Abstract
Background and Purpose
Sirtuin1 (SIRT1), the founding member of mammalian class III histone deacetylases, is reported to be a drug target involved in fibrotic diseases. However, whether it is an effective drug target in hypertrophic scar treatment is still not known.
Experimental Approach
In the present study, we observed that SIRT1 localized to both the epidermis and the dermis of skin tissues by immunohistochemistry. After knock‐down of SIRT1 by shRNA or up‐regulating SIRT1 by resveratrol, the expression of α‐SMA, Col1 and Col3 in fibroblasts were detected by western blots. A mouse excision wound healing model was used to observe the changes in collagen fibre associated with the different expression levels of SIRT1.
Key Results
SIRT1 expression was inhibited in hypertrophic scar tissue. The down‐regulation of SIRT1 resulted in an increased expression of α‐SMA, Col1 and Col3 in hypertrophic scar‐derived fibroblasts. In contrast, the up‐regulation of SIRT1 not only inhibited the expression of α‐SMA, Col1 and Col3 in hypertrophic scar‐derived fibroblasts but also blocked the activation of TGFβ1‐induced normal skin‐derived fibroblasts. In the mouse model of wound healing, the deletion of SIRT1 resulted in denser collagen fibres and a more disordered structure, whereas resveratrol treatment led to a more organized and thinner collagen fibre, which was similar to that observed during normal wound healing.
Conclusions and Implications
The results revealed that SIRT1 negatively regulates TGFβ1‐induced fibroblast activation and inhibits excessive scar formation and is, therefore, a promising drug target for hypertrophic scar formation.
Abbreviations
- α‐SMA
α‐smooth muscle actin
- Col1
collagen 1
- Col3
collagen 3
- DAB
3,3′‐diaminobenzidine
- ECM
extracellular matrix
- EMT
epithelial‐mesenchymal transition
- HDACs
histone deacetylases
- SIRT1
sirtuin 1
- TSA
trichostatin A
Tables of Links
| TARGETS | ||||||
|---|---|---|---|---|---|---|
| Collagenase 1 (MMP1) | ||||||
| HDACs | ||||||
| Sirtuin 1 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015).
Introduction
Hypertrophic scars are characterized by the excessive production and deposition of extracellular matrix (ECM) proteins. They usually develop after burn injury, trauma and surgery, and cause not only cosmetic but also functional problems (Gurtner et al., 2008; Hsu et al., 2010; Gauglitz et al., 2011; Tyack et al., 2012). Although extensively studied, the mechanism of hypertrophic scar formation is still currently unclear, and treatment remains a great challenge. It is widely accepted that the major characteristic of hypertrophic scars is the excessive deposition of collagen‐based ECM proteins. Under normal conditions, the dynamic balance between the synthesis and degradation of collagen is regulated by matrix metalloproteinase, tissue inhibitor of metalloproteinase and various cytokines, such as TGFβ1. The balance is broken after skin injuries, which leads to increased synthesis and deposition of collagen, and the skin then recovers and becomes normal after wound healing (Sternlicht and Werb, 2001; Park et al., 2004; Armour et al., 2007). However, prolonged inflammation and other chronic stimuli may result in the overproduction of collagen, finally leading to hypertrophic scar formation (Armour et al., 2007; van der Veer et al., 2009).
Among the various cell types in skin, fibroblasts are responsible for the synthesis of collagen and other ECM proteins and play a critical role in wound healing and scar formation. After skin injuries, fibroblasts transdifferentiate into myofibroblasts, which are characterized by the expression of α‐smooth muscle actin (α‐SMA) and an increased propensity to synthesize and secrete collagen and thus facilitate wound healing. However, the persistence of fibroblasts/myofibroblasts after wound healing may result in the formation of hypertrophic scars (Hinz, 2010; Sidgwick and Bayat, 2012; Klingberg et al., 2013). Although it is not fully understood, current evidence suggests that the fibroblast‐to‐myofibroblast transition is primarily regulated by TGFβ1 (Sarrazy et al., 2011; Pakyari et al., 2013). In addition to α‐SMA, various fibrosis‐related proteins are transcriptionally regulated by TGFβ1, including collagen 1 (Col1) and collagen 3 (Col3) (Kohan et al., 2010; Conte et al., 2011; Conte et al., 2013). Given their critical role in scar formation, myofibroblasts have been considered as an anti‐fibrosis/scar target (Penn et al., 2012). Recently, it was reported that histone deacetylases (HDACs) play an important role in the transcriptional regulation of collagen, partly by binding to the proximal promoters of the collagen gene with other transcription factors and co‐factors (Xia et al., 2012); thus, they might be a potential therapeutic strategy for hypertrophic scars (Li et al., 2010; Huang et al., 2014).
Sirtuins, the class III HDACs that function through the deacetylation of histone and non‐histone substrates, are involved in ageing, epigenetics, inflammation, cancer and a variety of other cellular processes (Michan and Sinclair, 2007). Among the seven members of the class III HDAC family, sirtuin 1 (SIRT1) is best characterized. SIRT1, the mammalian orthologue of yeast silent information regulator 2, is involved in a large number of cellular activities, including metabolism (Chang and Guarente, 2014), proliferation, differentiation, survival and apoptosis (Xia et al., 2011), and it plays a key role in chronic diseases, including diabetes (Kitada and Koya, 2013), chronic inflammatory pulmonary diseases (Arunachalam et al., 2010), neurodegeneration (Herskovits and Guarente, 2013) and chronic renal diseases (Kitada et al., 2014). Recently, it has been reported that SIRT1 might be involved in the formation and development of fibrotic diseases (Schuetze et al., 2014; Williams et al., 2014). In kidney epithelial cells, SIRT1 inhibits TGFβ‐driven epithelial‐mesenchymal transition (EMT) and suppresses kidney fibrosis (Simic et al., 2013). The overexpression of SIRT1 attenuates TGFβ1‐induced ECM expression, probably by binding to Smad3, as well as the deacetylation of PPAR‐γ coactivator‐1a (Planavila et al., 2011; Huang et al., 2014). Moreover, resveratrol (3,5,4′‐trihydroxy‐trans‐stilbene), the agonist of SIRT1, up‐regulates both the expression (Costa Cdos et al., 2010) and enzyme activity of SIRT1 (Howitz et al., 2003; Hubbard et al., 2013a), and has been reported to inhibit renal fibrosis, liver fibrosis and bleomycin‐induced pulmonary fibrosis (Lee et al., 2010; Akgedik et al., 2012; Liang et al., 2014). However, the expression pattern of SIRT1 in hypertrophic scars and its effects on the fibrotic responses of fibroblasts and scar formation are poorly understood.
In the present study, we investigated the role of SIRT1 during fibroblast activation and scar formation. We found that the expression of SIRT1 decreased in the hypertrophic scar tissues, and the up‐regulation of SIRT1 induced by resveratrol blocked TGFβ1‐induced dermal fibroblast transition and led to an improvement in the density and the arrangement of collagen fibres in a mouse model of wound healing. It was concluded that SIRT1 is a promising therapeutic target for the treatment of hypertrophic scars.
Methods
Hypertrophic scar tissues and cell culture
Hypertrophic scar samples and adjacent full‐thickness normal skin tissues from nine patients with no previous treatment were obtained during plastic surgery (Supporting Information Table S1). Diagnosis was confirmed by routine pathological examination. Before surgery, all of the patients were informed of the purpose and procedure of this study and agreed to donate excess tissue. Written informed consent was obtained from all participants involved in this study. All of the protocols were approved by the Ethics Committee of Xijing Hospital, affiliated with the Fourth Military Medical University (China). The protocol number is XJYYLL‐2013190. Each sample was divided into three parts for Western blot analysis, immunohistochemistry and cell culture. To isolate the fibroblasts from the tissue, the dermal parts of the tissues were rinsed repeatedly with PBS and were then minced and incubated at 37 °C for 3 h in a solution containing type I collagenase (0.1 mg · mL−1, Sigma, Germany). The fibroblasts were pelleted and grown in DMEM (Waltham, MA, Gibco, USA), supplemented with 10% fetal calf serum (Gibco), 100 U · mL−1 penicillin and 100 U · mL−1 streptomycin. The cells were incubated at 37 °C in a 5% (v · v−1) CO2 humidified atmosphere. All experiments were performed using cells between the 3rd and 5th passages.
