Abstract
The immune response of the host against invading pathogens is clinically manifested as sepsis. Sepsis is a complicated process characterized by distinct phases that usually occur in a sequential manner. The initial hyper-inflammation helps in elimination of the pathogen, but potentially may lead to excessive tissue injury. Hypo-inflammation helps in restoring immune homeostasis, but may lead to significant immune suppression and death from secondary infections if not appropriately controlled. Immune-modulating intervention in sepsis should be based on a balanced control of both the hyper and the hypo-inflammatory phase.
Keywords: Sepsis, janus kinase, inhibition, cytokines, immune-suppression
The immune response of the host against invading pathogens such as bacteria, fungi, and viruses is clinically manifested as sepsis. Sepsis is diagnosed when there is evidence for the presence of an infection and the host has clinical signs of the systemic inflammatory response syndrome (SIRS). Sepsis is characterized as severe when complicated by organ dysfunction, while septic shock is defined as sepsis with concurrent acute circulatory failure not responding to aggressive volume resuscitation.
Sepsis is the leading cause of death in intensive care unit (ICU) patients. Despite the significant improvements in supportive care and the prompt administration of broad spectrum empirical antimicrobial therapy, mortality rate is still around 30% (1).
Pathogenesis of sepsis
The pathogenesis of the septic process is extremely complex and involves a dynamic interplay between the pathogen and the host immune system. Although simplistic the immune response to sepsis can be divided in two distinct but overlapping phases (2). During the initial phase of sepsis, an over-activation of the immune system takes place in an effort to eliminate the invading pathogen. This hyper-inflammatory phase is mediated by a “cytokine storm’’ of pro-inflammatory cytokines such as TNF-α, IL-1, and IL-6, and if left uncontrolled results in excessive tissue damage manifesting as septic shock and/or multi-organ dysfunction (MOD). Termination of the inflammatory process and restoration of immune system homeostasis occurs immediately after control of infection and is mediated by secretion of anti-inflammatory cytokines such as IL-10 (3). Prolonged or intensive hypo-inflammatory state may lead to immune effector exhaustion finally resulting in immune-suppression that is frequently observed in the late phases of sepsis (2). During this late immune-suppressive phase, patients may develop secondary infections such as ventilator associated pneumonia or bloodstream and other organ specific infections by weakly virulent pathogens such as Acinetobacter, Stenotrophomonas, Enterococus and Candida (4). Secondary infections result in repeated cycles of hyper and hypo-inflammatory phases further complicating the septic process. Additional evidence for the sepsis-associated immunosuppression is the high incidence of herpes virus reactivation that occurs in patients with prolonged septic episodes (5). These clinical observations are supported from in vitro data showing impaired cytotoxicity and increased apoptosis of immune effectors from septic patients (6).
Trials targeting the hyper-inflammatory phase of sepsis
Interleukin-1 receptor antagonist (IL-1Ra) is a plasma protein that inhibits both IL-1a and IL-1b after binding to the type 1 IL-1 receptor (7). Anakinra is a recombinant human IL-1Ra. The efficacy of Anakinra as an immune-modulator in patients with sepsis was compared with placebo in a double blind phase III trial. In this trial anakinra failed to prolong 28-day survival, but in a subgroup analysis survival was improved in patients with septic shock or MOD in the arm of anakinra (8). Based on these data a phase III trial testing the efficacy of anakinra in patients with septic shock and/or severe sepsis was conducted, and again failed to show any survival benefit (9). However, in a subgroup analysis IL-1 inhibition improved survival in patients with severe sepsis and features of macrophage activation syndrome (MAS) (10). The beneficial efficacy of anakinra in this group of patients should be clarified in a prospective randomized trial.
Most of the clinical trials conducted in the past were based on the notion that sepsis mortality was mainly due to an uncontrolled inflammatory response, and therefore were focused on testing the effect of blocking the hyper-inflammatory phase by using various agents including corticosteroids, and anti-endotoxin or anti-cytokine antibodies. These studies failed to show therapeutic benefit, and moreover in many cases excessive anti-inflammatory inhibition had a negative impact on the outcome of septic patients (11-13).
