Abstract
Sporadic patients with acute respiratory distress syndrome (ARDS) caused by Mycoplasma pneumoniae have been reported. However, knowledge about the pathophysiology and pharmacological treatment of this condition is insufficient. Moreover, the pulmonary vascular permeability in ARDS related to M. pneumoniae infection has not been reported. We report a case of ARDS caused by Mycoplasma pneumoniae without elevated pulmonary vascular permeability, which was successfully treated using low-dose short-term hydrocortisone, suggesting that pulmonary infiltration in ARDS caused by Mycoplasma pneumoniae does not match the criteria of permeability edema observed in typical ARDS.
Keywords: Mycoplasma pneumoniae, acute respiratory distress syndrome (ARDS), transpulmonary thermodilution technique, pulmonary vascular permeability index (PVPI), corticosteroid treatment
Introduction
Mycoplasma pneumoniae is one of the most common pathogens in young adult cases of community-acquired pneumonia (CAP). M. pneumoniae pneumonia (MPP) manifests non-specific symptoms and findings such as fever, sore throat, persistent dry cough, weakness and hepatic disorder, which are sometimes self-limiting (1,2). However, life-threatening cases of MPP including acute respiratory distress syndrome (ARDS) as a clinical presentation have been reported (2,3). Although systemic steroid therapy with appropriate anti-mycoplasmal drugs may be successful for these cases (2,3), their pathophysiology has not been fully elucidated.
The pulmonary vascular permeability index (PVPI), the ratio of the extravascular lung water (EVLW) to the pulmonary blood volume, reflects alveolar-capillary barrier permeability and is now regarded as a useful marker to differentiate ARDS from hydrostatic edema, with high specificity. In ARDS patients, EVLW increases with elevated PVPI, while in hydrostatic edema, EVLW increases without elevated PVPI (4). However, to the best of our knowledge, EVLW and PVPI in ARDS related to M. pneumoniae infection have not been reported. We herein describe a patient with MPP who progressed to severe ARDS, prompting the need for mechanical ventilation without elevated pulmonary vascular permeability, who was successfully treated with low-dose short-term hydrocortisone and levofloxacin.
Case presentation
A 31-year-old male who was previously in good health was admitted to a local hospital with fever and nonproductive cough. Although he was treated for bacterial pneumonia with cefotiam and piperacillin for the first 3 days, and subsequently with meropenem (MEPM) and micafungin for 1 day, his clinical condition changed rapidly for the worse and progressive dyspnea was observed. Then, he was transferred to our hospital with oxygen inhalation. On physical examination, his temperature was 38.8 °C, pulse 100/min, blood pressure 120/70 mmHg and respiratory rate 24/min. Lung auscultation revealed decreased breath sounds in both lungs.
Laboratory data were as follows: white blood cell count, 4,320/µL (neutrophils, 89.4%; lymphocytes, 8.6%; eosinophils, 0.2%; monocytes, 1.6%; basophils, 0.2%); hemoglobin, 12.2 g/dL; platelet count, 12.2×104/μL; C-reactive protein, 19.55 mg/dL; AST, 59 IU/L; ALT, 60 IU/L; LDH, 412 IU/L; (1-3)-β-d-glucan, 8.3 pg/mL (<20); and KL-6, 141 U/mL (<500). Serum anti-M. pneumoniae, Chlamydophila pneumonia, human immunodeficiency virus antibodies, serum Aspergillus galactomannan and Cryptococcus neoformans antigens, and Streptococcus pneumoniae and Legionella pneumophila urinary antigens were all negative. Arterial blood gas values when oxygen was supplied at 8 L/min by a non-rebreather mask were pH 7.500, PaCO2 40.3 mmHg, PaO2 52.5 mmHg and oxygen saturation of 89.0%. Chest X-ray (Figure 1A) and computed tomography (CT) (Figure 1B) showed diffuse air-space consolidation predominantly in the right lung. Although treatment using levofloxacin (LVFX) (750 mg/day) with MEPM (3 g/day) was initiated for severe CAP of unknown origin, dyspnea and pulmonary infiltration developed, necessitating mechanical ventilation on the second hospital day (Figure 1C). The PaO2/FiO2 ratio at 10 cmH2O positive end-expiratory pressure (PEEP) was 89.4 (89.4/1.0) mmHg. A continuous cardiac output (CCO) monitoring system (VolumeViewTM/EV1000TM, Edwards Lifesciences, Irvine, CA, USA) used for evaluation of the patient’s hemodynamic status revealed a preserved cardiac index (4.4 L/min/m2) with elevated EVLW indexed for body weight (EVLWi) (12.1 mL/kg), leading to a diagnosis of ARDS. Nevertheless, PVPI (2.1) was not elevated as in ARDS (4).
