Abstract
Background
The aims of this study were to stratify the risk of recurrence based on the Masaoka-Koga stage and World Health Organization (WHO) classification systems after R0-resection for thymic epithelial tumors (TETs).
Methods
A retrospective analysis was conducted on 479 patients who underwent surgery between Jan 1994 and Feb 2014 for TETs. The study group comprised 251 males and 228 females, with a median age of 52 years (range, 15–84 years).
Results
Of the 479 patients, 406 (84.8%) patients underwent R0-resection. Recurrence after R0-resection occurred in 32 patients during a median follow-up of 53 months (range, 2–227 months). A multivariate analysis revealed that the preoperative treatment including chemotherapy (P=0.036), Masaoka-Koga stage (P=0.011) and the WHO classification (P=0.001) were predictors for recurrence after R0-resection. Patients were stratified into four risk groups using a potential model incorporating both the Masaoka-Koga stage and WHO classifications. Group 1 comprised WHO types A/AB/B1 in stage I/II; Group 2 comprised WHO type A/AB/B1 in stage III or WHO type B2/B3 in stage I/II or WHO type C in stage I; Group 3 comprised Type B2/B3/C in stage III, or WHO type C in stage II/III; and Group 4 comprised WHO type B2/B3/C in stage IV. The 5-year freedom-from-recurrence (FFR) rates were 99.4% for group 1, 84.7% for group 2, 63.7% for group 3, and less than 44.4% for group 4 (P<0.001). In group 3, the rate of locoregional recurrence of patients treated with postoperative radiation therapy was lower than patients treated without postoperative radiation therapy (P=0.032).
Conclusions
A risk model incorporating both Masaoka-Koga stage and WHO classification systems may provide multi-faceted information about recurrence and adjuvant treatment after R0-resection of TETs.
Keywords: Thymoma, thymic carcinoma, surgery, recurrence
Introduction
The Masaoka staging system and the World Health Organization (WHO) classification system are widely used to stage and to classify thymic epithelial tumors (TETs) including thymomas and thymic carcinomas, although the International Association for the Study of Lung Cancer (IASLC) and the International Thymic Malignancy Interest Group (ITMIG) proposed new TNM classification or TETs for forthcoming (8th) edition of the TNM classification of malignant tumors (1-3).
The Masaoka staging system, published in 1981, staged tumors on the basis of their anatomical extent (4). In 1994, Koga et al. suggested a modification of this system (5). The ITMIG opted to use the Masaoka-Koga system until a scientifically validated system is defined and remains the most widely used system currently (6,7). The WHO’s histologic typing system of tumors of the thymus, published in 1999, classifies TETs as type A, AB, B1, B2, B3, or C on the basis of the morphology of their epithelial cells and the ratio of lymphocytes to epithelial cells. The WHO classification system has been widely adopted because it enables simple comparisons of clinicopathological studies (8).
It has previously been suggested that the Masaoka stage and the WHO histological subtype may be independent factors for the prognosis of TETs (4,8-16), however, the potential advantages of using a combination of these two classification systems is still unclear. Therefore, the aims of this study were to evaluate prognostic factors after complete resection for TETs and to stratify the risk of recurrence after R0-resection based on the Masaoka-Koga stage and WHO classification systems.
Methods
Patients
A retrospective analysis of 479 patients who underwent surgical resection for TETs at Asan Medical Center between Jan 1994 and Feb 2014 was conducted. Patients with tumors that could not be classified according to the WHO system, those with thymic neuroendocrine carcinomas and those who underwent biopsy alone were excluded from this study. This study was approved by our institutional Ethics Committee/Review Board, which waived the requirement for informed patient consent because of the retrospective nature of this study.
Classification of thymic epithelial tumors (TETs)
TETs were histologically categorized according to the WHO classification system (17). Routine histological sections were stained with hematoxylin and eosin and examined by a pathologist. A second experienced pathologist then reviewed the histological classification and made the final decision on the histological type. The Masaoka-Koga stage was determined after review of the surgical and pathological reports. For patients who received preoperative chemotherapy, their staging was determined based on the post chemotherapy status.
Surgical treatment
The mainstay of treatment of TETs was surgery. The surgical procedures included tumor excision (including the partial thymus), total thymectomy, and extended thymectomy, which were achieved via transsternal thoracic surgery, video-assisted thoracic surgery (VATS), or robotic surgery. For each approach, the basic principles of total thymectomy included en bloc resection of the gland (including the cervical poles and adjacent mediastinal fat), protection of the phrenic nerves, and prevention of intrapleural dissemination whenever possible. For patients with myasthenia gravis (MG), extended thymectomy under a full median sternotomy was most commonly performed. Systematic mediastinal lymph node dissection was not routinely performed at our institution, although lymph node dissection was performed in cases of metastasis on preoperative evaluations. Of the 479 patients enrolled, 187 (39.0%) patients underwent lymph node sampling or dissection during surgery.
