Background and Purpose
Berberine, a small molecule derived from Coptidis rhizome, has been found to be potent at lowering blood glucose and regulating lipid metabolism. Recent clinical studies have shown that berberine reduces blood pressure and increases systemic insulin sensitivity in patients with metabolic syndrome; however, the underlying mechanism is still unclear. Here, we investigated the mechanism by which berberine improves vascular insulin sensitivity in diabetic rats.
Experimental Approach
Diabetes was induced in male Sprague–Dawley rats by feeding a high‐fat diet and administration of a low dose of streptozotocin. These diabetic rats were treated with berberine (200 mg·kg−1·day−1, gavage) for 4 weeks. Vascular dilation was determined in isolated mesenteric artery rings. Effects of berberine on insulin signalling were also studied in human artery endothelial cells cultured in high glucose (25 mmol·L−1) and palmitate (500 μmol·L−1).
Key Results
Berberine treatment for 4 weeks significantly restored the impaired ACh‐ and insulin‐induced vasodilatation of mesenteric arteries from diabetic rats. In isolated mesenteric artery rings, berberine (2.5–10 μmol·L−1) elicited dose‐dependent vasodilatation and significantly enhanced insulin‐induced vasodilatation. Mechanistically, berberine up‐regulated phosphorylation of the insulin receptor and its downstream signalling molecules AMPK, Akt and eNOS, and increased cell viability and autophagy in cultured endothelial cells. Moreover, down‐regulating insulin receptors with specific siRNA significantly attenuated berberine‐induced phosphorylation of AMPK.
Conclusions and Implications
Berberine improves diabetic vascular insulin sensitivity and mesenteric vasodilatation by up‐regulating insulin receptor‐mediated signalling in diabetic rats. These findings suggest berberine has potential as a preventive or adjunctive treatment of diabetic vascular complications.
Linked Articles
This article is part of a themed section on Chinese Innovation in Cardiovascular Drug Discovery. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2015.172.issue-23.
Abbreviations
- AMPK
AMP‐activated protein kinase
- eNOS
endothelial NOS
- IRS
insulin receptor substrate
- InsR
insulin receptor
- MTT
3‐[4,5 dimethylthiazol‐2‐yl]‐2,5‐diphenyl tetrazolium bromide thiazolyl blue
- PE
phenylephrine
- PI3K
phosphatidylinositol 3‐kinase
- SNP
sodium nitroprusside
Tables of Links
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,bAlexander et al., 2015a, 2015b).
Introduction
Type 2 diabetes characterized by insulin resistance is associated with impaired endothelium‐dependent vasodilatation (Steinberg et al., 1996). Berberine is a natural isoquinoline alkaloid, which is present in medicinal herbs Coptidis rhizome (Huanglian in Chinese). Recent clinical studies have shown that berberine reduces blood pressure and increases systemic insulin sensitivity in patients with metabolic syndrome (Perez‐Rubio et al., 2013). Berberine has been shown to lower blood glucose and modulate lipid metabolism by activating AMP‐activated protein kinase (AMPK) (Ko et al., 2005; Chang et al., 2013). Berberine has also vasodilator effects (Ko et al., 2000; Lau et al., 2001), preventing hyperglycaemia‐induced endothelial injury via AMPK (Wang et al., 2009). In a previous study, we showed that berberine protects the diabetic heart from ischaemic/reperfusion injury through activating the Akt‐eNOS signalling pathway and inhibiting the apoptosis of myocardium cells in rats (Chen et al., 2014). Therefore, we hypothesized that berberine may improve insulin sensitivity in diabetes by up‐regulating insulin receptor‐mediated signalling. The present study aimed to investigate the effects of berberine on insulin‐induced vasodilatation and its underlying mechanism in a streptozotocin (STZ)‐treated, high‐fat diet‐fed type 2 diabetes animal model. We found that berberine significantly ameliorated impaired endothelial function and improved insulin‐induced vasodilatation in diabetic mesentery arteries mainly through up‐regulating insulin receptor‐mediated signalling.
Methods
Male Sprague–Dawley rats, 120–150 g, were housed in a temperature controlled room with a 12 h light dark cycle. Rats were fed a casein‐cornstarch‐sucrose‐based diet containing a high‐fat content consisting of 20% fat, 62% carbohydrate and 17% protein, ad libitum, with free access to dwater. After 1 week of adaptation, rats were fasted overnight and then injected i.p. with 30 mg·kg−1 body weight of STZ (Sigma‐Aldrich, Shanghai, China) dissolved in citrate buffer (pH 4.5) to induce diabetes. Normal control animals (Control) were deprieved of food (fasted) overnight and injected with the citrate buffer vehicle. The fasting blood glucose of STZ‐treated rats was measured 3 days later, and a second injection was applied if rats were not diabetic. Fasting blood glucose was measured again 3 days after the first STZ injection. Rats with fasting blood glucose levels over 7.0 mmol·L−1 were randomly divided into two groups and continued to be fed the high‐fat diet for 8 weeks. These two groups were matched for body weight and blood glucose levels. One group was used as the diabetic control (Diabetes) and the other was treated with berberine chloride (gavage) dissolved in 0.9% saline solution at a dose of 200 mg·kg−1·day−1(Chen et al., 2014). The diabetic rats and the normal control rats were treated with the vehicle of 0.9% saline solution (gavage) as in our previous study (Chen et al., 2014).
At the end of the study, animals were fasted overnight and killed by administration of an overdose of anaesthetic, sodium pentobarbital (30 mg·kg−1, i.p.). All experimental procedures were performed in accordance with International Guidelines for the Care and Use of Laboratory Animals and were approved by the Animal Care and Research Ethics Committee of the Fourth Military Medical University Research Council. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath & Lilley, 2015).