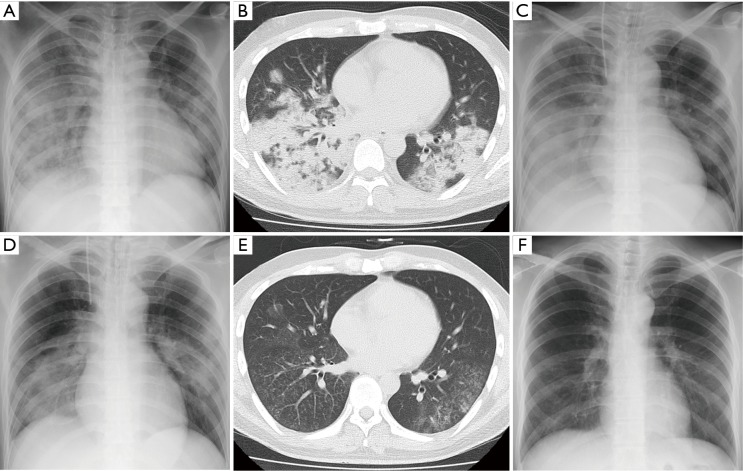
Figure 1.
Chest X-ray (A, C and D: antero-posterior; F: postero-anterior) and computed tomography (CT) (B and E) findings. (A) and (B): Diffuse air-space consolidation predominantly in the right lung on admission to our hospital (day 1); (C) Worsening of pre-existing lung infiltration one day after initiation of the treatment using levofloxacin (LVFX) (750 mg/day) with meropenem (3 g/day) (day 2); (D) Marked improvement during corticosteroid treatment (day 5). (E) and (F): Further improvements after administration of LVFX in a total period of two weeks (day 22). CT (E), but not X-ray (F), detected only indistinct micronodular infiltration.
Blood and sputum cultures ordered on admission were negative. However, M. pneumoniae DNA was detected in a bronchial lavage specimen obtained just after intubation by loop-mediated isothermal amplification assay. The patient was diagnosed with severe ARDS accompanied by mild hepatic disorder as a clinical presentation of fulminant MPP, and low-dose hydrocortisone infusion (300 mg/day) was started with the continuation of LVFX. MEPM was tapered and then stopped. After the initiation of corticosteroid treatment, the clinical course rapidly turned favorable. Pulmonary infiltration (Figure 1D) and oxygenation status improved in response to treatments. Hydrocortisone dose was tapered to 0 mg for 4 days (300 mg, 200 mg, 150 mg and 75 mg) and the endotracheal tube was removed after 4 days of mechanical ventilation (day 6). Then, oxygen therapy was stopped at day 12. After the administration of LVFX for a total period of two weeks, chest CT (Figure 1E), but not chest X-ray (Figure 1F), detected only indistinct micronodular infiltration, and hepatic disorder was not observed. EVLWi values declined with the improvement of the clinical course (Figure 2). However, PVPI values were 2.4 or less over the measurement period (Figure 2). The particle agglutination titer for M. pneumoniae rose from <1:40 to 1:2,560 during two weeks.
Figure 2.
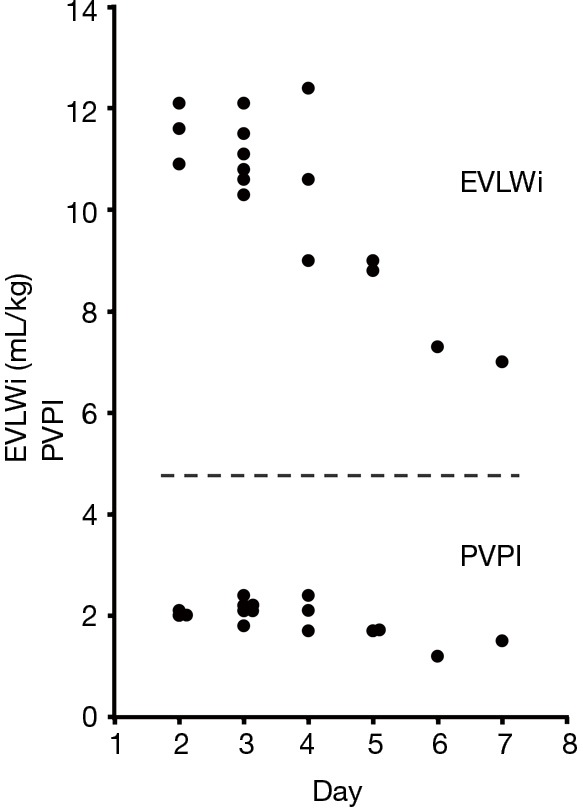
Time course of extravascular lung water indexed for body weight (EVLWi) and pulmonary vascular permeability index (PVPI) values. Until day 6, all values were measured using a continuous cardiac output monitoring system (VolumeViewTM/EV1000TM, Edwards Lifesciences, Irvine, CA, USA) at 10 cmH2O positive end-expiratory pressure (PEEP), whereas values of day 7 were measured during spontaneous respiration. EVLWi values decreased in response to treatments, while PVPI values were 2.4 or less over the entire measurement period.