Preoperative and postoperative treatments and surveillance
Preoperative chemotherapy (with or without radiation therapy) was administered to patients with advanced disease, such as non-resectable stage III or disseminated stage IV tumors. In patients whose tumors became resectable after preoperative treatment, curative-intent surgery was performed. Postoperative radiation therapy was recommended for patients with stage II or III disease and was not considered appropriate for patients who underwent complete resection for stage I disease. Of the 479 patients, 46 patients (9.6%) received preoperative chemotherapy or radiation therapy and 219 patients (45.7%) received postoperative radiation therapy with or without chemotherapy.
Recently, our institution’s radiation oncologists are recommending postoperative radiation therapy for patients with WHO-classified type B2, or B3 or type C tumors, even in the setting of R0-resection. The actual rates of patients who received postoperative radiation therapy with/without chemotherapy were 12.1% in type A (4/33), 28.6% in Type AB (30/105), 30.6% in type B1 (37/121), 50.0% in type B2 (41/82), 72.5% in type B3 (45/62), and 65.8% in type C (50/76).
Postoperative surveillance included routine annual computed tomography (CT) scans (performed with contrast) of the thorax for five years after surgical resection, followed by annual chest radiographs. In cases of resected stage III and IVa disease, WHO-classified type B3 or C, incomplete resection, or other high risk tumors, bi-annual CT scans were performed for three years after surgical resection. In cases of suspected recurrence on CT scans, patients were suggested to undergo positron emission tomography-computed tomography (PET-CT) to confirm the extent of recurrent disease.
Determination of the efficacy of thymic epithelial tumors (TETs) treatment
The efficacy of treatment of TETs was determined by measuring the rates of postoperative survival and tumor recurrence. Overall survival is easy to measure and is hence the most commonly used end point; however, it is not an ideal metric because many patients with thymoma die of unrelated causes and patients can survive for many years with recurrent disease (18). The ITMIG proposed that the rate of recurrence of tumors is the most appropriate measure of the efficacy of treatment of thymic malignancies (19). Here, the overall survival (OS) rate was used in all patients and the freedom-from-recurrence (FFR) rate was used as a measure of successful complete resection, and the definition of recurrence after R0-resection was based on the ITMIG proposed criteria (19).
Statistical analyses
Categorical variables were compared using Chi-squared or Fisher’s exact tests. Continuous variables are expressed as the median ± range. Kaplan-Meier curves were used to delineate the overall survival rate, the FFR rate, and the progression-free-rate. Log-rank tests were used to compare the between-group differences in these rates. For multivariable analyses, Cox-proportional hazards models were used to determine the risk factors for recurrence or death. Variables with P≤0.10 in univariate analyses were candidates for the multivariable Cox models. Multivariable analyses involved a backward elimination technique. All statistical analyses were performed using SPSS version 18.0 software (SPSS Inc., Chicago, Ill, USA). P value less than 0.05 was considered statistically significant.
Results
Patient and tumor characteristics
The baseline characteristics of the 479 patients are summarized in Table 1. The study group comprised 251 males and 228 females, with a median age of 52 years (range, 15–84 years). The most frequent histologic subtypes were B1 (25.3%), followed by type AB (21.9%), and B2 (17.1%) thymomas. A total of 93 patients (19.4%) were diagnosed with MG; the prevalence rates of MG were 0% in type A (0/33), 17.1% in type AB (18/105), 20.7% in type B1 (25/121), 32.9% in type B2 (27/82), 37.1% in type B3 (23/62), and 0% in type C (0/76).
Table 1. Baseline characteristics of the 479 patients who underwent surgical resection for thymic epithelial tumors.
| Variables | Number of patients (n=479) |
|---|---|
| Age in years (median, range) | 52 [15–84] |
| Sex (male:female) | 251:228 (52.4%:47.6%) |
| Association with MG, n (%) | 93 (19.4) |
| Preoperative treatment, n (%) | |
| None | 433 (90.4) |
| CTx | 44 (9.2) |
| RTx | 2 (0.4) |
| Surgical extent, n (%) | |
| Thymectomy/excision | 376 (78.5) |
| Extended thymectomy | 103 (21.5) |
| Surgical approaches, n (%) | |
| Transsternal | 340 (71.0) |
| VATS or robotic | 139 (29.0) |
| LN sampling or dissection, n (%) | |
| None | 292 (61.0) |
| Yes | 187 (39.0) |
| Number of retrieved LN (median, range) | 4 [1–30] |
| LN metastasis, n (%) | |
| None | 171 (91.4) |
| Yes | 16 (8.6) |
| Type of resection, n (%) | |
| R0-resection | 406 (84.8) |
| R1-resection | 64 (13.4) |
| R2-resection | 9 (1.9) |
| Maximal tumor size in cm (median, range) | 6.0 (0–20.0) |
| Masaoka-Koga stage, n (%) | |
| Stage I | 262 (54.7) |
| Stage IIa | 76 (15.9) |
| Stage IIb | 33 (6.9) |
| Stage III | 93 (19.4) |
| Stage IVa | 7 (1.5) |
| Stage IVb | 8 (1.7) |
| WHO classification, n (%) | |
| A | 33 (6.9) |
| AB | 105 (21.9) |
| B1 | 121 (25.3) |
| B2 | 82 (17.1) |
| B3 | 62 (12.9) |
| C | 76 (15.9) |
| Postoperative treatment, n (%) | |
| None | 260 (54.3) |
| CTx | 204 (42.6) |
| RTx | 12 (2.5) |
| CRTx | 3 (0.6) |
MG, myasthenia gravis; CTx, chemotherapy; RTx, radiation therapy; CRTx, chemoradiation therapy; VATS, video-assisted thoracoscopic surgery; LN, lymph node; WHO, World Health Organization.