Isometric force measurement
After the rats had been killed, the mesenteric artery (inner diameter 100 to 150 μm) was dissected out, any adhering connective tissues were removed, and then it was cut into ring segments (1 mm in length). Rings were suspended on a micromyograph in organ baths containing oxygenated (95% O2; 5% CO2) physiological saline solution (composition in mmol·L−1: NaCl 119, KCl 4.7, KH2PO4 1.18, MgSO4 · 7H2O 1.17, CaCl2 2H2O 2.5, NaHCO325, EDTA 0.03 and d‐glucose 5.5, pH 7.4, 37°C). Following equilibration for 90 min under a resting tension of 0.25 g, rings were twice contracted with KCl (60 mmol·L−1) (Xing et al., 2013a, 2013b). All rings were initially contracted by addition of 10 nmol·L−1 phenylephrine (PE) (to evaluate the contractility) and then relaxed to 1 mmol·L−1 ACh (to assess the endothelial integrity). Then a sustained contraction was induced by PE (10− 5 mol·L−1), and ACh was added cumulatively to evoke relaxations. Contraction to PE (10− 5 mol·L−1) and subsequent vasodilatation to insulin (10−1 0 to 10− 6 mol·L−1), ACh (10− 9 to 10− 4 mol·L−1) and sodium nitroprusside (SNP, 10−1 0 to 10− 5 mol·L−1) were tested in all vessels. Subsequently, we evaluated the vasodilator responses of mesentery artery rings preconstricted with PE; vasorelaxation was evoked by increasing doses of insulin (10−1 0 to 10− 6 mol·L−1). The maximal contraction induced by PE was considered as the baseline and vasorelaxant responses are expressed as % reduction in contraction. We obtained concentration‐response curves to ACh, insulin and SNP on different segments from the same artery.
Cell culture
Human artery endothelial cell line EA.hy 926 (Summers et al., 2014) and HUVECs (obtained from American Type Culture Collection) were used. Endothelial cells at passages 4–8 were cultured on gelatin‐coated flasks in Dulbecco's minimum essential medium supplemented with 10% FBS (Invitrogen, Carlsbad, CA, USA) under endotoxin‐free conditions (Armani et al., 2014). Cells were cultured in an atmosphere of 5% CO2 at 37°C. One day before treatment, cells were trypsinized and allowed to grow to about 80% confluence. Afterwards, fresh media supplemented with vehicle (DMSO) or berberine were added to the cells as indicated for 1 h. We used HG/HF (25 mmol·L−1 glucose + 500 μmol·L−1 palmitate)‐treated endothelial cells to simulate the in vivo diabetic cell condition. For the insulin stimulation experiments, 1 or 100 nmol·L−1 of bovine insulin was added to the culture medium (Tanigaki et al., 2009). Cells were treated for an additional 60 min before harvest. Berberine was dissolved in DMSO and was added to the medium at the indicated concentrations.
Cell viability
The 3‐[4,5 dimethylthiazol‐2‐yl]‐2,5‐diphenyl tetrazolium bromide thiazolyl blue (MTT) assay was conducted as described previously (Hao et al., 2011). Briefly, the MTT was dissolved in PBS at a concentration of 5 mg·mL−1 and sterilized by passage through a 0.22 μm filter. EA.hy 926 cells were seeded in wells of a 96‐well plate containing 200 μL of the culture medium, and 20 μL MTT stock solution was then added to each well. After incubation for 4 h at 37°C, 150 μL of a DMSO solution were added to all of the wells and mixed thoroughly to lyse the cells and dissolve the dark blue crystals. After 10 min, the absorbance was read on a microplate reader (Beckman DU640, Brea, CA, USA) at a wavelength of 570 nm.
Transmission electron microscopy
HUVECs were quickly fixed with 2.5% glutaraldehyde in 0.1 mol·L−1 phosphate buffer (pH 7.4) overnight at 4°C. After fixation, cells were then immersed in 1% osmium tetroxide for 2 h, dehydrated in graded ethanol and then embedded in epoxy resin. After that, cells were post‐stained with uranyl acetate and lead citrate, and then examined under a JEM‐1230 transmission electron microscope (JEOL, Tokyo, Japan).
Small interfering (si) RNA design and transfection
The cognate siRNA against insulin receptors (InsR‐homo‐3428) was designed as described previously and purchased from Genepharm (Shanghai, China) along with a scrambled control siRNA (siCONTROL non‐targeting siRNA, scrambled) (Xing et al., 2013a, 2013b). HUVECs were seeded and transfected with siRNA to a final concentration of 20 nmol·L−1 using RNA mate (Genepharma, Shanghai, China) when cells reached 30–50% confluence. Protein knockdown efficiencies were assessed at 72 h after transfection.
Western blot
HUVECs were washed twice with ice‐cold 1× PBS solution and lysed in ice‐cold lysis buffer and proteins were extracted as described previously (Dong et al., 2008). Protein samples were subjected to SDS‐PAGE and transferred to polyvinylidene difluoride membranes by a semi‐dry transfer cell (Bio‐Rad, Hercules, CA, USA). For detection of InsR‐β‐1150, Ser473‐phosphorylated Akt and total Akt, LC3B and p62 were detected by specific rabbit polyclonal antibodies (Cell Signaling Technology, Danvers, MA, USA). Anti‐phospho‐eNOS (Ser1177), anti‐eNOS antibodies, anti‐phospho‐AMPK and anti‐AMPK were obtained from Abcam (San Francisco, CA, USA) with GADPH as internal control.
Berberine, PE, acetylcholine, insulin, sodium palmitic acid, MTT and PI3K inhibitor wortmannin were obtained from Sigma‐Aldrich.
Statistical analysis
All values are presented as mean ± SEM. Differences were compared by ANOVA followed by Bonferroni correction for post hoc t‐test, where appropriate. Probabilities of <0.05 were considered to be statistically significant. All of the statistical tests were performed with the graphpad Prism software, version 5.0 (GraphPad Software, San Diego, CA, USA). The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).
Results
Effects of berberine on blood glucose in diabetic rats
To examine the effects of berberine on blood glucose responses to an oral glucose challenge, the oral glucose tolerance test (OGTT) was performed during the last week of berberine treatment. Diabetic rats (Diabetes) showed significantly increased blood glucose with fasting glucose level of 15.3 ± 1.9 mmol·L−1 and 2 h glucose level in the OGTT of 24.7 ± 1.3 mmol·L−1. Compared with the diabetic control group, the berberine‐treated diabetic rats showed a significant improvement in oral glucose tolerance. The fasting glucose level of the berberine‐treated diabetic rats group was 4.0 ± 0.2 mmol·L−1, and 2 h glucose level was 9.5 ± 1.6 mmol·L−1. Berberine administration significantly lowered the blood glucose in diabetic rats, especially the fasting glucose level and the 2 h glucose level in OGTT (n = 10–15, P < 0.01).
