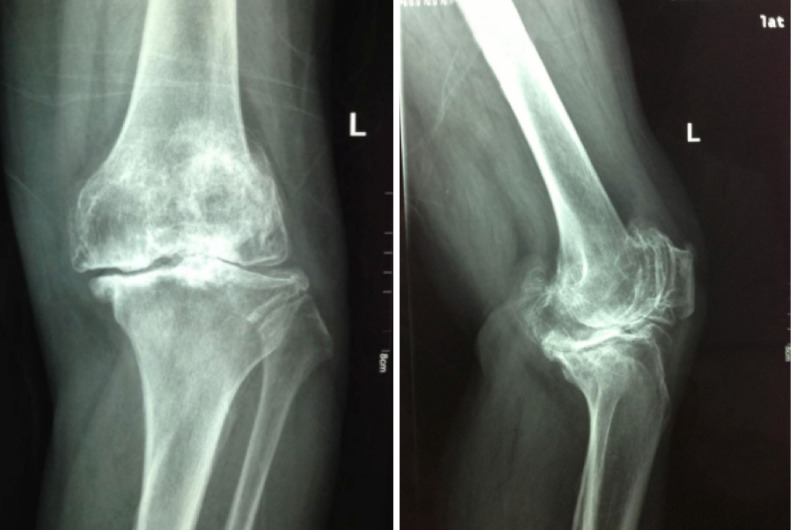
This study was conducted at a level 1 tertiary hospital. Seventy patients were included in the study, 58 females (83%) and 12 males with an age range of 50–75 years (mean, 63.63 years). Between October 2013 and March 2014, 75 consecutive patients who attended our institution for a total knee arthroplasty were considered for this study. The inclusion criteria for the case group were that all patients must meet the 1995 American College of Rheumatology (ACR) classification criteria for primary OA of the knee (13). All patients in the case group had Kellgren/Lawrence stage IV osteoarthritis (14). This was determined by radiographic evaluation by two experienced orthopaedic surgeons (MX, CY) and confirmed intra-operatively (Figure 1) (15). Exclusion criteria were recent medical infection in the preceding 12 weeks, current or previous joint infections, other inflammatory joint diseases (3 patients excluded with rheumatoid arthritis), comorbid autoimmune diseases, and all cases of secondary OA (2 patients excluded for trauma induced arthritis). Subjects were required to be able to understand the questions and be able to communicate as well as give informed consent prior to being included in this study.
Figure 1.
This figure demonstrates a Kellgren/Lawrence stage IV osteoarthritis of the knee typical of the patients undergoing total knee arthroplasty at our institution.
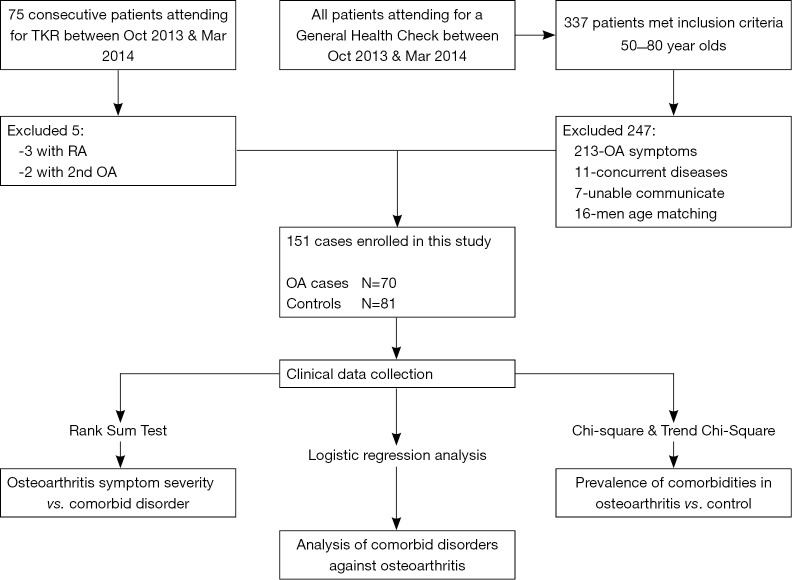
A control group, matched for age and gender was used for comparison. All consecutive patients aged between 50 and 80 (337 patients), who attended our institution for a routine general health examination between October 2013 and March 2014 were assessed against exclusion criteria. They were excluded if they had insufficient communication (n=7), had any symptomatology suggesting osteoarthritis (n=213) or meeting any of the exclusion criteria above (n=11). Matching was not on a 1:1 basis but rather both groups were concurrently recruited with all eligible control subjects included until additional male patients in a particular age bracket were no longer required. This resulted in 16 men not being assessed against inclusion/exclusion criteria based on their age (Figure 2). Eighty-one patients were included in the control group, 65 females and 16 males with an age range 50–80 years (mean, 64.11 years).