Median maximal tumor size was 6.0 cm (range, 0–20.0 cm). 406 (85.7%) of the surgical procedures were considered R0-resections. The rates of R0-resection were 95.8% in stage I, 85.3% in stage II, 60.2% in stage III, and 40.0% in stage IV. Of the 187 patients who underwent lymph node dissection or sampling (more than 1 retrieved lymph node), lymph node metastasis was confirmed in 16 patients (8.6%).
Analysis of prognostic variables
Overall survival analysis in all patients
The median follow-up duration of the 479 patients was 55 months (range, 2–227 months). Sixty seven patients died during the study period. Overall survival in this series was 90.1% at 5 years and 79.1% at 10 years. When classified according to the Masaoka-Koga system, the 5-year OS rates were 93.7%, 89.2%, 82.7%, and 82.5% for stage I, II, III, and IV patients, respectively (P<0.001). In univariate analyses, age (P<0.001), completeness of resection (P=0.019), Masaoka-Koga stage (P<0.001), and maximal diameter of tumor (P=0.009) were significant predictors. In multivariate regression analyses revealed that age (P<0.001), Masaoka-Koga stage (P<0.001) correlated significantly with overall survival (Table 2).
Table 2. Predictors of postoperative overall survival on 479 patients who underwent surgical resection for thymic epithelial tumors.
| Variables | Overall survival |
||||||
|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
||||||
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Age | 1.043 | 1.023–1.064 | <0.001 | 1.047 | 1.026–1.069 | <0.001 | |
| Sex | |||||||
| Female | 1 | – | – | – | |||
| Male | 1.530 | 0.936–2.502 | 0.090 | ||||
| Presence of MG | |||||||
| No | 1 | ||||||
| Yes | 0.886 | 0.503–1.559 | 0.674 | ||||
| Preoperative treatment | |||||||
| None | 1 | ||||||
| CTx or RTx | 1.253 | 0.537–2.923 | 0.602 | ||||
| Surgical approach | 0.063 | – | – | – | |||
| Transsternal | 1 | ||||||
| VATS or robotic | 0.448 | 0.192–1.043 | |||||
| Surgical extent | 0.084 | – | – | – | |||
| Excision/thymecomy | 1 | ||||||
| Extended thymectomy | 0.581 | 0.314–1.076 | |||||
| Resection status | 0.019 | – | – | – | |||
| R0-resection | 1 | ||||||
| R1-resection | 2.154 | 1.237–3.749 | 0.007 | ||||
| R2-resection | 2.108 | 0.511–8.705 | 0.303 | ||||
| Masaoka-Koga stage | <0.001 | <0.001 | |||||
| Stage I | 1 | 1 | |||||
| Stage II | 1.389 | 0.734–2.628 | 0.313 | 1.454 | 0.768–2.751 | 0.250 | |
| Stage III | 2.216 | 1.235–3.974 | 0.008 | 1.998 | 1.112–3.591 | 0.021 | |
| Stage IV | 6.847 | 2.789–16.812 | 0.000 | 9.761 | 3.889–24.501 | <0.001 | |
| Maximal tumor diameter | 1.107 | 1.026–1.195 | 0.009 | – | – | – | |
| WHO classification | 0.326 | ||||||
| Type A or AB or B1 | 1 | ||||||
| Type B2 or B3 | 1.073 | 0.630–1.828 | 0.794 | ||||
| Type C | 1.681 | 0.845–3.346 | 0.139 | ||||
| Postoperative treatment | 0.204 | ||||||
| None | 1 | ||||||
| Radiation | 1.011 | 0.614–1.666 | 0.964 | ||||
| Chemotherapy | 3.615 | 1.096–11.924 | 0.035 | ||||
OR, odds ratio; CI, confidence interval; MG, myasthenia gravis; CTx, chemotherapy; RTx, radiation therapy; CRTx, chemoradiation therapy; VATS, video-assisted thoracoscopic surgery; WHO, World Health Organization.
