Abstract
[Purpose] This study examined age-related differences in muscle control for support and propulsion during walking in both males and females in order to develop optimal exercise regimens for muscle control. [Subjects and Methods] Twenty elderly people and 20 young people participated in this study. Coordinates of anatomical landmarks and ground reaction force during walking were obtained using a 3D motion analysis system and force plates. Muscle forces during walking were estimated using OpenSim. Muscle modules were obtained by using non-negative matrix factorization analysis. A two-way analysis of covariance was performed to examine the difference between the elderly and the young in muscle weightings using walking speed as a covariate. The similarities in activation timing profiles between the elderly and the young were analyzed by cross-correlation analysis in males and females. [Results] In the elderly, there was a change in the coordination of muscles around the ankle, and muscles of the lower extremity exhibited co-contraction in late stance. Timing and shape of these modules were similar between elderly and young people. [Conclusion] Our results suggested that age-related alteration of muscle control was associated with support and propulsion during walking.
Key words: Elderly people, Muscle force, Non-negative matrix factorization
INTRODUCTION
As people age, age-related changes of physical function affect their ability to walk1). As the ability to walk in the elderly decreases, there is an associated decrease in balance ability2, 3) and independent daily living activities4) and increased risk of falling5). Therefore, it is necessary for the elderly to maintain the ability to walk. The ability to walk in the elderly is often represented by the walking speed. The elderly have low vertical and antero-posterior ground reaction force for support and propulsion related to slower walking speed, with a shorter stride length6). Thus, these forces can be considered to be factors affecting walking speed in elderly people.
The biomechanical features of walking change with age. In kinematic analysis, Murray and Kory7) and Finley et al.8) reported that the elderly demonstrate decreased joint angles of the hip, knee, and ankle. Through kinetic analysis, it has been reported that the hip and knee joint moments of the elderly were significantly lower than those of young people9). In addition, decline in plantar flexor moment and power in the elderly is related to slower walking speed10, 11). Moreover, there are also age-related changes in walking strategy in the elderly. DeVita and Hortobagyi12) reported that elderly people showed different distributions of joint moment and power as compared with young people. Toda et al.13) reported differences in joint moment, which were related to the profiles of ground reaction force between the elderly and young people. These results indicate that the biomechanical impact of aging is not solely represented as a reduction in motor function but also involves a change in control strategy for walking.
Local muscle movements are regulated by pools of motor neurons in the spinal cord, which are part of the dispersed locomotor central pattern generator (CPG) network14). This network in the central nervous system control motor behavior by modulating the appropriate amount, timing, and combination of muscle activation. Muscle activation patterns of the lower extremity during various motor tasks are produced by muscle modules. With regard to walking, each muscle module corresponds to a key phase of the walking cycle consistent with biomechanical subtasks in walking15). Muscle activation is managed by five basic modules in young people in walking16). Previous studies reported that there are age-related changes in muscle modules used in the sit to stand movement17) and in preparation to make a step18). Regarding walking, muscle activation patterns of the elderly change with age8), and differences in muscle synergy might be caused by aging19). Therefore, aging might be associated with the alternation of neural control in muscle activation in various movements. The targeted neuromotor and biomechanical function, including the timing function, can only be activated by performance of appropriate actions20). In order to develop optimal exercise regimens to prevent reduction of the ability to walk in the elderly, it is necessary to understand the differences in the timing and combinations of muscle activation during walking.
The purpose of this study was to examine age-related differences in muscle modules and to examine the role taken by these muscle modules during walking in both males and females. We hypothesized that there are age-related alterations of the muscle coordination during walking and that the support and propulsive force corresponding to these muscle modules are reduced with age.
SUBJECTS AND METHODS
Twenty elderly people, 65 years old or older and 20 younger people, aged 20 to 29 years, participated in this study. There were 10 males and 10 females in each group. The subjects were all right-handed. The exclusion criteria included neurologic disorders, osteoarthritis, rheumatic arthritis, joint pain affecting walking, and history of surgery in the lower extremities or spine. All procedures were approved by the Hiroshima International University Human Research Ethics Committee and all participants gave their written, informed consent prior to enrollment.
In the walking trials, all participants walked while barefoot to the end of a 7 m walkway at a self-selected preferred walking speed. A 3D motion analysis system with 8 infrared cameras (Vicon MX; Vicon Motion Systems, Oxford, UK) and 8 force plates (AMTI, Watertown, MA, USA) was used to record kinematic and kinetic data at sampling frequencies of 100 and 1,000 Hz, respectively. The force plate layout was designed so as to measure the ground reaction force of each limb using four force plates arranged in the longitudinal direction. Participants were instructed to walk such that in each step they placed a foot on the left or right force plate, avoiding stepping on two force plates simultaneously. Infrared-reflecting markers were attached to 30 landmarks according to our previous study13).
