Abstract
Despite considerable advancements that shattered previously held dogmas about the metastatic cascade, evolution of therapies to treat metastatic disease has not kept up. In this Opinion article, I argue that rather than waiting for metastases to emerge before initiating treatment, it would be more effective to target metastatic seeds before they sprout. Specifically, I advocate directing therapies towards the niches that harbor dormant disseminated tumor cells in order to render them susceptible to cytotoxic agents. Treatment sensitization achieved by disrupting reservoirs of leukemic stem cells and latent HIV argues that this approach, though unconventional, could succeed in improving patient survival by delaying or even preventing metastasis.
ToC
This Opinion article advocates therapeutically targeting the niches that harbor dormant disseminated tumor cells in order to render them susceptible to cytotoxic agents. Similar strategies have sensitized leukemic cells and latent HIV to therapy, and such an approach might delay or even prevent metastasis.
The concept of tumor dormancy was postulated nearly a century ago 1, but three findings within the last 25 years have led to a widespread appreciation for the clinical phenomenon of latent disease, motivating a desire to target tumor cells while they ‘sleep.’ The ability to detect disseminated tumor cells (DTCs)2 and their discovery in patients with no evidence of metastatic disease 2–4 indicated that tumor cells spread far earlier than thought previously (an idea substantiated in animal models 5, 6); establishment of their prognostic significance 3, 4, 7–13 suggested that these DTCs could be the origin of future metastases. Subsequent detection of circulating tumor cells (CTCs) in cancer survivors decades after successful treatment of their primary tumor14–16 demonstrated that: a) a fraction of DTCs survive therapy and remain despite no evidence of disease, and b) DTCs, though latent, are actively regulated based on their ability to consistently enter the circulation. Finally, the discovery that dormant DTCs persist at the single cell level 17, 18 and that like stem cells, their behavior is regulated by a niche 19 has raised the possibility that we can target dormant DTCs by altering their microenvironment. This approach could result in a means of metastasis prevention. The question is how to target dormant DTCs: Do we reinforce their niche in an attempt to keep them asleep forever? Or, do we draw dormant DTCs out of their niche to kill them at the potential risk of waking them up?
In this Opinion piece, I argue that addressing this dilemma requires a detailed understanding of the cellular and biochemical composition of the microenvironment harboring dormant DTCs: the ‘dormant niche.’ I posit that distinct cues within this niche regulate engraftment, quiescence, survival and chemoresistance of dormant DTCs, raising the possibility that these cells can be sensitized to systemic therapy without necessarily affecting their growth status. Examples primarily from hematopoietic cancers demonstrate how this paradigm has been applied successfully to eradicate putative leukemic stem cells from patients otherwise refractory to standard treatments, leading to substantially improved survival, whereas lessons from HIV research suggest practical reasons why an approach to eradicate dormant DTCs is superior to chronic maintenance of the dormant state.
Tumor cell dormancy: A singular focus
Biologically, tumor dormancy encompasses two distinct states: population-level dormancy and cellular dormancy 14, 20. Population-level dormancy, first proposed by Judah Folkman in the early 1970s 21, is a condition where tumor cell proliferation and death are balanced within micrometastatic foci due to diffusion limitations and/or immune surveillance 22, 23. On the other hand, cellular dormancy describes a state of mitotic arrest, in which cells have exited the cell cycle and entered the so-called G0 state 14, 18, 20. Although there is some debate as to which dormancy mechanism is more relevant, the reality is that these states are not mutually exclusive. Just as Folkman described the “angiogenic switch 24” as a barrier that must be overcome for tumor cell populations to progress to metastases 22, 24, a ‘dormancy switch’ likely serves as a preliminary hurdle that must be overcome for quiescent DTCs to proliferate initially into micrometastases 19. Therefore, both dormancy mechanisms and both switches bear consideration when devising therapeutic strategies. Since the vast majority of DTCs in bone marrow and other tissues are found in a state consistent with cellular dormancy 17, 25, and because anti-angiogenic strategies to maintain population-level dormancy have been discussed thoroughly elsewhere 26, 27, the focus of this Perspective is cellular dormancy. It is important to note here that although it is often assumed that late arising metastases originate from dormant DTCs, current evidence is circumstantial in nature. However, supportive data continue to accumulate; Box 1 presents this evidence and discusses how the strategies discussed in this article could result in direct proof of the clinical relevance of cellular dormancy.
Box 1. Do dormant DTCs initiate metastases?
Currently, it is not possible to prove directly that dormant disseminated tumor cells (DTCs) initiate metastases. We are not able to follow a single DTC for years in order to determine whether it ever awakens and colonizes tissue, nor are we able to eliminate DTCs in any organism to determine if progression is altered. However, there are a number of indirect lines of evidence supportive of a metastasis-initiating role of dormant DTCs. The first is that in contrast to circulating epithelial cells (a number of inflammatory syndromes cause epithelial cells to circulate in the blood 146–148), disseminated epithelial cells have not been uncovered in healthy individuals. However, when they are discovered in the bone marrow of breast, lung, prostate, colorectal, gastric and esophageal cancer patients, their presence correlates to significantly reduced metastasis-free survival 4, 7–9, 11, 12, 149. The second is that isolated DTCs have expansion potential, and can cause metastases once reinoculated into the host organism. DTCs have been isolated and expanded successfully in culture from mouse models and from clinical specimens 150, 151 (although immortalization was required for the latter). Further, DTCs isolated from bone marrow of immune-compromised mice formed primary tumors once re-implanted into the mammary fat pad, and went on to colonize the lymph nodes and lung 151. However, reinoculated cells remained dormant in bone marrow. Therefore, isolated and expanded DTCs cause metastatic disease, but may not gain the ability to colonize new sites without permissive changes in the microenvironment that trigger micrometastatic outgrowth 19, 121–125, 152–154.
As mentioned at the outset, all of these correlations are circumstantial. The ultimate proof will have to come from establishing a causal relationship between DTC elimination and extension of metastasis-free survival. Ironically, this will never happen without a treatment that successfully addresses dormant DTCs.
Properties of Dormant DTCs
Three essential properties define dormant DTCs: they persist within foreign microenvironments, they are reversibly growth-arrested and they resist targeted and cytotoxic treatments (Fig. 1a). The ability of DTCs to persist in foreign tissues for many years in a growth-arrested state is well documented, and it is increasingly appreciated that the microenvironment plays a role in conferring and maintaining these states 28, 29. On the other hand, the therapeutic resistant nature of dormant DTCs is a property that requires exploration.
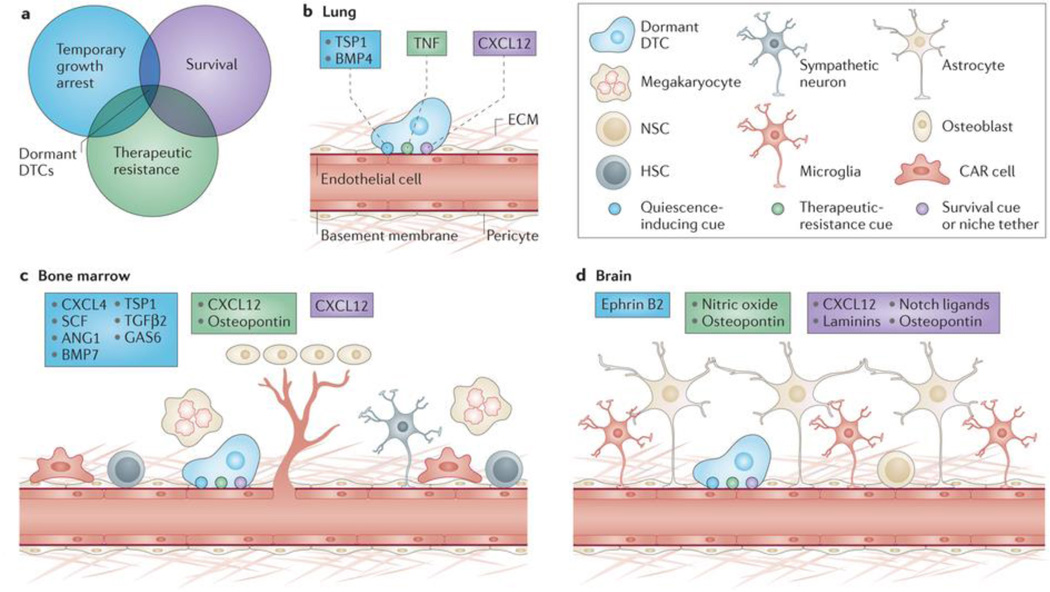
Figure 1. Properties of dormant disseminated tumor cells (DTCs) are conferred by tissue-specific perivascular niches.