Freedom-from recurrence analysis in patients who underwent R0-resection
Four hundred six patients underwent R0-resection; 32 patients (7.9%) of these patients recurred at a median of 24 months (range, 3–108 months). The sites of recurrence were the pleura in 14 patients, the lung in 7 patients, the anterior mediastinum in 3 patients, the bone in 2 patients, and multiple sites in 6 patients. Recurrences were classified as local recurrence in 3 patients, regional recurrence in 14 patients, distant recurrence in 11 patients, and mixed types (local with regional in 1, local with distant in 1, and regional with distant in 2) in 4 patients.
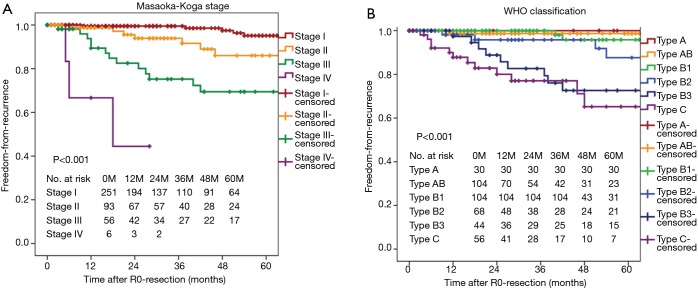
Freedom-from recurrence in these series was 88.0% at 5 years and 82.3% at 10 years. When classified according to the Masaoka-Koga system, the 5-year-FFR rates were 95.1% for stage I, 86.0% for stage II, and 69.4% for stage III. Two-year-FFR of stage IV was 44.4% (Figure 1A). When classified according to the WHO system, the 5-year-FFR rates were 100%, 98.7%, 95.8%, 87.6%, 72.6%, and 65.2% for types A, AB, B1, B2, B3, and C patients, respectively (Figure 1B). In univariate analyses, preoperative treatments (P<0.001), Masaoka-Koga stage (P<0.001), WHO classification (P<0.001), and postoperative treatments (P=0.002) were significant predictors of FFR. In multivariate analyses, preoperative treatments (P=0.016), Masaoka-Koga stage (P=0.008), WHO classification (P=0.001) were significant prognostic variables to predict recurrence after R0-resection (Table 3).
Figure 1.
Freedom-from-recurrence curves of patients stratified by the Masaoka-Koga stage (A) and by the WHO histological classification (B).
Table 3. Predictors of postoperative recurrence on 406 patients who underwent complete resection (R0 resection) for thymic epithelial tumors.
| Variables | Freedom from recurrence |
||||||
|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
||||||
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Age | 0.984 | 0.957–1.011 | 0.243 | ||||
| Sex | |||||||
| Female | 1 | ||||||
| Male | 1.473 | 0.727–2.984 | 0.282 | ||||
| Presence of MG | |||||||
| No | 1 | ||||||
| Yes | 0.951 | 0.410–2.205 | 0.906 | ||||
| Preoperative treatment | |||||||
| None | 1 | 1 | |||||
| CTx or RTx | 6.297 | 2.881–13.761 | <0.001 | 3.803 | 1.232–7.715 | 0.016 | |
| Surgical approach | |||||||
| Transsternal | 1 | ||||||
| VATS/Robotic | 0.461 | 0.161–1.319 | 0.149 | ||||
| Surgical extent | |||||||
| Excision/thymectomy | 1 | ||||||
| Extended thymectomy | 1.150 | 0.552–2.396 | 0.708 | ||||
| Masaoka-Koga stage | <0.001 | 0.008 | |||||
| Stage I | 1 | 1 | |||||
| Stage II | 3.426 | 1.186–9.897 | 0.023 | 2.669 | 0.906–7.868 | 0.075 | |
| Stage III | 10.227 | 3.966–26.368 | <0.001 | 4.775 | 1.793–12.713 | 0.002 | |
| Stage IV | 46.013 | 11.058–191.474 | <0.001 | 9.269 | 1.888–45.510 | 0.006 | |
| WHO classification | <0.001 | 0.001 | |||||
| Type A or AB or B1 | 1 | 1 | |||||
| Type B2 or B3 | 5.445 | 1.960–15.124 | 0.001 | 3.700 | 1.303–10.505 | 0.014 | |
| Type C | 14.093 | 4.999–39.734 | <0.001 | 8.089 | 2.740–23.884 | <0.001 | |
| Maximal tumor diameter | 1.109 | 0.990–1.241 | 0.074 | 1.107 | 0.988–1.241 | 0.080 | |
| Postoperative treatment | 0.002 | ||||||
| None | 1 | – | – | – | |||
| Radiation | 2.726 | 1.240–5.991 | 0.013 | – | – | – | |
| Chemotherapy | 12.570 | 3.381–46.728 | <0.001 | – | – | – | |
OR, odds ratio; CI, confidence interval; MG, myasthenia gravis; CTx, chemotherapy; RTx, radiation therapy; CRTx, chemoradiation therapy; VATS, video-assisted thoracoscopic surgery; WHO, World Health Organization.