Walking speed (m/s) was calculated from the position of the center of gravity (COG) of the whole body. Stride length (m) was measured as the antero-posterior distance of the position of the left calcaneal tuberosity marker at a heel contact and the next heel contact. Stride length was normalized to body height (%BH). Initial contact was assumed to occur when the vertical reaction force exceeded 10 N, and toe off was assumed to occur in the first frame following the initial contact where the vertical reaction force was <10 N.
Muscle forces during walking were estimated using OpenSim 3.221). The body was modeled as a 23 degree-of-freedom linkage actuated by 92 musculotendon actuators. Muscle parameters and path geometries were based on the data reported by Delp et al22). Optimal muscle fiber lengths and tendon slack lengths were scaled from a generic model to fit each subject using segment dimensions as scaling factors. Both ground reaction forces and marker trajectories were low-pass filtered with a fourth-order Butterworth filter (6 Hz). Data from the left leg were used in the analysis. OpenSim software24) was used to generate and analyze simulations of walking using a single representative gait trial of each subject. Inverse kinematics and inverse dynamics were subsequently applied, in conjunction with the measured marker trajectories and ground reaction forces and moments, to obtain a set of dynamically consistent joint angles and moments. The static optimization problem was solved within the OpenSim environment to determine the set of muscle forces corresponding to the prescribed motion. The objective was to minimize the sum of squares of all muscle activations in the model23). The gluteus maximus (GMAX), gluteus medius (GMED), gluteus minimus (GMIN), iliopsoas (IL), rectus femoris (RF), vastii muscles (VAS), hamstrings (HAM), gastrocnemius (GAS), soleus (SOL) and, tibialis anterior (TA) were analyzed.
The non-negative matrix factorization (NMF) algorithm24, 25) was used for analysis of muscle synergy modules. NMF defines the muscle activation modules by populating two matrixes indicating the relative weighting of each muscle within each module and reflecting the activation timing profile of the module across the gait cycle. Force values of each muscle were normalized to the maximum value for that muscle so that each value fit between 0 and 1. To select the number of muscle synergies that could best reproduce the recorded data, we extracted 1–7 synergy matrices and synergy activation profile matrices from the muscle force data matrices obtained for each subject. Then, we verified the goodness-of-fit between the original data (Do) and the reconstructed data (Dr) matrices, to select the smallest number of muscle synergies that result in an adequate reconstruction of muscle responses. We calculated the variability accounted for (VAF) as the ratio of the sum of squared error values to the sum of the squared original dataset values [VAF = 1 − (Do − Dr)2 / Do2 ], which was based on the entire dataset. VAF was sensitive to both shape and magnitude of the original and reconstructed data sets. Calculations of NMF were performed with Matlab R2013a (The MathWorks, Inc., Natick, MA, USA).
In order to evaluate the relationship between age-related difference in muscle modules and support and propulsive force during walking, the vertical and antero-posterior ground reaction forces corresponding to the peak value of the activation timing profile of each module were identified. To account for different builds, ground reaction force was normalized to each subject’s body mass (N/kg).
Because a previous study reported that there are gender differences in age-related changes in walking characteristics13), this study examined the gender differences according to age. A three-way analysis of variance with the number of synergies, age, and gender as factors was performed in order to determine the number of contributing synergies. A two-way analysis of covariance was performed to examine the difference between the elderly and the younger and the interaction between age and gender groups in muscle weightings using walking speed as a covariate. The similarities of the activation timing profiles between the elderly and the young were analyzed by cross-correlation analysis in males and females, respectively. The revel of statistical significance was set at p < 0.05. All data were analyzed with the SPSS 17.0 software (SPSS Japan Inc., Tokyo, Japan).
RESULTS
The baseline demographic characteristics and spatiotemporal parameters of the study subjects are presented in Table 1. The elderly participants were significantly shorter in body height and higher in BMI, but not significantly different in body mass, as compared with younger participants. The elderly males and females had significantly slower walking speeds, along with shorter stride lengths than the younger participants.