Berberine improved ACh‐induced vasodilatation of mesenteric arteries in diabetic rats
Berberine is known to have endothelial cell‐ and smooth muscle cell‐dependent vasodilator effects (Ko et al., 2000; Lau et al., 2001). Previous studies also showed that berberine prevented hyperglycaemia‐induced endothelial injury and enhanced vasodilatation via AMPK and eNOS(Wang et al., 2009). In the present study, as shown in Figure 1, the relaxation response induced by ACh in PE‐precontracted, mesenteric artery rings from diabetic rats was significantly impaired compared with that of the control group. The Emax fell to 19.10 ± 8.56% in diabetic rats compared with 93.94 ± 2.10% in that of the control. Four weeks treatment with berberine (200 mg·kg−1·day−1) (Cui et al., 2009; Chen et al., 2014) significantly improved ACh‐induced relaxation in the mesenteric arteries from diabetic rats. The Emax reached 71.09 ± 12.77% compared with that of the Diabetes group (n = 12, P < 0.01) (Figure 1A). Berberine (200 mg·kg−1·day−1) treatment had no effect on ACh‐induced relaxation in the control group. Moreover, SNP‐induced endothelium‐independent relaxation was not different among the four groups (Figure 1B). These results indicate that berberine improves ACh‐induced vasodilatation of mesenteric arteries in diabetic rats.
Figure 1.

Berberine improved ACh‐induced vasodilatation of mesenteric arteries in diabetic rats. Mesenteric artery rings were isolated from control and diabetic rats treated with berberine for 4 weeks. (A) Berberine improved ACh‐induced vasodilatation of mesenteric arteries in diabetic rats. ACh‐induced relaxation in age‐matched control, berberine‐treated control, diabetic rats and berberine‐treated diabetic rat mesenteric arteries. (B) Sodium nitroprusside (SNP)‐induced relaxation in mesenteric artery. Relaxation is expressed as a percentage relative to the contraction induced by PE. Data are expressed as mean ± SEM (n = 12). Control, control rats; Control + BBR, control rats treated with berberine (200 mg·kg−1·day−1, gavage), Diabetes, diabetic rats; Diabetes + BBR, diabetic rats treated with berberine (200 mg·kg−1·day−1, gavage). ** P < 0.01 versus Control; ## P < 0.01 versus Diabetes.
Berberine improved insulin‐induced vasodilatation in mesenteric arteries of diabetic rats through PI3K/Akt pathway
Insulin mainly combines with the insulin receptor to stimulate the intracellular signalling pathway and induce vasorelaxation of vessels (Zeng et al., 2000; Vicent et al., 2003). Interestingly, insulin‐evoked vasorelaxation was significantly improved in isolated mesentery arteries from diabetic rats treated with berberine (200 mg·kg−1·day−1) for 4 weeks. This indicates that berberine significantly improved the insulin‐mediated vasodilatation in diabetic rats. Furthermore, in mesenteric arteries isolated from the diabetic rats treated with both berberine and wortmannin (Wm, 15 mg·kg−1, i.p.), the inhibitor of PI3K/Akt, for 4 weeks, insulin‐evoked vasorelaxation almost disappeared (Figure 2), indicating that the effect of berberine on insulin‐induced vasorelaxation was mediated through the PI3K/Akt pathway.
Figure 2.

Berberine increased insulin‐induced vasodilatation in mesenteric arteries in diabetic rats via the PI3K/Akt pathway. Insulin‐induced relaxation was improved in berberine‐treated diabetic rat's mesenteric arteries. Relaxation is expressed as a percentage relative to the contraction induced by PE. Data are expressed as mean ± SEM (n = 12). Control, control rats; Diabetes, diabetic rats; Diabetes + BBR, diabetic rats treated with berberine (200 mg·kg−1·day−1, gavage); Diabetes + BBR + Wm, diabetic rats treated with berberine (200 mg·kg−1·day−1, gavage) and Wm (15 mg·kg−1·day−1, i.p.) for 4 weeks. ** P < 0.01 versus Control; ## P < 0.01 versus Diabetes; †† P < 0.01 versus Diabetes + BBR.
Berberine combined with insulin alleviated the impaired vasodilatation in isolated mesenteric arteries of diabetic rats ex vivo
We performed ex vivo experiments to further determine the effect of berberine on vasodilatation in isolated mesenteric arteries from normal, control, and diabetic rats. Berberine (from 2.5 to 10 μmol·L−1) was directly added to the bath solution of artery rings. It has been shown that berberine (from 2.5 to 10 μmol·L−1) elicits a vasodilator response in PE‐preconstricted mesenteric artery rings (Wang et al., 2009). As shown in Figure 3A, berberine (2.5 μmol·L−1) did not produce any significant constriction or relaxation in control and diabetic mesenteric arteries, whereas it caused significant relaxation at concentrations of 5 μmol·L−1, 76.11 ± 4.04% relaxation, and at 10 μmol·L−1, 89.05 ± 6.06% relaxation, in the control group. In contrast, in the diabetic group, the vasodilator effects of berberine were significantly decreased (P < 0.01, n = 5); it induced a relaxation response of 18.45 ± 4.60% at 5 μmol·L−1, and 62.10 ± 14.08% at 10 μmol·L−1. The vasodilatation evoked by berberine was concentration‐dependent in both the control and diabetic rat mesenteric arteries, as shown in Figure 3A.
Figure 3.

Berberine combined with insulin alleviated the vascular dysfunction in isolated mesenteric arteries of diabetic rats. Mesenteric artery rings were isolated and the vasodilator responses were assessed. (A) Berberine improved vasodilatation in mesentery arteries in control and diabetic rats in a dose‐dependent (2.5 μmol·L−1, 5 μmol·L−1 and 10 μmol·L−1) manner. Data are expressed as mean ± SEM (n = 5). ** P < 0.01 versus Control. (B) When combined with insulin (1 nmol·L−1), berberine (5 μmol·L−1) increased the vasodilatation of PE‐precontracted mesenteric artery rings isolated from control and diabetic rats. Data are expressed as mean ± SEM (n = 5). * P < 0.05 versus Control; ## P < 0.01 versus diabetic mesenteric artery rings treated with berberine (5 μmol·L−1).