Three properties that define dormant DTCs: survival, temporary growth arrest, and therapeutic resistance (a). Summaries of known and hypothesized mediators of these properties— survival (purple), quiescence (blue) and drug resistance (green) are shown for different tissue types. Lung (b), bone marrow (c) and brain (d) perivasculature exhibit tissue-specificity, and are a rich source of factors that confer dormancy properties. Whereas chemokine (C-X-C motif) ligand 12 (CXCL12)–chemokine (C-X-C motif) receptor 4 (CXCR4) signaling may mediate homing to and engraftment of each site, quiescence in lung is mediated by thrombospondin 1 (TSP-1) and bone morphogenetic protein 4 (BMP-4). Angiocrine tumor necrosis factor α (TNFα) drives chemoresistance of DTCs and may do the same for dormant DTCs. Conversely, TSP-1, BMP-7, transforming growth factor β2 (TGF-β2) and growth arrest-specific 6 (GAS-6) mediate DTC quiescence in bone marrow. Factors known to drive and maintain hematopoietic stem cell (HSC) quiescence (CXCL4, stem cell factor (SCF) and angiopoietin-1 (Ang-1)) may also act on DTCs in the bone marrow. Additionally, osteopontin may drive chemoresistance of solid tumor cells, as it does for leukemic cells. Finally, in the brain, osteopontin, laminins (primarily laminin-α2) and NOTCH ligands are niche constituents of stem-like glioblastoma cells, whereas ephrin-B2 is a known inducer of neural stem cell quiescence. These factors may also act on DTCs in brain, which require initial adhesion to the perivasculature to survive. Osteopontin and nitric oxide drive therapeutic resistance of glioblastoma cells, and these perivascular cues may function also to confer resistance to dormant DTCs. CAR, CXCL12-abundant reticular
The notion that dormant DTCs resist common therapies derives from their recovery from bone marrow aspirates of patients who underwent systemic treatment with chemotherapy regimens such as those containing doxorubicin or targeted agents like trastuzumab several years earlier 7, 30–32. Subsequent imaging-based studies in mice showed clearly that single DTCs persist despite doxorubicin treatment 33. Why these cells resist therapy has been based historically on two assumptions. One is that tumor progression is linear. According to this assumption, DTCs arise from cells that left the primary tumor at later stages, are further evolved than the primary tumor, and have accumulated mutations that confer resistance to the applied drug(s). However, comparative genomic hybridization studies comparing DTCs isolated from bone marrow with matched primary breast tumor revealed that matched DTCs have fewer genomic aberrations on a per cell basis than tumor cells from the primary site, and further that the majority of DTCs lack any detectable chromosomal abnormalities 34. These findings have been substantiated also in prostate cancer patients, using DTCs taken as far out as 8 years after radical prostatectomy 35. These studies support the notion that DTCs depart early in primary tumor development, and suggest that it is unlikely that these cells are inherently resistant to therapy.
A second assumption is that cytotoxic therapies, which target rapidly dividing cells, do not detrimentally affect dormant DTCs because they are not cycling. However, mitotic state is not the only factor that determines susceptibility to apoptosis-inducing agents 36. In particular, identification of discrete pathways regulating growth and polarity of mammary epithelium in three-dimensional culture allowed for demonstration that niche-mediated cellular architecture protects tumor cells from cytotoxic therapy. In these cultures, malignant cells form disorganized, continuously growing clusters that respond to both cytotoxic agents and tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) agonists, whereas tumor cells driven into growth-arrested and properly polarized three-dimensional structures by phenotypic reversion were resistant to each of the death-inducing agents applied 37. The key result is that forcing growth of organized colonies did not lead to enhanced chemotherapeutic efficacy as long as these colonies maintained their cellular architecture. Instead, disrupting polarity of quiescent colonies (without inducing growth) increased cytotoxicity dramatically 37.
One critical insight from this work is that chemoresistant cells can be made susceptible to cytotoxic agents without affecting their growth status. Another is that basement membrane is a principle effector of signaling that promotes drug resistance. This notion is supported by experiments conducted with multiple tumor types and different classes of cytotoxic agents 38–41. Taking these clues into account, we can pinpoint a niche that harbors DTCs, confers each of the essential properties listed above, and could be targeted for the purpose of treating dormant DTCs.
Defining the dormant niche
In addition to conferring drug resistance, basement membrane promotes survival and induces functional differentiation and growth arrest of epithelial cells. Merely disrupting adhesion of a single integrin subunit to laminin-111, a principle basement membrane component, causes mammary epithelial cell apoptosis 42, demonstrating that specific tethers to basement membrane are critical survival cues. Similarly, key features of the normal phenotype such as growth-arrest and functional differentiation are achieved only when mammary epithelial cells are adhered to basement membrane (principally laminin-111), or within a microenvironment that facilitates its endogenous production 43–47.
It is apparent then that basement membrane confers each of the properties associated with DTC dormancy when epithelial cells or epithelial-derived tumor cells are adhered to ‘native’ basement membrane. However, upon dissemination, DTCs are no longer able to interact with epithelial basement membrane from their tissue of origin. Instead, DTCs often take a hematogenous route to foreign tissues like lung, bone marrow, liver or brain 48, where vascular basement membrane is the first basement membrane they encounter. Therefore it is not surprising that dormant DTCs are found in close association with vascular basement membrane, a phenomenon observed in both spontaneous and experimental mouse models of breast tumor dissemination 19, and through live imaging studies tracking disseminated melanoma and lung cancer cells in brains of mice 49.
Indeed, evidence has accumulated over the last 15 years that the niche surrounding microvasculature (i.e., the perivascular niche, PVN) orchestrates a number of processes related to tissue growth, differentiation and regeneration 50. Key roles for endothelium in the regulation of pancreatic differentiation 51, liver growth 52 and regeneration 53, 54, glomerulogenesis and nephrogenesis in kidney 55, 56, lung regeneration 57, bone remodeling58, 59 and neuronal development and differentiation60 have been established. Even more germane to this discussion is the role played by the PVN in conferring essential properties of DTCs described above—principally survival and growth-arrest—to tissue-resident stem cells, a population of cells maintained in number until triggered to repopulate tissue, much like dormant DTCs.
The stem cell niche and the dormant niche: one and the same?
The idea of a specialized microenvironment regulating stem cell behavior is one that was first proposed formally by Schofield 61, who postulated that a niche restricts stem cell differentiation and cell cycle entry in order to maintain a tissue’s stem cell population. The vascular endothelium has emerged as a prominent component of the stem cell niche (Fig. 1b–d) 50, 62. Tissue-specific stem cells in brain, bone marrow, skeletal muscle and skin localize to hematogenous vasculature 63–66, and mesenchymal stem cells within muscle and pancreas reside on microvasculature as well 67. Endothelial cell-derived factors are key effectors of stem cell phenotype in each of these tissues 50.
Whereas stem cells and dormant DTCs both occupy PVNs, currently there is no direct evidence that these niches are identical. However, there are three pieces of evidence to suggest that this association is more than coincidental. The first is that careful analysis of efferent blood in rat models of mammary carcinoma established that primary tumor size correlates with the number of cells shed into circulation 68; however no such relationship between primary tumor size and the number of DTCs detected in bone marrow exists, as demonstrated in human cancer patients 5, 7, 69. This implies that CTCs can engraft only a limited number of niches in bone marrow 69, consistent with the idea that stem cell niches in bone marrow are limited in size and number 70, 71. Recent evidence supporting Schofield’s original assertion that stem cell niches can impose features of a stem cell on (non-stem) daughter cells 62, 72, 73 suggests that these specialized microenvironments could indeed support survival and impose growth restrictions on DTCs as well (Figure 2 illustrates this parallel). The last and perhaps strongest piece of evidence in support of this hypothesis is that the same cues used to mobilize hematopoietic stem cells (HSCs) from bone marrow result in mobilization of DTCs as well. Application of granulocyte colony-stimulating factor (G-CSF, also known as CSF3) or inhibition of the chemokine (C-X-C motif) ligand 12 (CXCL12)–chemokine (C-X-C motif) receptor 4 (CXCR4) signaling axis each result in mobilization of HSCs from bone marrow into the bloodstream 74. Growing concern over strategies used to collect HSCs for reintroduction into patients following high-dose chemotherapy led to the discovery in 1994 that blood from G-CSF-treated multiple myeloma patients was contaminated with tumor cells 75. This result has since been leveraged for solid tumor patients to increase CTC numbers for diagnostic purposes 76. Recently, Taichman and colleagues showed in mice that inhibition of CXCR4 releases disseminated prostate cancer cells from bone marrow into circulation, demonstrating that HSCs and prostate cancer cells are tethered to niches in bone marrow by the same signal 77. Whether dormant DTCs are tethered to bone marrow niches also via CXCR4, and whether stem cell niches and dormant niches overlap in other tissues remains to be seen.