Table 1. Characteristics of subjects.
| Variable | Elderly | Young | ||
|---|---|---|---|---|
| Males (n = 10) | Females (n = 10) | Males (n = 10) | Females (n = 10) | |
| Age (years) | 68.4 (3.0)* | 69.1 (4.3)* | 21.7 (2.1) | 24.1 (2.9) |
| Height (m) | 1.65 (0.07)* | 1.50 (0.03)* | 1.71 (0.07) | 1.57 (0.04) |
| Weight (kg) | 64.3 (10.0) | 53.9 (7.8) | 60.6 (5.1) | 48.3 (6.5) |
| BMI (kg/m2) | 23.5 (2.8)* | 23.8 (3.4)* | 20.6 (1.6) | 19.4 (1.7) |
| Walking speed (m/s) | 1.16 (0.12)* | 1.15 (0.13)* | 1.28 (0.14) | 1.27 (0.07) |
| Stride length (%BH) | 72.9 (5.7)* | 73.1 (5.0)* | 78.7 (5.0) | 81.1 (3.7) |
Value are shown as the mean (standard deviation: SD).
*Significant difference from the young at p < 0.05.
BMI: body mass index; %BH: percent body height
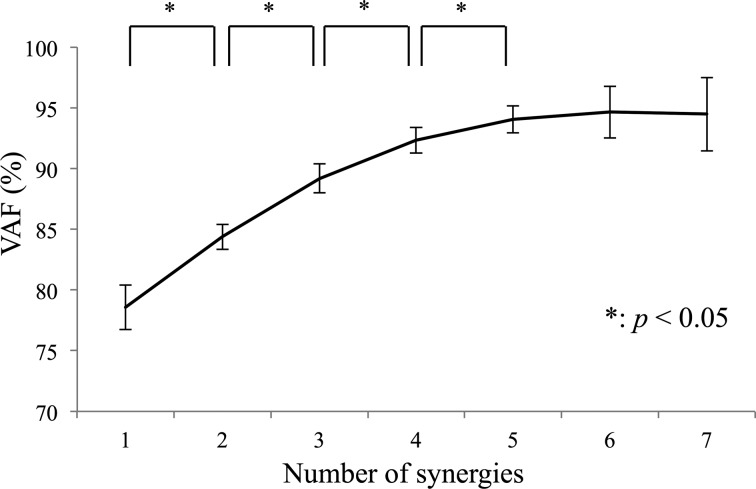
The main effect of the number of synergies was significant (F6, 252 = 454.52, p < 0.001), and NMF identified five muscle modules, as VAF significantly increased to the fifth of synergies (Fig. 1). There was no significant interaction between number of synergies and age (F6, 252 = 0.61, p = 0.72), number of synergies and gender (F6, 252 = 0.31, p = 0.57), and number of synergies, age, and gender (F6, 252 = 0.27, p = 0.94). When the module profiles and weightings were used to reconstruct the muscle force data, they accounted for more than 90% of the muscle force variance for all muscles and regions in the gait cycle.
Fig. 1.
Variability accounted for (VAF) in the determination of the number of synergies, averaged over the test subjects (n = 40)
VAF indicate the ratio of consistency of both the shape and magnitude of the original and reconstructed data sets. Error bars span ± one standard deviation
Muscle weightings during walking are shown in Table 2. Module 1 primarily contributes to weight acceptance in early stance. GMED, GMIN and VAS mainly act in module 1. The muscle weighting of the TA significantly increased in the elderly. Moreover, muscle weighting of SOL exhibited significant interaction between age and gender, and only elderly females had significantly lower muscle weighting as compared with young females. Module 2 contributes to propulsion in late stance. GAS and SOL mainly act in module 2. The muscle weightings of the GMAX, RF, and TA significantly increased in the elderly. Module 3 contributes to body support during the stance phase. The IL and RF mainly act in module 3. The muscle weighting of the IL significantly decreased in the elderly. In addition, there was significant interaction between age and gender in the muscle weighting of the GAS, and only elderly females had significantly higher muscle weighting as compared with young females. Module 4 contributes to deceleration of the leg motion and preparation for weight acceptance in late swing. The GMAX and HAM mainly act in module 4. The muscle weighting of the TA significantly increased in the elderly. The module 5 contributes to weight acceptances at initial contact. The TA mainly acts in module 5. The muscle weighting of the GAS significantly increased in the elderly, although the contribution was small.