Insulin at 100 nmol·L−1 induced a vasorelaxation response of 30% in mesenteric arteries, whereas a lower concentration of insulin (1 nmol·L−1) had little effect. In contrast and more importantly, insulin (1 nmol·L−1) combined with berberine (5 μmol·L−1) significantly ameliorated the impaired vascular relaxation of PE‐preconstricted arteries from diabetic rats, as shown in Figure 3B. This suggests that insulin combined with berberine exhibits synergistic beneficial effects on impaired vascular relaxation in diabetic rats.
Berberine increased cell viability and autophagy in HG/HF‐treated endothelial cells
Elevated plasma‐free fatty acid level is a common feature of both poorly‐controlled type 1 and type 2 diabetes, and is associated with obesity and metabolic syndrome (Yan et al., 2013). One of the most common dietary fatty acids is palmitate, a 16‐carbon saturated fatty acid. We used HG/HF‐treated EA.hy 926 cells as the simulated diabetic endothelial cell model and found that HG/HF (25 mmol·L−1 glucose + 500 μmol·L−1 palmitate) decreased cell viability. A low concentration of insulin, 1 nmol·L−1, and berberine at 50 μmol·L−1 had no significant effects on cell viability as assessed by the MTT assay. However, interestingly, exposure of the cells to insulin (1 nmol·L−1) combined with berberine (50 μmol·L−1) resulted in a significant increase in cell viability (101 ± 9.19% vs. 71 ± 8.49%, n = 6, P < 0.01) compared with insulin (1 nmol·L−1) (Figure 4A).
Figure 4.

Berberine increased cell viability and autophagy in HG/HF‐treated endothelial cells. EA.hy 926 cells were incubated for 24 h in medium supplemented with HG/HF (25 mmol·L−1 glucose + 500 μmol·L−1 palmitate) and 0.5% FBS in the presence or absence of insulin (1 nmol·L−1 or 100 nmol·L−1), or berberine (50 μmol·L−1). (A) Berberine increased the viability of HG/HF‐treated endothelial cells. Cell viability was assessed with MTT assay. Data are expressed as mean ± SEM (n = 6). * P < 0.05 versus Control; # P < 0.05 versus HG/HF; † P < 0.05 versus HG/HF + insulin (1 nmol·L−1). (B) Berberine increased autophagy in HG/HF‐treated HUVECs. HUVECs were fixed and examined using transmission electron microscopy for autophagosomes. Control, control culture medium; Control + BBR, control culture medium + berberine (50 μmol·L−1); HG/HF, HG/HF (25 mmol·L−1 glucose + 500 μmol·L−1 palmitate) culture medium; HG/HF + BBR, HG/HF culture medium treated with berberine (50 μmol·L−1). (C) Berberine increased autophagy‐related protein expression as analysed by western blots. Quantification of band intensity was normalized to GAPDH. The relative quantification of the Western blot band intensities are presented as mean ± SEM (n = 5–7). * P < 0.05 versus HG/HF.
Autophagy is involved in insulin resistance (Lumeng and Saltiel, 2006; Yang et al., 2010). We assessed whether berberine inhibits autophagy in HG/HF‐treated HUVECs. There was no difference in the autophagosome formation between the control and control + berberine groups, whereas berberine increased the formation of autophagosomes in HG/HF‐treated endothelial cells (Figure 4B). Then western blot analysis was carried out to measure autophagy‐associated biomarkers LC3B (microtubule‐associated protein 1 light chain 3). Conversion of the non‐lipidated form of LC3 (LC3‐I to the autophagosome membrane‐associated lipidated form, LC3‐II) and degradation of the autophagy substrate protein p62 were tested. The results showed that berberine increased autophagic activity, as demonstrated by an increase in LC3B‐II/LC3B‐I and decrease in p62 expression (Figure 4C).
Co‐treatment with berberine and insulin increased the phosphorylation of InsR, Akt and eNOS in HG/HF‐treated HUVECs
Previous studies have indicated that insulin triggers the phosphorylation of cardiovascular Akt‐eNOS (Gao et al., 2002). In the present study, we determined whether berberine could affect the phosphorylation of the downstream molecules of insulin receptor‐mediated signalling. Non‐stimulated endothelial cells had either no or only a low level of phosphorylated InsR, AMPK, Akt and eNOS. Berberine treatment (50 μmol·L−1) for 1 h increased InsR, AMPK, Akt and eNOS phosphorylation 1.5–2.5 fold compared with their corresponding basal levels, as shown in Figure 5A. Insulin (at both 1 nmol·L−1 and 100 nmol·L−1) increased the phosphorylation of InsR, Akt and eNOS. The combination of insulin (1 nmol·L−1) with berberine (50 μmol·L−1) resulted in a significant increase in phosphorylated InsR, Akt and eNOS, as shown in Figure 5B. These findings suggest that berberine improves insulin receptor‐mediated signalling.
Figure 5.

Co‐treatment with berberine and insulin increased the HG/HF‐induced phosphorylation of InsR, Akt and eNOS in HG/HF‐treated endothelial cells. Berberine increased the protein expression of insulin receptor‐mediated signalling as analysed by western blots. (A) The effects of berberine on the phosphorylation of InsR, AMPK, Akt and eNOS. Quantification of band intensity was normalized to the corresponding total protein levels. The relative quantification of the western blot band intensities are presented as mean ± SEM (n = 5–9). * P < 0.05, ** P < 0.01 versus HG/HF. (B) Co‐treatment with berberine and insulin increased HG/HF‐induced phosphorylation of InsR, Akt and eNOS in HG/HF‐treated endothelial cells. Representative bands are shown. Quantification of band intensity of p‐InsR, p‐Akt and p‐eNOS was normalized to InsR, Akt and eNOS protein levels respectively. The relative quantification of the western blot band intensities are presented as mean ± SEM (n = 5). ** P < 0.01 versus Control; # P < 0.05, ## P < 0.01 versus HG/HF; † P < 0.05, †† P < 0.01 versus HG/HF + Ins (1 nmol·L−1).