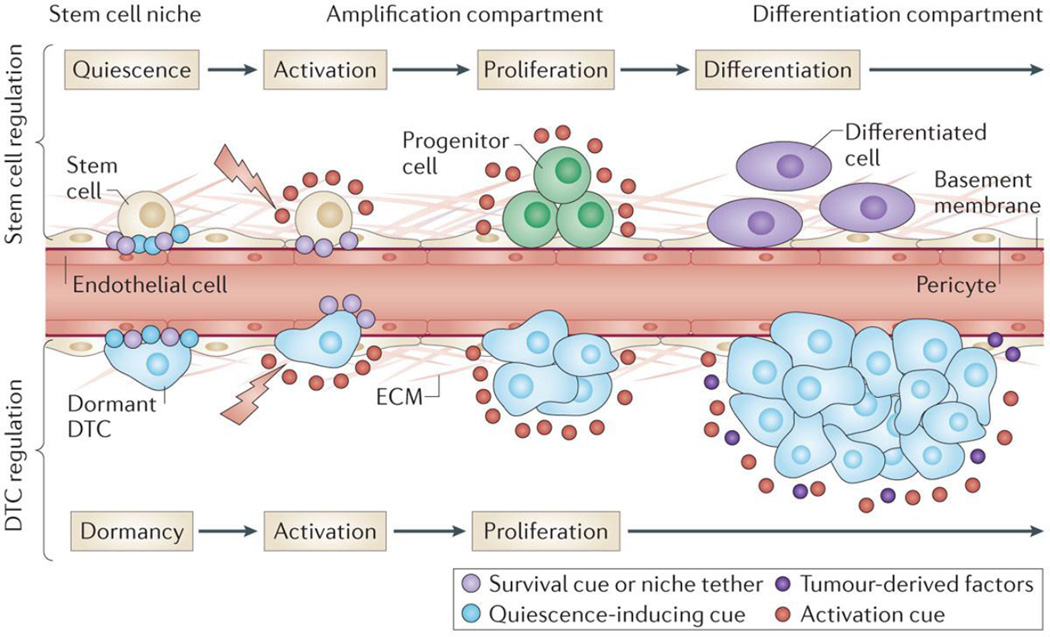
Figure 2. Parallels between stem cell- and dormant disseminated tumor cell (DTC)-regulation by the perivascular niche.
Stem cell survival and growth-arrest are maintained in a number of tissues by the perivascular niche. Upon activation of the niche (e.g., by wounding), growth-arrest cues are lost and/or overcome by proliferation-inducing signals, some of which are derived from endothelium. Stem cells give rise to rapidly dividing tissue-specific progenitors, which are ultimately steered towards differentiated cell types found in that tissue. DTCs are regulated in a similar fashion. Stable microvasculature maintains DTCs in a quiescent state; upon disruption of tissue homeostasis, activation cues (e.g., those derived from nascent, sprouting endothelium) spark DTC proliferation. Here, however, proliferating DTCs ignore differentiation cues and secrete a number of factors that lure resident- and circulating-cells to aid metastatic progression. Whether the parallels between tissue-specific stem cells and dormant DTCs end here, or whether dormant DTCs are indeed regulated by native stem cell niches within each tissue they occupy, remains to be seen.
Dormant DTCs: A product of their microenvironment
Dormant DTCs may or may not occupy de facto stem cell niches on vasculature. In either case, it is clear that perivascular tumor cells exhibit each of the properties stem cells and dormant DTCs share in common (Fig. 1a). Intravital imaging to track individual DTCs within mouse brains showed that adhesion to the basal side of microvasculature is an absolute pre-requisite for DTC survival 49, and studies in zebrafish and chicken embryos suggest that connexin-mediated signaling between endothelium and DTCs mediate this effect 78. Growth-arrested DTCs persist on or very close to lung and bone marrow microvasculature also, and recreating the microvascular niche of these tissues with human cells revealed that stable microvascular endothelium induces and sustains breast tumor cell quiescence 19. Endothelial-derived thrombospondin-1 (TSP-1), which associates with mature microvascular basement membrane, is a prime effector of this phenotype 19.
Given the complexity of basement membrane and the matricellular proteins that associate with it 79, a number of additional factors within the PVN likely play direct and indirect roles in sustaining DTC quiescence. To this end, additional constituents of the dormant niche have been identified. An emerging theme is that these molecules signal in a tissue-specific manner (Fig. 1b–d).
Using experimental metastasis assays in syngeneic mice, the bone morphogenetic protein (BMP) antagonist COCO (also known as DAND5) was pinpointed as a factor that enables dormant DTCs to colonize lung 80. This finding implies that BMPs play a key role in restricting DTC growth in lung, with BMP-4 the most likely effector of dormancy 80. Whereas an angiocrine source of BMP-4 was not tested, it is worth noting that dormant cells resided exclusively on lung microvasculature in this study, and more recent work showed that autocrine BMP-4 signaling by lung endothelium results in TSP-1 expression that drives alveolar differentiation of lung stem cells 81.
Notably, dormancy-associated functions of BMP-4 may not translate to other tissues like bone marrow or brain 80, 82. Instead, mouse xenograft models have demonstrated a clear role for CXCL12–CXCR4 signaling in attracting prostate cancer cells to engraft bone marrow 77, where stromal BMP-7 and transforming growth factor (TGF)-β2 induce prostate cancer cells and head and neck squamous cell carcinoma cells to enter a dormant state 82, 83. The intertwining of PVN and endosteum84 raises the possibility that osteoblast-derived effectors of quiescence (e.g., growth arrest-specific 6 (GAS6)) 85 may also act on perivascular DTCs.
What role any of these factors play in mediating chemoresistance of dormant DTCs beyond inducing growth-arrest is unknown; currently, there is no evidence that perivascular cues specifically mediate therapeutic resistance of dormant DTCs. However, support for this concept continues to accumulate in the setting of both primary tumors and distant metastases. For instance, glioblastoma cells residing on brain microvasculature acquire stem cell-like properties 86; perivascular nitric oxide, Notch signaling, and CD44- and integrin α6 subunit-mediated binding to PVN components (e.g., osteopontin and laminin α2 chain) mediate this phenotype 87–91. Perivascular glioblastoma cells resist radiotherapy92 and upregulate expression of the drug transporter ABC transporter G family member 2 (ABCG2) 87, which prevents intracellular accumulation of chemotherapeutics such as methotrexate, mitoxantrone and anthracyclins 93.
The PVN provides a therapeutic safe haven in the metastatic context as well. Colorectal cancer cells expressing the putative cancer stem cell marker CD133 are found concentrated on microvasculature of liver metastases in human specimens. Medium conditioned by liver endothelial cells enriches for CD133-positive tumor cells that are highly resistant to chemotherapeutics 5-fluorouracil and oxaliplatin. An endothelial cell-derived proteolytically shed form of the Notch ligand Jagged-1 is the principal effector of this phenotype 94. Liver endothelium is implicated also in lymphoma cell survival, and angiocrine Jagged-1 is the culprit here as well, driving doxorubicin resistance and manifesting in a more aggressive form of the disease in mice 95. Finally, chemotherapies also alter the angiocrine profile of endothelium directly; lung endothelial cells trigger a signaling cascade through the release of TNF-α in response to taxol, doxorubicin and cyclophosphamide that directly and indirectly sustains DTC survival 96.
The message is not only that the PVN elicits each of the properties associated with dormant DTCs, but that this occurs through mechanisms specific to each tissue’s vascular bed (Fig. 1b–d) 97. This reflects a functional diversity amongst the cells, basement membrane molecules and soluble effectors that comprise each organ’s PVN. Therefore, understanding tissue-specific perivascular heterogeneity between tissues will be necessary to determine whether there are universal properties of the PVN that can be exploited to sustain tumor dormancy or eradicate dormant tumor cells, or whether a more tailored approach that takes specific organ PVNs into account (and perhaps even their sub-niches; see Box 2) is required.