Prognostic stratification based on a potential risk model incorporating both Masaoka-Koga stage and WHO classification systems
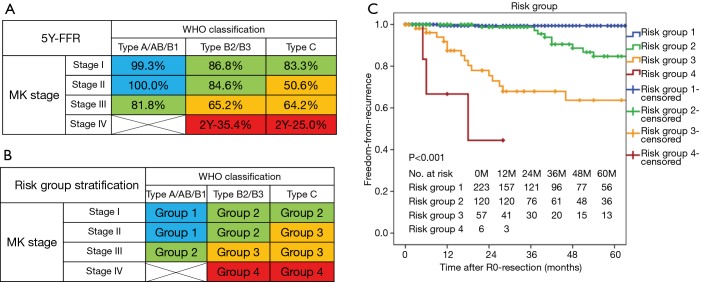
A cross-comparison of the WHO classifications and Masaoka-Koga stages of the 406 patients who underwent complete resection for TETs is shown in Figure 2A. A multivariate analysis of prognostic factors for recurrence subdivided the 406 patients into 12 subpopulations based on the basis of combinations of Masaoka-Koga stage and WHO histological classifications (the subtypes were grouped into three categories; type A or AB or B1, type B2 or B3, and type C).
Figure 2.
The risk group model classifying the risk of recurrence of thymic epithelial tumors after complete resection. The model incorporate both the Masaoka-Koga stage and WHO histological classification systems, which were grouped into four categories as indicated. The 406 patients who underwent complete resection for TETs were initially classified into nine subgroups. (A) The freedom-from-recurrence rates of patients in the nine initial subgroups at the 5-year time point; (B) the proposed grouping system to assess the risk of recurrence after complete resection; (C) freedom-from-recurrence curves stratified by recurrence risk groups. 5Y-FFR, 5-year-freedom-from-recurrence rate.
According to results of the 5-year-FFR rate of the each subpopulation (Figure 2A), the subpopulation comprising type A/AB/B1 in stage I or stage II was labeled as ‘Group 1’. And the subpopulations comprising type B2/B3/C in stage I, or type B2/B3 in stage II, or type A/AB/B1 in stage III were labeled as ‘Group 2’. And the subpopulations comprising type C in stage II/III, or type B2/B3 in stage III were labeled as ‘Group 3’. And the subpopulations comprising type B2/B3/C in stage IV were labeled as ‘Group 4’ (Figure 2B).
The 5-year FFR rates were 99.4% for group 1, 84.7% for group 2, 63.7% for group 3, and less than 44.4% for group 4 (P<0.001, Figure 2C). In a multivariate regression adjusting for preoperative treatments and maximal tumor size, risk groups were significant factors predicting recurrence after R0-resection (data not shown).
The effect of postoperative radiation therapy on the recurrence after R0-resection
The rates of patients who received postoperative radiation therapy were 19.3% in group 1 (43/223), 53.3% in group 2 (64/120), 82.5% in group 3 (47/57), and 16.7% in group 4 (1/6). All types of recurrence occurred in 3 out of 223 patients in group 1, 9 out of 120 patients in group 2, and 17 out of 57 patients in group 3, and 3 out of 6 patients in group 4. In group 1, 2, and 3, the rates of all type of recurrence were not different between in patients treated with postoperative radiation therapy and in those treated without postoperative radiation therapy).
Locoregional recurrences occurred in 2 patients in group 1, 7 patients in group 2, and 7 patients in group 3. In group 1 or group 2, the rates of locoregional recurrence of patients treated with postoperative radiation therapy was not significantly different from patients treated without postoperative radiation therapy (P=0.414 for group 1, P=0.151 for group 2, Figure 3A,B). On the contrary, in group 3, the rate of locoregional recurrence of patients treated with postoperative radiation therapy was lower than patients treated without postoperative radiation therapy (P=0.032, Figure 3C).
Figure 3.