Reasons for failure of agents targeting the hyper-inflammatory phase
Failure of pro-inflammatory cytokine inhibition to improve the outcome of septic patients might be attributed to several reasons:
Inhibition of the hyper-inflammatory phase needs to occur at the right time. Anti-inflammatory blockade should occur at the very beginning of the septic process and not at later stages where patients are already in a state of immune-suppression. Previous trials included patients admitted to ICU. Usually patients admitted to ICU are already in a late phase of the septic process with most of them having survived the initial hyper-inflammatory phase (14). Inhibition of pro-inflammatory cytokines in patients already in the late immunosuppressive phase might be detrimental. In accordance with the previous hypothesis is the fact that clinical trials using agents with the aim to block the initial pro-inflammatory phase showed some benefit in patients treated in early phase of sepsis, while the impact was detrimental in patients treated during the late imuno-supressed phase of sepsis (15);
Moreover, inhibition of a single cytokine may not be effective simply because the function of one cytokine is counterbalanced by one or more of the other pro-inflammatory mediators acting in parallel;
Another important issue is the intensity of inhibition, since a certain degree of inflammation is required for the effective clearance of the pathogen. Complete blockade of the hyper-inflammatory phase might have detrimental effects because of the inability to eliminate the pathogen. In conclusion, absence of fine control and monitoring of the inhibition in a time specific manner may contribute to the failure of anti-inflammatory trials.
The significance of a fine balance between pro and anti-inflammatory phases, and the association with outcome has been shown convincingly in an animal model of sepsis induced by cecal ligation and puncture (CLP). A hyper-inflammatory immune response mediated by an excessive amount of pro-inflammatory cytokines is observed in severe sepsis and is associated with increased mortality, whereas a more balanced immune response with a more pronounced anti-inflammatory phase is associated with less severe sepsis and reduced mortality. Increasing the pro/anti-inflammatory ratio by administering an anti-IL-10 antibody increases death rate, while decreasing the pro/anti-inflammatory ratio by exogenous administration of IL-10 results in increase survival. Thus, the fine balance between the pro and anti-inflammatory mediators is closely related to severity and outcome of sepsis (16).
The significance of the balance between pro and anti-inflammatory cytokines in the outcome of sepsis has been shown also in human studies. In a previous study including patients with sepsis a TNF-α/IL-10 ratio during the first 48 hours equal to one is a good predictor of improved outcome (17).
Prevention of immune suppression associated with sepsis
As already mentioned most of the patients admitting in ICU have survived the hyper-inflammatory stage and are already in a state of immune-suppression and in risk for secondary infections. Since immune-suppression is considered as a significant contributor of sepsis mortality, harnessing the host immunity by various agents is a reasonable alternative. Based on this notion, previous trials attempted to shorten the degree and the length of the immune-suppressive phase by using immune-stimulators such as interferon-gamma (IFN-gamma), granulocyte macrophage colony stimulating factor (GM-CSF), and granulocyte colony stimulating factor (G-CSF). However, the clinical benefit produced by these agents was modest (18,19).
A promising cytokine with an immune stimulating effect and the ability to restore immune function in various diseases is interleukin-7 (IL-7). IL-7 is a cytokine with a pleotropic action on various immune cell subsets including proliferation of both naïve and memory T-cells. Administration of IL-7 to patients with cancer and HIV infected patients resulted in significant increase of peripheral blood CD4 and CD8 without expansion of the T-regulatory cell pool (20). The immune stimulating effect and the good safety clinical profile makes IL-7 a promising agent that need to be tested in future clinical trials in sepsis patients.
Recently the scientific interest has focused on the manipulation of immune checkpoint inhibitors for the treatment of patients with cancer. An interesting observation in patients with sepsis is the increased expression of PD-1 on T-cells as an important mediator of the immune-suppressive phase. Indeed, animal studies have already shown that blocking of PD-1/PD-L checkpoint results in improved survival of the septic mice (21). Moreover, human trials have shown that PD-1 overexpression is associated with impaired T-cell function and mortality from secondary infections in patients with established sepsis (22). These data suggest that blocking immune checkpoints with the aim to prevent sepsis-associated immune-suppression is a promising therapeutic option that deserves testing in human clinical trials.
Balanced monitoring of both hyper-inflammatory and immunosuppressive phase
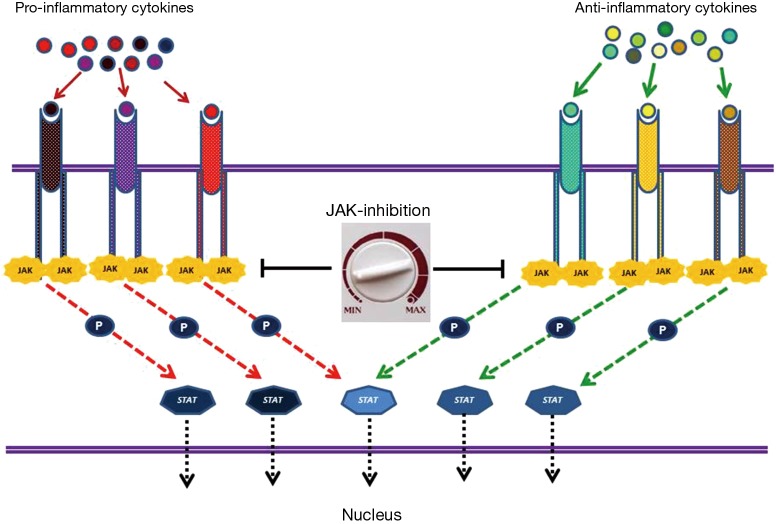
Pro-inflammatory and anti-inflammatory cytokines mediate their effect through a common downstream signaling pathway that consists of different tyrosine kinase of Janus family (JAKs) interacting with signal transducer and activator of transcription (STAT) proteins. Inhibition of JAK proteins theoretically offers the potential for a global immune-modulation affecting both phases of sepsis, e.g., preventing the excessive tissue damage associated with hyper-inflammation and the over expression of anti-inflammatory cytokines resulting in immune paresis and death from secondary infections. Ruxolitinib (Ruxo), a JAK1 and JAK2 small molecule inhibitor recently approved for the treatment of patients with primary and secondary myelofibrosis, is a candidate drug for testing in future clinical trials (23).