Berberine‐induced phosphorylation of AMPK, Akt and eNOS were blunted following insulin receptor knockdown using small interfering RNA (siRNA)
To further investigate the mechanism underlying berberine's effect on the insulin receptor, HUVECs were transfected with pre‐designed siRNA for insulin receptors or scrambled control RNA for insulin receptors. When cells had reached 30–50% confluence, they were seeded and transfected with siRNA to a final concentration of 20 nmol·L−1 using RNA mate (Genepharma, Shanghai, China). Then the cells were exposed to HG/HF for 24 h and treated with berberine (50 μmol·L−1) for 1 h. The results show that the expression of InsRs was decreased by ~85% in the insulin receptor knockdown group, as shown in Figure 6. This knockdown of insulin receptors significantly reduced insulin receptor protein expression and blunted berberine‐induced phosphorylation of AMPK, Akt and eNOS, as shown in Figure 6.
Figure 6.

Berberine‐induced phosphorylations of AMPK, Akt and eNOS were blunted following insulin receptor knockdown using siRNA in HG/HF‐treated endothelial cells. Semi‐quantification of p‐AMPK was normalized to the total AMPK protein levels and presented as mean ± SEM (n = 5). * P < 0.05 versus siRNA for InsR + BBR.
Discussion
Our findings demonstrate that berberine improves vascular insulin sensitivity of the mesenteric arteries and protects against endothelial dysfunction in diabetic rats mainly through up‐regulation of insulin receptor‐mediated signalling.
The extent of endothelium‐dependent vasodilatation in obese and insulin‐resistant subjects correlates with their individual insulin sensitivity (Steinberg et al., 1996; Rask‐Madsen and King, 2007). Berberine has a wide range of pharmacological actions; it has been shown to have anti‐diarrheic, anti‐inflammatory (Amin et al., 1969; Anis et al., 2001) and anti‐metabolic (Kong et al., 2004; Turner et al., 2008) effects, and protect against hypertrophy (Hong et al., 2003) or ischaemia reperfusion injury (Zeng et al., 2003). In the present study, we aimed to evaluate whether berberine can ameliorate endothelial dysfunction of mesenteric arteries in diabetic rats. The behaviour of mesenteric artery rings is thought to be a useful proxy for the function of vascular beds that contribute more directly to blood pressure. We found that diabetic rats treated for 4 weeks with berberine exhibited substantially improved vasodilatation, particularly vasodilator responses to insulin compared with vehicle‐treated diabetic animals. Notably, the magnitude of this improvement mediated by berberine was blunted by pretreatment with wortmannin. Although wortmannin is not a totally specific inhibitor for PI3K/Akt, its ability to completely abolish the vasodilatation induced by berberine strongly suggests that the vasodilator effects of berberine are mediated via a PI3K/Akt‐dependent mechanism. Previous studies have suggested that berberine protects against endothelial injury and enhances vasodilatation through activation of the AMPK signalling cascade (Wang et al., 2009). Our results indicate that in diabetic rats the PI3K‐Akt signalling pathway is involved in the vasodilator effects of berberine. This discrepancy may be related to differences in the experimental systems studied, including the animal models. In the present study, we used mesenteric artery rings isolated from STZ‐induced diabetic rats instead of high glucose‐perfused artery rings.
To further assess berberine's vasodilator action and its synergistic effects with insulin, we performed an ex vivo experiment in isolated mesenteric arteries and found that infusion of berberine in combination with a low concentration of insulin significantly ameliorated the impaired vasodilatation of mesenteric arteries isolated from diabetic rats (Figure 3), suggesting synergistic effects between insulin and berberine. We found that diabetic rats exhibited a reduced vasorelaxant response to insulin, while berberine treatment enhanced these insulin‐induced endothelium‐dependent vasorelaxant responses, which is regarded as one of the important indicators reflecting vascular insulin sensitivity (Xing et al., 2013a, 2013b). Insulin has important vascular actions; it stimulates the production of nitric oxide from endothelium, leading to capillary recruitment, vasodilatation, increased blood flow and subsequent augmentation of glucose disposal in classic insulin target tissues, while in diabetic animals, the reduced insulin sensitivity often leads to a reduced vasorelaxant effect of insulin (Muniyappa et al., 2007).
In diabetes, the relative ineffectiveness of insulin and hyperglycaemia act together to reduce the activity of InsR/PI3K/Akt signalling, resulting in an impaired InsR/Akt‐mediated endothelial function. Studies have reported that berberine is a potent sensitizer of the insulin signal and is derived from anti‐diabetic plants (Chen et al., 2009; Chen et al., 2010; Di Pierro et al., 2012; Gomes et al., 2012). Our data revealed that phosphorylation of the InsR, AMPK, Akt and eNOS increased in response to a combined treatment of insulin with berberine (Figure 5). Previous study showed that insulin receptor substrates IRS‐1 and IRS‐2 activate the Akt‐mTOR signalling pathway. At the same time, an increased AMP/ATP ratio activates AMPK. Increased AMPK activity further antagonizes mTOR/Raptor‐mediated protein synthesis and up‐regulation of fatty acid oxidation (Long et al., 2011). In our study, the down‐regulation of insulin receptor expression significantly reduced berberine‐stimulated phosphorylation of the insulin receptor, AMPK, Akt and eNOS (Figure 6), suggesting that berberine improves insulin sensitivity by up‐regulating insulin receptor‐mediated signalling in diabetic rats. Many studies have shown that insulin suppresses rather than activates AMPK (Witters and Kemp, 1992; Gamble and Lopaschuk, 1997; Winder and Holmes, 2000), whereas insulin has been shown to activate the AMPK signalling pathway in adipocytes and that this is associated with an elevation of the AMP/ATP ratio (Liu et al., 2010). AMPK is also known to regulate insulin sensitivity by inhibiting IRS‐1 (Jakobsen et al., 2001; Qiao et al., 2002; Ning and Clemmons, 2010). Our data provide novel evidence that berberine improves endothelium‐mediated vasodilatation in diabetes via a mechanism involving both Akt and AMPK activation through the up‐regulation of the insulin receptor.