Discussion
MMP usually follows a favorable clinical course and only a few percent of all patients with MPP need hospitalization (5). However, about 6% of inpatients with MPP require ventilatory support (2). Although the prognosis of MPP with hypoxia is not always poor (6,7), knowledge of the pathophysiology and pharmacological treatment of ARDS in MPP is insufficient due to its rarity.
ARDS is a condition designated with clinical diagnostic criteria and alveolocapillary hyperpermeability is regarded as its physiological hallmark. However, a clinical diagnosis of ARDS does not necessarily coincide with the presence of DAD, the histological hallmark of the syndrome, suggesting the heterogeneity of this clinical condition (8,9).
Sepsis due to common bacterial pathogens is a leading cause of ARDS and the inflammatory response under this condition that leads to ARDS mainly results from marked accumulation of neutrophils in the lung. It was thought that activated neutrophils induce an increase of pulmonary vascular permeability through the release of reactive oxygen species, proteases, leukotrienes and other molecules (10,11). Recently, the CCO monitoring system, which combines a transpulmonary thermodilution (TPTD) technique and continuous pulse contour analysis, has enabled the real-time evaluation of patients’ hemodynamic status including pulmonary vascular permeability, and been used for fluid management in critical care settings (12).
In contrast, pulmonary infiltration in MPP is considered to be associated with excessive cell-mediated immunity. After M. pneumoniae infection, alveolar macrophages and T cells synergistically induce the cellular immune response by the release of proinflammatory cytokines leading to ARDS in individuals with hyperresponsiveness (13-15). Although open lung biopsy in ARDS is impracticable, autopsy cases of MPP with fatal respiratory failure have shown that mycoplasma organisms localized on the surface of bronchiolar epithelium, whereas the tissue inflammation and damage extended into deeper parts of the lungs (16), suggesting host immune responses to highly immunogenic molecules associated with M. pneumoniae. However, in spite of the above-mentioned differences of pathophysiology, the pulmonary vascular permeability in ARDS related to M. pneumoniae infection has not been reported, except for this manuscript.
The characteristics of our patient at the beginning of ventilatory support represented severe ARDS as described by the Berlin definition (PaO2/FiO2 <100 mmHg with PEEP ≥5 cmH2O) (17). Concerning the CCO monitoring system, previous studies have suggested that EVLWi (>10 mL/kg) should be included in the definition of ARDS (18). Moreover, PVPI was higher in ARDS patients than in patients with hydrostatic pulmonary edema (4.7±1.8 vs. 2.1±0.5) (4). A PVPI of 3.0 was proposed as the cut-off value to allow the diagnosis of ARDS with sensitivity of 85% and specificity of 100% (4). The EVLWi value of our patient was more than 10 mL/kg, which was consistent with ARDS, and decreased in response to treatments. However, PVPI values were not equivalent to those of ARDS patients over the measurement period. Although there is no report regarding a correlation in clinical course between improvements of PVPI values and those of pulmonary infiltrations, we thought it was unlikely that PVPI was initially at a high value and had returned to normal by the time the patient was monitored because dyspnea and pulmonary infiltration kept getting worse until the initiation of PVPI monitoring. These data suggest that pulmonary infiltration caused by M. pneumoniae does not match the criteria of permeability edema observed in typical ARDS, reflecting the differences of pathophysiology.
Randomized controlled studies have revealed that high-dose short-term corticosteroid is ineffective for patients with sepsis-related ARDS, suggesting that neutrophilic lung inflammation with increased pulmonary vascular permeability is steroid-resistant (19-21). However, corticosteroids may have a desirable effect on MPP by down-regulating cell-mediated immunity. Clinical evidence supporting this hypothesis has been reported by several groups (2,3,6,7). Although about half of previously reported patients with fulminant MPP were treated with methylprednisolone pulse therapy probably following typical ARDS (6), the optimal dose and duration of steroid therapy remain unclear.