Figure 2.
This flow diagram summarizes the patient recruitment process as well as the statistical tests applied to that population.
Metabolic syndrome was defined using the Chinese Diabetes Society (CDS) 2004 recommendations combined with the 2013 People’s Republic of China Ministry of Health and Family Planning Commission guidelines (16,17). This provided a diagnosis of metabolic syndrome as the presence of three or more of the following: (I) obesity = body mass index (BMI) ≥24.0 (kg/m2), or central obesity as waist circumference (WC) men ≥85 cm, females ≥80 cm; (II) hyperglycemia = fasting plasma glucose (FPG) ≥6.1 mmol/L or previous diagnosis of diabetes with treatment; (III) hypertension = systolic blood pressure (SBP)/diastolic blood pressure (DBP) ≥140/90 mmHg, or previous diagnosis of hypertension with treatment; (IV) dyslipidemia = fasting serum triglyceride (TG) ≥1.7 mmol/L or fasting plasma high density lipoprotein (HDL) <0.9 mmol/L (men) or <1.0 mmol/L (female).
Each patient underwent a physical assessment mapping his or her age, gender, height and weight with resultant BMI, in addition to WC and blood pressure measurements. Blood pressure was classified into four groups, normal, stage 1, stage 2, and stage 3 as per the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (18). Every patient was also required to undergo a pathology test to assess the lipid profile and blood glucose levels.
All patients completed a visual analogue scale (VAS) for pain, the hospital for special surgery knee score (HSS) for knee function and the Hamilton depression rating scale (HAMD) for depression screening (19,20). Two independent evaluators assessed each patient on separate occasions and differences in scores were averaged. The HSS is a measure of knee disability assessed by an interview with the patient resulting in a score out of 100 where a lower score indicates more serious disability (19). The HAMD score consists of 24 questions resulting in a score out of 76, where a score less than 8 indicates no depression, 8–20 possible depression, 20–34 mild-moderate depression, >35 severe depression (20).
Data was collected using Epidata 3.1 software (Copyright EpiData Association, Denmark 2003–2008) to ensure accurate entry of data. Statistical analysis was performed using IBM SPSS 19.0 software [Copyright IBM Corporation and others(s) 1989, 2010]. Descriptive statistics included adoption rates, percentages, distributions, and averages. The univariate analysis statistical tests performed included the Wilcoxon rank sum test, count column χ2 test and a multivariate logistic regression analysis for correlations (Figure 2). The level of significance was defined as P<0.05.
Results
The osteoarthritis case group consisted of 58 females and 12 males with an age range of 50–75 years (mean, 63.63 years). The control group consisted of 65 females, 16 males with an age range 50–80 years (mean, 64.11 years). There was no difference between the two groups in terms of age, gender, and hobbies (smoking status or alcohol consumption) with P values of 0.979, 0.681 and 0.410 respectively.
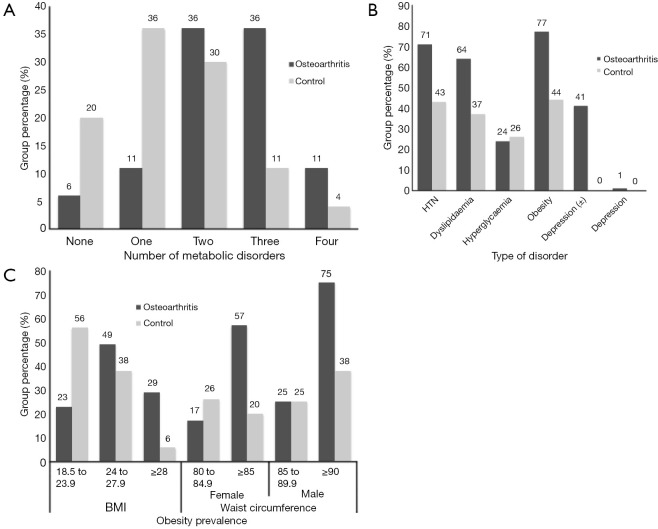
The prevalence of metabolic syndrome, the number of comorbidities, and obese patients were all greater in the OA group compared with the control group each with a P value of <0.000 (Table 1, Figure 3). Hypertension was also more prevalent in the OA group with a P value of 0.015 (Table 1, Figure 3B). Lipid metabolism indicators TC & HDL-C were both abnormal in the OA group (P=0.001) whereas TG and LDL-C levels were not different between groups (P=0.711 and 0.059 respectively). The prevalence of diabetes was equal across both groups (P=0.817) (Table 1, Figure 3B).
