Abstract
Hedgehog (HH) signaling is known to be essential during the embryonal development of the pituitary gland but the knowledge about its role in the adult pituitary and in associated tumors is sparse. In this report we investigated the effect of excess Hh signaling activation in murine pituitary explants and analyzed the HH signaling status of human adenopituitary lobes and a large cohort of pituitary adenomas. Our data show that excess Hh signaling led to increased proliferation of Sox2+ and Sox9+ adult pituitary stem cells and to elevated expression levels of adrenocorticotropic hormone (Acth), growth hormone (Gh) and prolactin (Prl) in the adult gland. Inhibition of the pathway by cyclopamine reversed these effects indicating that active Hh signaling positively regulates proliferative processes of adult pituitary stem cells and hormone production in the anterior pituitary. Since hormone producing cells of the adenohypophysis as well as ACTH-, GH- and PRL-immunopositive adenomas express SHH and its target GLI1, we furthermore propose that excess HH signaling is involved in the development/maintenance of hormone-producing pituitary adenomas. These findings advance the understanding of physiological hormone regulation and may open new treatment options for pituitary tumors.
The Hedgehog (HH) signaling pathway is closely linked to developmental processes, organ patterning, tissue and stem cell maintenance, cell differentiation processes, cell proliferation, regenerative responses after injury and cancer formation1,2,3. Binding of the ligand HH to its receptor Patched 1 (PTCH) results in activation of the pathway by suspension of the PTCH-dependent inhibition of Smoothened (SMO). Subsequently the GLI transcription factors (GLI2, GLI3) transfer the activation signal into the nucleus and regulate the expression of several GLI target genes. In a positive and negative feedback loop these targets encompass GLI1 and PTCH, respectively. Abnormal decrease of HH signaling results in developmental defects whereas an increase results in cancer formation (reviewed in1).
HH signaling is also essential for the development of the pituitary. For example, holoprosencephaly patients with inactivating mutations in Sonic HH (SHH) suffer from an agenesis of this organ4. Similar observations have been made in Shh knockout mice5,6. Additionally, inactivating GLI2 mutations have been associated with hypopituitarism and pituitary malformations7,8. In contrast excess Hh signaling activity due to overexpression of Shh results in pituitary hyperplasia in mice9. Moreover, inactivating PTCH mutations may affect the hormone homeostasis of the pituitary since patients with heterozygous PTCH germline mutation (Gorlin-Goltz-Syndrome) as well as heterozygous Ptch knockout mice occasionally develop acromegaly-like symptoms10,11,12,13,14,15,16,17,18,19.
Besides the involvement of HH signaling pathway in pituitary development, several links indicate that this pathway is also involved in the maintenance and hormone homeostasis of this organ. Thus, the human anterior pituitary expresses SHH and GLI1, which suggest that HH signaling plays a role in hormone secretion20,21.
Hormone producing cells of the frontal pituitary lobe are the origin of the vast majority of pituitary adenomas (PA). These tumors constitute about 10 to 15% of all intracranial neoplasms and in general represent benign epithelial lesions22. Recently it has been suggested that SHH maintain pituitary tumor cells in a non-proliferative state. Consequently, HH pathway activity was proposed to prevent the development of PA21. However, the proof of this hypothesis is missing.
We here assessed the effect of activation and inactivation of Hh signaling on morphology, hormone expression/release and proliferation of pituitary explants and single cells isolated from Ptchflox/flox CreERT2 mice23. In addition, we analyzed the activation status of HH pathway in the human adenopituitary (qRT-PCR: n = 12, specific immunohistological stainings: n = 15) and in a large cohort of human pituitary tumors (qRT-PCR: n = 48, specific immunohistological stainings: n = 96). In contrast to former reports, our results demonstrate that active Hh signaling induces proliferation of Sox2+ and Sox9+ adult pituitary stem cells and hormone release in the adult pituitary gland. Finally our data suggest that activation of the HH pathway may be involved in the formation and/or maintenance of pituitary tumors. Therefore, inhibition of Hh signaling could be a promising new target for the treatment of aggressive PA.