Table 2. Comparison of muscle weightings indicating the relative contribution of each muscle within the corresponding module.
| Module | Age | Gender | GMAX | GMED | GMIN | IL | RF | VAS | HAM | GAS | SOL | TA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Elderly | Males (n=10) | 0.99 (0.83) | 2.22 (0.92) | 2.24 (0.72) | 0.10 (0.24) | 1.00 (0.75) | 2.30 (0.91) | 0.20 (0.50) | 0.06 (0.09) | 0.52 (0.63) | 1.27 (1.00)* |
| Females (n=10) | 1.55 (1.17) | 2.07 (1.07) | 2.11 (1.01) | 0.34 (0.74) | 1.57 (0.65) | 2.14 (0.98) | 0.17 (0.18) | 0.02 (0.09) | 0.42 (0.56)† | 1.02 (1.13)* | ||
| Young | Males (n=10) | 0.68 (0.51) | 2.58 (0.93) | 2.50 (0.76) | 0.24 (0.50) | 1.53 (0.83) | 1.86 (0.85) | 0.01 (0.26) | 0.07 (0.09) | 0.44 (0.59) | 0.83 (0.63) | |
| Females (n=10) | 0.86 (1.01) | 2.35 (0.81) | 2.11 (0.74) | 0.02 (0.06) | 1.66 (0.73) | 2.02 (0.53) | 0.10 (0.26) | 0.13 (0.25) | 1.28 (0.66) | 0.41 (0.51) | ||
| 2 | Elderly | Males (n=10) | 0.95 (0.73)* | 1.27 (0.58) | 1.39 (0.48) | 1.50 (1.16) | 0.56 (0.50)* | 0.05 (0.11) | 0.17 (0.20) | 3.72 (0.37) | 3.92 (0.34) | 0.84 (0.80)* |
| Females (n=10) | 1.32 (0.61)* | 0.87 (0.71) | 0.80 (0.60) | 1.89 (1.54) | 1.12 (0.78)* | 0.65 (1.25) | 0.18 (0.27) | 3.24 (1.18) | 4.11 (0.74) | 1.64 (1.05)* | ||
| Young | Males (n=10) | 0.43 (0.29) | 0.94 (0.60) | 1.21 (0.40) | 2.15 (0.88) | 0.57 (0.45) | 0.02 (0.07) | 0.11 (0.14) | 3.14 (0.95) | 3.60 (0.56) | 0.21 (0.31) | |
| Females (n=10) | 0.95 (0.70) | 0.84 (0.45) | 0.91 (0.51) | 1.76 (0.72) | 0.46 (0.33) | 0.11 (0.17) | 0.28 (0.44) | 3.69 (0.69) | 3.90 (0.37) | 0.87 (0.66) | ||
| 3 | Elderly | Males (n=10) | 0.08 (0.17) | 0.16 (0.34) | 0.26 (0.33) | 1.97 (0.82)* | 2.61 (0.25) | 1.34 (0.79) | 0.05 (0.08) | 0.02 (0.04) | 0.30 (0.29) | 0.38 (0.45) |
| Females (n=10) | 0.46 (0.65) | 1.02 (1.49) | 1.03 (1.39) | 2.19 (1.28)* | 2.25 (1.11) | 1.37 (0.78) | 0.09 (0.09) | 0.53 (0.64)† | 0.80 (1.21) | 0.87 (0.62) | ||
| Young | Males (n=10) | 0.41 (1.30) | 0.10 (0.23) | 0.47 (0.33) | 2.75 (1.31) | 2.63 (1.22) | 0.73 (0.75) | 0.32 (0.65) | 0.17 (0.36) | 0.38 (0.32) | 0.26 (0.66) | |
| Females (n=10) | 0.14 (0.20) | 0.45 (0.68) | 0.40 (0.31) | 2.63 (0.63) | 2.54 (0.51) | 1.34 (0.42) | 0.11 (0.12) | 0.04 (0.12) | 0.26 (0.31) | 0.37 (0.44) | ||
| 4 | Elderly | Males (n=10) | 1.51 (1.17) | 0.32 (0.11) | 0.27 (0.16) | 0.84 (1.19) | 0.11 (0.23) | 0.04 (0.09) | 2.15 (1.17) | 0.73 (0.51) | 0.06 (0.06) | 1.57 (1.19)* |
| Females (n=10) | 2.07 (0.86) | 0.09 (0.21) | 0.27 (0.21) | 0.36 (0.33) | 0.02 (0.01) | 0.04 (0.10) | 2.87 (0.29) | 2.08 (1.13) | 0.29 (0.35) | 1.28 (1.03)* | ||
| Young | Males (n=10) | 1.74 (1.03) | 0.26 (0.07) | 0.45 (0.33) | 0.18 (0.21) | 0.01 (0.04) | 0.00 (0.00) | 2.71 (0.51) | 1.58 (1.26) | 0.19 (0.37) | 0.58 (0.60) | |
| Females (n=10) | 2.70 (0.53) | 0.08 (0.11) | 0.55 (0.74) | 0.48 (0.32) | 0.03 (0.07) | 0.01 (0.04) | 2.82 (0.34) | 1.77 (0.90) | 0.13 (0.21) | 0.95 (1.21) | ||
| 5 | Elderly | Males (n=10) | 2.01 (1.09) | 0.74 (1.00) | 1.00 (0.82) | 0.95 (0.93) | 0.11 (0.19) | 0.49 (0.43) | 0.86 (1.13) | 0.31 (0.25)* | 0.32 (0.59) | 2.42 (1.65) |