Mouse cutaneous excision model
All animal experiments were performed in accordance with the guidelines from the Administration of Animal Experiments for Medical Research Purposes issued by the Ministry of Health of China, which is in accordance with the principle of ARRIVE (Kilkenny et al., 2010; McGrath and Lilley, 2015). The protocol was approved by the Animal Experiment Administration Committee of the Fourth Military Medical University (no: XJYYLL‐2013190). All surgical procedures were performed under 1% sodium pentobarbital anaesthesia (0.5 mL · 100 g−1 bodyweight) and in a clean surgical room with sterilized instruments. All efforts were made to minimize the suffering of the mice during the experiments. The hair was shaved from the dorsum of the mice, and the skin was treated with iodine solution. Full‐thickness excisional wounds of 1 cm × 1 cm were created using a template on the dorsal skin of 7‐week‐old Balb/C mice (Outtz et al., 2010). No antibiotics were used as the incision was clean. No analgesics were used as the mice seemed active after resuscitation. One hundred microlitres of SIRT1 shRNA at 100 nM or resveratrol (4.4 mM, 0.5 mL · 100 g−1 bodyweight) was administered i.d. and circumferentially around the wounds at 1, 3, 5 and 7 days after the wound was established. After 4 weeks, the wound tissues and the normal skin around the wounds were harvested and fixed in 10% formaldehyde for Masson's trichrome staining to assess wound healing, collagen production and distribution. Twenty‐four Balb/C mice were used in the experiment.
Lentiviral transfection in vivo
The recombinant lentiviral vector for silencing of SIRT1 expression (SIRT1 shRNA), negative control lentiviral vector containing non‐specific shRNA (mock), lentiviral expressing SIRT1 (Lv‐SIRT1) and control lentiviral (Lv‐NC, mock) were purchased from Shanghai Gene Chemistry Company (Shanghai, China). All vectors were labelled with GFP, which served as a marker. Hypertrophic scar‐derived fibroblasts, grown to 70–80% confluence, were incubated for 12 h in serum‐depleted medium and transfected with SIRT1 shRNA (33 nM) for 48 h to generate stable SIRT1 knock‐down cells. The efficiency of shRNA infection was measured by Western blot analysis.
Real‐time PCR
Total RNA from cultured cells was extracted using an RNA‐isolation kit (Takara, Kusatsu, Japan). Five hundred nanograms of total RNA was reverse‐transcribed using a PrimeScript™ RT reagent Kit (Takara). The cDNA obtained was then amplified by the Bio‐Rad IQ5 real‐time system (Bio‐Rad, Hercules, CA, USA), using SYBR®Premix Ex Taq™ Kit (Takara) with specific primers (Supporting Information Table S2). The PCR conditions were 95 °C for 30 s, followed by 40 cycles of 95 °C for 30 s and 60 °C for 10 s, and elongation at 72 °C for 15 s. The results from three independent vials were used to determine the relative expression levels of the target genes, which were normalized against the expression level of GAPDH.
Western blotting
Fifty micrograms of total protein was subjected to SDS‐PAGE and transferred onto PVDF membranes. The membranes were blocked with 5% non‐fat milk at room temperature for 3 h and were then incubated with rabbit anti‐human α‐SMA (1:1000, Epitomics, Burlingame, CA, USA), rabbit anti‐human Col1 (Col1α2, 1:1000, Abcam, Cambridge, UK), rabbit anti‐human Col3 (1:1000, Abcam), mouse anti‐human SIRT1(1:800, Abcam) and rabbit anti‐human β‐actin (1:1000, Abcam) antibodies at 4 °C overnight. Then, the membranes were incubated with HRP‐conjugated secondary antibodies diluted at 1:3000 (Boster, Wuhan, China) at 37 °C for 1 h. The proteins were visualized with an ECL Kit (Millipore, Billerica, MA, USA) and FluorChemFC (Alpha Innotech, San Leandro, CA, USA).
Immunohistochemistry and immunocytochemistry
Hypertrophic scar and normal skin tissues were fixed in 10% formaldehyde. The paraffin‐embedded sections were dewaxed, and endogenous peroxidase activity was quenched with 3% hydrogen peroxide. After being blocked with goat serum for 30 min, the sections were incubated at 4 °C overnight with mouse anti‐human SIRT1 antibody (1:100, Abcam). Then, the sections were incubated with biotinylated antibody, and streptavidin‐biotin‐HRP and 3,3′‐diaminobenzidine (DAB) were used for signal amplification and staining, respectively. Finally, the sections were counterstained with haematoxylin. A negative control was achieved using an isotype‐matched IgG in each of the immunostaining conditions.
Fibroblast samples were fixed in 4% formaldehyde and were permeabilized with 1% Triton X‐100. After being blocked with 1% BSA, the samples were incubated with rabbit anti‐human α‐SMA antibodies (1:500, Epitomics). Cy3‐conjugated goat anti‐rabbit IgG antibody (1:100, Cwbiology, Beijing, China) was used as a secondary antibody, and DAPI was used for nuclear staining. An image was obtained and analysed using the Image‐Pro Plus system.
Statistical analysis
The results are presented as the mean ± SEM of six independent experiments. Statistical analysis was performed using ANOVA or Student's t‐test as appropriate using the spss (Statistical Package for the Social Sciences) 13.0 programme (IBM, Chicago, IL, USA). P < 0.05 was considered statistically significant. The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).
Results
The expression of SIRT1 is decreased in hypertrophic scars
To determine the localization and expression level of SIRT1 in hypertrophic scars and normal skin tissues, immunohistochemistry was performed. The results showed that SIRT1 was localized to both the epidermal and dermal layers of skin tissues. DAB staining showed that the intensity of SIRT1 in hypertrophic scar tissues was lower than that in normal skin tissues (Figure 1A). To further validate these observations, the mRNA and protein levels of SIRT1 were analysed by real‐time PCR and western blot. As shown in Figure 1B and Figure 1C, similar to the immunochemistry results, the mRNA and protein levels of SIRT1 decreased significantly in hypertrophic scars compared with normal skin tissues (P < 0.05).
Figure 1.
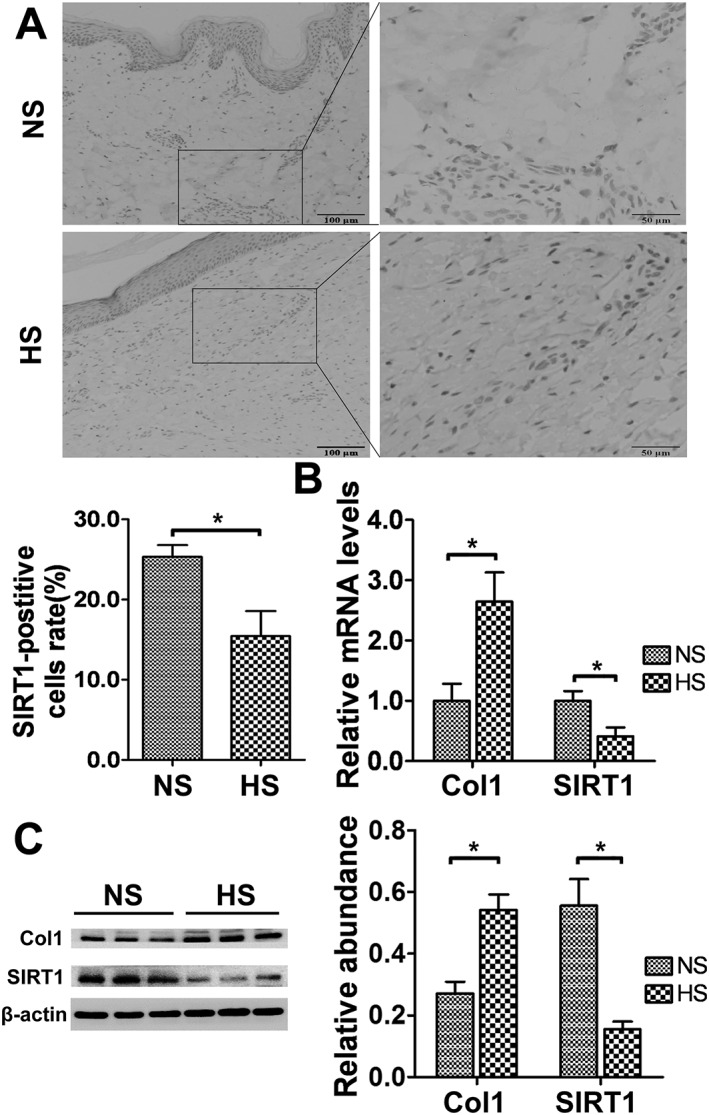
Decreased SIRT1 expression in hypertrophic scar tissues. (A) Representative immunohistochemical staining for SIRT1 in normal skin (NS) and hypertrophic scar (HS) tissues (scale bar = 50 μm). The average SIRT1‐positive cell rate was calculated (n = 6). (B) SIRT1 and Col1 mRNA expression levels in NS and HS tissues were analysed by real‐time PCR and normalized to GAPDH (n = 6). (C) Representative western blot analysis for SIRT1 and Col1 in NS and HS tissues (n = 6). The results represent the mean ± SEM of six independent experiments. *P < 0.05.