Results
Hh signaling activation induces hormone secretion and proliferation of Sox2+ and Sox9+ adult pituitary stem cells in murine pituitaries
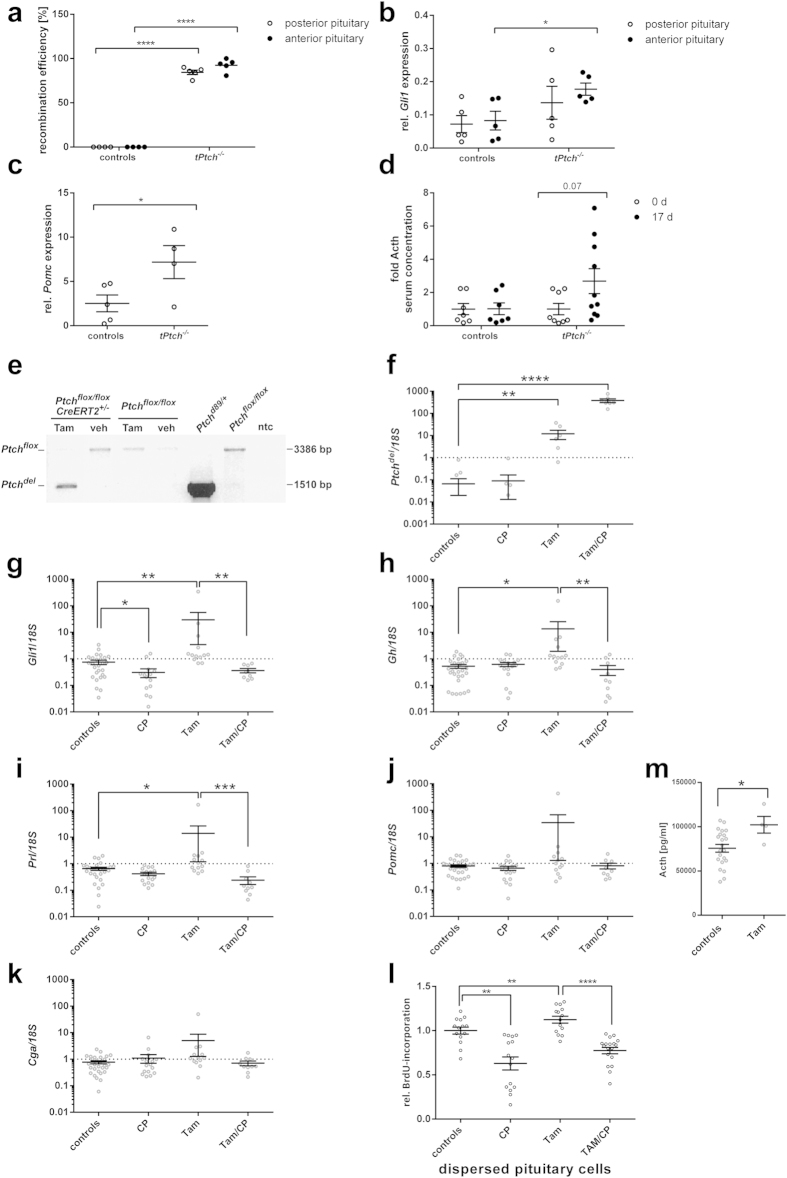
To investigate if activation of Hh signaling triggers expression of pituitary hormones, we analyzed pituitaries from Ptchflox/floxCreERT2+/− mice 17 d after tamoxifen-mediated induction of a biallelic Ptch deletion (tPtch−/− mice)23. The floxed Ptchflox loci of the posterior and the anterior tPtch−/− pituitaries were efficiently deleted (Fig. 1a), which resulted in significantly elevated Gli1 transcription (Fig. 1b, p = 0.023) and thus in activation of Hh signaling in the respective glands. Additionally, the Pomc expression levels of the pituitaries and the Acth serum levels of tPtch−/− mice were increased compared to the controls (Fig. 1c,d).
Figure 1. Ptch depletion leads to activation of Hh signaling and to increased expression/release of pituitary hormones and proliferation.