| Females (n=10) | 1.25 (0.87) | 0.40 (0.58) | 0.55 (0.59) | 0.91 (1.17) | 0.44 (0.80) | 0.60 (0.84) | 0.34 (0.57) | 0.41 (0.43)* | 0.13 (0.25) | 2.74 (1.28) | ||
| Young | Males (n=10) | 1.39 (1.05) | 0.60 (0.83) | 1.01 (0.86) | 0.45 (0.39) | 0.30 (0.52) | 0.95 (0.79) | 0.21 (0.23) | 0.11 (0.17) | 0.00 (0.00) | 2.56 (1.03) | |
| Females (n=10) | 2.12 (1.32) | 0.51 (0.51) | 0.85 (0.41) | 1.03 (0.77) | 0.34 (0.90) | 0.70 (0.47) | 0.43 (0.80) | 0.18 (0.23) | 0.16 (0.37) | 3.18 (1.11) |
Value are shown as the mean (standard deviation: SD).
*Significant difference from the young at p < 0.05
†Significant difference from the young females at p < 0.05
The cross-correlation coefficient between the elderly and young people was large (r > 0.70) in all modules in both males and females, and the activation timing profiles of the elderly and young people were highly similar (Table 3).
Table 3. Similarity of activation patterns of muscle modules between the elderly and the young.
| Module 1 | Module 2 | Module 3 | Module 4 | Module 5 | |
|---|---|---|---|---|---|
| Males | 0.97* | 0.97* | 0.79* | 0.94* | 0.78* |
| Females | 0.95* | 0.97* | 0.79* | 0.97* | 0.75* |
Values are cross-correlation coefficients of activation profiles. The correlation coefficient (r) is interpreted as large (r > 0.7).*p < 0.05
The ground reaction force profiles corresponding to each muscle module are presented in Table 4. Both the vertical and antero-posterior components corresponding to module 1 were significantly lower in the elderly as compared with younger people. Antero-posterior component corresponding to module 3 of the elderly was significantly lower than that of the young. In addition, the antero-posterior component corresponding to module 2 was significantly lower in the elderly males than that in the young males.
Table 4. Comparison of vertical and antero-posterior ground reaction force profiles corresponding to each muscle module.
| Ground reaction force (N/kg) | Module | Elderly | Young | ||
|---|---|---|---|---|---|
| Males (n = 10) | Females (n = 10) | Males (n = 10) | Females (n = 10) | ||
| Vertical | 1 | 8.41 (1.79)* | 8.67 (1.63)* | 9.68 (1.76) | 10.26 (0.86) |
| 2 | 8.93 (0.72) | 9.36 (0.43) | 9.49 (0.98) | 8.81 (0.70) | |
| 3 | 7.95 (2.21) | 7.85 (2.09) | 8.38 (3.18) | 9.40 (0.78) | |
| 4 | 0.08 (0.08) | 0.06 (0.09) | 0.04 (0.06) | 0.08 (0.06) | |
| 5 | 3.74 (3.86) | 5.20 (3.15) | 5.45 (1.00) | 5.92 (2.56) | |
| Antero-posterior | 1 | −1.49 (0.55)* | −1.56 (0.36)* | −1.85 (0.37) | −1.93 (0.28) |
| 2 | 0.24 (0.13)† | 0.61 (0.41) | 0.61 (0.37) | 0.42 (0.25) | |
| 3 | 1.61 (0.30)* | 1.57 (0.37)* | 1.92 (0.73) | 2.32 (0.35) | |
| 4 | 0.00 (0.01) | 0.00 (0.04) | 0.00 (0.03) | 0.03 (0.03) | |
| 5 | −0.54 (0.69) | −0.54 (0.46) | −0.86 (0.46) | −0.80 (0.44) | |
Value are shown as the mean (standard deviation: SD). Positive and negative values of antero-posterior ground reaction force indicate anterior and posterior components, respectively. *Significant difference from the young at p < 0.05. †Significant difference from the young males at p < 0.05.