Table 1. Distribution of metabolic comorbidities across the study cohort.
| Investigated field | N | OA |
Chi squared (χ2) |
|||
|---|---|---|---|---|---|---|
| Case (+) | Control (−) | χ2 | P value (P) | |||
| HTN | ||||||
| Normal | 66 | 20 (13.2%) | 46 (30.5%) | 5.934 | 0.015 | |
| Stage 1 | 38 | 16 (10.6%) | 22 (14.6%) | |||
| Stage 2 | 31 | 20 (13.2%) | 11 (7.3%) | |||
| Stage 3 | 16 | 14 (9.3%) | 2 (1.3%) | |||
| Obesity | ||||||
| BMI (kg/m2) | ||||||
| 18.5≤ BMI <24.0 | 61 | 16 (10.6%) | 45 (29.8%) | |||
| 24.0≤ BMI <28 | 65 | 34 (22.5%) | 31 (20.5%) | 22.242 | 0.000 | |
| BMI ≥28.0 | 25 | 20 (13.2%) | 5 (3.3%) | |||
| WC (cm) | ||||||
| M: WC <85; F: WC <80 | 56 | 15 (9.9%) | 41 (27.2%) | |||
| M: 85≤ WC <90; F: 80≤ WC <85 | 31 | 13 (8.6%) | 18 (11.9%) | 18.424 | 0.000 | |
| M: WC ≥90; F: WC ≥85 | 64 | 42 (27.8%) | 22 (14.6%) | |||
| Blood glucose | ||||||
| Normal | 113 | 53 (35.1%) | 60 (39.7%) | 0.054 | 0.817 | |
| Diabetes | 38 | 17 (11.3%) | 21 (13.9%) | |||
| Lipid metabolism | ||||||
| Normal | 76 | 25 (16.6%) | 51 (33.8%) | 11.153 | 0.001 | |
| Dyslipidaemia | 75 | 45 (29.8%) | 30 (19.9%) | |||
| Lipid parameters | ||||||
| TC | ||||||
| Normal | 96 | 37 (24.5%) | 59 (39.1%) | 6.475 | 0.011 | |
| Elevated | 55 | 33 (21.9%) | 22 (14.6%) | |||
| TG | ||||||
| Normal | 129 | 59 (39.1%) | 70 (46.4%) | 0.137 | 0.711 | |
| Elevated | 22 | 11 (7.3%) | 11 (7.3%) | |||
| HDL-C | ||||||
| Normal | 116 | 44 (29.1%) | 72 (47.7%) | 15.163 | 0.001 | |
| Elevated | 3 | 3 (2.0%) | 0 (0%) | |||
| Lower | 32 | 23 (15.2%) | 9 (6.0%) | |||
| LDL-C | ||||||
| Normal | 122 | 52 (34.4%) | 70 (46.4%) | 3.563 | 0.059 | |
| Elevated | 29 | 18 (11.9%) | 11 (7.3%) | |||
| N of metabolic disorders | ||||||
| 0 | 20 | 4 (2.6%) | 16 (10.6%) | |||
| 1 | 37 | 8 (5.3%) | 29 (19.2%) | |||
| 2 | 49 | 25 (16.6%) | 24 (15.9%) | 28.290 | 0.000 | |
| 3 | 34 | 25 (16.6%) | 9 (6.0%) | |||
| 4 | 11 | 8 (5.3%) | 3 (2.0%) | |||
This table highlights the differences between the distributions of various metabolic disorders between the two groups. N, number; OA, osteoarthritis; HTN, hypertension; BMI, body mass index; WC, waist circumference; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol.
Figure 3.
These bar graphs demonstrate the greater prevalence and severity of metabolic syndrome comorbidities in the osteoarthritis case group. (A) More patients in the osteoarthritis group had metabolic syndrome defined as 3 or more metabolic disorders; (B) the prevalence of comorbidities was greater in the osteoarthritis group for all comorbidities except diabetes. Depression (±) = HAMD score of 8–20 while depression was defined as a HAMD score >20; (C) there were more overweight patients in the osteoarthritis group in terms of both BMI and WC. HTN, hypertension; BMI, body mass index; WC, waist circumference; HAMD, Hamilton depression score.