(a–d) In vivo analyses: (a) Recombination efficiencies at the genomic Ptchflox locus, (b) Gli1 expression levels in posterior (open circles) and anterior pituitary glands (black circles) and (c) Pomc expression levels in anterior pituitary glands of tPtch−/− (2 females, 2 males) and control mice (2 females, 3 males) 17 days after the first tamoxifen-injection. (d) Fold change of Acth blood serum concentration of tPtch−/− mice before (4 females, 4 males; open circles) and 17 days after the first tamoxifen-injection (5 females, 5 males; black circles) in comparison to control mice (3 females, 4 males; tamoxifen-treated Ptchflox/flox and vehicle-treated Ptchflox/floxCreERT2+/− mice). (e–m) ex vivo analyses: (e) PCR-based recombination analysis of the genomic Ptchflox locus, (f–k) relative Ptchdel, Gli1, Gh, Prl, Pomc and Cga expression levels and (l) BrdU incorporation assays of tamoxifen-treated Ptchflox/floxCreERT2+/− (Tam) and control (e–k) pituitary explants or (l) dispersed cells with or without cyclopamine treatment (CP). (e) Amplification of the Ptchflox locus results in a 3386 bp fragment. Recombination of the Ptchflox locus leads to amplification of a 1510 bp fragment due to the deletion of Ptch exons 8 and 923. ntc, no template control. (f–k) ncontrols = 28 (14 females, 14 males), nCP = 16 (7 females, 9 males), nTam = 13 (7 females, 6 males), nTam/CP = 10 (5 females, 5 males). Data shown in (l) represent 6 independent experiments. (f–l) Controls include vehicle-treated Ptchflox/flox CreERT2+/−, tamoxifen- or vehicle-treated Ptchflox/flox and untreated pituitary glands/cells of both genotypes. (m) Quantification of the Acth concentration in supernatants of ex vivo recombined Ptchflox/floxCreERT2+/− (Tam) and control pituitaries. Circles indicate Acth concentrations of supernatant conditioned from 3 pituitaries of the same genotype. Controls include vehicle-treated Ptchflox/floxCreERT2+/− and tamoxifen- or vehicle-treated Ptchflox/flox pituitary glands. Acth serum levels in (d), Gli1, Gh, Prl, Pomc and Cga expression levels and BrdU incorporation of the controls were set to 1. (a–d,f–l) Circles indicate biological replicates measured in triplicates. Horizontal lines, mean+/− standard error of the mean (SEM); *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Since the animals are in a very poor general condition 17 d after induction of the Ptch deletion23, the elevated Acth levels in tPtch−/− mice could be stress-induced or correlated with activation of the Hh signaling pathway. To prevent potential hormonal feed-back loops that may have distorted the results concerning the direct influence of Hh signaling on hormone production, we isolated Ptchflox/floxCreERT2+/− pituitaries and induced the Ptch mutation ex vivo by administrating tamoxifen (for verification of successful culture see Fig. 2 showing immunohistological stainings of cultured explants). This resulted in recombination of the Ptchflox loci (Ptchdel) (Fig. 1e), a significant increase of mutant Ptch transcripts (Ptchdel) (Fig. 1f) (p = 0.0013) and a loss of wt Ptch expression in Ptchflox/floxCreERT2+/− compared to control pituitaries (Fig. 2). As expected, the mutation furthermore resulted in elevated Gli1 expression levels and thus activation of Hh signaling in Ptchflox/floxCreERT2+/− glands (Fig. 1g). Similar results were obtained after incubation of normal murine pituitaries with rShh-N, which likewise resulted in an upregulation of Gli1 and wt Ptch transcription, however to a much lesser extent (Suppl. Fig. S1a,b). When the glands were treated with cyclopamine, the Gli1 expression levels were considerably reduced in tamoxifen-treated Ptchflox/floxCreERT2+/−, in rShh-N-treated and solvent-treated organs (Fig. 1g; Suppl. Fig. S1b). Most interestingly, Ptch deletion also resulted in a tendency towards increased Pomc and Glycoprotein hormones alpha chain (Cga) transcript levels (Fig. 1j,k), in a significantly elevated Acth release (Fig. 1m) (p = 0.023) and upregulation of Gh and Prl expression (Fig. 1h,i). These effects were abrogated by cyclopamine treatment (Fig. 1h–k), indicating a direct involvement of Hh signaling in the transcriptional activation of these genes. In contrast, the expression levels of Oxt, Tshβ, Lhβ and Fshβ were not altered by Ptch depletion, cyclopamine or rShh-N treatment (Suppl. Fig. S2).