DISCUSSION
This study investigated age-related differences in the muscle control for support and propulsion during walking in both males and females. This study revealed that, in the elderly, there were age-related changes in the coordination of muscles around the ankle and that muscles of the lower extremity exhibited co-contraction in late stance. In addition, these age-related alterations of muscle control might be associated with support and propulsive forces during walking. These findings support our hypothesis that there are age-related alterations in muscle coordination during walking, and that the support and propulsive force corresponding to these muscle modules are reduced in the elderly.
The timing and composition of each module obtained in this study corresponded to key phases of the gait cycle, consistent with the biomechanical subtasks of walking. In this study, five muscle modules were obtained in all groups. The activation pattern in each module was approximately consistent with previous studies16, 26). Moreover, the timing and shape of these modules were similar between the elderly and young people in both males and females. Ivanenko et al.16) reported that there were common basic patterns, even when walking conditions were changed. The age-related change of the underlying activation timing profile might be small too. On the other hand, there were age-related alternations in the contribution of muscles to each module. The pattern and combination of muscle activation are regulated by interneurons in the spinal cord that are part of the dispersed CPG14). Elderly people had changed the combinations of muscles rather than the activation pattern in order to achieve the walking subtasks.
The GMED, GMIN, and VAS contributed to weight acceptance in early stance. These muscles generate vertical and backward accelerations of the center of gravity and contribute to support and braking of the body27). Moreover, the contribution of the TA significantly increased in the elderly. The TA also generates vertical and backward accelerations of the center of gravity in early stance27). Elderly people have muscle weakness of the lower extremity, especially in the leg extensors28). Consequently, muscles of the ankle joint as well as the hip and knee joints also participate in weight acceptance. In addition, the contribution of the SOL was large only in young females and significantly decreased in elderly females. The SOL also generates only a small amount of vertical and backward accelerations of the center of gravity in early stance27) and may assist support and braking of the body in young females. Especially in females, there are age-related changes in the muscle coordination of the ankle in early stance. Both the vertical and antero-posterior components corresponding to this module were significantly lower in the elderly as compared to the younger people. The age-related alteration of ankle control in early stance may affect the support and braking force.
The GAS and SOL contributed in late stance to generate vertical and forward accelerations of the center of gravity, and they contribute to support and propulsion on the body27). Moreover both elderly males and females had significantly high muscle weightings of the GMAX, RF, and TA. This result suggested that many muscles in the lower extremity in late stance were co-contracting in the elderly. Co-contraction of lower extremity muscles can be predictive of performance in a dynamic balance test29) and risk of falling during walking30) in the elderly. Co-contraction of lower extremity muscles in late stance in the elderly may compensate for low balance ability. Only elderly males had a significantly lower anterior component of ground reaction force corresponding to this module compared with young males. Therefore, co-contraction in late stance may lead to low propulsive force with a slow walking speed in elderly males. On the other hand, there was no difference in this force between the elderly and young females. The elderly females may have generated propulsive force using various muscles, and there was a gender difference in the effect of co-contraction in late stance on propulsive force.
The RF and IL contributed to support, braking, and propulsion of the body in early and late stance. Neptune et al.31) found that the hip flexors, IL, and RF made larger contributions to swing initiation and trunk propulsion, respectively. The muscle weighting of the IL in the elderly significantly decreased for this module, and the antero-posterior component corresponding to this module in the elderly was significantly lower than that in the young. Therefore, functional decline of the IL in the elderly affected propulsion in late stance, and the elderly depended on the RF in performance of the stance-to-swing transition. Moreover, only elderly females had a large muscle weighting of the GAS. Support, braking, and propulsion due to this module was performed by co-contraction between the hip flexor and the ankle plantar flexor in the elderly females.
The GMAX and HAM mainly act in late swing in module 4. The ground reaction force corresponding to this module was very small, and this module was not directly involved in the generation of ground reaction force. The HAM is activated to decelerate the leg during the swing phase in preparation for foot contact27). Moreover, both elderly males and females had significantly high muscle weightings of the TA. The TA generates vertical and backward accelerations of the center of gravity in early stance30). The TA in module 1 of the elderly also had significantly high muscle weightings. It follows that the TA of the elderly greatly contributed to preparation of foot contact from late stance to early stance.
The TA contributes to weight acceptance at initial contact through co-activation of the GMAX. These muscles have role in shock absorption at initial contact. Age-related alteration of this module did not affect ground reaction force. The muscle weighting of the GAS of the elderly was significantly greater. The ankle of the elderly accepted their body weight by co-contraction between dorsiflexors and plantar flexor at initial contact.