An increased number of comorbidities, the presence of metabolic syndrome as well as the increasing severity of hypertension, diabetes and lipid metabolism were all associated with increasing levels of pain, disability and depression each with a P value lower than 0.036 (Tables 2,3). While both an increased BMI and WC were associated with increased pain and disability only those with increased WC reported higher levels of depression (P value, 0.021) (Table 3, Figure 3C).
Table 2. Osteoarthritis symptom severity vs. comorbid severity.
| Disorder | Severity of disorder |
P | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| Classification of hypertension | ||||||
| VAS | 5.25±0.44 | 6.81±0.83 | 7.30±0.66 | 7.43±0.51 | n/a | 0.000 |
| HSS | 49.80±2.67 | 45.06±5.62 | 43.30±5.86 | 43.79±3.02 | n/a | 0.000 |
| HAMD | 6.35±2.70 | 7.88±3.61 | 9.75±4.76 | 9.71±3.24 | n/a | 0.009 |
| Number of metabolic disorders | ||||||
| VAS | 5.00±0.00 | 5.25±0.46 | 6.28±0.84 | 7.36±0.70 | 7.63±0.52 | 0.000 |
| HSS | 53.50±1.29 | 49.63±2.13 | 48.00±1.94 | 41.76±5.49 | 42.63±3.29 | 0.000 |
| HAMD | 5.25±0.500 | 5.75±1.49 | 6.32±2.43 | 10.28±4.07 | 12.75±2.71 | 0.000 |
An association between severity of hypertension and the number of metabolic disorders is clearly evident. A higher VAS and HAMD indicates worse symptoms while the inverse is true for the HSS. P, P value; VAS, visual analogue scale; HSS, hospital for special surgery knee score; HAMD, Hamilton depression score.
Table 3. Comorbid disorder and osteoarthritis symptom severity.
| Disorder | Osteoarthritis |
P | |
|---|---|---|---|
| Present | Absent | ||
| Hypertension | |||
| VAS | 7.18±0.72 | 5.25±0.44 | 0.000 |
| HSS | 44.0±5.10 | 49.80±2.67 | 0.000 |
| HAMD | 9.14±4.04 | 6.35±2.70 | 0.003 |
| Diabetes | |||
| VAS | 7.12±0.93 | 6.47±1.10 | 0.035 |
| HSS | 42.76±5.53 | 46.58±4.83 | 0.004 |
| HAMD | 11.64±4.66 | 7.28±2.96 | 0.000 |
| Waist circumference | |||
| VAS | 6.82±1.07 | 5.93±0.88 | 0.005 |
| HSS | 44.93±5.39 | 48.33±3.64 | 0.019 |
| HAMD | 8.91±4.07 | 6.27±2.25 | 0.021 |
| BMI | |||
| VAS | 6.83±1.02 | 5.94±1.06 | 0.005 |
| HSS | 44.19±4.52 | 47.25±7.08 | 0.036 |
| HAMD | 8.59±3.70 | 7.50±4.53 | 0.142 |
| Dyslipidaemia | |||
| VAS | 6.84±1.09 | 6.24±1.01 | 0.022 |
| HSS | 44.11±5.50 | 48.44±3.25 | 0.001 |
| HAMD | 9.62±4.09 | 6.04±2.13 | 0.000 |
Each of these comorbidities was associated with greater levels of pain, disability and depression. P, P value; VAS, visual analogue scale (0= no pain); HSS, hospital for special surgery knee score (0= complete disability); HAMD, Hamilton depression scale (0= no depression); BMI, body mass index.
Low HDL-C levels, hypertension, and an increasing number of co-morbidities were all positive risk factors for increased OA symptoms with P values of 0.044, 0.026, and 0.001 respectively (Table 4). This was measured using multivariate logistic regression analysis after controlling for possible confounding factors such as age, smoking, and alcohol consumption. The obesity index, blood glucose, and the remaining lipid profiles, apart from HDL-C, were not found to be positive risk factors all with P values greater than 0.05.