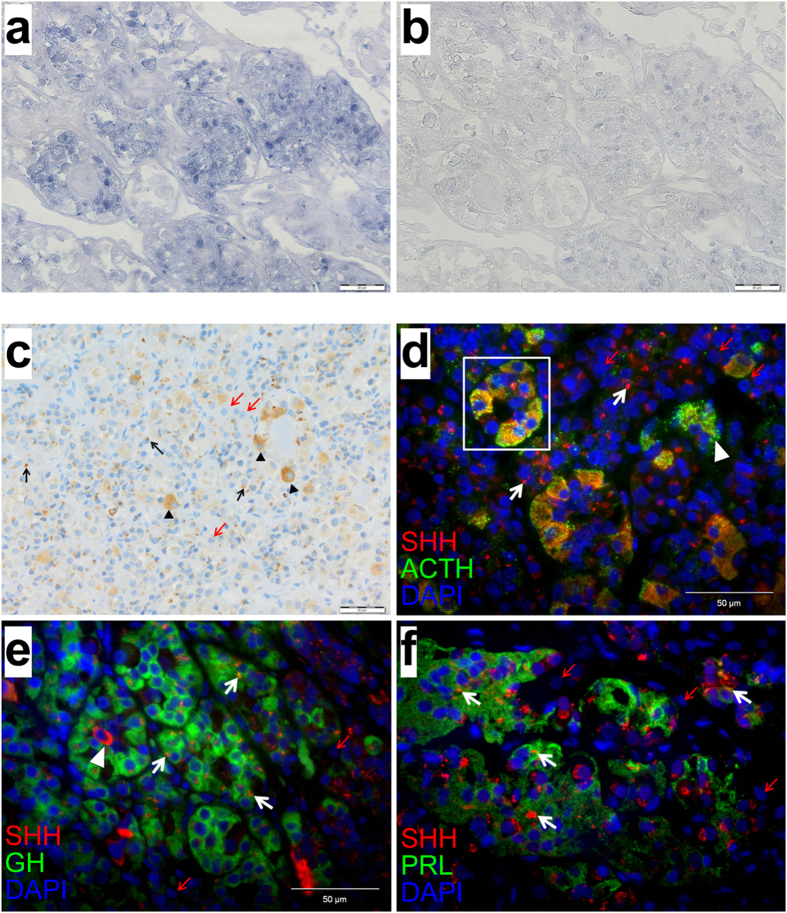
Figure 2. Distribution pattern of Gh, Prl, Acth, Sox2, Sox9, Gli1 and Ptch in ex vivo cultured Ptchflox/flox CreERT2+/− and control pituitary glands.
Immunohistochemical analyses of Gh, Prl, Acth, Sox2, Sox9 and Gli1 and in situ hybridization for detection of Ptch transcripts on serial sections of control and Ptch-mutant (tamoxifen-treated Ptchflox/flox CreERT2+/−) ex vivo cultured murine pituitary explants. Arrows indicate overlapping Sox2, Sox9, Gli1 and Ptch expression pattern in the intermediate lobe (IL) and the marginal zone (squares). Dotted arrows indicate overlapping Sox2, Sox9, Gli1 and Ptch expression pattern in the anterior lobe (AL). For detection of whole Ptch transcripts a 477 bp riboprobe was used, which identifies mutant and wt Ptch transcripts simultaneously. A second 250 bp riboprobe detects exclusively wt Ptch transcripts48. Tamoxifen-treated Ptchflox/flox CreERT2+/− glands express only mutant Ptch transcripts, as signals were exclusively obtained using the Ptch 477 bp riboprobe. PL, posterior lobe; Acth, adrenocorticotrophic hormone; Gh, growth hormone; Prl, prolactin. Scale bar: 100 μm.
Immunohistological examinations of short-term cultured explants of tamoxifen-treated Ptchflox/floxCreERT2+/− and control pituitaries revealed no obviously changed expression patterns of Gh, Prl and Acth, the adult pituitary stem cell marker Sox2 and Sox924, Gli1, and Ptch (Fig. 2). Remarkably, the expression of Sox2, Sox9, Gli1 and Ptch overlapped in cells of the intermediate zone, the marginal zone and the anterior lobe although Gli1 or Ptch positivity was not exclusively restricted to Sox2+ or Sox9+ cells (Fig. 2). Beyond that double immunofluorescent stainings against Sox2 and Gli1 revealed that all Sox2+ cells of the intermediate lobe, the marginal zone (data not shown) and the anterior lobe also are Gli1+ (Fig. 3a,b). However Gli1 expression was not exclusively restricted to Sox2+ cells (Fig. 3a,b).
Figure 3. Ex vivo Ptch depletion in murine pituitaries results in proliferation of Sox2+/Gli1+ and Sox9+ adult pituitary stem cells.