A deficiency in autophagy leads to a deterioration of whole‐body energy metabolism and the development of metabolic disorders (Zhang et al., 2014a, 2014b), and impaired autophagy in endothelial cells is believed to contribute to endothelial dysfunction and diabetes (Ho et al., 2000; Cifarelli et al., 2011). Recent studies have shown that a chronic high‐fat diet or obesity impairs autophagy in various cells and tissues by blocking the formation of autophagosome and fusion between autophagosome and lysosome (Guo et al., 2013; Armani et al., 2014). Notably, inhibition of autophagy subsequently leads to the development of insulin resistance (Liu et al., 2009; Yang et al., 2010). Zhang et al. have reported that berberine promotes autophagy, subsequently attenuating left ventricular remodelling and cardiac dysfunction after myocardial ischaemia (Zhang et al., 2014a, 2014b). Similar results have also been obtained indicating that berberine attenuates left ventricular remodelling and cardiomyocyte apoptosis through an autophagy‐dependent mechanism in a rat model of cardiac hypertrophy, which is, at least in part, associated with enhanced autophagy through inhibition of the mTOR, p38 and ERK1/2 MAPK signalling pathways (Li et al., 2014). We found that berberine treatment enhanced autophagy in HG/HF‐induced diabetic endothelial cells as demonstrated by the increase in autophagosomes and protein expression of LC3B‐II/LC3B‐I (Figure 4B, C). Autophagy is a complex process that involves numerous signalling molecules and one of the most important positive regulated mechanisms is AMPK signalling in endothelial cells (Goyal et al., 2014). It has also been reported that AMPK phosphorylation directly leads to autophagosome formation (Guo and Ren, 2012).
The mechanism underlying the hypoglycaemic effect of berberine remains largely unclear. Berberine has been found to activate insulin signalling through the inhibition of PTP1B activity (Chen et al., 2010), and has, more recently, been shown to increase insulin secretion from islets of diabetic mice via the activation of AMPK and uncoupling protein‐2 (UCP2) (Liu et al., 2014). Another reasonable explanation for the effects of berberine is through restoration of uncoupling protein‐2 expression in high glucose (HG)‐treated INS‐1E cells and rat islets or in db/db mouse islets, and UCP2 may regulate glucose‐stimulated insulin secretion (Liu et al., 2014). Also, it has been suggested that berberine reduces endothelium‐dependent contractions by activating AMPK, thus inhibiting ER stress and subsequently scavenging ROS leading to COX‐2 down‐regulation in SHR carotid arteries (Liu et al., 2015).
In conclusion, our data show that berberine improves insulin‐mediated vasodilatation of mesenteric arteries in diabetic rats through up‐regulating insulin receptor‐mediated signalling and increasing vascular insulin sensitivity. These findings suggest that berberine has the potential to have a beneficial role in the preventive and adjunctive treatment of vascular complications associated with diabetes (summarized in Figure 7).
Figure 7.
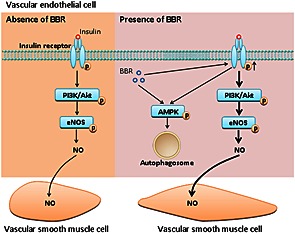
Schematic figure illustrating the enhancement by berberine of vascular insulin‐mediated signalling and vasodilatation. In addition to increasing AMPK‐induced autophagy, berberine up‐regulates the phosphorylation of the insulin receptor, resulting in enhanced activation of insulin‐mediated signalling and subsequent endothelial NO production with resultant vasodilatation in diabetic rats.
Author contributions
F‐H.G., G‐H.L., Z‐J.Z. and Y.Z. carried out the laboratory experiments, collected and analysed the data and interpreted the results. X.Z., P.Z. and M‐Q.D. analysed and interpreted the data. L.D. and F.G. designed the experiments, supervised the study, drafted and revised the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organizations engaged with supporting research.
Acknowledgements
This work was supported by grants from the National Nature Science Foundation of China (nos. 81470412, 81270301).
Geng, F.‐H. , Li, G.‐H. , Zhang, X. , Zhang, P. , Dong, M.‐Q. , Zhao, Z.‐J. , Zhang, Y. , Dong, L. , and Gao, F. (2016) Berberine improves mesenteric artery insulin sensitivity through up‐regulating insulin receptor‐mediated signalling in diabetic rats. British Journal of Pharmacology, 173: 1569–1579. doi: 10.1111/bph.13466.