In a previous study performed by our team, we examined the effect of Ruxo in a mouse model of sepsis due to Candida albicans. Fungal loads and inflammation scores were determined in various affected organs, while levels of pro and anti-inflammatory cytokines were measured in the serum of infected animals. Mice infected with Candida were treated with increasing doses of Ruxo (1.5–50 mg/kg). An inverted-U correlation between Ruxo dosing and median survival time (MST) was observed. High dose Ruxo was associated with the worst survival, while progressive de-escalation of dosing resulted in gradual increase of MST. Low dose (6.25 mg/kg) Ruxo produced the best survival and mice treated with this dose had increased survival as compared with control animals. The therapeutic benefit of Ruxo was lost after further reduction of dose. Thus, low dose Ruxo (6.25 mg/kg) was found as the optimal dose for treatment of septic mice. Mice treated with high dose Ruxo had the higher fungal loads and lower inflammation scores as compared with control mice, meaning that intensive abrogation of the inflammatory phase results in overwhelming infection. On the contrary, mice treated with the optimal dose of Ruxo had the same fungal load but lower inflammation score as compared with placebo, meaning that increased survival is due to prevention of excessive tissue injury and not to an antifungal effect of the study drug. Interestingly, mice treated with the optimal dose of Ruxo had a balanced serum TNF-α/IL-10 ratio equal to one, as a further proof of the concept that a balance between pro- and anti-inflammatory signalling is required for a successful outcome (24).
Conclusions
Data from animal studies support the concept that any immune-modulating intervention in sepsis should also take into account the immunosuppressive phase that may be exacerbated by an uncontrolled inhibition of the early hyper-inflammatory phase. Balanced monitoring of both phases may result in prevention of excessive tissue damage through control of hyper-inflammation and without leading to significant immune-suppression (Figure 1). Administration of agents with immune-stimulating activity such as IL-7 and immune checkpoint inhibitors, with the aim to alleviate immune-suppression should be tested in clinical trials. Inhibition of the JAK-STAT pathway is another therapeutic target with promising efficacy in animal studies. The efficacy of Ruxolitinib as an immune-modulator in sepsis should be further tested in human trials.
Figure 1.
Binding of pro and anti-inflammatory cytokines to specific receptors expressed on the cell surface results in activation of JAK-STAT signal transduction pathway. Janus tyrosine kinase family consists of four non-receptor kinases (Jak1, Jak2, Jak3, and Tyk2). Activated JAKs recruit and phosphorylate STAT proteins. STAT family consists of seven functionally related proteins (Stat1, Stat2, Stat3, Stat4, Stat5a, Stat5b, and Stat6). Activated STATs dissociate from the receptor, dimerize and translocate to nucleus and regulate gene expression. Various cytokines activate different JAK-STAT complexes and result in characteristic gene expression patterns. JAK inhibition results in downstream signaling blockade of all pro and anti-inflammatory cytokines. Moreover, escalating or de-escalating the dose of JAK-inhibitor offers the unique possibility of balanced monitoring of both pro and anti-inflammatory phases in sepsis. JAK, kinase of Janus; STAT, signal transducer and activator of transcription.
Acknowledgements
None.