(a,b) Representative picture of a wt murine pituitary explant stained against Sox2 and Gli1. (a) Overlay of Sox2 (green color) and Gli1 (red color). (c–h and k–p) Representative pictures of wt murine pituitary explants stained against BrdU and (c,d) Sox2, (e,f) Sox9, (g,h) Gli1, (k,l) Gh, (m,n) Prl, (o,p) or Acth. (c,e,g,k,m,o) Overlays of BrdU (green color) and staining against the indicated secondary antigen (red color). (b,d,f,h,l,n,p) Same pictures as in (a,c,e,g,k,m,o) including the DAPI-channel (blue color). White arrows in (a–h) indicate Sox2+/Gli1+ cells or BrdU+ proliferating Sox2+, Sox9+ and Gli1+ cells respectively. (i,j) Relative quantification of proliferating (white bars, BrdU/Sox2+ or BrdU/Sox9+) and non-proliferating (grey bars, Sox2+ or Sox9+) (i) Sox2+ and (j) Sox9+ cells in the anterior pituitary of double immunofluorescent stained tamoxifen-treated Ptchflox/floxCreERT2+/− (Tam) and control pituitary gland explants. Analysis is based on the absolute cell numbers given in Suppl. Fig. S3. (i) ncontrols = 4 (1 female, 3 males), nTam = 7 (6 females, 1 male), (j) ncontrols = 4 (1 female, 3 males), nTam = 6 (5 females, 1 male). Mean+/−SEM; *p < 0.05. Scale bar: 50 μm.
5′-bromo-2′-deoxyuridine (BrdU) incorporation assays of single pituitary cell cultures revealed a considerably higher proliferation rate of tamoxifen-treated Ptchflox/floxCreERT2+/− but not of rShh-N-treated cells compared to the controls (Fig. 1l, Suppl. Fig. S1g). Moreover, cyclopamine inhibited the proliferation of tamoxifen-treated Ptchflox/floxCreERT2+/− and control cells (Fig. 1l, Suppl. Fig. S1g).
To further characterize the proliferating cell population, tamoxifen-treated Ptchflox/floxCreERT2+/− explants were cultured in BrdU-containing medium and double immunoflourescent stainings against BrdU in combination with anti-Sox2, anti-Sox9, anti-Gli1, anti-Gh, anti-Prl anti-Acth and anti-Pomc antibodies were conducted. This approach revealed that all BrdU+ cells of the intermediate lobe, the marginal zone (data not shown) and the anterior lobe showed a distinct co-expression of Sox2, Sox9 or Gli1 (Fig. 3c–h). In contrast, endocrine cells (e.g. Gh+, Prl+, Acth+ and Pomc+) never showed BrdU positivity (Fig. 3k–p for BrdU/Gh, BrdU/Prl, BrdU/Acth staining). Quantification of the proliferating cells revealed more BrdU+ Sox2+ or BrdU+ Sox9+ cells in the anterior lobe of tamoxifen-treated Ptchflox/floxCreERT2+/− explants (Fig. 3i,j, Suppl. Fig. S3c,f), although the absolute numbers of Sox2+ and Sox9+ proliferating cells did not change (Suppl. Fig. 3a,d). Due to the fact that our experimental setup is sufficient to induce a statistically significant increase in the numbers of pituitary stem cells, but not of differentiated progeny25 these data exclude that enhanced Acth, Gh and Prl expression levels of Ptch-depleted pituitary glands result from elevated numbers of endocrine cells. It furthermore stresses our conclusion that Hedgehog signaling not only induces proliferation of Sox2+ cell but also activates the expression of Acth, Gh and Prl in already existing differentiated endocrine cells.
Gli-dependent activation of the murine and human Pomc/POMC promoter
Gli1 and Sox2 expression patterns largely overlap in the intermediate lobe, the marginal zone and the anterior lobe, but Gli1 positivity was not restricted to Sox2+ cells (see Fig. 3a,b). This suggests that Hh signaling might be also implicated in the function of endocrine cells. Sequence analyses of the mouse, rat and human Pomc/POMC promoter revealed two Gli binding sites upstream of the first ATG (Suppl. Fig. S4a). To validate our finding from the ex vivo explant experiments we tested whether activation of Hh signaling directly induces Acth-secretion in the murine PA cell line AtT-20 that has been widely used as a model system for Acth-expressing pituitary cells20,21,26,27,28. However, AtT-20 cells express only low levels of Ptch and no Gli1 or Gli2, and an induction of these genes upon Shh stimulation was not possible (data not shown). Nevertheless Gli1 or Gli2 overexpression resulted in transcriptional activation of the Ptch gene (Suppl. Fig. S4b), higher Pomc transcription and activation of the human POMC promoter (Suppl. Fig. S4c,e). Moreover Gli1 or Gli2 overexpression – contrary to Shh treatment – did not alter the proliferative capacity of AtT-20 cells (Suppl. Fig. S4d) which is in line with the observation that Ptch depletion does not induce proliferation of Acth-expressing cells in pituitary explants (see Fig. 3o,p). Besides, these results indicate that Shh induces proliferation in AtT-20 cells in a Gli1/Gli2-independent manner.