Table 4. Risk factors for osteoarthritis in this population.
| Variable | P | OR | OR (95.0% CI) |
|---|---|---|---|
| HDL-C (normal-controlled) | 0.044 | ||
| Normal | |||
| Lower | 0.012 | 4.584 | 1.389–15.125 |
| Hypertension (normal-controlled) | 0.026 | ||
| Normal | |||
| Stage 1 | 0.122 | 0.376 | 0.108–1.301 |
| Stage 2 | 0.576 | 1.457 | 0.401–5.285 |
| Stage 3 | 0.058 | 6.749 | 0.936–48.668 |
| The number of metabolic disorders | 0.001 | ||
| 0 | |||
| 1 | 0.979 | 1.020 | 0.222–4.700 |
| 2 | 0.008 | 8.011 | 1.705–37.638 |
| 3 | 0.000 | 63.895 | 6.351–642.865 |
| 4 | 0.008 | 57.407 | 2.932–1,123.913 |
Within this population lower HDL-C, hypertension and an increasing number of metabolic disorders were positive risk factors for osteoarthritis. P, P value; OR, odds ratio; CI, confidence interval; HDL-C, high density lipoprotein cholesterol.
Discussion
With the aging population the prevalence of osteoarthritis and metabolic syndrome is increasing (21). Not only are the individual components of metabolic syndrome troublesome for their symptoms but they also increase the risk of cerebrovascular and cardiovascular insults (22,23). Orthopedic surgery has proven to be an effective way of treating osteoarthritis especially with the introduction of total knee arthroplasty (24,25). However, in countries like China where access to health care is limited and not all patients can afford to pay for surgical interventions other less expensive treatment options are required (26). The treatment of metabolic disorders has been suggested as a possible avenue to retard the progression of osteoarthritic degeneration (27-29). Thus, identifying and further clarifying possible contributing factors in the pathogenesis and progression of symptomatic osteoarthritis is an ongoing requirement.
The limited sample size collected from one institution over a short time period limits the application of these results to the general population. This study population may not be an accurate representation of the whole Chinese community due to the exclusivity of access to health care, naturally biasing this population towards the affluent. Despite our best efforts in matching the groups, not all possible confounding factors could be accounted for.
This study supports the notion that metabolic syndrome and its comorbidities, except diabetes, are more common in patients with osteoarthritis symptomatology. Metabolic syndrome and osteoarthritis share many of the same pathophysiological mechanisms such as inflammatory aging, oxidative stress, lipid metabolism disorders, and vascular endothelial cell dysfunction leading to the damage of cartilage, subchondral bone, and mitochondrial DNA (4,5). Obesity promotes increased expression of pro-inflammatory cytokines and degrading enzymes, inhibiting cartilage matrix synthesis and potentially contributing to the formation of osteoarthritis (12,30-34). Hypertension, dyslipidemia and hyperglycemia are also more prevalent in the obese patient indicating that the existence of these metabolic disorders is intrinsically linked (6). It is no surprise therefore, that the prevalence of obesity, as well as hypertension and dyslipidemia were all more prevalent in the osteoarthritic patients. Hyperglycemia was the first metabolic condition implicated in the pathophysiology of osteoarthritis (32). Since then many scholars have found a positive correlation between hyperglycemia and osteoarthritis (33-35). This study was unable to demonstrate a higher prevalence of hyperglycemia in the osteoarthritic patients (P value, 0.817), which is in line with two large historical studies that also found no correlation (8,36).
Not only is there an association between the presence of metabolic disorders amongst the osteoarthritic patients but also the severity of the conditions are also linked. Hypertension induced subchondral bone ischemia can affect the supply of nutrients to the cartilage and hinder bone remodeling (5,37). In addition, endothelial cell damage promotes the secretion of prostaglandins increasing arterial inflammation and elevating blood pressure (38). This may account for the increased pain and disability in the patients with increasing hypertension. Dyslipidemia is also implicated in the pathogenesis of osteoarthritis (39). Increased blood viscosity and micro fat emboli as well as the deposition of lipids into the chondrocytes may be triggering events for osteoarthritis (40,41). Dyslipidemia inhibits nitric oxide synthesis allowing the local microenvironment to produce oxidative damage, along with cytokines released from the infrapatella fat pad, resulting in inflammatory mediated osteoarthritis progression (42,43). While the prevalence of hyperglycemia was not greater in the osteoarthritic group, its presence was associated with increased symptoms of pain, disability and depression (P value, 0.035, 0.004 and 0.000 respectively) (Table 3). It has been suggested that the damage caused by hyperglycemia is both direct and indirect via its effect on chondrocyte metabolism imbalance and sensitivity to matrix metalloproteinase promoting cartilage matrix degeneration and apoptosis (5,44,45). Hyperglycemia provides a chronic pro-inflammatory environment and further interferes with the insulin receptor pathway, both of which result in reactive oxygen species plausibly contributing to osteoarthritis progression (5,45).