SIRT1 shRNA up‐regulated the expression of Col1, Col3 and α‐SMA in hypertrophic scar‐derived fibroblasts
To investigate the role of SIRT1 during hypertrophic scar formation, shRNA was used to reduce SIRT1 expression. The inhibitory effects of four types of SIRT1 shRNA are shown in Supporting Information Fig. S1. The shRNA No.2214 was chosen in the following experiments. Twenty‐four hours after the transfection, the relative SIRT1 mRNA level decreased hypertrophic scar‐derived fibroblasts compared with the non‐specific shRNA (mock) group (P < 0.05, Figure 2A). Furthermore, the mRNA levels of Col1, Col3 and α‐SMA were up‐regulated in both kinds of fibroblasts (P < 0.05) (Figure 2A). Similarly, 48 h after SIRT1 shRNA transfection, the protein levels of SIRT1 decreased significantly, whereas the protein levels of Col1, Col3 and α‐SMA were markedly up‐regulated (P < 0.05, Figure 2B).
Figure 2.
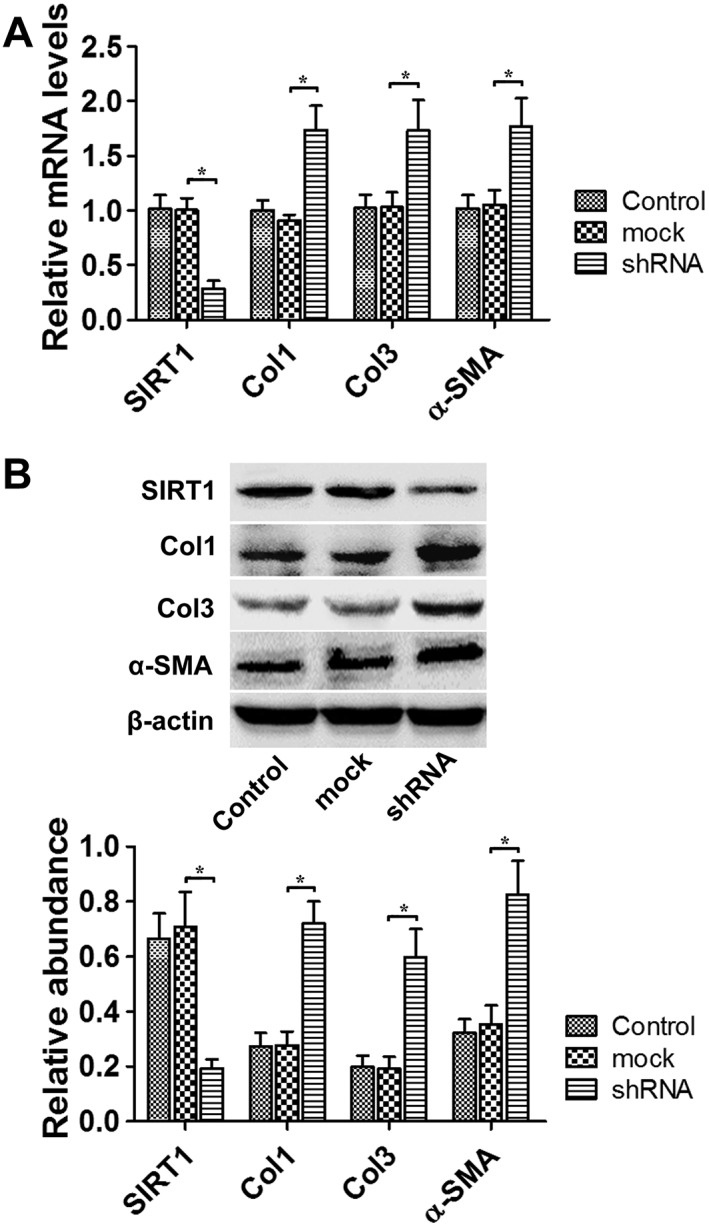
SIRT1 shRNA treatment up‐regulated Col1, Col3 and α‐SMA expression in hypertrophic scar fibroblasts. (A) The effects of SIRT1 shRNA on SIRT1, Col1, Col3 and α‐SMA mRNA levels in fibroblasts derived from hypertrophic scars were analysed by real‐time PCR and normalized to GAPDH (n = 6). (B) Representative western blot analysis for SIRT1, Col1, Col3 and α‐SMA protein levels in hypertrophic scar‐derived fibroblasts after SIRT1 down‐regulation by shRNA treatment (n = 6). The results represent the mean ± SEM of six independent experiments. *P < 0.05.
The up‐regulation of SIRT1 suppressed the expression of α‐SMA, Col1 and Col3 in hypertrophic scar‐derived fibroblasts
As the expression of SIRT1 was decreased in the hypertrophic scar tissue, we further investigated whether the up‐regulation of SIRT1 by its agonist resveratrol as well as Lv‐SIRT1 could reverse the elevated expression of α‐SMA, Col1 and Col3 in hypertrophic scar‐derived fibroblasts. After stimulating the fibroblasts with resveratrol at doses of 2.5, 5, 10, 20 and 40 μM, the protein levels of SIRT1 were detected by western blot. With increasing resveratrol concentrations, the protein level of SIRT1 was up‐regulated and reached a maximum elevation at 20 μM (Supporting Information Fig. S2). Hence in the following experiments, the concentration of resveratrol used was 20 μM. We further observed that 24 h after 20 μM resveratrol was added, the mRNA levels of Col1, Col3 and α‐SMA were significantly down‐regulated (Figure 3A). The relative protein levels were also down‐regulated at 48 h after resveratrol administration or Lv‐SIRT1 transfection (Figures 3B and 4A).
Figure 3.
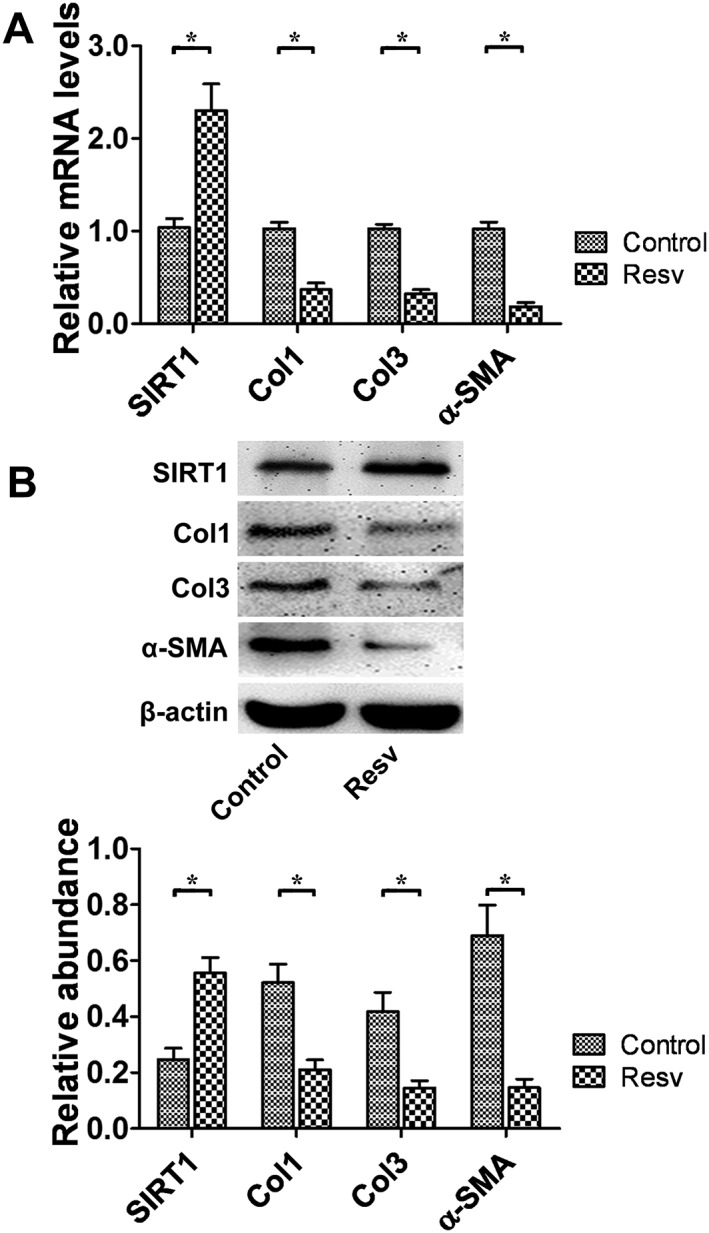
SIRT1 up‐regulation by resveratrol treatment down‐regulated Col1 Col3, and α‐SMA expression in hypertrophic scar fibroblasts. (A) The effects of resveratrol on the mRNA levels of SIRT1, Col1, Col3 and α‐SMA were analysed by real‐time PCR and normalized to GAPDH (n = 6). (B) Representative western blot analysis for SIRT1, Col1, Col3 and α‐SMA protein levels after the up‐regulation of SIRT1 by resveratrol (n = 6). Resv, resveratrol. The results represent the mean ± SEM of six independent experiments. *P < 0.05.