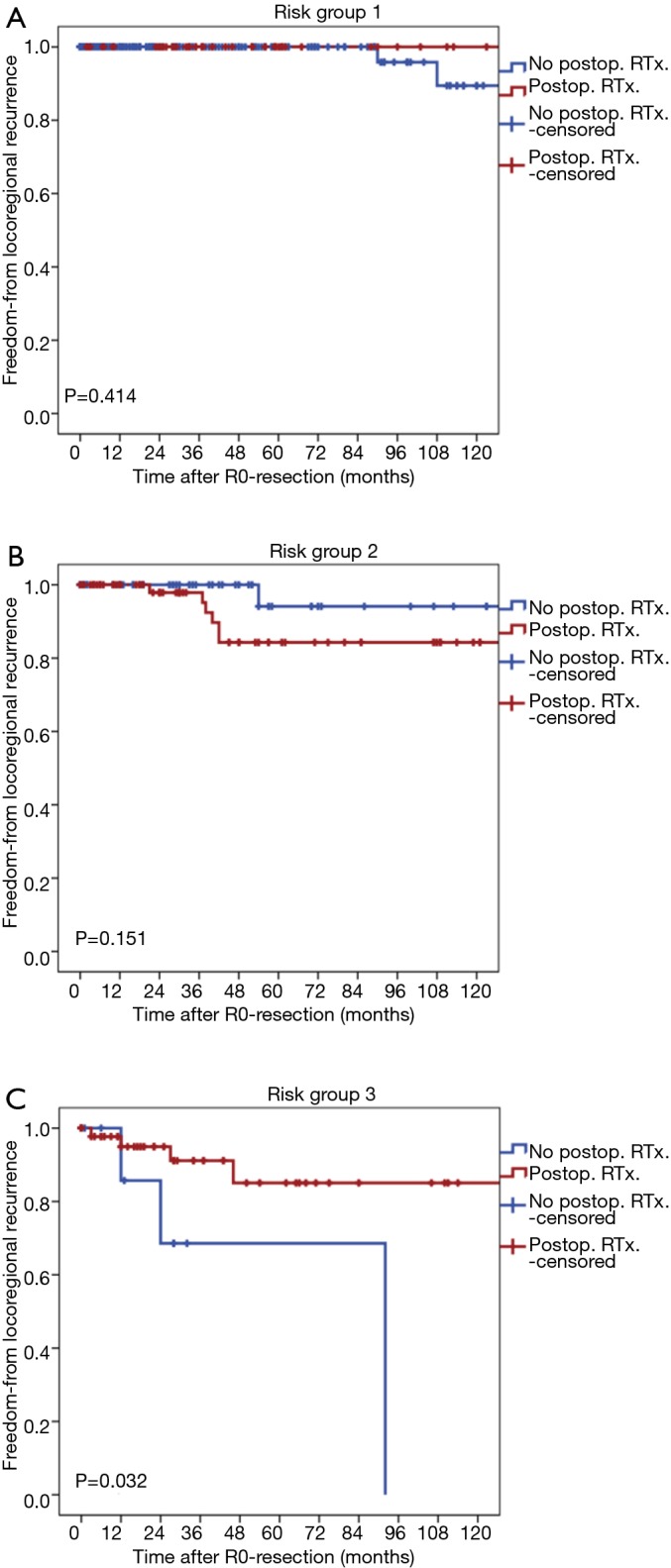
Freedom-from-locoregional recurrence curves stratified by the postoperative radiation therapy in each risk groups. (A) Risk group 1; (B) risk group 2; (C) risk group 3. RTx, postoperative radiation therapy.
Discussion
The tumor-nodes-metastasis (TNM) classification system has been used to stage malignant tumors of multiple organs; however, there is currently no authorized TNM system for TETs, although IASLC/ITMIG is currently proposed evidence-based TNM-based stage classification system of TETs for 8th edition of the stage classification manuals (20). We fully recognize that it is necessary to apply and to validate a newly proposed system, however, it is difficult to apply this system to our clinicopathologic data, because of the lack of detailed data for lymph node status. Therefore, we decided to stratify the prognosis of TETs based on the currently using systems including Masaoka-Koga stage and WHO classifications of TETs.
Masaoka-Koga stage and WHO histological classification are important variables for disease recurrence after R0-resection. In this study, Kaplan-Meier curves demonstrated a clear distinction between the recurrence rates after complete resection in Masaoka-Koga stage I and stage II patients, which supports Masaoka’s original opinion that these patients should be individually grouped (21). However, in accordance with previous studies (10,11,22-24), the multivariate analysis did not identify any significant prognostic differences between stage I and II patients. Controversies regarding proposals to combine stages I and II will need to be resolved by additional large cohort studies with long-term follow-up periods.
Consistent with recent reports (10,11,22,24), the WHO histological classification was also a prognostic factor for TET recurrence in this study. Although the official WHO classification subdivides TETs into three major categories (A, B, and C), the Kaplan-Meier curves presented here enabled division of these tumors into different three categories on the basis of their recurrence rate: type A or AB or B1, type B2 or B3, and thymic carcinomas. Regarding type B1 thymoma, among the 104 patients proved type B1 thymoma after complete resection in this study, only 4 patients (3.8%) experiences recurrence after R0-resection; One of these 4 patient underwent extended thymectomy at the age of 61 for type B1 thymoma with regional lymph node metastasis (four of nine lymph nodes) and received adjuvant radiation therapy. Recurrent pleural seeding (regional recurrence) was identified in the left pleural cavity 40 months after the initial operation and chemotherapy was administered. The patient died 93 months after surgical resection (at the age of 69) due to pneumonia. Consequently, it seems that B1 thymomas follow a favorable clinical course; even at stage IV they may not be fatal due to the very slow progression of the disease. These results are consistent with those of previous study, which reported that WHO type A and AB thymomas are considered benign and type B1 thymomas have a very low malignant potential, although local recurrence or late metastasis may occur rarely (25). On the contrary, type B2 or type B3 thymomas had a greater degree of malignancy, which is consistent with a previous report that showed a clear association between malignancy and type B2 or B3 thymomas and thymic carcinomas (26).