Expression of SHH and GLI1 in corticotropic, somatotropic and lactotropic cells of the human adenohypophysis and related adenoma subtypes
To transfer our results to the human situation we analyzed the HH signaling activity in 15 normal adenopituitaries by GLI1 in situ hybridization (Fig. 4a,b) and/or anti-SHH immunostaining using an antibody generated against the N-terminal part of SHH protein (Fig. 4c–f). Cells throughout the adenohypophysis homogenously expressed GLI1 (Fig. 4a,b) and stained positive for SHH to, however, variable extent. We found cell populations displaying an intense cytoplasmic and predominantly granular SHH staining pattern as well as cells with restricted immunoreactivity to particularly perinuclear structures (Fig. 4c). Double immunofluorescent stainings using antibodies against SHH and several hormones produced in the adenohypophysis (ACTH, GH and PRL) support former studies21 that ACTH-expressing (corticotropic) cells show a distinct, homogenous SHH immunoreactivity (Fig. 4d). However, besides co-localization of SHH and corticotrophs circumscribed, perinuclear, dot-like SHH positivity in obviously lactrotrophs and somatotrophs was observed (Fig. 4e,f). Since binding of SHH to PTCH results in internalization of both proteins to perinuclear lysosomes or endosomes29,30,31 the distinct SHH signals in these endocrine active cells (Fig. 4e,f) might reflect its localization to the respective vesicles and therefore might stain SHH-responding cells. In contrast, SHH might co-localize to ACTH storing vesicles in corticotropic cells (Fig. 4d).
Figure 4. GLI1 expression and SHH protein localization in the human pituitary gland.
(a,b) Representative GLI1 in situ hybridization, (c) SHH immunohistochemical staining and (d–f) double immunofluorescent stainings against SHH and (d) ACTH, (e) GH or (f) PRL in the human adenohypophysis. (a) GLI1 is expressed in several endocrine cells of the human adenohypophysis indicating activation of the HH signaling pathway. Cells are organized in small lobules, being characteristic for the physiological anatomy. (b) Serial section hybridized with GLI1 sense riboprobes served as negative control. (c–f) Particular cells of the human adenohypophysis either show a distinct, homogenous and particularly granular SHH staining pattern (arrowheads), or a circumscribed and dot-like pattern (black arrows in a and white arrows in (d–f). SHH negative cells are exemplarily marked with red arrows. (d–f) The majority of ACTH expressing cells is SHH positive (yellow color, square, granular staining pattern). Several GH or and PRL positive cells (green color) show a distinct perinuclear, dot-like localization of SHH (red color, white arrows). ACTH, adreno-corticotrophic hormone; GH, growth hormone; PRL, prolactin. Scale bar in (a–c) 20 μm; Scale bar in (d–f) 50 μm.
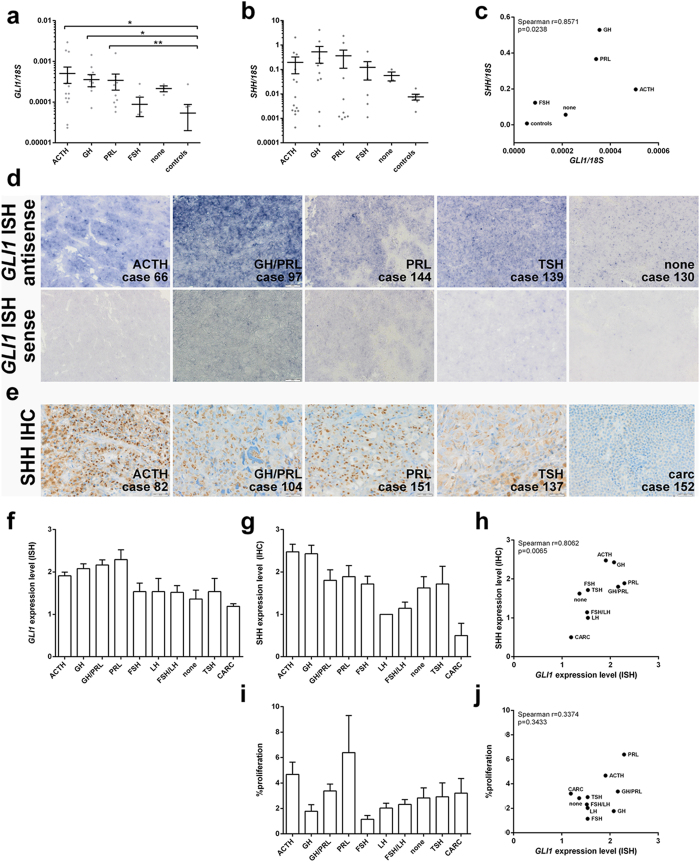
We next quantified the SHH and GLI1 transcript levels of 12 normal adenopituitaries and 48 PA by qRT-PCR (Table 1, Fig. 5a,b). GLI1 expression levels were significantly elevated in ACTH-, GH- and PRL-expressing adenomas (p = 0.026; p = 0.035; p = 0.0059, respectively) whereas null cell adenomas and FSH-expressing tumors showed similar GLI1 expression levels compared to normal adenopituitary lobes (Fig. 5a). Although SHH expression levels of the tumors did not reach significance a generally higher SHH expression of all PA in comparison to the controls was detected (Fig. 5b). Correlation analysis revealed that the SHH and GLI1 expression levels significantly correlate on transcript level throughout the tumor collection (Fig. 5c; Spearman r-coefficient 0.8571; p = 0.0238).