Figure 4.
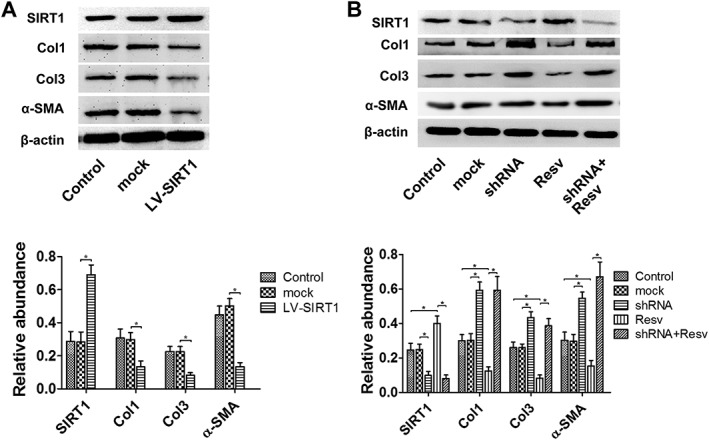
The overexpression of SIRT1 inhibited the expression the Col1, Col3 and α‐SMA. After the infection of SIRT1 shRNA, resveratrol did not change the expression of the three molecules. (A) The effects of Lv‐SIRT1 infection on the expression of Col1, Col3 and α‐SMA in hypertrophic scar‐derived fibroblasts were detected by western blot (n = 6). (B) Representative western blot analysis for Col1, Col3 and α‐SMA protein levels after treatment with SIRT1 shRNA and resveratrol (n = 6). Resv, resveratrol. The results represent the mean ± SEM of six independent experiments. *P < 0.05.
Resveratrol, the agonist of SIRT1, down‐regulates the expression of Col1, Col3 and α‐SMA through up‐regulating SIRT1
SIRT1 shRNA were first added into the medium of hypertrophic scar‐derived fibroblasts for 48 h, followed by the application of resveratrol. Forty‐eight hours later, the protein levels of SIRT1, Col1 and Col3 were detected. As shown in Figure 4B, compared with the control and mock group, the resveratrol group showed a significant increasing in SIRT1 and decrease in Col1, Col3 and SMA. Whereas in the shRNA + resveratrol group, the expression of SIRT1 was inhibited, and the expressions of Col1, Col3 and SMA were similar to those in the shRNA group (Figure 4B).
The expression of SIRT1 was elevated during the TGF‐β1‐induced fibroblast‐to‐myofibroblast transition
To measure the expression of SIRT1 during TGFβ1‐induced fibroblast activation, normal skin‐derived fibroblasts were seeded in 60 mm culture dishes and stimulated with TGFβ1 (5 ng · mL−1) for 48 h. After TGFβ1 stimulation, the relative mRNA levels of Col1, Col3 and α‐SMA were significantly up‐regulated (Supporting Information Fig. S3), which indicated the transdifferentiation of fibroblasts into myofibroblasts. Additionally, the relative SIRT1 mRNA level was increased by approximately 55% (P < 0.05, Supporting Information Fig. S3) compared with the control group.
SIRT1 shRNA led to a further increase in Col1, Col3 and α‐SMA in the TGFβ1‐induced fibroblasts
SIRT1 shRNA was used to investigate the role of SIRT1 in the TGFβ1‐induced fibroblast‐to‐myofibroblast transition. Normal skin‐derived fibroblasts at 70–80% confluence were divided into five groups. Fibroblasts were treated with vehicle, SIRT1 shRNA (33 nM), mock RNA, TGFβ1 (5 ng · mL−1) + SIRT1 shRNA (33 nM) and TGFβ1 (5 ng mL−1) + mock RNA, respectively. Twenty‐four hours after TGFβ1 application, the mRNA levels were detected by real‐time PCR. Forty‐eight hours after TGFβ1 application, the protein levels were detected by western blot, and the intracellular expression of α‐SMA was observed by immunofluorescence.
As shown in Figure 5A, compared with the control and mock groups, SIRT1 shRNA led to a further increase in the mRNA levels of Col1, Col3 and α‐SMA, with increases of approximately 81% (P < 0.05), 48% (P < 0.05) and 39% (P < 0.05), respectively. The Col1, Col3 and α‐SMA protein expression levels were also significantly elevated after SIRT1 knock‐down (P < 0.05, Figure 5B). In addition, immunocytochemistry showed that compared with the control group, TGFβ1 led to a significant increase in α‐SMA‐positive fibroblasts, indicating the fibroblast‐to‐myofibroblast transition. Moreover, SIRT1 shRNA resulted in a further elevation of the optical density of the immunocytochemical staining for α‐SMA compared with TGFβ1 alone (Figure 5C, Supporting Information Fig. S4).
Figure 5.
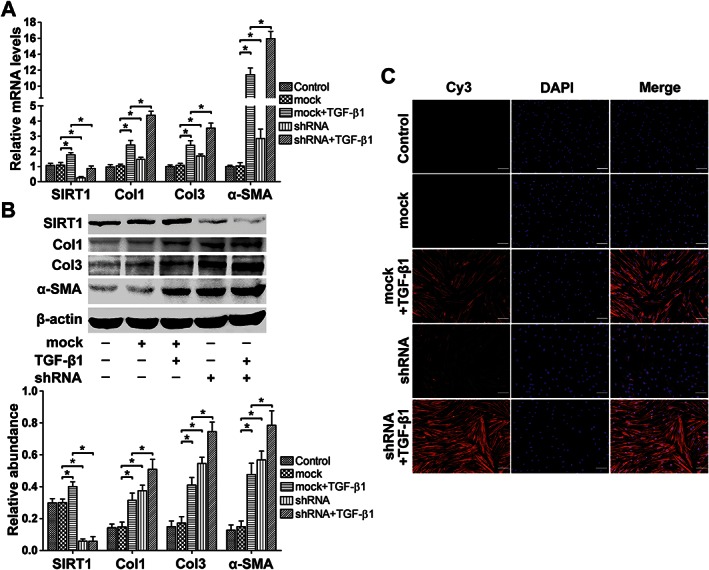
SIRT1 shRNA treatment led to a further increase in Col1, Col3 and α‐SMA expression in TGFβ1‐induced fibroblasts. (A) The effects of SIRT1 knock‐down on TGFβ1‐induced Col1, Col3 and α‐SMA transcription were analysed by real‐time PCR and normalized to GAPDH (n = 6). (B) Representative western blot analysis for Col1, Col3 and α‐SMA protein levels in the presence of SIRT1 shRNA with or without TGFβ1 (n = 6). (C) Representative immunocytochemical staining for α‐SMA shows that SIRT1 down‐regulation by SIRT1 shRNA treatment led to a further increase in TGF‐β1‐induced α‐SMA expression (scale bar = 100 μm) (n = 6). The results represent the mean ± SEM of six independent experiments. *P < 0.05.
Resveratrol inhibited the TGFβ1‐induced transdifferentiation of fibroblasts into myofibroblasts
To further validate the role of SIRT1 during the TGFβ1‐induced fibroblast‐to‐myofibroblast transition, resveratrol was used in combination with or without TGFβ1. Real‐time PCR and western blot were used to evaluate the mRNA and protein expression levels of Col1 and Col3 and α‐SMA. Immunofluorescence was used for the detection of α‐SMA expression. Resveratrol treatment led to a significant increase in SIRT1 expression at both the mRNA and protein levels (Figure 6A, B). When used in combination with TGFβ1, resveratrol inhibited the TGFβ1‐induced increase in mRNA (P < 0.05, Figure 6A) and protein levels (P < 0.05, Figure 6B) of Col1, Col3 and α‐SMA. In addition, immunocytochemistry showed that the optical density of α‐SMA decreased after the up‐regulation of SIRT1 by resveratrol (Figure 6C, Supporting Information Fig. S4).
