Abstract
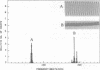
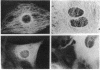
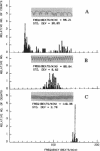
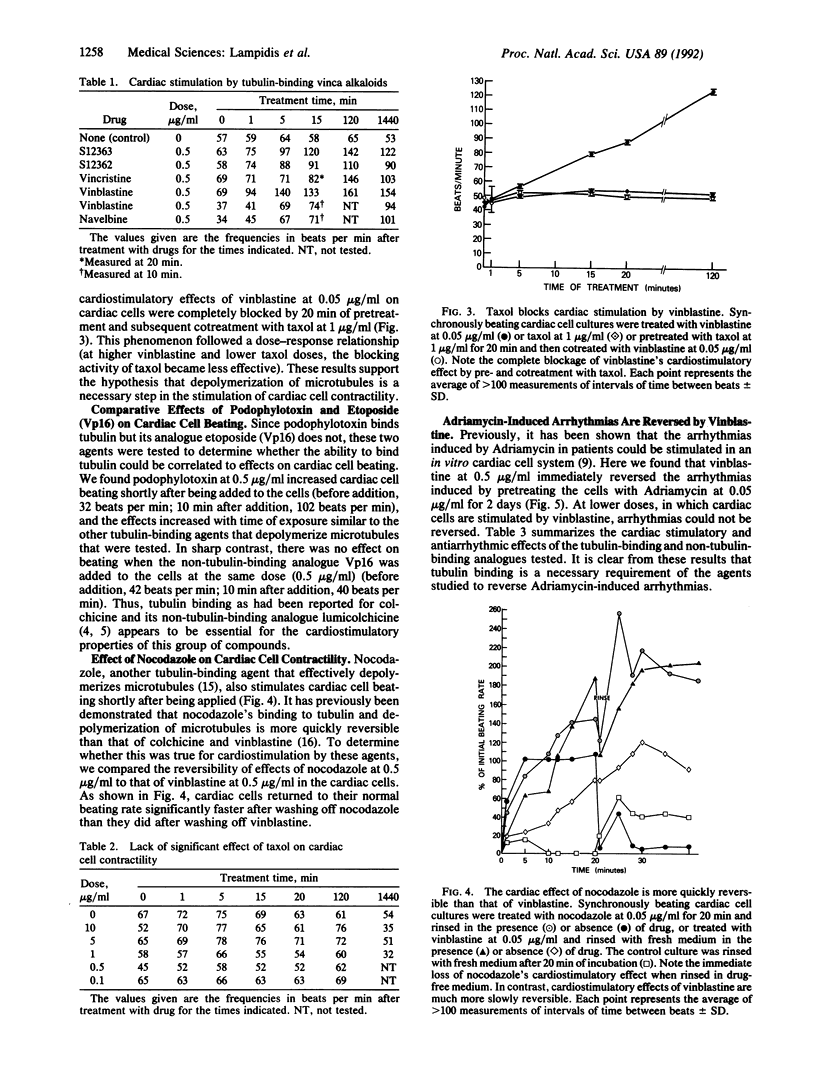
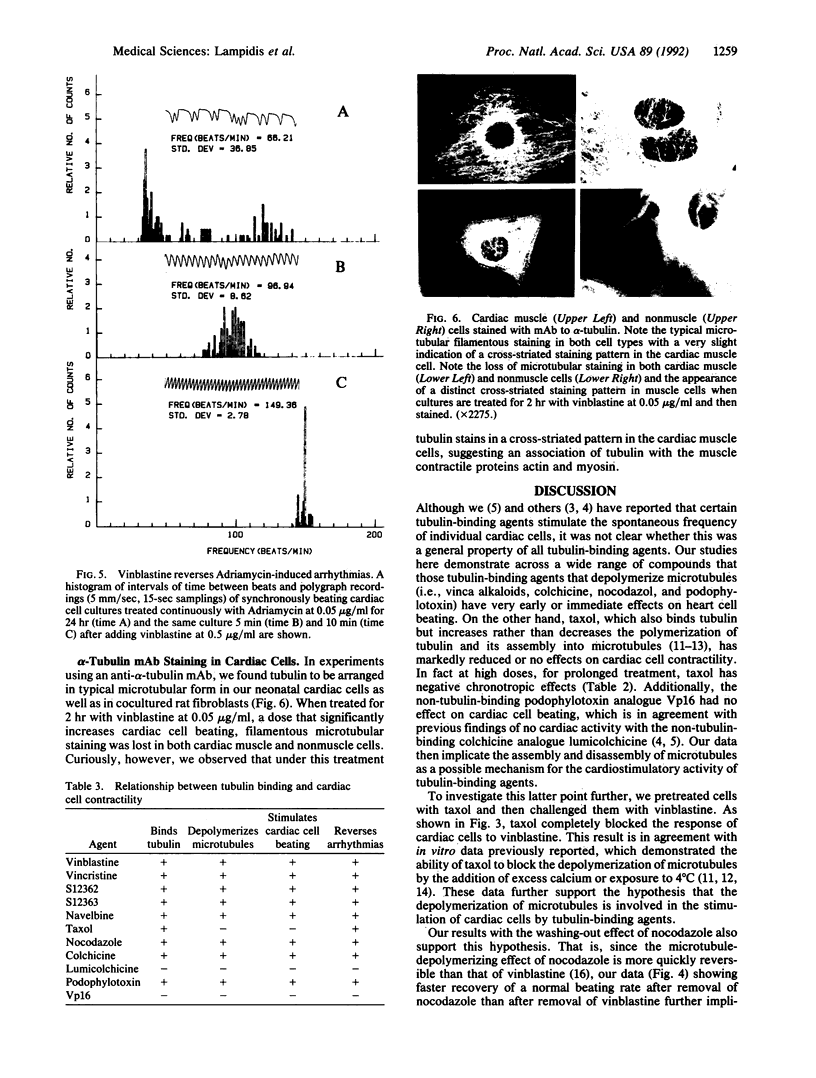
Rhythmic, spontaneously pulsating cardiac cells cultured from newborn rats are immediately stimulated to beat faster by addition of a number of tubulin-binding agents but not by their non-tubulin-binding analogues. The tubulin-binding agents tested include vinblastine, vincristine, navelbine, two analogs of vinblastine (S12362 and S12363), nocodazole, colchicine, and podophylotoxin. In addition to binding tubulin, all of the above agents also depolymerize microtubules. In contrast, taxol, a tubulin-binding agent that stabilizes microtubules, does not stimulate cardiac cells. Moreover, the immediate and ensuing cardiac stimulation by vinblastine at 0.05 microgram/ml is completely blocked by pre- and cotreatment with taxol at 1.0 microgram/ml. The time necessary to reverse the cardiostimulatory effect of vinblastine is significantly longer than that required for nocodazole, further implicating depolymerization of microtubules in the cardiac activity of these agents. All of the tubulin-binding agents tested (including taxol) also immediately reverse adriamycin-induced arrhythmias. By using a monoclonal antibody to alpha-tubulin, typical filamentous microtubules are visualized in cardiac muscle and cocultured non-muscle cells by immunofluorescence. When cells are treated for 2 hr with vinblastine at 0.05 microgram/ml, fluorescence is detected in cross-striated patterns in cardiac muscle cells. Overall, these data open the possibility of uncovering an additional relationship between cytoskeletal elements (other than actin and myosin) and the contractility of cardiac muscle. They also suggest an alternative mechanism for affecting cardiac cell function in vitro (namely, by tubulin-binding agents). If these agents are shown to be cardioactive in vivo, they may provide another approach to the treatment and management of cardiac arrhythmias.