References
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin AH, Subbaiah TV, Abbasi KM (1969). Berberine sulfate: antimicrobial activity, bioassay, and mode of action. Can J Microbiol 15: 1067–1076. [DOI] [PubMed] [Google Scholar]
- Anis KV, Rajeshkumar NV, Kuttan R (2001). Inhibition of chemical carcinogenesis by berberine in rats and mice. J Pharm Pharmacol 53: 763–768. [DOI] [PubMed] [Google Scholar]
- Armani A, Cinti F, Marzolla V, Morgan J, Cranston GA, Antelmi A et al. (2014). Mineralocorticoid receptor antagonism induces browning of white adipose tissue through impairment of autophagy and prevents adipocyte dysfunction in high‐fat‐diet‐fed mice. FASEB J 28: 3745–3757. [DOI] [PubMed] [Google Scholar]
- Chang W, Zhang M, Li J, Meng Z, Wei S, Du H et al. (2013). Berberine improves insulin resistance in cardiomyocytes via activation of 5′‐adenosine monophosphate‐activated protein kinase. Metabolism 62: 1159–1167. [DOI] [PubMed] [Google Scholar]
- Chen C, Zhang Y, Huang C (2010). Berberine inhibits PTP1B activity and mimics insulin action. Biochem Biophys Res Commun 397: 543–547. [DOI] [PubMed] [Google Scholar]
- Chen K, Li G, Geng F, Zhang Z, Li J, Yang M et al. (2014). Berberine reduces ischemia/reperfusion‐induced myocardial apoptosis via activating AMPK and PI3K‐Akt signaling in diabetic rats. Apoptosis 19: 946–957. [DOI] [PubMed] [Google Scholar]
- Chen TC, Lai KC, Yang JS, Liao CL, Hsia TC, Chen GW et al. (2009). Involvement of reactive oxygen species and caspase‐dependent pathway in berberine‐induced cell cycle arrest and apoptosis in C6 rat glioma cells. Int J Oncol 34: 1681–1690. [DOI] [PubMed] [Google Scholar]
- Cifarelli V, Geng X, Styche A, Lakomy R, Trucco M, Luppi P (2011). C‐peptide reduces high‐glucose‐induced apoptosis of endothelial cells and decreases NAD(P)H‐oxidase reactive oxygen species generation in human aortic endothelial cells. Diabetologia 54: 2702–2712. [DOI] [PubMed] [Google Scholar]
- Cui G, Qin X, Zhang Y, Gong Z, Ge B, Zang YQ (2009). Berberine differentially modulates the activities of ERK, p38 MAPK, and JNK to suppress Th17 and Th1 T cell differentiation in type 1 diabetic mice. J Biol Chem 284: 28420–28429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pierro F, Villanova N, Agostini F, Marzocchi R, Soverini V, Marchesini G (2012). Pilot study on the additive effects of berberine and oral type 2 diabetes agents for patients with suboptimal glycemic control. Diabetes Metab Syndr Obes 5: 213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, Zheng YM, Van Riper D, Rathore R, Liu QH, Singer HA et al. (2008). Functional and molecular evidence for impairment of calcium‐activated potassium channels in type‐1 diabetic cerebral artery smooth muscle cells. J Cereb Blood Flow Metab 28: 377–386. [DOI] [PubMed] [Google Scholar]
- Gamble J, Lopaschuk GD (1997). Insulin inhibition of 5′ adenosine monophosphate‐activated protein kinase in the heart results in activation of acetyl coenzyme A carboxylase and inhibition of fatty acid oxidation. Metabolism 46: 1270–1274. [DOI] [PubMed] [Google Scholar]
- Gao F, Gao E, Yue TL, Ohlstein EH, Lopez BL, Christopher TA et al. (2002). Nitric oxide mediates the antiapoptotic effect of insulin in myocardial ischemia–reperfusion: the roles of PI3‐kinase, Akt, and endothelial nitric oxide synthase phosphorylation. Circulation 105: 1497–1502. [DOI] [PubMed] [Google Scholar]
- Gomes AP, Duarte FV, Nunes P, Hubbard BP, Teodoro JS, Varela AT et al. (2012). Berberine protects against high fat diet‐induced dysfunction in muscle mitochondria by inducing SIRT1‐dependent mitochondrial biogenesis. Biochim Biophys Acta 1822: 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal A, Neill T, Owens RT, Schaefer L, Iozzo RV (2014). Decorin activates AMPK, an energy sensor kinase, to induce autophagy in endothelial cells. Matrix Biol 34: 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R, Ren J (2012). Deficiency in AMPK attenuates ethanol‐induced cardiac contractile dysfunction through inhibition of autophagosome formation. Cardiovasc Res 94: 480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R, Zhang Y, Turdi S, Ren J (2013). Adiponectin knockout accentuates high fat diet‐induced obesity and cardiac dysfunction: role of autophagy. Biochim Biophys Acta 1832: 1136–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao M, Li SY, Sun CK, Xu J, Lin Y, Liu KX et al. (2011). Amelioration effects of berberine on diabetic microendothelial injury model by the combination of high glucose and advanced glycation end products in vitro . Eur J Pharmacol 654: 320–325. [DOI] [PubMed] [Google Scholar]
- Ho FM, Liu SH, Liau CS, Huang PJ, Lin‐Shiau SY (2000). High glucose‐induced apoptosis in human endothelial cells is mediated by sequential activations of c‐Jun NH(2)‐terminal kinase and caspase‐3. Circulation 101: 2618–2624. [DOI] [PubMed] [Google Scholar]
- Hong Y, Hui SS, Chan BT, Hou J (2003). Effect of berberine on catecholamine levels in rats with experimental cardiac hypertrophy. Life Sci 72: 2499–2507. [DOI] [PubMed] [Google Scholar]
- Jakobsen SN, Hardie DG, Morrice N, Tornqvist HE (2001). 5′‐AMP‐activated protein kinase phosphorylates IRS‐1 on Ser‐789 in mouse C2C12 myotubes in response to 5‐aminoimidazole‐4‐carboxamide riboside. J Biol Chem 276: 46912–46916. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko BS, Choi SB, Park SK, Jang JS, Kim YE, Park S (2005). Insulin sensitizing and insulinotropic action of berberine from Cortidis rhizoma . Biol Pharm Bull 28: 1431–1437. [DOI] [PubMed] [Google Scholar]
- Ko WH, Yao XQ, Lau CW, Law WI, Chen ZY, Kwok W et al. (2000). Vasorelaxant and antiproliferative effects of berberine. Eur J Pharmacol 399: 187–196. [DOI] [PubMed] [Google Scholar]
- Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C et al. (2004). Berberine is a novel cholesterol‐lowering drug working through a unique mechanism distinct from statins. Nat Med 10: 1344–1351. [DOI] [PubMed] [Google Scholar]
- Lau CW, Yao XQ, Chen ZY, Ko WH, Huang Y (2001). Cardiovascular actions of berberine. Cardiovasc Drug Rev 19: 234–244. [DOI] [PubMed] [Google Scholar]
- Li MH, Zhang YJ, Yu YH, Yang SH, Iqbal J, Mi QY et al. (2014). Berberine improves pressure overload‐induced cardiac hypertrophy and dysfunction through enhanced autophagy. Eur J Pharmacol 728: 67–76. [DOI] [PubMed] [Google Scholar]