Figure 6.
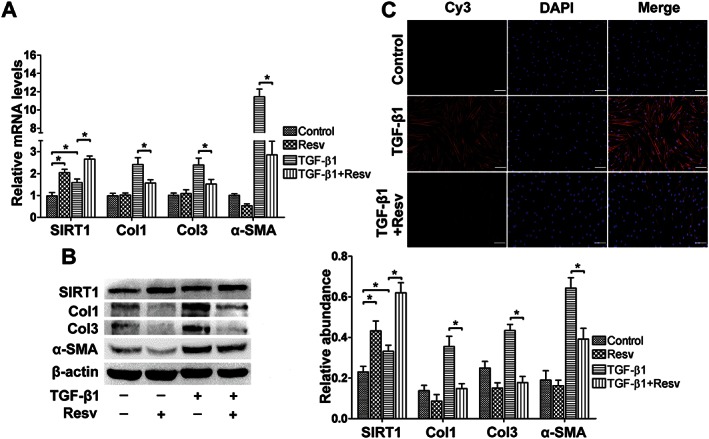
Resveratrol treatment inhibited the TGFβ1‐induced transdifferentiation of fibroblasts into myofibroblasts. (A) Resveratrol up‐regulated SIRT1 expression and inhibited TGFβ1‐induced increase in Col1, Col3 and α‐SMA mRNA expression, as analysed by real‐time PCR and normalized to GAPDH (n = 6). (B) Representative western blot analysis for Col1, Col3 and α‐SMA protein levels in the presence of resveratrol with or without TGFβ1 (n = 6). (C) Representative immunocytochemical staining for α‐SMA shows that SIRT1 up‐regulation by resveratrol treatment blocked TGFβ1‐induced increase in α‐SMA expression (scale bar = 100 μm) (n = 6). Resv, resveratrol. The results represent the mean ± SEM of six independent experiments. *P < 0.05.
Resveratrol improved the dermal architecture in a mouse cutaneous excision model
To assess the effects of SIRT1 on the continuous process of wound healing and scar formation, cutaneous excision wound models were established in Balb/C mice, which were treated with i.d. injections of either SIRT1 shRNA or resveratrol. The pictures of wound healing process are shown in Supporting Information Fig. S6. The wound tissues were harvested 4 weeks later and subjected to Masson's staining. As shown in Figure 7, treatment with SIRT1 shRNA resulted in a more disordered structure and denser collagen fibres compared with the control group. In contrast, the up‐regulation of SIRT1 by resveratrol led to more neatly arranged and thinner collagen fibres compared with the SIRT1 shRNA group. The former was similar to the normal tissue. We detected the phosphorylation of Smad2 through immunohistochemistry. As shown in Figure 7D–F, SIRT1 shRNA significantly inhibited the expression of SIRT1 and increase the expression of α‐SMA, and simultaneously, the phosphorylation of Smad2 was increased. In contrast, resveratrol increased the expression of SIRT1 and decreased the expression of α‐SMA, during which the phosphorylation of Smad2 was significantly decreased.
Figure 7.
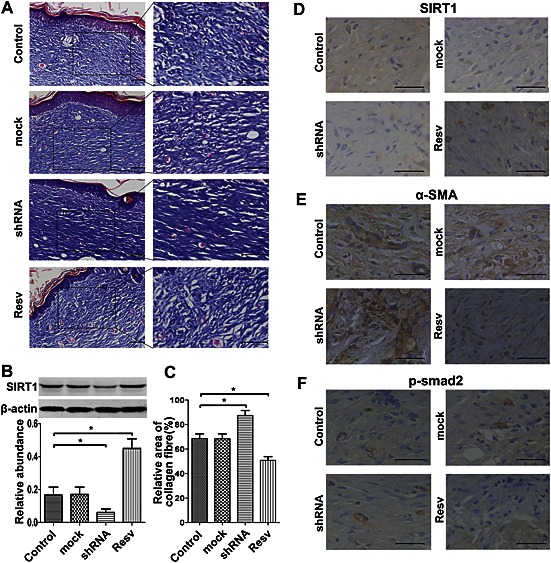
Resveratrol treatment improved the dermal architecture after wound healing. (A) As described in the Methods section, a mouse excision model was established, and either SIRT1 shRNA or resveratrol was administered i.d. and circumferentially around the wounds. The wound tissues and the adjacent normal skin were harvested 4 weeks later. Representative Masson's trichrome staining is shown (n = 6) (Scale bar = 50 μm). (B) Representative western blot analysis for SIRT1 at 4 weeks after wound healing (n = 6). (C) Histogram quantifying the relative area of collagen fibres in (A) using Image‐Pro Plus 6.0 software (n = 6). (D) Representative immunohistochemical staining for SIRT1 in hypertrophic scar tissue. SIRT1 shRNA led to the inhibition of SIRT1 while resveratrol led to an up‐regulation of SIRT1 (n = 6). (E) Representative immunohistochemical staining for α‐SMA in hypertrophic scar tissue. The inhibition of SIRT1 led to increased expression of α‐SMA while resveratrol led to decreased expression of α‐SMA (n = 6). (F) Representative immunohistochemical staining for p‐Smad in hypertrophic scar tissue. The inhibition of SIRT1 led to increased phosphorylation of Smad2. In contrast, the up‐regulation of SIRT1 led to decreased phosphorylation of Smad2 (n = 6). Resv, resveratrol. The results represent the mean ± SEM of six independent experiments. *P < 0.05.
Discussion and conclusion
Hypertrophic scarring after burn injury and trauma is a fibrotic disease that is characterized by the excessive deposition of ECM components (Gurtner et al., 2008; Hsu et al., 2010; Gauglitz et al., 2011; Tyack et al., 2012). So far, there is no satisfactory therapeutic option for hypertrophic scar. Recently, it has been reported that SIRT1 plays a role in ameliorating fibrotic disease and may thus represent a therapeutic target in anti‐fibrosis treatment (Li et al., 2010; Xia et al., 2012; Huang et al., 2014). Our study was aimed at determining whether SIRT1 is a potential therapeutic target for hypertrophic scars. Fibroblasts are the main components that synthesize and secrete collagen in skin (Hinz, 2010; Sidgwick and Bayat, 2012; Klingberg et al., 2013). During the normal wound healing process, fibroblasts around the wound are activated and transdifferentiate into myofibroblasts. Then they migrate into the wound area, and facilitate wound healing by increasing the synthesis and secretion of collagen. After wound healing, myofibroblasts are removed by apoptosis (van der Veer et al., 2009; Hinz, 2010; Klingberg et al., 2013). However, under pathological conditions, such as prolonged inflammation and infection, myofibroblasts persist in the wound, which results in the excessive deposition of ECM proteins and hypertrophic scar formation (van der Veer et al., 2009; Hinz, 2010; Klingberg et al., 2013). Thus, the inhibition of collagen synthesis and secretion is of great importance for the treatment of hypertrophic scars. Among the various cytokines that are secreted during wound healing, TGFβ1 plays a key role in scar formation by regulating the transcription of collagen (Sarrazy et al., 2011; Pakyari et al., 2013).
HDACs are a group of enzymes that catalyse the removal of acetyl from the lysine residues of histone and non‐histone proteins, thereby regulating their function. There are 18 different HDACs in humans, and they can be divided into four separate classes depending on their sequence similarity and cofactor dependency (Michan and Sinclair, 2007; Shakespear et al., 2011; Chang and Guarente, 2014). Class I, II and IV HDACs are zinc‐dependent and share a similar structure. In contrast, the class III HDACs, which belong to the sirtuin family, contain seven members (SIRT1‐7) that show no sequence resemblance to the other three classes and require nicotinamide adenine dinucleotide as a cofactor. HDACs have multiple functions in many cellular processes, such as the cell cycle, apoptosis, proliferation and differentiation, and may be involved in various pathological processes, including cancer, inflammatory disorders, cardiovascular diseases and lung diseases (Shakespear et al., 2011; Reichert et al., 2012). Recently, it has been reported that HDACs play a key role in fibrotic diseases, including systemic sclerosis and cardiac fibrosis, and thus represent a potential therapeutic target for fibrotic diseases (Li et al., 2010; Van Beneden et al., 2013; Huang et al., 2014; Schuetze et al., 2014). Simic et al. demonstrated that in HMLER breast cancer, SIRT1 inhibited the EMT whereas the loss of SIRT1 in the tubular epithelial cells aggravated injury‐induced renal fibrosis (Simic et al., 2013). In addition, SIRT1 and resveratrol counteract age‐related diseases, and could therefore underlie changes in wound healing that occur during natural ageing (Bhullar and Hubbard, 2015). The up‐regulation of SIRT1 by its agonist resveratrol has been shown to inhibit the renal fibrosis, liver fibrosis and pulmonary fibrosis induced by bleomycin (Lee et al., 2010; Akgedik et al., 2012; Liang et al., 2014). Furthermore, Ikeda et al. (2013) reported that in keloid‐derived fibroblasts, resveratrol significantly down‐regulates the expression of Col1, α‐SMA and TGF‐β1. However, the proliferation of fibroblasts also decreased after stimulation with resveratrol (Ikeda et al., 2013). Hence, the role of SIRT1 in skin fibroblast activation after skin injury and the pathological process of hypertrophic scar formation remain unclear.