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blose S. H., Meltzer D. I., Feramisco J. R. 10-nm filaments are induced to collapse in living cells microinjected with monoclonal and polyclonal antibodies against tubulin. J Cell Biol. 1984 Mar;98(3):847–858. doi: 10.1083/jcb.98.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller G. M., Brinkley B. R. Structure and control of assembly of cytoplasmic microtubules in normal and transformed cells. J Supramol Struct. 1976;5(4):497(349)–514(366). doi: 10.1002/jss.400050407. [DOI] [PubMed] [Google Scholar]
- Harris C. C., Frank A. L., van Haaften C., Kaufman D. G., Connor R., Jackson F., Barrett L. A., McDowell E. M., Trump B. F. Binding of (3H)benzo(a)pyrene to DNA in cultured human bronchus. Cancer Res. 1976 Mar;36(3):1011–1018. [PubMed] [Google Scholar]
- Klein I. Colchicine stimulates the rate of contraction of heart cells in culture. Cardiovasc Res. 1983 Aug;17(8):459–465. doi: 10.1093/cvr/17.8.459. [DOI] [PubMed] [Google Scholar]
- Lampidis T. J., Henderson I. C., Israel M., Canellos G. P. Structural and functional effects of adriamycin on cardiac cells in vitro. Cancer Res. 1980 Nov;40(11):3901–3909. [PubMed] [Google Scholar]
- Lampidis T. J., Trevorrow K. W., Rubin R. W. Effects of colchicine on cardiac cell function indicate possible role for membrane surface tubulin. Exp Cell Res. 1986 Jun;164(2):463–470. doi: 10.1016/0014-4827(86)90044-3. [DOI] [PubMed] [Google Scholar]
- Mark G. E., Strasser F. F. Pacemaker activity and mitosis in cultures of newborn rat heart ventricle cells. Exp Cell Res. 1966 Nov-Dec;44(2):217–233. doi: 10.1016/0014-4827(66)90427-7. [DOI] [PubMed] [Google Scholar]