Box 2. The Niche within the Niche: Sub-Niche Heterogeneity.
A theme of this article is that one cannot assume a one-size fits all approach exists to targeting dormant disseminated tumor cells (DTCs) because tissue-specific differences could underlie how DTCs resist chemotherapy in different organs (Fig. 1). But an emerging theme in both the stem cell and dormancy literature is that sub-niches within tissues exhibit heterogeneity in terms of how their molecular repertoire drives cells into or out of quiescence. For instance, painstaking analysis of tissue-specific knockouts identified the hematopoietic stem cell (HSC) niche in bone marrow as a perivascular one 74, 155, but recent work by Frenette and colleagues has elaborated two additional perivascular locales (periarteriolar niches and megakaryocytic niches) that maintain populations of dormant HSCs through distinct molecular mechanisms 156, 157. Even two cells on the same microvessel can experience distinct signaling milieus. Endothelial stalks are surrounded by mature basement membrane that steers cells towards a quiescent state, whereas endothelial tip cells deposit a host of mitogenic factors that drive growth 19. This signaling paradigm extends to epithelial tubes of the embryonic mouse lung 158. The bottom line: location matters. Determining exactly how much could be the difference in developing a therapy that works against some DTCs, or all.
Targeting dormant DTCs
Clinical and experimental evidence supports the therapeutic potential of treating dormant DTCs (Box 1), and a role for the PVN in conferring crucial properties of dormant DTCs. The question now is how to leverage this knowledge to target dormant DTCs: should we try to keep them asleep by supplementation of key niche factors or should we instead kill them by disrupting interactions with supportive microenvironmental cues? The HIV field is similarly plagued by the issue of minimal residual disease, and provides a striking and potentially instructive parallel to this dilemma.
Antiretroviral therapy (ART) is an extremely effective treatment regimen that drives viremia (concentration of virus in plasma) down to undetectable levels in HIV-infected individuals 98. However, ART does not eradicate the virus altogether. HIV persists in a latent but replication-competent form in a small subset of extremely long-lived resting memory CD4+ T-lymphocytes 99–101. Perpetual application of ART to keep HIV in a dormant state is now in its second decade; however, concerns with its long-term toxicity, cost and reemergence of HIV once ART is halted prevent it from being a permanent solution 102. Perhaps this is a cautionary tale for strategies aimed at keeping dormant DTCs at bay indefinitely.
Instead, researchers have pursued an alternative strategy aimed at disrupting HIV latency in CD4+ cells so ART can target the replicative virus successfully. In this context, the recent discoveries that protein kinase C activators and histone deacetylase inhibitors significantly upregulate HIV transcript expression in resting CD4+ T cells derived from HIV-infected, ART-treated patients 103, 104 is promising. However, a lingering concern is whether eradicating HIV from resting memory CD4+ T-lymphocytes will result in total depletion of HIV throughout the body 102. Researchers may effectively eliminate residual virus within the CD4+ T-lymphocyte population only to find that there are other reservoirs of latent HIV. This provides a point of caution for approaches aimed at targeting dormant DTCs as well: even a strategy that is successful in one tissue must be tested extensively to determine whether it eliminates dormant DTCs in others. Below, these two strategies are explored in the context of dormant DTCs.
Option 1: Keeping dormant tumor cells ‘asleep’
The idea of treating dormant DTCs by keeping them ‘asleep’ is attractive on many fronts. Most alluring is that it could obviate the need for chemotherapy, and all of its associated side effects, in certain patient populations. Tumor dormancy models have facilitated the identification of a number of signaling pathways that, when suppressed, drive tumor cells into a dormant state. For instance, reduced urokinase receptor- and integrin-mediated ERK signaling is exhibited by dormant head and neck squamous cell carcinoma cells 105. A model of breast tumor dormancy predicted also that suppression of MAPK signaling would maintain dormancy, and offered a number of other targets (e.g., integrin-β1, epidermal growth factor receptor (EGFR), matrix metalloproteinase-9, amphiregulin and PI3K) that, when suppressed, steer malignant cells into a dormant state 46, 106–109. SRC family kinases are another potential target 110, and transcript profiling of single or small pools of DTCs promises to reveal more potential targets 111–114.
An alternative to chronic suppression of pathways activated typically in malignant cells is to instead induce expression of dormancy initiating or maintenance factors in DTCs (Fig. 3b). For instance, the stress-activated protein kinase p38 and homeobox transcription factor D10 (HOXD10) are known drivers of dormancy in different cancers 115, 116. Therefore, strategies aimed at inducing and/or sustaining p38 or HOXD10 signaling in dormant DTCs could theoretically result in maintenance of tumor dormancy.
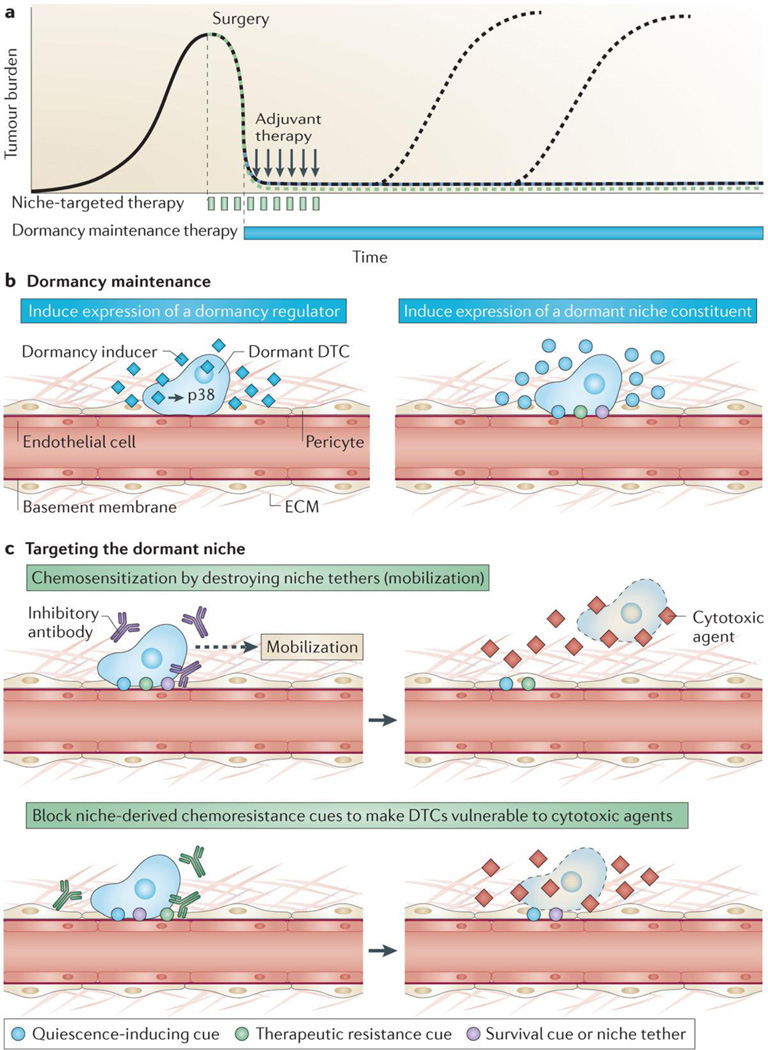
Figure 3. Two strategies to treat disseminated tumor cells.
A. Primary tumors are typically treated by neoadjuvant or adjuvant therapy (black arrows) around the time of surgery, and exhibit variable and unpredictable relapse dynamics. Two types of treatment strategies meant to address latent disseminated disease could be employed peri-operatively; chronic dormancy maintenance therapy (blue) or niche-targeted agents meant to sensitize dormant DTCs to cytotoxic treatment (green). Either strategy could result in metastasis prevention (green and blue dashed lines, respectively) B. Examples of chronic dormancy maintenance include: inducing expression of a global regulator of tumor dormancy (e.g., p38) (left panel) or using small molecules to induce expression of key dormant niche constituents, such as those that mediate DTC quiescence (e.g., thrombospondin 1 (TSP-1)) (blue circles, right panel). C. Alternatively, niche-targeted chemo-sensitization could be achieved by targeting either molecules that tether DTCs to the niche in order to induce DTC mobilization (purple circle, top panel) or cues that confer therapeutic resistance to dormant DTCs within the niche (green circle, bottom panel).