The weightings of muscles in the ankle were significantly different between the elderly and the young in all modules. There was an age-related change in the coordination of the ankle. De Vita and Hortobagyi12) reported that the elderly showed a redistribution of joint moments and powers, which emphasized proximal extensors and de-emphasized distal extensors compared with the young. Judge et al. 32), reported that peak ankle joint power generation at pre-swing decreased in the elderly. It is necessary for improvement of the elderly’s ability to walk to inprove ankle control in addition to muscle strengthening, because the influence of functional decline with age is readily apparent in the ankle joint during walking.
In this study, analysis of muscle tension force was performed using musculoskeletal simulation. Generally, surface electromyography (sEMG) is often used for the analysis of muscle activity during motion. However, the results of sEMG are affected by not only the amount of muscle activity but also the thickness and conductivity of the skin and subcutaneous fat. Basmajian et al.33) reported that the integrated value of muscle activity potentials and the muscle force during isometric contraction have a linear or curvilinear relationship. On the other hand, the muscle tension force determined by musculoskeletal simulation in the present study was calculated from kinematics data, kinetic data, and physical parameters obtained by the three-dimensional motion analysis system, with the uncertainties inherent to the used model. As estimated muscle tension forces were analyzed, it is necessary to confirm the reliability of the estimated data. The waveform of the muscle force obtained in this study is similar to the waveform of the muscle activity of the lower limbs reported by Winter34). Therefore, the reliability for estimated data of the muscle tension force used in this study was assumed to be good.
The present study has several limitations. First, the model used in this study was identical of the young and the elderly. The influence of muscle mass difference between the young and the elderly can be removed by normalization according to the subject’s body weight, and the dimensions of the segments can be scaled to match the subject in OpenSim. However, viscoelastic changes in muscles and deformation of the spine could not be incorporated into the model. In the future, in order to perform musculoskeletal simulation with higher accuracy, it is necessary to develop a model and simulation system incorporating the physical features of the subject, such as age, gender, and joint deformity. Second, this study showed that muscle modules during walking changed with age, but we were unable to identify the causative factor of the difference in muscle modules between the elderly and young people. Elderly people experience a functional decline in the musculoskeletal system and nervous system with aging. Further studies are required to determine the relationship between muscle control during walking and physical function in the elderly.
REFERENCES
- 1.Steffen TM, Hacker TA, Mollinger L: Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys Ther, 2002, 82: 128–137. [DOI] [PubMed] [Google Scholar]
- 2.Miyabara H, Nishi M: The relation of walking velocity with motor ability and functional capacity in the community dwelling elderly. J Phys Ther Sci, 2008, 20: 59–62. [Google Scholar]
- 3.Shimada H, Kim H, Yoshida H, et al. : Factors associated with the timed up and go test score in elderly women. J Phys Ther Sci, 2010, 22: 273–278. [Google Scholar]
- 4.Potter JM, Evans AL, Duncan G: Gait speed and activities of daily living function in geriatric patients. Arch Phys Med Rehabil, 1995, 76: 997–999. [DOI] [PubMed] [Google Scholar]
- 5.Huo M, Maruyama H, Akiyama S: An approach to assessment of the fall risk for the elderly by probe reaction time during walking. J Phys Ther Sci, 2009, 21: 311–316. [Google Scholar]
- 6.Yamada T, Maie K: The characteristics of walking in old men analyzed from the ground reaction force. J Anthrop Soc, 1988, 96: 7–15. [Google Scholar]
- 7.Murray MP, Kory RC, Clarkson BH: Walking patterns in healthy old men. J Gerontol, 1969, 24: 169–178. [DOI] [PubMed] [Google Scholar]
- 8.Finley FR, Cody KA, Finizie RV: Locomotion patterns in elderly women. Arch Phys Med Rehabil, 1969, 50: 140–146. [PubMed] [Google Scholar]
- 9.Kerrigan DC, Todd MK, Della Croce U, et al. : Biomechanical gait alterations independent of speed in the healthy elderly: evidence for specific limiting impairments. Arch Phys Med Rehabil, 1998, 79: 317–322. [DOI] [PubMed] [Google Scholar]