In the study cohort we identified hypertension, reduced HDL-C levels and the total number of metabolic co-morbidities as positive risk factors for the development and progression of osteoarthritis and its symptoms. Age, obesity, female gender, race, genetic predisposition, and occupation have all previously been shown to be risk factors for the development of osteoarthritis (9,10,46). Diabetes and obesity (BMI and WC) were not identified as positive risk factors in this study, but rather the comorbidities that are often concurrent in these patients were identified as the positive risk factors. While some of these components of metabolic syndrome were not independent positive risk factors they did contribute to the combined total number of metabolic conditions in the patient, which was identified as a positive risk factor for osteoarthritis (P value, 0.001).
Many of the components of metabolic disorders are involved in self-propagating cycles resulting in both systemic and intra-articular inflammation and oxidative damage (5,7). Diabetes was equally prevalent in both groups but associated with increased severity of symptoms consistent with previous reports (47). There is a strong correlation between the severity of hypertension, the presence of dyslipidemia or hyperglycemia and severity of osteoarthritis symptoms of pain, disability and depression in this Chinese cohort. In addition to the number of metabolic disorders, the comorbidities of obesity, hypertension and decreased HDL-C, rather than obesity itself (BMI, WC) were positive risk factors for osteoarthritis. While the link between BMI and osteoarthritis has clearly been demonstrated we have found that it is the comorbidities that come with an increasing BMI that more closely correlate with the severity of osteoarthritis symptoms. Metabolic syndrome has been shown to be more commonly associated with osteoarthritis in Asian populations than Caucasian and our results may have more significance to these populations (3). Orthopedic surgeons play a key role in breaking the pain and disability cycle through surgical interventions (24,25). In countries where access to surgical options is limited other treatment modalities are required to break these vicious cycles.
This study has demonstrated a further correlation between the components of metabolic syndrome and the symptoms of osteoarthritis. While proposed mechanisms for how these components could lead to worsening symptoms has been provided the direction of the association has not been shown and the reverse is also plausible. Increasing osteoarthritis symptoms may lead to worsening of metabolic disorders through pain, stress and immobility. Future research should therefore investigate the direction of these links by demonstrating the role of early novel medical interventions in retarding the development and progression of osteoarthritis and its symptomatology or by demonstrating a reduction in metabolic syndrome after the reduction of osteoarthritis symptoms through surgery.
Acknowledgements
We thank Professor Chen You, MD, PhD, Department of Orthopaedic Surgery, 2nd Xiangya Hospital for her assistance in the radiological staging of the osteoarthritis patients. We would also like to thank Professor Wanchun Wang, MD, PhD, Department of Orthopaedic Surgery, 2nd Xiangya Hospital for his support and intellectual contributions.
Funding: This work was supported by the National Natural Science Foundation of China (81371997) for research on osteoarthritis.
Ethical Review Committee Statement: Ethical review and approval was given from the Medical Ethics Committee of Xiangya 2nd Hospital, Central South University. The original, Chinese, document is attached as well as a certified translation into English.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Grotle M, Hagen KB, Natvig B, et al. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord 2008;9:132. 10.1186/1471-2474-9-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hochberg MC. New paradigms in the management of osteoarthritis patients with hypertension. Osteoarthritis Cartilage 2010;18:S1-2. 10.1016/j.joca.2010.04.001 [DOI] [PubMed] [Google Scholar]