A tactic different than altering DTC physiology directly is to deliver constituents of the dormant niche in an attempt to maintain DTC growth-arrest permanently. Ostensibly, this ‘niche-based’ approach would cause minimal side effects since it involves supplementation of naturally occurring factors. However, delivery of TSP-1 and other ECM-derived glycoproteins that could mediate DTC quiescence presents a substantial hurdle because of their size 117. One strategy to overcome this hurdle is to induce expression of these molecules instead (Fig. 3b). Watnick and colleagues identified a five-amino acid peptide derived from the TSP-1-inducing glycoprotein prosaposin 117, 118_ENREF_114 that stimulates TSP-1 expression systemically. In mice bearing tumors from orthotopic injection of breast cancer cells, those treated with the peptide exhibited significantly reduced lung metastatic burden as compared to scrambled peptide treated controls 117, demonstrating the promise of a prophylactic, niche-based approach to prevent outgrowth of dormant DTCs.
What are the drawbacks? Toxicity associated with sustained inhibition of key signaling proteins like p38 or sustained induction of dormant niche factors like TSP-1 would need to be defined rigorously. For example, TSP-1 has potent anti-angiogenic activity in addition to its anti-tumor functions 119; how would its chronic, systemic induction impact physiologic processes? Another consideration is that the dormant niche can be subverted; that is, by definition dormant DTCs can eventually ‘wake up.’ Whereas supplementing niche-derived factors may prevent this from happening due to natural degradation of key niche factors (e.g., due to aging), this strategy may fail against strong and sustained induction (e.g., by wounding or inflammation) that could overwhelm the dormant niche 19, 120. Accordingly, a parallel line of investigation is to identify extracellular factors that promote DTC outgrowth; i.e., metastatic niche components 121. These constituents include ECM proteins such as fibronectin 122, periostin 123, versican 124 and tenascin-C 125 that are expressed abundantly during development, but subside in the adult organism. Ectopic re-expression by activated stroma or by endothelial tip cells19 drives metastatic outgrowth. Thus, in theory sustained inhibition of critical receptors for metastatic niche components (e.g., integrin αvβ3) could be a viable strategy— perhaps in combination with induction of dormancy-initiating factors— to prevent activation of the dormant switch.
While promising, practical issues like clinical trial enrollment and expense, patient adherence and ultimately patient expenditure have to be factored into the cost-benefit analysis of any dormancy maintenance strategy. Convincing patients who already have a good prognosis that they should take a drug indefinitely poses a challenge, and funding a clinical trial based on the endpoint of extension of metastasis-free survival—many years down the road for this patient population— could prove troublesome given that costs increase with time. Additionally, treatment adherence and reporting are expected management burdens that become increasingly problematic with time due to difficulty tracking patients and maintaining standards for dosing frequency. Indeed, persistence of patient adherence to a prescribed regimen for management of a chronic condition drops off strikingly after the first 6 months, a challenge not faced by more acute treatments 126. This could pose a serious hurdle to arriving at a clinically meaningful, statistically significant conclusion about the efficacy of a dormancy-maintenance regimen 127.
Lastly, even if successful, whether the patient is willing and able to pay for a given treatment is an additional consideration. For instance, despite findings of the ATLAS trial, which showed significant reduction in recurrence and mortality when breast cancer survivors continued tamoxifen treatment for 10-years vs. the usual 5-year period 128, some health care providers have reportedly balked at paying the costs of tamoxifen for the additional 5-years. Knowing the expense of a dormancy maintaining drug are likely, at some point, to be passed onto the patient could limit the market, and thus interest, of drug companies willing to explore this treatment avenue. Taking all of these practical concerns into account, one could argue that developing a strategy to kill dormant DTCs is the preferable option.
Option 2: Killing dormant DTCs
A therapy that eradicates dormant DTCs would not suffer from the same practical concerns as chronic dormancy-maintenance therapy. Patient enrollment in trials would be simpler because treatment would be employed acutely around the time of surgery, when systemic neoadjuvant or adjuvant therapies are already administered (Fig. 3a). Whereas both strategies would ultimately rely on a long-term indicator (metastasis-free survival) for proof of efficacy, elimination of DTCs from bone marrow aspirates within a year of treatment offers a near-term indicator of therapeutic efficacy, which does not exist for chronic dormancy-maintenance. Importantly, a smaller scale trial has already established a basis for using DTC presence as a surrogate for long-term survival 129. Importantly, data on the ability of highly-promising candidate drugs to sensitize DTCs to therapy could be gathered initially even in patients with metastatic disease, provided their bone marrow is DTC-positive but free of metastases. Thus, enrollment criteria would be less stringent than for dormancy maintenance therapies, which would be limited to early-stage patients by necessity.
How could we selectively induce death of dormant DTCs? The evidence presented in the prior section implicates the PVN in endowing DTCs with dormant properties. Thus, a viable strategy could entail a combinatorial approach that prefaces chemotherapeutic delivery with targeted disruption of key tethers to mobilize DTCs from the PVN (Fig. 3c). Successful salvage regimens used for hematopoietic cancers provide a blueprint for this approach.
Most acute myeloid leukemia (AML) patients eventually become refractory to standard cytotoxic therapy. Treatment-resistant leukemic stem cells (LSCs) persist and ultimately reconstitute the disease. Their adhesion to chemo-protective niches is thought to play a major role 130. LSCs are found in both osteoblastic niches and PVNs in bone marrow 95, 131–134. Factors derived from these niches; namely CXCL12 and osteopontin, play major roles in mediating adhesion, temporary cell cycle arrest and chemoresistance of LSCs 135, 136. Accordingly, an alternative therapeutic strategy was formulated for AML patients refractory to standard treatments that involved evicting LSCs from their therapeutic safe havens prior to cytotoxic therapy administration. G-CSF was the first mobilizing agent used and quickly gained traction as the FLAG (a combination of fludarabine and ara-c with G-CSF priming) salvage regimen 137, 138. More recently, G-CSF priming in combination with other cytotoxic agents has resulted in a complete remission rate of 46% in refractory AML patient populations 139. Current approaches employ a more niche-targeted approach, using antagonists to receptors for niche constituents CXCL-12 (CXCR4 antagonists) and osteopontin (CD44 and integrin α4β1 antagonists) to mobilize resistant leukemic cells. In fact, the use of CXCR4 inhibitors to sensitize refractory AML cells to chemotherapy has entered clinical trials already 140, and inhibition of osteopontin has proven highly effective in reducing minimal residual disease burden in preclinical models 135. These results demonstrate the viability and promise of strategies aimed at displacing tumor cells from a protective niche in order to eradicate them altogether.
A principle consideration is how to apply such a strategy to DTCs of solid tumor origin. Owing to its critical role in directing and retaining both HSCs and tumor cells to bone marrow 77, 141, 142, the CXCL12–CXCR4 axis clearly overlaps amongst some tumor types. Provocative data suggests that marrow-engrafted DTCs are mobilized into blood by application of G-CSF 76 and via inhibition of CXCL12–CXCR4 signaling 77, providing proof-of-principle that DTCs can be mobilized from their niche. However, it remains to be seen whether these approaches a) sensitize mobilized DTCs to chemotherapy and b) do not exacerbate HSC mobilization and/or depletion (which would present a major complication). This, however, is one promising avenue of exploration.
The specter that looms over this debate is whether mobilizing DTCs from the dormant niche will result in their awakening, an unpalatable and potentially undesirable side effect. Here, two points should be considered. The first is that this result would not necessarily be the disaster some predict it to be. Employment of mathematical models analogous to those pioneered by Norton and Simon143 for treatment of invasive breast cancer could define reliable windows to deploy cytotoxic therapy and kill vulnerable DTCs before they undergo multiple cell divisions. Such a model would have to account for DTC mobilization rates and cell cycle dynamics, which would require careful measurement. The second is the distinct possibility that DTC mobilization will not trigger growth. Evidence suggests mobilization and proliferation are regulated by distinct factors. For example, proliferation of dormant HSCs can be stimulated directly by interferon-α without displacing HSCs from their niche, or indirectly by G-CSF, which cleaves a number of niche tethers, resulting in mobilization of HSCs from the stem cell niche and proliferation 144. However, interfering with a growth-suppressive microenvironment does not necessarily mean that a cell will proliferate, as often assumed. Here, the role of osteopontin in leukemic cell engraftment is illustrative. Dormant leukemia cells colocalize with osteopontin in the osteoblastic niche. Targeting osteopontin receptors or osteopontin itself results in sensitization of dormant leukemic cells to the cytotoxin ara-C 135. Interestingly, unlike G-CSF145 osteopontin inhibition does not directly alter cell cycle progression of leukemia cells 135, suggesting that its role is chemo-protecting leukemic cells within a dormancy-inducing microenvironment, where other factors induce quiescence.