In the present study, the expression pattern of SIRT1 in hypertrophic scars, normal skin tissues as well as TGFβ1‐induced fibroblast‐to‐myofibroblast transition was analysed, and we observed that SIRT1 localized to both the epidermal and dermal parts of the skin tissues. Compared with normal skin tissues, a decreased expression level of SIRT1 in hypertrophic scars was observed by immunohistochemistry and western blot. In addition, the mRNA and protein levels of SIRT1 also increased significantly after TGFβ1 stimulation. These observations indicate that SIRT1 is involved in the formation of hypertrophic scars. To elucidate the role of SIRT1 during scar formation, SIRT1 shRNA was transduced into hypertrophic scar‐derived fibroblasts. Notably, the expression levels of α‐SMA, Col1 and Col3 were significantly up‐regulated after the depletion of SIRT1. Moreover, SIRT1 knock‐down led to a further increase in Col1, Col3 and α‐SMA expression in TGFβ1‐induced fibroblasts, which suggests that SIRT1 may negatively regulate the expression of these fibrotic makers.
Previous studies have shown that HDAC inhibitors can block tissue fibrosis in multiple organs. Williams et al. reported that selective inhibition of class I HDACs potently suppresses angiotensin II‐mediated cardiac fibrosis by targeting cardiac fibroblasts and bone marrow‐derived fibrocytes. (Liu et al., 2008; Williams et al., 2014). In liver fibrosis, the HDAC inhibitor trichostatin A (TSA) suppressed α‐SMA, Col1 and Col3 expression and reduced the migration of stellate cells (Niki et al., 1999). TSA also inhibited mesangial cell proliferation and the synthesis of collagen and α‐SMA in renal fibrosis (Freidkin et al., 2010). Furthermore, a recent study using a rabbit ear model showed that TSA led to decreased Col1 and fibronectin synthesis and that it inhibited hypertrophic scar formation (Diao et al., 2013). These results indicate that HDAC inhibitors could be used to treat fibrotic diseases. However, as a member of the Class III HDACs, the activity of sirtuins was not affected by TSA or the other inhibitors used in the above studies. Thus, in the present study, SIRT1 shRNA was used to knock‐down SIRT1, and the results suggest that SIRT1 may have different effects from those of other HDACs on scar formation.
To further verify this hypothesis, resveratrol, which has been found to be an agonist of SIRT1, up‐regulates both the expression (Costa Cdos et al., 2010) and enzyme activity (Howitz et al., 2003; Hubbard et al., 2013b) of SIRT1, and was used in the present study. The anti‐fibrotic effects of resveratrol have been reported in organ fibrosis, including hepatic fibrosis, pulmonary fibrosis and renal fibrosis (Chavez et al., 2008; Lee et al., 2010; Akgedik et al., 2012; Liang et al., 2014). However, there are no reports regarding its effects on hypertrophic scar formation. In the present study, we observed that resveratrol led to an obvious decline in the expression of α‐SMA, Col1 and Col3 in hypertrophic scar‐derived fibroblasts. To confirm that resveratrol plays a role in anti‐scar formation through the up‐regulation of SIRT1, the expression of SIRT1 was inhibited through shRNA, and it was found that after the transfection of SIRT1 shRNA, resveratrol did not change the expression of Col1, Col3 and α‐SMA. To further confirm these results, SIRT1 was up‐regulated through Lv‐SIRT1 in hypertrophic scar‐derived fibroblasts. It was shown that the up‐regulation of SIRT1 through Lv‐SIRT1 led to the inhibition of the expression of Col1, Col3 and α‐SMA. Furthermore, resveratrol blocked TGFβ1‐induced transdifferentiation of fibroblasts into myofibroblasts, as seen by the significant inhibition of α‐SMA, Col1 and Col3 expression. To investigate the effects of up‐regulating SIRT1 in vivo, a cutaneous excision model was established in mice, and both SIRT1 shRNA and resveratrol were injected i.d. around the wounds. SIRT1 shRNA resulted in a more disordered structure and denser collagen fibres in the tissue after wound healing compared with the mice that were injected with resveratrol instead. So far, there are no widely‐accepted animal models that perfectly mimic human hypertrophic scars, as hypertrophic scar tissue seldom forms in other mammals (Ramos et al., 2008). The rabbit ear model (Morris et al., 1997; Zhu et al., 2008) and nude mouse model of transplanted human skin (Honardoust et al., 2013) have been used to investigate hypertrophic scarring and the effects anti‐fibrotic drugs. An ideal animal model should match human hypertrophic scarring in its clinical appearance, histological aspects, biochemistry, immunology, molecular biology and clinical behaviour. Despite the large number of scientific publications using mice, rabbits and other animals to study hypertrophic scarring, the wound healing process in these species presents significant differences when compared with human scarring (Ramos et al., 2008). In our experiments, the mouse cutaneous excision model was used, as we focused on the continuous process of wound healing and scar formation. The most important pathological characteristic of the hypertrophic scar is the overexpression of ECM as well as the transdifferentiation of fibroblasts into myofibroblasts (Hinz, 2010). The mouse cutaneous excision model benefits from similar pathological changes to those of wound healing and hypertrophic scars (Shi et al., 2013). That is why we choose this model in our experiments. Notably, resveratrol could improve wound healing in animals (Yaman et al., 2013), shown as an increased tensile strength of the wound, and an up‐regulated expression of Col1 and other ECM proteins. During this period, resveratrol reduced the inflammation of the wound, which is supposed to be the key effect for improving wound healing. It is known that rapid and efficient wound healing reduces scar formation. Hence, it is reasonable to assume that increasing the level of SIRT1 has beneficial effects on wound healing and can inhibit scar formation. Although the up‐regulation of SIRT1 by resveratrol improved wound healing in the mouse cutaneous excision model, the anti‐scarring properties of SIRT1 still need to be confirmed in more animal models in the future.
It is known that the TGFβ1/Smad pathway is one of the most important pathways involved in scar formation (Profyris et al., 2012). We detected the phosphorylation of Smad2 through immunohistochemistry, which showed that the phosphorylation of Smad2 could be suppressed by resveratrol but increased by SIRT1 shRNA. These results indicate that the TGFβ1/Smad pathway is affected by SIRT1. However, further exploration of this effect is still needed.
Taken together, our present results demonstrate that the expression of SIRT1 is decreased in hypertrophic scar tissues compared with normal skin. SIRT1 may negatively regulate TGFβ1‐induced fibroblast‐to‐myofibroblast transition, as demonstrated by the decreased expression of α‐SMA, Col1 and Col3 after resveratrol treatment. In addition, treatment with resveratrol in the mouse cutaneous excision model improved the dermal structure, as shown by the appearance of more organized and thinner collagen fibres. Future studies will focus on the exact molecular mechanisms of the anti‐fibrotic properties of SIRT1.
Author contributions
X.B., J.L., L.Y. and D.H. designed the research; X.B., L.F. and L.Y. performed the experiments; T.H., L.Y., X.B. and L.S. analysed the data; X.B., L.Y. and J.L. wrote the manuscript; L.F., L.S. and J.S. performed the statistical analysis; X.B., C.T., L.Y. and J.S. contributed reagents, materials and analysis tools; Z.Z. and D.H. revised and approved the final submission. All authors discussed the results and reviewed the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organizations engaged with supporting research.
Supporting information
Figure S1 The inhibition effects of four types of SIRT1 shRNA. To investigate the role of SIRT1 during hypertrophic scar formation, the inhibition effects of four types of SIRT1 shRNA including shRNA1075, shRNA1251, shRNA1961 and shRNA2214 were examined by western blot. The results represent the mean ± SEM of six independent experiments. *P < 0.05.
Figure S2 Resveratrol promotes the expression of SIRT1 in hypertrophic scar fibroblasts. The protein levels of SIRT1 in hypertrophic scar fibroblasts after administration of different doses of resveratrol were determined by western blot analysis. The results represent the mean ± SEM of six independent experiments. *P < 0.05.