- 3.Gandhi R, Razak F, Tso P, et al. Asian ethnicity and the prevalence of metabolic syndrome in the osteoarthritic total knee arthroplasty population. J Arthroplasty 2010;25:416-9. 10.1016/j.arth.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 4.Velasquez MT, Katz JD. Osteoarthritis: another component of metabolic syndrome? Metab Syndr Relat Disord 2010;8:295-305. 10.1089/met.2009.0110 [DOI] [PubMed] [Google Scholar]
- 5.Zhuo Q, Yang W, Chen J, et al. Metabolic syndrome meets osteoarthritis. Nat Rev Rheumatol 2012;8:729-37. 10.1038/nrrheum.2012.135 [DOI] [PubMed] [Google Scholar]
- 6.Katz JD, Agrawal S, Velasquez M. Getting to the heart of the matter: osteoarthritis takes its place as part of the metabolic syndrome. Curr Opin Rheumatol 2010;22:512-9. 10.1097/BOR.0b013e32833bfb4b [DOI] [PubMed] [Google Scholar]
- 7.Sellam J, Berenbaum F. Is osteoarthritis a metabolic disease? Joint Bone Spine 2013;80:568-73. 10.1016/j.jbspin.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 8.Anderson JJ, Felson DT. Factors associated with osteoarthritis of the knee in the first national Health and Nutrition Examination Survey (HANES I). Evidence for an association with overweight, race, and physical demands of work. Am J Epidemiol 1988;128:179-89. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med 2010;26:355-69. 10.1016/j.cger.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neogi T, Zhang Y. Epidemiology of osteoarthritis. Rheum Dis Clin North Am 2013;39:1-19. 10.1016/j.rdc.2012.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carman WJ, Sowers M, Hawthorne VM, et al. Obesity as a risk factor for osteoarthritis of the hand and wrist: a prospective study. Am J Epidemiol 1994;139:119-29. [DOI] [PubMed] [Google Scholar]
- 12.Yusuf E, Nelissen RG, Ioan-Facsinay A, et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis 2010;69:761-5. 10.1136/ard.2008.106930 [DOI] [PubMed] [Google Scholar]
- 13.Hochberg MC, Altman RD, Brandt KD, et al. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. American College of Rheumatology. Arthritis Rheum 1995;38:1541-6. 10.1002/art.1780381104 [DOI] [PubMed] [Google Scholar]
- 14.Kellgren JH, Lawrence JS. Rheumatism in miners. II. X-ray study. Br J Ind Med 1952;9:197-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riddle DL, Jiranek WA, Hull JR. Validity and reliability of radiographic knee osteoarthritis measures by arthroplasty surgeons. Orthopedics 2013;36:e25-32. 10.3928/01477447-20121217-14 [DOI] [PubMed] [Google Scholar]
- 16.Chinese Medical Association Diabetes Society metabolic syndrome Study Group. Chinese Medical Association Diabetes Society recommendations for the metabolic syndrome. Chinese Journal of Diabetes 2004;(3):5-10.
- 17.Chen C, Zhao W, Yang X, et al, editors. Health industry standards of the people's Republic of China: Criteria of weight for adults. Beijing, China: China Quality Press, 2013:1-2. [Google Scholar]
- 18.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560-72. 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- 19.Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med 1982;10:150-4. 10.1177/036354658201000306 [DOI] [PubMed] [Google Scholar]
- 20.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56-62. 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen US, Zhang Y, Zhu Y, et al. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Ann Intern Med 2011;155:725-32. 10.7326/0003-4819-155-11-201112060-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab 2004;89:2595-600. 10.1210/jc.2004-0372 [DOI] [PubMed] [Google Scholar]
- 23.Hawker GA, Croxford R, Bierman AS, et al. All-cause mortality and serious cardiovascular events in people with hip and knee osteoarthritis: a population based cohort study. PLoS One 2014;9:e91286. 10.1371/journal.pone.0091286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richmond JC. Surgery for osteoarthritis of the knee. Rheum Dis Clin North Am 2013;39:203-11. 10.1016/j.rdc.2012.10.008 [DOI] [PubMed] [Google Scholar]
- 25.Jevsevar DS, Brown GA, Jones DL, et al. The American Academy of Orthopaedic Surgeons evidence-based guideline on: treatment of osteoarthritis of the knee, 2nd edition. J Bone Joint Surg Am 2013;95:1885-6. [DOI] [PubMed] [Google Scholar]
- 26.Wang HM, Liu JN, Zhao Y. Progress on integrated Chinese and Western medicine in the treatment of osteoarthritis. Chin J Integr Med 2010;16:378-84. 10.1007/s11655-010-0512-2 [DOI] [PubMed] [Google Scholar]