Table 1. Relative quantification of GLI1 and SHH expression levels in human pituitary adenomas and adenohypophyses.
| patient no. | age | sex | IHC | GLI1/18S (mean ± SEM × 10−4) | SHH/18S (mean ± SEM × 10−4) | ||
|---|---|---|---|---|---|---|---|
| 1 | 30 | m | ACTH | 0.12 | 0.003 | 0.00 | 0.000 |
| 2 | 44 | f | ACTH | 0.00 | 0.000 | 0.00 | 0.000 |
| 3 | 47 | f | ACTH | 0.16 | 0.053 | 2.13 | 0.317 |
| 4 | 49 | m | ACTH | 0.28 | 0.091 | 2.67 | 0.442 |
| 5 | 36 | f | ACTH | 1.86 | 0.509 | 1.46 | 0.162 |
| 6 | 62 | f | ACTH | 0.00 | 0.000 | 3.41 | 0.790 |
| 7 | 39 | f | ACTH | 2.95 | 0.290 | 489.94 | 33.993 |
| 8 | 64 | f | ACTH | 0.02 | 0.002 | 31.06 | 5.662 |
| 9 | 69 | m | ACTH | 1.73 | 0.041 | 38.41 | 4.019 |
| 10 | 33 | f | ACTH | 0.03 | 0.002 | 0.42 | 0.034 |
| 11 | 51 | m | ACTH | 0.31 | 0.040 | 2064.58 | 139.327 |
| 12 | 50 | f | ACTH | 0.16 | 0.011 | 1.99 | 0.130 |
| 13 | 65 | m | ACTH | 0.24 | 0.040 | 412.76 | 69.239 |
| 14 | 21 | f | ACTH | 0.00 | 0.000 | 2.01 | 0.353 |
| 15 | 66 | m | ACTH | 0.12 | 0.019 | 101.15 | 4.176 |
| 16 | 58 | m | ACTH | 0.10 | 0.018 | 7.42 | 0.214 |
| 17 | 53 | f | GH | 0.00 | 0.000 | 0.00 | 0.000 |
| 18 | 40 | m | GH | 0.33 | 0.016 | 0.00 | 0.000 |
| 19 | 45 | m | GH | 0.23 | 0.041 | 1.85 | 0.466 |
| 20 | 32 | f | GH | 0.07 | 0.003 | 146.92 | 12.587 |
| 21 | 33 | m | GH | 0.53 | 0.081 | 1415.44 | 53.226 |
| 22 | 57 | f | GH | 0.72 | 0.085 | 37.07 | 2.046 |
| 23 | 36 | m | GH | 0.00 | 0.000 | 288.98 | 9.869 |
| 24 | 65 | f | GH | 0.45 | 0.029 | 4167.47 | 7.086 |
| 25 | 39 | m | GH | 0.19 | 0.015 | 71.83 | 6.497 |
| 26 | 25 | f | GH | 0.00 | 0.000 | 0.48 | 0.028 |
| 27 | 35 | m | GH | 1.44 | 0.082 | 178.02 | 6.736 |
| 28 | 41 | f | GH | 0.30 | 0.010 | 41.33 | 2.662 |
| 29 | 20 | f | PRL | 0.15 | 0.014 | 1235.95 | 66.472 |
| 30 | 69 | m | PRL | 0.06 | 0.015 | 1.11 | 0.099 |
| 31 | 18 | m | PRL | 1.59 | 0.118 | 1.21 | 0.202 |
| 32 | 45 | f | PRL | 0.26 | 0.041 | 2372.95 | 188.663 |
| 33 | 34 | f | PRL | 0.30 | 0.028 | 0.00 | 0.000 |
| 34 | 55 | f | PRL | 0.42 | 0.024 | 0.93 | 0.048 |
| 35 | 53 | f | PRL | 0.10 | 0.004 | 1.19 | 0.292 |
| 36 | 34 | f | PRL | 0.11 | 0.021 | 6.10 | 0.703 |
| 37 | 47 | f | PRL | 0.08 | 0.007 | 44.43 | 2.871 |
| 38 | 50 | m | PRL | 0.35 | 0.028 | 1.72 | 0.270 |
| 39 | 47 | f | PRL | 0.43 | 0.102 | 1937.14 | 243.703 |
| 40 | 45 | m | FSH | 0.00 | 0.000 | 1.11 | 0.122 |
| 41 | 71 | f | FSH | 0.00 | 0.000 | 142.39 | 16.203 |
| 42 | 76 | m | FSH | 0.28 | 0.064 | 540.21 | 42.347 |
| 43 | 47 | m | FSH | 0.14 | 0.013 | 41.89 | 0.255 |
| 44 | 61 | f | FSH | 0.05 | 0.002 | 9.01 | 0.338 |
| 45 | 44 | m | FSH | 0.06 | 0.008 | 3.88 | 1.116 |