Figure S3 TGF‐β1 upregulates the mRNA expression levels of Col1, Col3, α‐SMA and SIRT1 in normal skin fibroblasts. Normal skin derived fibroblasts were treated with or without TGF‐β1, the mRNA levels of Col1, Col3, α‐SMA and SIRT1 were determined by real‐time PCR. The results represent the mean ± SEM of six independent experiments. *P < 0.05.
Figure S4 Effects of SIRT1 shRNA on the expression of α‐SMA induced by TGF‐β1. Normal skin derived fibroblasts were treated with or without TGF‐β1 and SIRT1 shRNA, the expression of α‐SMA were determined by immunocytochemical staining. The positive cells rate and optical density mean were calculated by using the Image‐Pro Plus system. The results represent the mean ± SEM of six independent experiments. *P < 0.05.
Figure S5 Resveratrol inhibits the TGF‐β1 induced expression of α‐SMA. Normal skin derived fibroblasts were treated with or without TGF‐β1 and resveratrol, the expression of α‐SMA were determined by immunocytochemical staining, The positive cells rate and optical density mean were calculated by using the Image‐Pro Plus system. The results represent the mean ± SEM of six independent experiments. *P < 0.05.
Figure S6 The wound healing process of mouse cutaneous excision model. To assess the effects of SIRT1 on the continuous process of wound healing and scar formation, cutaneous excision wound models were established in Balb/C mice, which were treated with intradermal injections of either SIRT1 shRNA or resveratrol. Data shown are the wound manifestation among different groups at 0, 7, 14 and 16 days after the operation.
Table S1 Demographic data of patient samples used.
Table S2 Primer sequences used for real‐time‐PCR analysis
Supporting info item
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant nos: 81372069, 81201470, 81171811).
Bai, X.‐Z. , Liu, J.‐Q. , Yang, L.‐L. , Fan, L. , He, T. , Su, L.‐L. , Shi, J.‐H. , Tang, C.‐W. , Zheng, Z. , and Hu, D.‐H. (2016) Identification of sirtuin 1 as a promising therapeutic target for hypertrophic scars. British Journal of Pharmacology, 173: 1589–1601. doi: 10.1111/bph.13460.
References
- Akgedik R, Akgedik S, Karamanli H, Uysal S, Bozkurt B, Ozol D, et al. (2012). Effect of resveratrol on treatment of bleomycin‐induced pulmonary fibrosis in rats. Inflammation 35: 1732–1741. [DOI] [PubMed] [Google Scholar]
- Armour A, Scott PG, Tredget EE (2007). Cellular and molecular pathology of HTS: basis for treatment. Wound Repair Regen 15 (Suppl 1): S6–17. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. (2015). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunachalam G, Yao H, Sundar IK, Caito S, Rahman I (2010). SIRT1 regulates oxidant‐ and cigarette smoke‐induced eNOS acetylation in endothelial cells: role of resveratrol. Biochem Biophys Res Commun 393: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhullar KS, Hubbard BP (2015). Lifespan and healthspan extension by resveratrol. Biochim Biophys Acta 1852: 1209–1218. [DOI] [PubMed] [Google Scholar]
- Chang HC, Guarente L (2014). SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab 25: 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez E, Reyes‐Gordillo K, Segovia J, Shibayama M, Tsutsumi V, Vergara P, et al. (2008). Resveratrol prevents fibrosis, NF‐kappaB activation and TGF‐beta increases induced by chronic CCl4 treatment in rats. J Appl Toxicol 28: 35–43. [DOI] [PubMed] [Google Scholar]
- Conte E, Fruciano M, Fagone E, Gili E, Caraci F, Iemmolo M, et al. (2011). Inhibition of PI3K prevents the proliferation and differentiation of human lung fibroblasts into myofibroblasts: the role of class I P110 isoforms. PLoS One 6 e24663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte E, Gili E, Fruciano M, Korfei M, Fagone E, Iemmolo M, et al. (2013). PI3K p110gamma overexpression in idiopathic pulmonary fibrosis lung tissue and fibroblast cells: in vitro effects of its inhibition. Lab Invest 93: 566–576. [DOI] [PubMed] [Google Scholar]
- Costa Cdos S, Hammes TO, Rohden F, Margis R, Bortolotto JW, Padoin AV, et al. (2010). SIRT1 transcription is decreased in visceral adipose tissue of morbidly obese patients with severe hepatic steatosis. Obes Surg 20: 633–639. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA, et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao JS, Xia WS, Yi CG, Yang Y, Zhang X, Xia W, et al. (2013). Histone deacetylase inhibitor reduces hypertrophic scarring in a rabbit ear model. Plast Reconstr Surg 132: 61e–69e. [DOI] [PubMed] [Google Scholar]
- Freidkin I, Herman M, Tobar A, Chagnac A, Ori Y, Korzets A, et al. (2010). Effects of histone deacetylase inhibitors on rat mesangial cells. Am J Physiol Renal Physiol 298: F426–F434. [DOI] [PubMed] [Google Scholar]
- Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG (2011). Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med 17: 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008). Wound repair and regeneration. Nature 453: 314–321. [DOI] [PubMed] [Google Scholar]
- Herskovits AZ, Guarente L (2013). Sirtuin deacetylases in neurodegenerative diseases of aging. Cell Res 23: 746–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B (2010). The myofibroblast: paradigm for a mechanically active cell. J Biomech 43: 146–155. [DOI] [PubMed] [Google Scholar]
- Honardoust D, Kwan P, Momtazi M, Ding J, Tredget EE (2013). Novel methods for the investigation of human hypertrophic scarring and other dermal fibrosis. Methods Mol Biol 1037: 203–231. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. (2003). Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425: 191–196. [DOI] [PubMed] [Google Scholar]
- Hsu YC, Chen MJ, Yu YM, Ko SY, Chang CC (2010). Suppression of TGF‐beta1/SMAD pathway and extracellular matrix production in primary keloid fibroblasts by curcuminoids: its potential therapeutic use in the chemoprevention of keloid. Arch Dermatol Res 302: 717–724. [DOI] [PubMed] [Google Scholar]
- Huang X‐Z, Wen D, Zhang M, Xie Q, Ma L, Guan Y, et al. (2014). Sirt1 activation ameliorates renal fibrosis by inhibiting the TGF‐β/Smad3 pathway. J Cell Biochem 115: 996–1005. [DOI] [PubMed] [Google Scholar]
- Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, et al. (2013a). Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science 339: 1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, et al. (2013b). Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science 339: 1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Torigoe T, Matsumoto Y, Fujita T, Sato N, Yotsuyanagi T (2013). Resveratrol inhibits fibrogenesis and induces apoptosis in keloid fibroblasts. Wound Repair Regen 21: 616–623. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada M, Koya D (2013). SIRT1 in type 2 diabetes: mechanisms and therapeutic potential. Diabetes Metabol J 37: 315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada M, Kume S, Koya D (2014). Role of sirtuins in kidney disease. Curr Opin Nephrol Hypertens 23: 75–79. [DOI] [PubMed] [Google Scholar]
- Klingberg F, Hinz B, White ES (2013). The myofibroblast matrix: implications for tissue repair and fibrosis. J Pathol 229: 298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohan M, Muro AF, White ES, Berkman N (2010). EDA‐containing cellular fibronectin induces fibroblast differentiation through binding to alpha4beta7 integrin receptor and MAPK/Erk 1/2‐dependent signaling. FASEB J 24: 4503–4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ES, Shin MO, Yoon S, Moon JO (2010). Resveratrol inhibits dimethylnitrosamine‐induced hepatic fibrosis in rats. Arch Pharm Res 33: 925–932. [DOI] [PubMed] [Google Scholar]
- Li J, Qu X, Ricardo SD, Bertram JF, Nikolic‐Paterson DJ (2010). Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. Am J Pathol 177: 1065–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Tian S, Han J, Xiong P (2014). Resveratrol as a therapeutic agent for renal fibrosis induced by unilateral ureteral obstruction. Ren Fail 36: 285–291. [DOI] [PubMed] [Google Scholar]
- Liu F, Levin MD, Petrenko NB, Lu MM, Wang T, Yuan LJ, et al. (2008). Histone‐deacetylase inhibition reverses atrial arrhythmia inducibility and fibrosis in cardiac hypertrophy independent of angiotensin. J Mol Cell Cardiol 45: 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michan S, Sinclair D (2007). Sirtuins in mammals: insights into their biological function. Biochem J 404: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DE, Wu L, Zhao LL, Bolton L, Roth SI, Ladin DA, et al. (1997). Acute and chronic animal models for excessive dermal scarring: quantitative studies. Plast Reconstr Surg 100: 674–681. [DOI] [PubMed] [Google Scholar]