- 27.Liu FC, Huang HS, Huang CY, et al. A benzamide-linked small molecule HS-Cf inhibits TNF-α-induced interferon regulatory factor-1 in porcine chondrocytes: a potential disease-modifying drug for osteoarthritis therapeutics. J Clin Immunol 2011;31:1131-42. 10.1007/s10875-011-9576-9 [DOI] [PubMed] [Google Scholar]
- 28.Fahmi H, Martel-Pelletier J, Pelletier JP, et al. Peroxisome proliferator-activated receptor gamma in osteoarthritis. Mod Rheumatol 2011;21:1-9. 10.3109/s10165-010-0347-x [DOI] [PubMed] [Google Scholar]
- 29.Yudoh K, Karasawa R. Statin prevents chondrocyte aging and degeneration of articular cartilage in osteoarthritis (OA). Aging (Albany NY) 2010;2:990-8. 10.18632/aging.100213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gosset M, Berenbaum F, Levy A, et al. Mechanical stress and prostaglandin E2 synthesis in cartilage. Biorheology 2008;45:301-20. [PubMed] [Google Scholar]
- 31.Pottie P, Presle N, Terlain B, et al. Obesity and osteoarthritis: more complex than predicted! Ann Rheum Dis 2006;65:1403-5. 10.1136/ard.2006.061994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waine H, Nevinny D, Rosenthal J, et al. Association of osteoarthritis and diabetes mellitus. Tufts Folia Med 1961;7:13-9. [PubMed] [Google Scholar]
- 33.Cimmino MA, Cutolo M. Plasma glucose concentration in symptomatic osteoarthritis: a clinical and epidemiological survey. Clin Exp Rheumatol 1990;8:251-7. [PubMed] [Google Scholar]
- 34.Schett G, Kiechl S, Bonora E, et al. Vascular cell adhesion molecule 1 as a predictor of severe osteoarthritis of the hip and knee joints. Arthritis Rheum 2009;60:2381-9. 10.1002/art.24757 [DOI] [PubMed] [Google Scholar]
- 35.Stürmer T, Brenner H, Brenner RE, et al. Non-insulin dependent diabetes mellitus (NIDDM) and patterns of osteoarthritis. The Ulm osteoarthritis study. Scand J Rheumatol 2001;30:169-71. 10.1080/030097401300162969 [DOI] [PubMed] [Google Scholar]
- 36.Frey MI, Barrett-Connor E, Sledge PA, et al. The effect of noninsulin dependent diabetes mellitus on the prevalence of clinical osteoarthritis. A population based study. J Rheumatol 1996;23:716-22. [PubMed] [Google Scholar]
- 37.Findlay DM. Vascular pathology and osteoarthritis. Rheumatology (Oxford) 2007;46:1763-8. 10.1093/rheumatology/kem191 [DOI] [PubMed] [Google Scholar]
- 38.Appenzeller O. Pathogenesis of migraine. Med Clin North Am 1991;75:763-89. 10.1016/S0025-7125(16)30448-5 [DOI] [PubMed] [Google Scholar]
- 39.Masuko K, Murata M, Suematsu N, et al. A metabolic aspect of osteoarthritis: lipid as a possible contributor to the pathogenesis of cartilage degradation. Clin Exp Rheumatol 2009;27:347-53. [PubMed] [Google Scholar]
- 40.Javaid MK, Lynch JA, Tolstykh I, et al. Pre-radiographic MRI findings are associated with onset of knee symptoms: the most study. Osteoarthritis Cartilage 2010;18:323-8. 10.1016/j.joca.2009.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gkretsi V, Simopoulou T, Tsezou A. Lipid metabolism and osteoarthritis: lessons from atherosclerosis. Prog Lipid Res 2011;50:133-40. 10.1016/j.plipres.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 42.Xia W, Szomor Z, Wang Y, et al. Nitric oxide enhances collagen synthesis in cultured human tendon cells. J Orthop Res 2006;24:159-72. 10.1002/jor.20060 [DOI] [PubMed] [Google Scholar]
- 43.Bastiaansen-Jenniskens YM, Clockaerts S, Feijt C, et al. Infrapatellar fat pad of patients with end-stage osteoarthritis inhibits catabolic mediators in cartilage. Ann Rheum Dis 2012;71:288-94. 10.1136/ard.2011.153858 [DOI] [PubMed] [Google Scholar]
- 44.Rosa SC, Gonçalves J, Judas F, et al. Impaired glucose transporter-1 degradation and increased glucose transport and oxidative stress in response to high glucose in chondrocytes from osteoarthritic versus normal human cartilage. Arthritis Res Ther 2009;11:R80. 10.1186/ar2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saudek DM, Kay J. Advanced glycation endproducts and osteoarthritis. Curr Rheumatol Rep 2003;5:33-40. 10.1007/s11926-003-0081-x [DOI] [PubMed] [Google Scholar]
- 46.Srikanth VK, Fryer JL, Zhai G, et al. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage 2005;13:769-81. 10.1016/j.joca.2005.04.014 [DOI] [PubMed] [Google Scholar]
- 47.Schett G, Kleyer A, Perricone C, et al. Diabetes is an independent predictor for severe osteoarthritis: results from a longitudinal cohort study. Diabetes Care 2013;36:403-9. 10.2337/dc12-0924 [DOI] [PMC free article] [PubMed] [Google Scholar]