Besides M. pneumoniae infection, excessive cell-mediated immunity is observed upon immune reconstitution after the initiation of anti-HIV therapy, pregnancy, withdrawal from immunosuppressive agents and the initiation of antimicrobial treatment for patients with pathogen-induced immunosuppression resulting in various infectious diseases, such as Pneumocystis jirovecii pneumonia, cryptococcal disease and active tuberculosis (22-24). Generally speaking, the therapeutic dose of corticosteroids (prednisolone) for the treatment of these diseases is 0.5-1.5 mg/kg/day or less (25-27). Moreover, a recent study has shown that low-dose hydrocortisone treatment (10 mg/hour for 7 days) for severe CAP was related to decreased durations of mechanical ventilation and hospital stay, as well as risk of mortality (28). For these reasons, low-dose hydrocortisone infusion was introduced for our patient, and resulted in prompt clinical improvement. The hydrocortisone dose could be decreased from 300 mg to 0 mg for 4 days. The requirement for a sufficient dose and duration of corticosteroid treatment of ARDS in MPP may differ from that of sepsis-related ARDS.
Our data from previous reports indicate that the presence of alveolocapillary hyperpermeability or DAD is not a requirement for the diagnosis of a clinical syndrome of ARDS. The accumulation of data from the CCO monitoring system may contribute to narrowing down the heterogeneous conditions clinically diagnosed with ARDS to a typical phenotype. However, this system requires specialized devices and invasive procedures. More research is needed to find serum markers that can reflect alveolocapillary hyperpermeability or DAD.
Conclusions
In summary, our patient with MPP presented severe ARDS without elevated pulmonary vascular permeability, and was successfully treated with low-dose short-term hydrocortisone and anti-mycoplasmal drug. These facts suggested that the real-time monitoring of PVPI in ARDS related to M. pneumoniae infection may provide useful information about the pathophysiology and pharmacological treatment of individual patients. Further clinical studies on PVPI in ARDS related to M. pneumoniae infection are needed in order to establish the standard treatment of this rare condition.
Acknowledgements
We thank Yukihisa Komatsu (Division of Radiology, National Hospital Organization Kochi Hospital) for his contribution to this article.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Atkinson TP, Balish MF, Waites KB. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol Rev 2008;32:956-73. 10.1111/j.1574-6976.2008.00129.x [DOI] [PubMed] [Google Scholar]
- 2.Miyashita N, Obase Y, Ouchi K, et al. Clinical features of severe Mycoplasma pneumoniae pneumonia in adults admitted to an intensive care unit. J Med Microbiol 2007;56:1625-9. 10.1099/jmm.0.47119-0 [DOI] [PubMed] [Google Scholar]
- 3.Radisic M, Torn A, Gutierrez P, et al. Severe acute lung injury caused by Mycoplasma pneumoniae: potential role for steroid pulses in treatment. Clin Infect Dis 2000;31:1507-11. 10.1086/317498 [DOI] [PubMed] [Google Scholar]
- 4.Monnet X, Anguel N, Osman D, et al. Assessing pulmonary permeability by transpulmonary thermodilution allows differentiation of hydrostatic pulmonary edema from ALI/ARDS. Intensive Care Med 2007;33:448-53. 10.1007/s00134-006-0498-6 [DOI] [PubMed] [Google Scholar]
- 5.Foy HM. Infections caused by Mycoplasma pneumoniae and possible carrier state in different populations of patients. Clin Infect Dis 1993;17:S37-46. 10.1093/clinids/17.Supplement_1.S37 [DOI] [PubMed] [Google Scholar]
- 6.Izumikawa K, Izumikawa K, Takazono T, et al. Clinical features, risk factors and treatment of fulminant Mycoplasma pneumoniae pneumonia: a review of the Japanese literature. J Infect Chemother 2014;20:181-5. 10.1016/j.jiac.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 7.Miyashita N, Kawai Y, Inamura N, et al. Setting a standard for the initiation of steroid therapy in refractory or severe Mycoplasma pneumoniae pneumonia in adolescents and adults. J Infect Chemother 2015;21:153-60. 10.1016/j.jiac.2014.10.008 [DOI] [PubMed] [Google Scholar]
- 8.Thille AW, Esteban A, Fernández-Segoviano P, et al. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am J Respir Crit Care Med 2013;187:761-7. 10.1164/rccm.201211-1981OC [DOI] [PubMed] [Google Scholar]