- Niki T, Rombouts K, De Bleser P, De Smet K, Rogiers V, Schuppan D, et al. (1999). A histone deacetylase inhibitor, trichostatin A, suppresses myofibroblastic differentiation of rat hepatic stellate cells in primary culture. Hepatology 29: 858–867. [DOI] [PubMed] [Google Scholar]
- Outtz HH, Wu JK, Wang X, Kitajewski J (2010). Notch1 deficiency results in decreased inflammation during wound healing and regulates vascular endothelial growth factor receptor‐1 and inflammatory cytokine expression in macrophages. J Immunol 185: 4363–4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakyari M, Farrokhi A, Maharlooei MK, Ghahary A (2013). Critical role of transforming growth factor beta in different phases of wound healing. Adv Wound Care 2: 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Lee MJ, Ahn J, Kim S, Kim HH, Kim KH, et al. (2004). Heat shock‐induced matrix metalloproteinase (MMP)‐1 and MMP‐3 are mediated through ERK and JNK activation and via an autocrine interleukin‐6 loop. J Invest Dermatol 123: 1012–1019. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. (2014). The IUPHAR/BPS guide to PHARMACOLOGY: an expert‐driven knowledge base of drug targets and their ligands. Nucleic Acids Res 42: D1098–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn JW, Grobbelaar AO, Rolfe KJ (2012). The role of the TGF‐beta family in wound healing, burns and scarring: a review. Int J Burns Trauma 2: 18–28. [PMC free article] [PubMed] [Google Scholar]
- Planavila A, Iglesias R, Giralt M, Villarroya F (2011). Sirt1 acts in association with PPARalpha to protect the heart from hypertrophy, metabolic dysregulation, and inflammation. Cardiovasc Res 90: 276–284. [DOI] [PubMed] [Google Scholar]
- Profyris C, Tziotzios C, Do Vale I (2012). Cutaneous scarring: pathophysiology, molecular mechanisms, and scar reduction therapeutics Part I. The molecular basis of scar formation. J Am Acad Dermatol 66: 1–10; quiz 11‐12. [DOI] [PubMed] [Google Scholar]
- Ramos ML, Gragnani A, Ferreira LM (2008). Is there an ideal animal model to study hypertrophic scarring? J Burn Care Res 29: 363–368. [DOI] [PubMed] [Google Scholar]
- Reichert N, Choukrallah MA, Matthias P (2012). Multiple roles of class I HDACs in proliferation, differentiation, and development. Cell Mol Life Sci 69: 2173–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrazy V, Billet F, Micallef L, Coulomb B, Desmouliere A (2011). Mechanisms of pathological scarring: role of myofibroblasts and current developments. Wound Repair Regen 19 (Suppl 1): s10–s15. [DOI] [PubMed] [Google Scholar]
- Schuetze KB, McKinsey TA, Long CS (2014). Targeting cardiac fibroblasts to treat fibrosis of the heart: focus on HDACs. J Mol Cell Cardiol 70C: 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakespear MR, Halili MA, Irvine KM, Fairlie DP, Sweet MJ (2011). Histone deacetylases as regulators of inflammation and immunity. Trends Immunol 32: 335–343. [DOI] [PubMed] [Google Scholar]
- Shi JH, Guan H, Shi S, Cai WX, Bai XZ, Hu XL, et al. (2013). Protection against TGF‐beta1‐induced fibrosis effects of IL‐10 on dermal fibroblasts and its potential therapeutics for the reduction of skin scarring. Arch Dermatol Res 305: 341–352. [DOI] [PubMed] [Google Scholar]
- Sidgwick GP, Bayat A (2012). Extracellular matrix molecules implicated in hypertrophic and keloid scarring. J Eur Acad Dermatol Venereol 26: 141–152. [DOI] [PubMed] [Google Scholar]
- Simic P, Williams EO, Bell EL, Gong JJ, Bonkowski M, Guarente L (2013). SIRT1 suppresses the epithelial‐to‐mesenchymal transition in cancer metastasis and organ fibrosis. Cell Reprogram 3: 1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z (2001). How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17: 463–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyack Z, Simons M, Spinks A, Wasiak J (2012). A systematic review of the quality of burn scar rating scales for clinical and research use. Burns 38: 6–18. [DOI] [PubMed] [Google Scholar]
- Van Beneden K, Mannaerts I, Pauwels M, Van den Branden C, van Grunsven LA (2013). HDAC inhibitors in experimental liver and kidney fibrosis. Fibrogenesis Tissue Repair 6: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veer WM, Bloemen MC, Ulrich MM, Molema G, van Zuijlen PP, Middelkoop E, et al. (2009). Potential cellular and molecular causes of hypertrophic scar formation. Burns 35: 15–29. [DOI] [PubMed] [Google Scholar]
- Williams SM, Golden‐Mason L, Ferguson BS, Schuetze KB, Cavasin MA, Demos‐Davies K, et al. (2014). Class I HDACs regulate angiotensin II‐dependent cardiac fibrosis via fibroblasts and circulating fibrocytes. J Mol Cell Cardiol 67: 112–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Wu X, Yang Y, Zhao Y, Fang M, Xie W, et al. (2012). SIRT1 deacetylates RFX5 and antagonizes repression of collagen type I (COL1A2) transcription in smooth muscle cells. Biochem Biophys Res Commun 428: 264–270. [DOI] [PubMed] [Google Scholar]
- Xia L, Ding F, Zhu JH, Fu GS (2011). Resveratrol attenuates apoptosis of pulmonary microvascular endothelial cells induced by high shear stress and proinflammatory factors. Hum Cell 24: 127–133. [DOI] [PubMed] [Google Scholar]
- Yaman I, Derici H, Kara C, Kamer E, Diniz G, Ortac R, et al. (2013). Effects of resveratrol on incisional wound healing in rats. Surg Today 43: 1433–1438. [DOI] [PubMed] [Google Scholar]
- Zhu GY, Xu B, Cai JL (2008). Experimental research of correlation between anatomy structure of rabbit ear and creating hypertrophic scar animal model. Zhonghua Zheng Xing Wai Ke Za Zhi 24: 216–219. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The inhibition effects of four types of SIRT1 shRNA. To investigate the role of SIRT1 during hypertrophic scar formation, the inhibition effects of four types of SIRT1 shRNA including shRNA1075, shRNA1251, shRNA1961 and shRNA2214 were examined by western blot. The results represent the mean ± SEM of six independent experiments. *P < 0.05.
Figure S2 Resveratrol promotes the expression of SIRT1 in hypertrophic scar fibroblasts. The protein levels of SIRT1 in hypertrophic scar fibroblasts after administration of different doses of resveratrol were determined by western blot analysis. The results represent the mean ± SEM of six independent experiments. *P < 0.05.
Figure S3 TGF‐β1 upregulates the mRNA expression levels of Col1, Col3, α‐SMA and SIRT1 in normal skin fibroblasts. Normal skin derived fibroblasts were treated with or without TGF‐β1, the mRNA levels of Col1, Col3, α‐SMA and SIRT1 were determined by real‐time PCR. The results represent the mean ± SEM of six independent experiments. *P < 0.05.
Figure S4 Effects of SIRT1 shRNA on the expression of α‐SMA induced by TGF‐β1. Normal skin derived fibroblasts were treated with or without TGF‐β1 and SIRT1 shRNA, the expression of α‐SMA were determined by immunocytochemical staining. The positive cells rate and optical density mean were calculated by using the Image‐Pro Plus system. The results represent the mean ± SEM of six independent experiments. *P < 0.05.
Figure S5 Resveratrol inhibits the TGF‐β1 induced expression of α‐SMA. Normal skin derived fibroblasts were treated with or without TGF‐β1 and resveratrol, the expression of α‐SMA were determined by immunocytochemical staining, The positive cells rate and optical density mean were calculated by using the Image‐Pro Plus system. The results represent the mean ± SEM of six independent experiments. *P < 0.05.
Figure S6 The wound healing process of mouse cutaneous excision model. To assess the effects of SIRT1 on the continuous process of wound healing and scar formation, cutaneous excision wound models were established in Balb/C mice, which were treated with intradermal injections of either SIRT1 shRNA or resveratrol. Data shown are the wound manifestation among different groups at 0, 7, 14 and 16 days after the operation.
Table S1 Demographic data of patient samples used.
Table S2 Primer sequences used for real‐time‐PCR analysis
Supporting info item