The implication is that distinct molecules within the dormant niche convey each of the dormancy-related properties described in Figure 1. Thus, identifying dormant niche constituents that independently regulate DTC proliferation and chemosensitivity should result in viable strategies to chemosensitize dormant DTCs without eliciting their growth (Fig. 3c). Successful implementation of such an approach will result in a means to eliminate the risk of metastatic relapse in patients with early disseminated disease.
Concluding Perspectives
The above argues that those researching solid tumors should take a cue from colleagues researching HIV and hematopoietic cancers, and invest more effort in identifying therapies that target the dormant niche. Our goal should be to sensitize the ‘sleeping giants’ these niches protect from current therapies. Because the dormant niche is perivascular and the PVN provides critical survival and therapeutic resistance cues to dormant DTCs, strategies to mobilize DTCs from this microenvironment are necessary.
Which PVNs do we target? Because most of our clinical understanding about the behavior of dormant DTCs derives from bone marrow aspirates, which sample only ~0.4% of one potential metastatic site— we have little idea of what the typical DTC burden of an early stage cancer patient is. Therefore, it is important to understand how far and wide dormant DTCs have spread. It is also important to determine whether the mechanisms governing resistance of dormant DTCs are tissue-specific, or whether unifying principles exist that will enable us to target dormant DTCs universally. This will require a comprehensive molecular description of the PVN in each tissue, which will have to be crosschecked against a catalogue of functionally relevant stem cell niche anchors so that collateral damage to stem cell populations is prevented.
The final hurdle will be to determine which patients receive dormant niche-targeted agents prior to chemotherapeutic administration. The immediate inclination would be to administer this regimen to all early-stage patients with DTC-positive bone marrow aspirates. But, we do not know whom within this patient population will progress. Approximately two-thirds of breast cancer patients with DTC-positive bone marrow have a good prognosis 7; is this because their DTCs have a functional shortcoming that prevents them from proliferating in a foreign tissue, or is it because their DTCs never see microenvironmental triggers that awaken them? The answer will determine whether the combinatorial strategy proposed is unnecessary for some or appropriate for all.
Acknowledgments
I apologize to those whose invaluable contributions to the field were not cited and assure that this was due to space limitations and not because of oversight. I am indebted to M.J. Bissell for critical insights and endless conversation that formed the basis of this article, and for funding that supported my time in her laboratory from the National Cancer Institute (Award Number U54CA143836), U.S. Department of Defense Innovator Award (W81XWH0810736); and The Breast Cancer Research Foundation. My laboratory is partially supported by the National Cancer Institute (Award Number P50CA097186) and by the Cuyamaca Foundation. I am very grateful to D. Lyden, C. Li, J. Bielas, and A. Bruni-Cardoso for their critical feedback on this manuscript, and to C.A. Grzelak for her advice on figures.
Biography
Cyrus Ghajar directs the Laboratory for the Study of Metastatic Microenvironments (LSM2) (URL: http://research.fhcrc.org/ghajar/en.html) in Fred Hutchinson Cancer Research Center’s Translational Research Program. Broadly, he is interested in how “foreign” tissue microenvironments influence the behavior of disseminated tumor cells (DTCs). Specifically, his laboratory is working to understand how tissues regulate DTC dormancy, how the dormant niche contributes to therapeutic resistance, and how local and systemic changes impact the dormant niche and foster DTC re-emergence.
Footnotes
Competing Interests Statement.
The author declares no competing financial interests.
References
- 1.Willis RA. The spread of tumours in the human body. London: J. & A. Chuchill; 1934. [Google Scholar]
- 2.Schlimok G, et al. Micrometastatic cancer cells in bone marrow: in vitro detection with anti-cytokeratin and in vivo labeling with anti-17-1A monoclonal antibodies. Proc Natl Acad Sci U S A. 1987;84:8672–8676. doi: 10.1073/pnas.84.23.8672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cote RJ, et al. Monoclonal antibodies detect occult breast carcinoma metastases in the bone marrow of patients with early stage disease. Am J Surg Pathol. 1988;12:333–340. doi: 10.1097/00000478-198805000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Pantel K, et al. Frequency and prognostic significance of isolated tumour cells in bone marrow of patients with non-small-cell lung cancer without overt metastases. Lancet. 1996;347:649–653. doi: 10.1016/s0140-6736(96)91203-9. [DOI] [PubMed] [Google Scholar]
- 5.Husemann Y, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Rhim AD, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun S, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353:793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 8.Cote RJ, Rosen PP, Lesser ML, Old LJ, Osborne MP. Prediction of early relapse in patients with operable breast cancer by detection of occult bone marrow micrometastases. J Clin Oncol. 1991;9:1749–1756. doi: 10.1200/JCO.1991.9.10.1749. [DOI] [PubMed] [Google Scholar]
- 9.Mansi JL, et al. Bone marrow micrometastases in primary breast cancer: prognostic significance after 6 years’ follow-up. Eur J Cancer. 1991;27:1552–1555. doi: 10.1016/0277-5379(91)90413-8. [DOI] [PubMed] [Google Scholar]
- 10.Pantel K, et al. Immunocytochemical detection of isolated tumour cells in bone marrow of patients with untreated stage C prostatic cancer. Eur J Cancer. 1995;31A:1627–1632. doi: 10.1016/0959-8049(95)00290-y. [DOI] [PubMed] [Google Scholar]
- 11.Thorban S, et al. Immunocytochemical detection of disseminated tumor cells in the bone marrow of patients with esophageal carcinoma. J Natl Cancer Inst. 1996;88:1222–1227. doi: 10.1093/jnci/88.17.1222. [DOI] [PubMed] [Google Scholar]
- 12.Schlimok G, et al. Micrometastatic tumour cells in bone marrow of patients with gastric cancer: methodological aspects of detection and prognostic significance. Eur J Cancer. 1991;27:1461–1465. doi: 10.1016/0277-5379(91)90032-9. [DOI] [PubMed] [Google Scholar]
- 13.Weckermann D, et al. Disseminated cytokeratin positive tumor cells in the bone marrow of patients with prostate cancer: detection and prognostic value. J Urol. 2001;166:699–703. [PubMed] [Google Scholar]
- 14.Goss PE, Chambers AF. Does tumour dormancy offer a therapeutic target? Nat Rev Cancer. 2010;10:871–877. doi: 10.1038/nrc2933. [DOI] [PubMed] [Google Scholar]
- 15.Meng S, et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004;10:8152–8162. doi: 10.1158/1078-0432.CCR-04-1110. [DOI] [PubMed] [Google Scholar]
- 16.Uhr JW, Pantel K. Controversies in clinical cancer dormancy. Proc Natl Acad Sci U S A. 2011;108:12396–12400. doi: 10.1073/pnas.1106613108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang Y, Pantel K. Tumor cell dissemination: emerging biological insights from animal models and cancer patients. Cancer Cell. 2013;23:573–581. doi: 10.1016/j.ccr.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naumov GN, et al. Persistence of solitary mammary carcinoma cells in a secondary site: a possible contributor to dormancy. Cancer Res. 2002;62:2162–2168. [PubMed] [Google Scholar]
- 19.Ghajar CM, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15:807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gimbrone MA, Jr, Leapman SB, Cotran RS, Folkman J. Tumor dormancy in vivo by prevention of neovascularization. J Exp Med. 1972;136:261–276. doi: 10.1084/jem.136.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 23.Rakhra K, et al. CD4(+) T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell. 2010;18:485–498. doi: 10.1016/j.ccr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 25.Noltenius C, Noltenius H. Dormant tumor cells in liver and brain An autopsy study on metastasizing tumors. Pathol Res Pract. 1985;179:504–511. doi: 10.1016/S0344-0338(85)80191-6. [DOI] [PubMed] [Google Scholar]
- 26.Naumov GN, Folkman J, Straume O. Tumor dormancy due to failure of angiogenesis: role of the microenvironment. Clin Exp Metastasis. 2009;26:51–60. doi: 10.1007/s10585-008-9176-0. [DOI] [PubMed] [Google Scholar]
- 27.O’Reilly MS, Holmgren L, Chen C, Folkman J. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat Med. 1996;2:689–692. doi: 10.1038/nm0696-689. [DOI] [PubMed] [Google Scholar]
- 28.Giancotti FG. Mechanisms governing metastatic dormancy and reactivation. Cell. 2013;155:750–764. doi: 10.1016/j.cell.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14:611–622. doi: 10.1038/nrc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braun S, et al. Lack of effect of adjuvant chemotherapy on the elimination of single dormant tumor cells in bone marrow of high-risk breast cancer patients. J Clin Oncol. 2000;18:80–86. doi: 10.1200/JCO.2000.18.1.80. [DOI] [PubMed] [Google Scholar]
- 31.Krawczyk N, et al. HER2 status on persistent disseminated tumor cells after adjuvant therapy may differ from initial HER2 status on primary tumor. Anticancer Res. 2009;29:4019–4024. [PubMed] [Google Scholar]