- 9.Kao KC, Hu HC, Chang CH, et al. Diffuse alveolar damage associated mortality in selected acute respiratory distress syndrome patients with open lung biopsy. Crit Care 2015;19:228. 10.1186/s13054-015-0949-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson ER, Matthay MA. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv 2010;23:243-52. 10.1089/jamp.2009.0775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujishima S. Pathophysiology and biomarkers of acute respiratory distress syndrome. J Intensive Care 2014;2:32. 10.1186/2052-0492-2-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oren-Grinberg A. The PiCCO Monitor. Int Anesthesiol Clin 2010;48:57-85. 10.1097/AIA.0b013e3181c3dc11 [DOI] [PubMed] [Google Scholar]
- 13.Waites KB, Balish MF, Atkinson TP. New insights into the pathogenesis and detection of Mycoplasma pneumoniae infections. Future Microbiol 2008;3:635-48. 10.2217/17460913.3.6.635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimizu T, Kida Y, Kuwano K. Cytoadherence-dependent induction of inflammatory responses by Mycoplasma pneumoniae. Immunology 2011;133:51-61. 10.1111/j.1365-2567.2011.03408.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka H, Narita M, Teramoto S, et al. Role of interleukin-18 and T-helper type 1 cytokines in the development of Mycoplasma pneumoniae pneumonia in adults. Chest 2002;121:1493-7. 10.1378/chest.121.5.1493 [DOI] [PubMed] [Google Scholar]
- 16.Kannan TR, Hardy RD, Coalson JJ, et al. Fatal outcomes in family transmission of Mycoplasma pneumoniae. Clin Infect Dis 2012;54:225-31. 10.1093/cid/cir769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ARDS Definition Task Force , Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [DOI] [PubMed] [Google Scholar]
- 18.LeTourneau JL, Pinney J, Phillips CR. Extravascular lung water predicts progression to acute lung injury in patients with increased risk*. Crit Care Med 2012;40:847-54. 10.1097/CCM.0b013e318236f60e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernard GR, Luce JM, Sprung CL, et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med 1987;317:1565-70. 10.1056/NEJM198712173172504 [DOI] [PubMed] [Google Scholar]
- 20.Bone RC, Fisher CJ, Jr, Clemmer TP, et al. Early methylprednisolone treatment for septic syndrome and the adult respiratory distress syndrome. Chest 1987;92:1032-6. 10.1378/chest.92.6.1032 [DOI] [PubMed] [Google Scholar]
- 21.Luce JM, Montgomery AB, Marks JD, et al. Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis 1988;138:62-8. 10.1164/ajrccm/138.1.62 [DOI] [PubMed] [Google Scholar]
- 22.Achenbach CJ, Harrington RD, Dhanireddy S, et al. Paradoxical immune reconstitution inflammatory syndrome in HIV-infected patients treated with combination antiretroviral therapy after AIDS-defining opportunistic infection. Clin Infect Dis 2012;54:424-33. 10.1093/cid/cir802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh N, Perfect JR. Immune reconstitution syndrome and exacerbation of infections after pregnancy. Clin Infect Dis 2007;45:1192-9. 10.1086/522182 [DOI] [PubMed] [Google Scholar]
- 24.Breen RA, Smith CJ, Bettinson H, et al. Paradoxical reactions during tuberculosis treatment in patients with and without HIV co-infection. Thorax 2004;59:704-7. 10.1136/thx.2003.019224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montaner JS, Lawson LM, Levitt N, et al. Corticosteroids prevent early deterioration in patients with moderately severe Pneumocystis carinii pneumonia and the acquired immunodeficiency syndrome (AIDS). Ann Intern Med 1990;113:14-20. 10.7326/0003-4819-113-1-14 [DOI] [PubMed] [Google Scholar]
- 26.Lortholary O, Fontanet A, Mémain N, et al. Incidence and risk factors of immune reconstitution inflammatory syndrome complicating HIV-associated cryptococcosis in France. AIDS 2005;19:1043-9. 10.1097/01.aids.0000174450.70874.30 [DOI] [PubMed] [Google Scholar]
- 27.Meintjes G, Wilkinson RJ, Morroni C, et al. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS 2010;24:2381-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Confalonieri M, Urbino R, Potena A, et al. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med 2005;171:242-8. 10.1164/rccm.200406-808OC [DOI] [PubMed] [Google Scholar]