This study demonstrates that a potential risk model incorporating both the Masaoka-Koga stage and WHO classification systems can be used to stratify the recurrence risk after R0-resection for TETs. This concept is in close agreement with those of other clinicians or researchers who tried to establish a simplified tool for the stratification of patients with TETs and to propose rationale for adjuvant therapies (27-30). This study tried to assess the effect of postoperative radiation therapy in group 1, 2, and 3. Because it is obvious that systemic chemotherapy is needed in group 4. Our results demonstrated that the postoperative radiation therapy could not reduce overall recurrence in risk group 1, 2, and 3. However, the postoperative radiation therapy could reduce the locoregional recurrence in group 3, although it is not effective in group 1 and 2. The goal of postoperative radiation therapy is to reduce not distant recurrences but the locoregional recurrence, because the radiation field was localized in anterior mediastinum. These results suggest that postoperative radiation therapy might be necessary to reduce the rate of locoregional recurrence in group 3, but it might not be needed in group 1 and 2.
This study has important limitations stemming from its limited number of events (such as death or recurrence) and a retrospective analysis of observational data from a single institution. The major shortcoming of the study is the approaches to reclassify the patients required the use of somewhat biased and potentially inappropriate statistical methods and the lack of a confirmatory subgroup of patients. Our results should be considered preliminary until confirmed in an independent validation cohort. Nevertheless, we have reported the retrospective outcomes of our cohort, because the risk grouping method based on a combination of the Masaoka-Koga and WHO classification systems takes into account the tumor extent and histology after complete resection of TETs and may aid the formulation of hypotheses required to develop future studies.
In conclusion, the Masaoka-Koga stage and WHO histological classifications were independent predictors of recurrence after R0-resection of TETs. A potential risk stratification model incorporating both classification systems may provide multi-faceted information about recurrence and adjuvant treatment after R0-resection of TETs.
Acknowledgements
The authors thank Hwa Jung Kim, MD, from the Department of Biostatistics for statistical analysis.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Marom EM, Detterbeck FC. Overview. J Thorac Oncol 2014;9:S63-4. 10.1097/JTO.0000000000000289 [DOI] [PubMed] [Google Scholar]
- 2.Nicholson AG, Detterbeck FC, Marino M, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the T Component for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S73-80. 10.1097/JTO.0000000000000303 [DOI] [PubMed] [Google Scholar]
- 3.Kondo K, Van Schil P, Detterbeck FC, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the N and M components for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S81-7. 10.1097/JTO.0000000000000291 [DOI] [PubMed] [Google Scholar]
- 4.Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [DOI] [PubMed] [Google Scholar]
- 5.Koga K, Matsuno Y, Noguchi M, et al. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int 1994;44:359-67. 10.1111/j.1440-1827.1994.tb02936.x [DOI] [PubMed] [Google Scholar]
- 6.Detterbeck FC, Nicholson AG, Kondo K, et al. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. J Thorac Oncol 2011;6:S1710-6. 10.1097/JTO.0b013e31821e8cff [DOI] [PubMed] [Google Scholar]
- 7.Detterbeck FC, Asamura H, Crowley J, et al. The IASLC/ITMIG thymic malignancies staging project: development of a stage classification for thymic malignancies. J Thorac Oncol 2013;8:1467-73. 10.1097/JTO.0000000000000017 [DOI] [PubMed] [Google Scholar]
- 8.Hasserjian RP, Ströbel P, Marx A. Pathology of thymic tumors. Semin Thorac Cardiovasc Surg 2005;17:2-11. 10.1053/j.semtcvs.2004.12.002 [DOI] [PubMed] [Google Scholar]
- 9.Singhal S, Shrager JB, Rosenthal DI, et al. Comparison of stages I-II thymoma treated by complete resection with or without adjuvant radiation. Ann Thorac Surg 2003;76:1635-41; discussion 1641-2. 10.1016/S0003-4975(03)00819-1 [DOI] [PubMed] [Google Scholar]