- 32.Janni W, et al. Persistence of disseminated tumor cells in the bone marrow of breast cancer patients predicts increased risk for relapse--a European pooled analysis. Clin Cancer Res. 2011;17:2967–2976. doi: 10.1158/1078-0432.CCR-10-2515. [DOI] [PubMed] [Google Scholar]
- 33.Naumov GN, et al. Ineffectiveness of doxorubicin treatment on solitary dormant mammary carcinoma cells or late-developing metastases. Breast Cancer Res Treat. 2003;82:199–206. doi: 10.1023/B:BREA.0000004377.12288.3c. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt-Kittler O, et al. From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc Natl Acad Sci U S A. 2003;100:7737–7742. doi: 10.1073/pnas.1331931100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weckermann D, et al. Perioperative activation of disseminated tumor cells in bone marrow of patients with prostate cancer. J Clin Oncol. 2009;27:1549–1556. doi: 10.1200/JCO.2008.17.0563. [DOI] [PubMed] [Google Scholar]
- 36.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 37.Weaver VM, et al. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999;93:1658–1667. [PMC free article] [PubMed] [Google Scholar]
- 39.Fridman R, et al. Reconstituted basement membrane (matrigel) and laminin can enhance the tumorigenicity and the drug resistance of small cell lung cancer cell lines. Proc Natl Acad Sci U S A. 1990;87:6698–6702. doi: 10.1073/pnas.87.17.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korah R, Boots M, Wieder R. Integrin alpha5beta1 promotes survival of growth-arrested breast cancer cells: an in vitro paradigm for breast cancer dormancy in bone marrow. Cancer Res. 2004;64:4514–4522. doi: 10.1158/0008-5472.CAN-03-3853. [DOI] [PubMed] [Google Scholar]
- 41.Sethi T, et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med. 1999;5:662–668. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- 42.Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gudjonsson T, et al. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci. 2002;115:39–50. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee EY, Parry G, Bissell MJ. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol. 1984;98:146–155. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Streuli CH, et al. Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol. 1995;129:591–603. doi: 10.1083/jcb.129.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weaver VM, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weir ML, et al. Dystroglycan loss disrupts polarity and beta-casein induction in mammary epithelial cells by perturbing laminin anchoring. J Cell Sci. 2006;119:4047–4058. doi: 10.1242/jcs.03103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 49.Kienast Y, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16:116–122. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- 50.Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer. 2010;10:138–146. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 52.Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 53.Ding BS, et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505:97–102. doi: 10.1038/nature12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ding BS, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lindahl P, et al. Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development. 1998;125:3313–3322. doi: 10.1242/dev.125.17.3313. [DOI] [PubMed] [Google Scholar]
- 56.Tufro A, Norwood VF, Carey RM, Gomez RA. Vascular endothelial growth factor induces nephrogenesis and vasculogenesis. J Am Soc Nephrol. 1999;10:2125–2134. doi: 10.1681/ASN.V10102125. [DOI] [PubMed] [Google Scholar]
- 57.Ding BS, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147:539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramasamy SK, Kusumbe AP, Wang L, Adams RH. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507:376–380. doi: 10.1038/nature13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 61.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 62.Scadden DT. Nice neighborhood: emerging concepts of the stem cell niche. Cell. 2014;157:41–50. doi: 10.1016/j.cell.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goldman SA, Chen Z. Perivascular instruction of cell genesis and fate in the adult brain. Nat Neurosci. 2011;14:1382–1389. doi: 10.1038/nn.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 65.Xiao Y, et al. Perivascular hair follicle stem cells associate with a venule annulus. J Invest Dermatol. 2013;133:2324–2331. doi: 10.1038/jid.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Christov C, et al. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell. 2007;18:1397–1409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crisan M, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 68.Butler TP, Gullino PM. Quantitation of cell shedding into efferent blood of mammary adenocarcinoma. Cancer Res. 1975;35:512–516. [PubMed] [Google Scholar]
- 69.Klein CA. Selection and adaptation during metastatic cancer progression. Nature. 2013;501:365–372. doi: 10.1038/nature12628. [DOI] [PubMed] [Google Scholar]
- 70.Calvi LM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 71.Zhang J, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 72.Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature. 2013;502:513–518. doi: 10.1038/nature12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tata PR, et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vora AJ, Toh CH, Peel J, Greaves M. Use of granulocyte colony-stimulating factor (G-CSF) for mobilizing peripheral blood stem cells: risk of mobilizing clonal myeloma cells in patients with bone marrow infiltration. Br J Haematol. 1994;86:180–182. doi: 10.1111/j.1365-2141.1994.tb03271.x. [DOI] [PubMed] [Google Scholar]
- 76.Fischer JC, et al. Diagnostic leukapheresis enables reliable detection of circulating tumor cells of nonmetastatic cancer patients. Proc Natl Acad Sci U S A. 2013;110:16580–16585. doi: 10.1073/pnas.1313594110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shiozawa Y, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121:1298–1312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stoletov K, et al. Role of connexins in metastatic breast cancer and melanoma brain colonization. J Cell Sci. 2013;126:904–913. doi: 10.1242/jcs.112748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Resovi A, Pinessi D, Chiorino G, Taraboletti G. Current understanding of the thrombospondin-1 interactome. Matrix Biol. 2014 doi: 10.1016/j.matbio.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 80.Gao H, et al. The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell. 2012;150:764–779. doi: 10.1016/j.cell.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee JH, et al. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell. 2014;156:440–455. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kobayashi A, et al. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp Med. 2011;208:2641–2655. doi: 10.1084/jem.20110840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bragado P, et al. TGF-beta2 dictates disseminated tumour cell fate in target organs through TGF-beta-RIII and p38alpha/beta signalling. Nat Cell Biol. 2013;15:1351–1361. doi: 10.1038/ncb2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shiozawa Y, et al. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia. 2010;12:116–127. doi: 10.1593/neo.91384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Calabrese C, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 87.Charles N, et al. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell. 2010;6:141–152. doi: 10.1016/j.stem.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lathia JD, et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6:421–432. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lathia JD, et al. Laminin alpha 2 enables glioblastoma stem cell growth. Ann Neurol. 2012;72:766–778. doi: 10.1002/ana.23674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pietras A, et al. Osteopontin-CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth. Cell Stem Cell. 2014;14:357–369. doi: 10.1016/j.stem.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu TS, et al. Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells. Cancer Res. 2011;71:6061–5072. doi: 10.1158/0008-5472.CAN-10-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hambardzumyan D, et al. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22:436–448. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer. 2010;10:147–156. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- 94.Lu J, et al. Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1. Cancer Cell. 2013;23:171–185. doi: 10.1016/j.ccr.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cao Z, et al. Angiocrine factors deployed by tumor vascular niche induce B cell lymphoma invasiveness and chemoresistance. Cancer Cell. 2014;25:350–365. doi: 10.1016/j.ccr.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Acharyya S, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–178. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aird WC. Endothelial cell heterogeneity. Cold Spring Harb Perspect Med. 2012;2:a006429. doi: 10.1101/cshperspect.a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blankson JN, Persaud D, Siliciano RF. The challenge of viral reservoirs in HIV-1 infection. Annu Rev Med. 2002;53:557–593. doi: 10.1146/annurev.med.53.082901.104024. [DOI] [PubMed] [Google Scholar]