- Masson-Pévet M., Jongsma H. J., De Bruijne J. Collagenase- and trypsin-dissociated heart cells: a comparative ultrastructural study. J Mol Cell Cardiol. 1976 Oct;8(10):747–757. doi: 10.1016/0022-2828(76)90082-1. [DOI] [PubMed] [Google Scholar]
- Nath K., Shay J. W., Bollon A. P. Relationship between dibutyryl cyclic AMP and microtubule organization in contracting heart muscle cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):319–323. doi: 10.1073/pnas.75.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Microtubules. Annu Rev Biochem. 1973;42:507–540. doi: 10.1146/annurev.bi.42.070173.002451. [DOI] [PubMed] [Google Scholar]
- Rappaport L., Samuel J. L. Microtubules in cardiac myocytes. Int Rev Cytol. 1988;113:101–143. doi: 10.1016/s0074-7696(08)60847-5. [DOI] [PubMed] [Google Scholar]
- Rowinsky E. K., McGuire W. P., Guarnieri T., Fisherman J. S., Christian M. C., Donehower R. C. Cardiac disturbances during the administration of taxol. J Clin Oncol. 1991 Sep;9(9):1704–1712. doi: 10.1200/JCO.1991.9.9.1704. [DOI] [PubMed] [Google Scholar]
- Schiff P. B., Fant J., Horwitz S. B. Promotion of microtubule assembly in vitro by taxol. Nature. 1979 Feb 22;277(5698):665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- Schiff P. B., Horwitz S. B. Taxol assembles tubulin in the absence of exogenous guanosine 5'-triphosphate or microtubule-associated proteins. Biochemistry. 1981 May 26;20(11):3247–3252. doi: 10.1021/bi00514a041. [DOI] [PubMed] [Google Scholar]
- Schiff P. B., Horwitz S. B. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. C., Wilson L., Purich D. L. Taxol induces microtubule assembly at low temperature. Cell Motil. 1981;1(4):445–454. doi: 10.1002/cm.970010405. [DOI] [PubMed] [Google Scholar]
- Watkins S. C., Samuel J. L., Marotte F., Bertier-Savalle B., Rappaport L. Microtubules and desmin filaments during onset of heart hypertrophy in rat: a double immunoelectron microscope study. Circ Res. 1987 Mar;60(3):327–336. doi: 10.1161/01.res.60.3.327. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]