Footnotes
Provenance: This is an invited Perspective commissioned by the Section Editor Zhongheng Zhang (Department of Critical Care Medicine, Jinhua Municipal Central Hospital, Jinhua Hospital of Zhejiang University, Jinhua, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Gaieski DF, Edwards JM, Kallan MJ, et al. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 2013;41:1167-74. 10.1097/CCM.0b013e31827c09f8 [DOI] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003;348:138-50. 10.1056/NEJMra021333 [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am J Emerg Med 2008;26:711-5. 10.1016/j.ajem.2007.10.031 [DOI] [PubMed] [Google Scholar]
- 4.Kollef KE, Schramm GE, Wills AR, et al. Predictors of 30-day mortality and hospital costs in patients with ventilator-associated pneumonia attributed to potentially antibiotic-resistant gram-negative bacteria. Chest 2008;134:281-7. 10.1378/chest.08-1116 [DOI] [PubMed] [Google Scholar]
- 5.Heininger A, Haeberle H, Fischer I, et al. Cytomegalovirus reactivation and associated outcome of critically ill patients with severe sepsis. Crit Care 2011;15:R77. 10.1186/cc10069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unsinger J, Kazama H, McDonough JS, et al. Sepsis-induced apoptosis leads to active suppression of delayed-type hypersensitivity by CD8+ regulatory T cells through a TRAIL-dependent mechanism. J Immunol 2010;184:6766-72. 10.4049/jimmunol.0904054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granowitz EV, Clark BD, Mancilla J, et al. Interleukin-1 receptor antagonist competitively inhibits the binding of interleukin-1 to the type II interleukin-1 receptor. J Biol Chem 1991;266:14147-50. [PubMed] [Google Scholar]
- 8.Fisher CJ, Jr, Dhainaut JF, Opal SM, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA 1994;271:1836-43. 10.1001/jama.1994.03510470040032 [DOI] [PubMed] [Google Scholar]
- 9.Opal SM, Fisher CJ, Jr, Dhainaut JF, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med 1997;25:1115-24. 10.1097/00003246-199707000-00010 [DOI] [PubMed] [Google Scholar]
- 10.Shakoory B, Carcillo JA, Chatham WW, et al. Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit Care Med 2016;44:275-81. 10.1097/CCM.0000000000001402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vincent JL, Sun Q, Dubois MJ. Clinical trials of immunomodulatory therapies in severe sepsis and septic shock. Clin Infect Dis 2002;34:1084-93. 10.1086/339549 [DOI] [PubMed] [Google Scholar]
- 12.Abraham E, Laterre PF, Garbino J, et al. Lenercept (p55 tumor necrosis factor receptor fusion protein) in severe sepsis and early septic shock: a randomized, double-blind, placebo-controlled, multicenter phase III trial with 1,342 patients. Crit Care Med 2001;29:503-10. 10.1097/00003246-200103000-00006 [DOI] [PubMed] [Google Scholar]
- 13.Panacek EA, Marshall JC, Albertson TE, et al. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab')2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit Care Med 2004;32:2173-82. 10.1097/01.CCM.0000145229.59014.6C [DOI] [PubMed] [Google Scholar]
- 14.Otto GP, Sossdorf M, Claus RA, et al. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit Care 2011;15:R183. 10.1186/cc10332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis 2013;13:260-8. 10.1016/S1473-3099(13)70001-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walley KR, Lukacs NW, Standiford TJ, et al. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun 1996;64:4733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gogos CA, Drosou E, Bassaris HP, et al. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis 2000;181:176-80. 10.1086/315214 [DOI] [PubMed] [Google Scholar]
- 18.Giamarellos-Bourboulis EJ, Raftogiannis M. The immune response to severe bacterial infections: consequences for therapy. Expert Rev Anti Infect Ther 2012;10:369-80. 10.1586/eri.12.2 [DOI] [PubMed] [Google Scholar]
- 19.Dries DJ, Jurkovich GJ, Maier RV, et al. Effect of interferon gamma on infection-related death in patients with severe injuries. A randomized, double-blind, placebo-controlled trial. Arch Surg 1994;129:1031-41; discussion 1042. 10.1001/archsurg.1994.01420340045008 [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg SA, Sportès C, Ahmadzadeh M, et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother 2006;29:313-9. 10.1097/01.cji.0000210386.55951.c2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Zhou Y, Lou J, et al. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit Care 2010;14:R220. 10.1186/cc9354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guignant C, Lepape A, Huang X, et al. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care 2011;15:R99. 10.1186/cc10112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mesa RA, Gotlib J, Gupta V, et al. Effect of ruxolitinib therapy on myelofibrosis-related symptoms and other patient-reported outcomes in COMFORT-I: a randomized, double-blind, placebo-controlled trial. J Clin Oncol 2013;31:1285-92. 10.1200/JCO.2012.44.4489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsirigotis P, Papanikolaou N, Elefanti A, et al. Treatment of Experimental Candida Sepsis with a Janus Kinase Inhibitor Controls Inflammation and Prolongs Survival. Antimicrob Agents Chemother 2015;59:7367-73. 10.1128/AAC.01533-15 [DOI] [PMC free article] [PubMed] [Google Scholar]