- 10.Judge JO, Davis RB, 3rd, Ounpuu S: Step length reductions in advanced age: the role of ankle and hip kinetics. J Gerontol A Biol Sci Med Sci, 1996, 51: M303–M312. [DOI] [PubMed] [Google Scholar]
- 11.Winter DA: Overall principle of lower limb support during stance phase of gait. J Biomech, 1980, 13: 923–927. [DOI] [PubMed] [Google Scholar]
- 12.DeVita P, Hortobagyi T: Age causes a redistribution of joint torques and powers during gait. J Appl Physiol 1985, 2000, 88: 1804–1811. [DOI] [PubMed] [Google Scholar]
- 13.Toda H, Nagano A, Luo Z: Age and gender differences in the control of vertical ground reaction force by the hip, knee and ankle joints. J Phys Ther Sci, 2015, 27: 1833–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goulding M: Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci, 2009, 10: 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neptune RR, Clark DJ, Kautz SA: Modular control of human walking: a simulation study. J Biomech, 2009, 42: 1282–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivanenko YP, Poppele RE, Lacquaniti F: Five basic muscle activation patterns account for muscle activity during human locomotion. J Physiol, 2004, 556: 267–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi A, Ikemoto Y, Asama H: Muscle synergy analysis between young and elderly people in standing-up motion. J Roboti Mech, 2013, 25: 1038–1049. [Google Scholar]
- 18.Wang Y, Zatsiorsky VM, Latash ML: Muscle synergies involved in preparation to a step made under the self-paced and reaction time instructions. Clin Neurophysiol, 2006, 117: 41–56. [DOI] [PubMed] [Google Scholar]
- 19.Monaco V, Ghionzoli A, Dario P, et al. : Muscle synergies during walking: comparison between young and elderly people. Preliminary results. In: Proceedings of 30th Annual International IEEE EMBS conference, 2008, pp 5370–5373. [DOI] [PubMed]
- 20.Car JH, Shepherd RB: Neurological Rehabilitation, Optimal Motor Performance. London: Churchill Livingstone, 2010, pp 15–55. [Google Scholar]
- 21.Delp SL, Anderson FC, Arnold AS, et al. : OpenSim: open-source software to create and analyze dynamic simulations of movement. IEEE Trans Biomed Eng, 2007, 54: 1940–1950. [DOI] [PubMed] [Google Scholar]
- 22.Delp SL, Loan JP, Hoy MG, et al. : An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Trans Biomed Eng, 1990, 37: 757–767. [DOI] [PubMed] [Google Scholar]
- 23.Crowninshield RD, Johnston RC, Andrews JG, et al. : A biomechanical investigation of the human hip. J Biomech, 1978, 11: 75–85. [DOI] [PubMed] [Google Scholar]
- 24.Lee DD, Seung HS: Learning the parts of objects by non-negative matrix factorization. Nature, 1999, 401: 788–791. [DOI] [PubMed] [Google Scholar]
- 25.Ting LH, Macpherson JM: A limited set of muscle synergies for force control during a postural task. J Neurophysiol, 2005, 93: 609–613. [DOI] [PubMed] [Google Scholar]
- 26.Ivanenko YP, Cappellini G, Dominici N, et al. : Coordination of locomotion with voluntary movements in humans. J Neurosci, 2005, 25: 7238–7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu MQ, Anderson FC, Pandy MG, et al. : Muscles that support the body also modulate forward progression during walking. J Biomech, 2006, 39: 2623–2630. [DOI] [PubMed] [Google Scholar]
- 28.Moreland JD, Richardson JA, Goldsmith CH, et al. : Muscle weakness and falls in older adults: a systematic review and meta-analysis. J Am Geriatr Soc, 2004, 52: 1121–1129. [DOI] [PubMed] [Google Scholar]
- 29.Nelson-Wong E, Appell R, McKay M, et al. : Increased fall risk is associated with elevated co-contraction about the ankle during static balance challenges in older adults. Eur J Appl Physiol, 2012, 112: 1379–1389. [DOI] [PubMed] [Google Scholar]
- 30.Nagai K, Yamada M, Uemura K, et al. : Effects of fear of falling on muscular coactivation during walking. Aging Clin Exp Res, 2012, 24: 157–161. [DOI] [PubMed] [Google Scholar]
- 31.Neptune RR, Sasaki K, Kautz SA: The effect of walking speed on muscle function and mechanical energetics. Gait Posture, 2008, 28: 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Judge JO, Davis RB, 3rd, Ounpuu S: Step length reductions in advanced age: the role of ankle and hip kinetics. J Gerontol A Biol Sci Med Sci, 1996, 51: M303–M312. [DOI] [PubMed] [Google Scholar]
- 33.Basmajian JV, Carlo J: Muscle Alive, 5th ed. Baltimore: Williams & Wilkins, 1985. [Google Scholar]
- 34.Winter DA: The Biomechanics and Motor Control of Human Gait: Normal, Elderly and Pathological. Waterloo: Waterloo Biomechanics Press, 1991. [Google Scholar]