- Liu HY, Han J, Cao SY, Hong T, Zhuo D, Shi J et al. (2009). Hepatic autophagy is suppressed in the presence of insulin resistance and hyperinsulinemia: inhibition of FoxO1‐dependent expression of key autophagy genes by insulin. J Biol Chem 284: 31484–31492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Liu J, Gao Y, Yu X, Xu G, Huang Y (2014). Uncoupling protein‐2 mediates the protective action of berberine against oxidative stress in rat insulinoma INS‐1E cells and in diabetic mouse islets. Br J Pharmacol 171: 3246–3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Liu J, Huang Z, Yu X, Zhang X, Dou D et al. (2015). Berberine improves endothelial function by inhibiting endoplasmic reticulum stress in the carotid arteries of spontaneously hypertensive rats. Biochem Biophys Res Commun 458: 796–801. [DOI] [PubMed] [Google Scholar]
- Liu Q, Gauthier MS, Sun L, Ruderman N, Lodish H (2010). Activation of AMP‐activated protein kinase signaling pathway by adiponectin and insulin in mouse adipocytes: requirement of acyl‐CoA synthetases FATP1 and Acsl1 and association with an elevation in AMP/ATP ratio. FASEB J 24: 4229–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long YC, Cheng Z, Copps KD, White MF (2011). Insulin receptor substrates Irs1 and Irs2 coordinate skeletal muscle growth and metabolism via the Akt and AMPK pathways. Mol Cell Biol 31: 430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Saltiel AR (2006). Insulin htts on autophagy. Autophagy 2: 250–253. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL (2010). Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol 160: 1573–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniyappa R, Montagnani M, Koh KK, Quon MJ (2007). Cardiovascular actions of insulin. Endocr Rev 28: 463–491. [DOI] [PubMed] [Google Scholar]
- Ning J, Clemmons DR (2010). AMP‐activated protein kinase inhibits IGF‐I signaling and protein synthesis in vascular smooth muscle cells via stimulation of insulin receptor substrate 1 S794 and tuberous sclerosis 2 S1345 phosphorylation. Mol Endocrinol 24: 1218–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP et al. (2014). The IUPHAR/BPS Guide to PHARMACOLOGY: an expert‐driven knowledgebase of drug targets and their ligands. Nucleic Acids Res 42: D1098–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez‐Rubio KG, Gonzalez‐Ortiz M, Martinez‐Abundis E, Robles‐Cervantes JA, Espinel‐Bermudez MC (2013). Effect of berberine administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab Syndr Relat Disord 11: 366–369. [DOI] [PubMed] [Google Scholar]
- Qiao LY, Zhande R, Jetton TL, Zhou G, Sun XJ (2002). In vivo phosphorylation of insulin receptor substrate 1 at serine 789 by a novel serine kinase in insulin‐resistant rodents. J Biol Chem 277: 26530–26539. [DOI] [PubMed] [Google Scholar]
- Rask‐Madsen C, King GL (2007). Mechanisms of Disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab 3: 46–56. [DOI] [PubMed] [Google Scholar]
- Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD (1996). Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest 97: 2601–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers CM, Hammons AL, Arora J, Zhang S, Jochems J et al. (2014). Methotrexate modulates folate phenotype and inflammatory profile in EA.hy 926 cells. Eur J Pharmacol 732: 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigaki K, Mineo C, Yuhanna IS, Chambliss KL, Quon MJ, Bonvini E et al. (2009). C‐reactive protein inhibits insulin activation of endothelial nitric oxide synthase via the immunoreceptor tyrosine‐based inhibition motif of FcgammaRIIB and SHIP‐1. Circ Res 104: 1275–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N, Li JY, Gosby A, To SW, Cheng Z, Miyoshi H et al. (2008). Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP‐activated protein kinase and improve insulin action. Diabetes 57: 1414–1418. [DOI] [PubMed] [Google Scholar]
- Vicent D, Ilany J, Kondo T, Naruse K, Fisher SJ, Kisanuki YY et al. (2003). The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J Clin Invest 111: 1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Huang Y, Lam KS, Li Y, Wong WT, Ye H et al. (2009). Berberine prevents hyperglycemia‐induced endothelial injury and enhances vasodilatation via adenosine monophosphate‐activated protein kinase and endothelial nitric oxide synthase. Cardiovasc Res 82: 484–492. [DOI] [PubMed] [Google Scholar]
- Winder WW, Holmes BF (2000). Insulin stimulation of glucose uptake fails to decrease palmitate oxidation in muscle if AMPK is activated. J Appl Physiol (1985) 89: 2430–2437. [DOI] [PubMed] [Google Scholar]
- Witters LA, Kemp BE (1992). Insulin activation of acetyl‐CoA carboxylase accompanied by inhibition of the 5′‐AMP‐activated protein kinase. J Biol Chem 267: 2864–2867. [PubMed] [Google Scholar]
- Xing W, Li Y, Zhang H, Mi C, Hou Z, Quon MJ et al. (2013a). Improvement of vascular insulin sensitivity by downregulation of GRK2 mediates exercise‐induced alleviation of hypertension in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 305: H1111–H1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing W, Yan W, Liu P, Ji L, Li Y, Sun L et al. (2013b). A novel mechanism for vascular insulin resistance in normotensive young SHRs: hypoadiponectinemia and resultant APPL1 downregulation. Hypertension 61: 1028–1035. [DOI] [PubMed] [Google Scholar]
- Yan W, Zhang H, Liu P, Wang H, Liu J, Gao C et al. (2013). Impaired mitochondrial biogenesis due to dysfunctional adiponectin‐AMPK‐PGC‐1alpha signaling contributing to increased vulnerability in diabetic heart. Basic Res Cardiol 108: 329. [DOI] [PubMed] [Google Scholar]
- Yang L, Li P, Fu S, Calay ES, Hotamisligil GS (2010). Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab 11: 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng G, Nystrom FH, Ravichandran LV, Cong LN, Kirby M, Mostowski H et al. (2000). Roles for insulin receptor, PI3‐kinase, and Akt in insulin‐signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation 101: 1539–1545. [DOI] [PubMed] [Google Scholar]
- Zeng XH, Zeng XJ, Li YY (2003). Efficacy and safety of berberine for congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 92: 173–176. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Han X, Hu N, Huff AF, Gao F, Ren J (2014a). Akt2 knockout alleviates prolonged caloric restriction‐induced change in cardiac contractile function through regulation of autophagy. J Mol Cell Cardiol 71: 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang YJ, Yang SH, Li MH, Iqbal J, Bourantas CV, Mi QY et al. (2014b). Berberine attenuates adverse left ventricular remodeling and cardiac dysfunction after acute myocardial infarction in rats: role of autophagy. Clin Exp Pharmacol Physiol 41: 995–1002. [DOI] [PubMed] [Google Scholar]