- 99.Chun TW, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 100.Finzi D, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 101.Wong JK, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 102.Richman DD, et al. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 103.Archin NM, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kulkosky J, et al. Prostratin: activation of latent HIV-1 expression suggests a potential inductive adjuvant therapy for HAART. Blood. 2001;98:3006–3015. doi: 10.1182/blood.v98.10.3006. [DOI] [PubMed] [Google Scholar]
- 105.Aguirre Ghiso JA, Kovalski K, Ossowski L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J Cell Biol. 1999;147:89–104. doi: 10.1083/jcb.147.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Beliveau A, et al. Raf-induced MMP9 disrupts tissue architecture of human breast cells in three-dimensional culture and is necessary for tumor growth in vivo. Genes Dev. 2010;24:2800–2811. doi: 10.1101/gad.1990410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kenny PA, Bissell MJ. Targeting TACE-dependent EGFR ligand shedding in breast cancer. J Clin Invest. 2007;117:337–345. doi: 10.1172/JCI29518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang F, et al. Phenotypic reversion or death of cancer cells by altering signaling pathways in three-dimensional contexts. J Natl Cancer Inst. 2002;94:1494–1503. doi: 10.1093/jnci/94.19.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang F, et al. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci U S A. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.El Touny LH, et al. Combined SFK/MEK inhibition prevents metastatic outgrowth of dormant tumor cells. J Clin Invest. 2014;124:156–168. doi: 10.1172/JCI70259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chery L, et al. Characterization of single disseminated prostate cancer cells reveals tumor cell heterogeneity and identifies dormancy associated pathways. Oncotarget. 2014;5:9939–9951. doi: 10.18632/oncotarget.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim RS, et al. Dormancy signatures and metastasis in estrogen receptor positive and negative breast cancer. PLoS One. 2012;7:e35569. doi: 10.1371/journal.pone.0035569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Klein CA, et al. Combined transcriptome and genome analysis of single micrometastatic cells. Nat Biotechnol. 2002;20:387–392. doi: 10.1038/nbt0402-387. [DOI] [PubMed] [Google Scholar]
- 114.Welty CJ, et al. Single cell transcriptomic analysis of prostate cancer cells. BMC Mol Biol. 2013;14:6. doi: 10.1186/1471-2199-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aguirre-Ghiso JA, Liu D, Mignatti A, Kovalski K, Ossowski L. Urokinase receptor and fibronectin regulate the ERK(MAPK) to p38(MAPK) activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol Biol Cell. 2001;12:863–879. doi: 10.1091/mbc.12.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen A, et al. Endothelial cell migration and vascular endothelial growth factor expression are the result of loss of breast tissue polarity. Cancer Res. 2009;69:6721–6729. doi: 10.1158/0008-5472.CAN-08-4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Catena R, et al. Bone marrow-derived Gr1+ cells can generate a metastasis-resistant microenvironment via induced secretion of thrombospondin-1. Cancer Discov. 2013;3:578–589. doi: 10.1158/2159-8290.CD-12-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kang SY, et al. Prosaposin inhibits tumor metastasis via paracrine and endocrine stimulation of stromal p53 and Tsp-1. Proc Natl Acad Sci U S A. 2009;106:12115–12120. doi: 10.1073/pnas.0903120106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med. 2002;6:1–12. doi: 10.1111/j.1582-4934.2002.tb00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jones FS, Rous P. On the Cause of the Localization of Secondary Tumors at Points of Injury. J Exp Med. 1914;20:404–412. doi: 10.1084/jem.20.4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Malanchi I, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481:85–89. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 124.Kim S, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oskarsson T, et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med. 2011;17:867–874. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 127.Schmidt D, Leppik IE. Compliance in epilepsy (Elsevier ; Sole distributors for the USA and Canada. New York New York, NY, USA: Elsevier Science Pub. Co., Amsterdam; 1988. [Google Scholar]
- 128.Davies C, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Aft R, et al. Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial. Lancet Oncol. 2010;11:421–428. doi: 10.1016/S1470-2045(10)70054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Doan PL, Chute JP. The vascular niche: home for normal and malignant hematopoietic stem cells. Leukemia. 2012;26:54–62. doi: 10.1038/leu.2011.236. [DOI] [PubMed] [Google Scholar]
- 131.Cogle CR, et al. Functional integration of acute myeloid leukemia into the vascular niche. Leukemia. 2014 doi: 10.1038/leu.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Colmone A, et al. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322:1861–1865. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- 133.Ishikawa F, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25:1315–1321. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- 134.Krause DS, et al. Differential regulation of myeloid leukemias by the bone marrow microenvironment. Nat Med. 2013;19:1513–1517. doi: 10.1038/nm.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Boyerinas B, et al. Adhesion to osteopontin in the bone marrow niche regulates lymphoblastic leukemia cell dormancy. Blood. 2013;121:4821–4831. doi: 10.1182/blood-2012-12-475483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sipkins DA, et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Estey E, et al. Use of granulocyte colony-stimulating factor before, during, and after fludarabine plus cytarabine induction therapy of newly diagnosed acute myelogenous leukemia or myelodysplastic syndromes: comparison with fludarabine plus cytarabine without granulocyte colony-stimulating factor. J Clin Oncol. 1994;12:671–678. doi: 10.1200/JCO.1994.12.4.671. [DOI] [PubMed] [Google Scholar]
- 138.Visani G, et al. FLAG (fludarabine + high-dose cytarabine + G-CSF): an effective and tolerable protocol for the treatment of ‘poor risk’ acute myeloid leukemias. Leukemia. 1994;8:1842–1846. [PubMed] [Google Scholar]
- 139.Becker PS, et al. Clofarabine with high dose cytarabine and granulocyte colony-stimulating factor (G-CSF) priming for relapsed and refractory acute myeloid leukaemia. Br J Haematol. 2011;155:182–189. doi: 10.1111/j.1365-2141.2011.08831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Becker PS. Dependence of acute myeloid leukemia on adhesion within the bone marrow microenvironment. ScientificWorldJournal. 2012;2012:856467. doi: 10.1100/2012/856467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Muller A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 142.Peled A, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 143.Norton L, Simon R. Tumor size, sensitivity to therapy, and design of treatment schedules. Cancer Treat Rep. 1977;61:1307–1317. [PubMed] [Google Scholar]
- 144.Trumpp A, Essers M, Wilson A. Awakening dormant haematopoietic stem cells. Nat Rev Immunol. 2010;10:201–209. doi: 10.1038/nri2726. [DOI] [PubMed] [Google Scholar]
- 145.Saito Y, et al. Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nat Biotechnol. 2010;28:275–280. doi: 10.1038/nbt.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hardingham JE, et al. Molecular detection of blood-borne epithelial cells in colorectal cancer patients and in patients with benign bowel disease. Int J Cancer. 2000;89:8–13. [PubMed] [Google Scholar]
- 147.Pantel K, et al. Circulating epithelial cells in patients with benign colon diseases. Clin Chem. 2012;58:936–940. doi: 10.1373/clinchem.2011.175570. [DOI] [PubMed] [Google Scholar]
- 148.Rhim AD, et al. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology. 2014;146:647–651. doi: 10.1053/j.gastro.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lindemann F, Schlimok G, Dirschedl P, Witte J, Riethmuller G. Prognostic significance of micrometastatic tumour cells in bone marrow of colorectal cancer patients. Lancet. 1992;340:685–689. doi: 10.1016/0140-6736(92)92230-d. [DOI] [PubMed] [Google Scholar]
- 150.Putz E, et al. Phenotypic characteristics of cell lines derived from disseminated cancer cells in bone marrow of patients with solid epithelial tumors: establishment of working models for human micrometastases. Cancer Res. 1999;59:241–248. [PubMed] [Google Scholar]
- 151.Suzuki M, Mose ES, Montel V, Tarin D. Dormant cancer cells retrieved from metastasis-free organs regain tumorigenic and metastatic potency. Am J Pathol. 2006;169:673–681. doi: 10.2353/ajpath.2006.060053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gil-Bernabe AM, et al. Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood. 2012;119:3164–3175. doi: 10.1182/blood-2011-08-376426. [DOI] [PubMed] [Google Scholar]
- 153.Peinado H, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Barkan D, et al. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. 2010;70:5706–5716. doi: 10.1158/0008-5472.CAN-09-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bruns I, et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med. 2014;20:1315–1320. doi: 10.1038/nm.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kunisaki Y, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kumar ME, et al. Mesenchymal cells. Defining a mesenchymal progenitor niche at single-cell resolution. Science. 2014;346:1258810. doi: 10.1126/science.1258810. [DOI] [PMC free article] [PubMed] [Google Scholar]