- 10.Park MS, Chung KY, Kim KD, et al. Prognosis of thymic epithelial tumors according to the new World Health Organization histologic classification. Ann Thorac Surg 2004;78:992-7; discussion 997-8. 10.1016/j.athoracsur.2004.03.097 [DOI] [PubMed] [Google Scholar]
- 11.Kondo K, Yoshizawa K, Tsuyuguchi M, et al. WHO histologic classification is a prognostic indicator in thymoma. Ann Thorac Surg 2004;77:1183-8. 10.1016/j.athoracsur.2003.07.042 [DOI] [PubMed] [Google Scholar]
- 12.Fang W, Chen W, Chen G, et al. Surgical management of thymic epithelial tumors: a retrospective review of 204 cases. Ann Thorac Surg 2005;80:2002-7. 10.1016/j.athoracsur.2005.05.058 [DOI] [PubMed] [Google Scholar]
- 13.Korst RJ, Kansler AL, Christos PJ, et al. Adjuvant radiotherapy for thymic epithelial tumors: a systematic review and meta-analysis. Ann Thorac Surg 2009;87:1641-7. 10.1016/j.athoracsur.2008.11.022 [DOI] [PubMed] [Google Scholar]
- 14.Hosaka Y, Tsuchida M, Toyabe S, et al. Masaoka stage and histologic grade predict prognosis in patients with thymic carcinoma. Ann Thorac Surg 2010;89:912-7. 10.1016/j.athoracsur.2009.11.057 [DOI] [PubMed] [Google Scholar]
- 15.Okereke IC, Kesler KA, Freeman RK, et al. Thymic carcinoma: outcomes after surgical resection. Ann Thorac Surg 2012;93:1668-72; discussion 1672-3. [DOI] [PubMed]
- 16.Weksler B, Shende M, Nason KS, et al. The role of adjuvant radiation therapy for resected stage III thymoma: a population-based study. Ann Thorac Surg 2012;93:1822-8; discussion 1828-9. [DOI] [PubMed]
- 17.Travis WD, Brambilla E, Müller-Hermelink HK, et al, editors. Pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon; [Great Britain]: IARC Press, 2004. [Google Scholar]
- 18.Detterbeck F, Youssef S, Ruffini E, et al. A review of prognostic factors in thymic malignancies. J Thorac Oncol 2011;6:S1698-704. 10.1097/JTO.0b013e31821e7b12 [DOI] [PubMed] [Google Scholar]
- 19.Huang J, Detterbeck FC, Wang Z, et al. Standard outcome measures for thymic malignancies. J Thorac Oncol 2010;5:2017-23. 10.1097/JTO.0b013e3181f13682 [DOI] [PubMed] [Google Scholar]
- 20.Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S65-72. 10.1097/JTO.0000000000000290 [DOI] [PubMed] [Google Scholar]
- 21.Masaoka A. Staging system of thymoma. J Thorac Oncol 2010;5:S304-12. 10.1097/JTO.0b013e3181f20c05 [DOI] [PubMed] [Google Scholar]
- 22.Okumura M, Ohta M, Tateyama H, et al. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma: a clinical study of 273 patients. Cancer 2002;94:624-32. 10.1002/cncr.10226 [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa K, Asamura H, Matsuno Y, et al. Thymoma: a clinicopathologic study based on the new World Health Organization classification. J Thorac Cardiovasc Surg 2003;126:1134-40. 10.1016/S0022-5223(03)00798-0 [DOI] [PubMed] [Google Scholar]
- 24.Wright CD, Wain JC, Wong DR, et al. Predictors of recurrence in thymic tumors: importance of invasion, World Health Organization histology, and size. J Thorac Cardiovasc Surg 2005;130:1413-21. 10.1016/j.jtcvs.2005.07.026 [DOI] [PubMed] [Google Scholar]
- 25.Chalabreysse L, Roy P, Cordier JF, et al. Correlation of the WHO schema for the classification of thymic epithelial neoplasms with prognosis: a retrospective study of 90 tumors. Am J Surg Pathol 2002;26:1605-11. 10.1097/00000478-200212000-00008 [DOI] [PubMed] [Google Scholar]
- 26.Suster S, Rosai J. Thymic carcinoma. A clinicopathologic study of 60 cases. Cancer 1991;67:1025-32. [DOI] [PubMed] [Google Scholar]
- 27.Pescarmona E, Rendina EA, Venuta F, et al. Analysis of prognostic factors and clinicopathological staging of thymoma. Ann Thorac Surg 1990;50:534-8. 10.1016/0003-4975(90)90185-9 [DOI] [PubMed] [Google Scholar]
- 28.Chen G, Marx A, Chen WH, et al. New WHO histologic classification predicts prognosis of thymic epithelial tumors: a clinicopathologic study of 200 thymoma cases from China. Cancer 2002;95:420-9. 10.1002/cncr.10665 [DOI] [PubMed] [Google Scholar]
- 29.Ströbel P, Bauer A, Puppe B, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol 2004;22:1501-9. 10.1200/JCO.2004.10.113 [DOI] [PubMed] [Google Scholar]
- 30.D'Angelillo RM, Trodella L, Ramella S, et al. Novel prognostic groups in thymic epithelial tumors: assessment of risk and therapeutic strategy selection. Int J Radiat Oncol Biol Phys 2008;71:420-7. 10.1016/j.ijrobp.2007.10.006 [DOI] [PubMed] [Google Scholar]