| 46 | 49 | m | none | 0.15 | 0.011 | 31.60 | 0.694 |
| 47 | 42 | f | none | 0.27 | 0.072 | 100.81 | 3.977 |
| 48 | 77 | m | none | 0.22 | 0.016 | 36.44 | 0.820 |
| 49 | 80 | f | control | 0.08 | 0.003 | 2.10 | 0.739 |
| 50 | 59 | m | control | 0.08 | 0.025 | 0.69 | 0.068 |
| 51 | 42 | m | control | 0.00 | 0.000 | 34.70 | 1.329 |
| 52 | 89 | f | control | 0.00 | 0.000 | 0.89 | 0.186 |
| 53 | 54 | m | control | 0.00 | 0.000 | n.d. | n.d. |
| 54 | 72 | m | control | 0.27 | 0.004 | 4.96 | 0.157 |
| 55 | 87 | f | control | 0.00 | 0.000 | 0.00 | 0.000 |
| 56 | 54 | f | control | 0.00 | 0.000 | 1.83 | 0.032 |
| 57 | 45 | f | control | n.d. | n.d. | 5.82 | 0.100 |
| 58 | 91 | f | control | n.d. | n.d. | 16.80 | 2.175 |
| 59 | 74 | m | control | n.d. | n.d. | 7.42 | 0.387 |
| 60 | 62 | f | control | n.d. | n.d. | 9.19 | 0.535 |
Given are age and sex of patients with hormone-active or -inactive pituitary adenomas and of donors of adenohypophysis control tissue (control). The GLI1 and SHH expression levels of the samples were quantified by qRT-PCR as described in the Material and Methods section. m, male; f, female; ACTH, adrenocorticotrophic hormone; GH, growth hormone; PRL, prolactin; FSH, follicle stimulating hormone. n.d. not determined.
Figure 5. GLI1 and SHH/SHH expression levels and proliferation index of human pituitary tumors.
qRT-PCR-based analyses of (a) GLI1 and (b) SHH expression levels and (c) nonparametric Spearman correlation analyses of GLI1 and SHH expression levels in human PA and normal adenopituitary lobes (control) listed in Table 1. (d) Representative GLI1 in situ hybridization and (e) immunohistological SHH staining of pituitary tumors. (d) ACTH positive, mixed GH/PRL positive and PRL positive adenomas showed highest GLI1 expression level (Score 2.5–3.5) whereas TSH positive and null cell adenomas were moderately positive for GLI1 (Score 1–2.75). (e) Within the group of pituitary tumors, distribution of distinct cytoplasmic SHH immunostaining (brown color) reached high levels in ACTH positive adenomas (Score 4), moderate levels in mixed GH/PRL positive (atypical PA), PRL positive and TSH positive adenomas (Score 3). Lowest SHH staining scores were observed in pituitary carcinoma (carc) and associated metastases (Score 0). For case numbers see Table 2. (f) In situ hybridization-based analyses of GLI1 expression levels, (g) immunohistology-based analyses of SHH expression levels and (i) percentage of Ki67+ proliferative tumor cells in human PA listed in Table 2 (for scoring see Material and Method section). (h,j) nonparametric Spearman correlation analyses of (h) GLI1 and SHH expression levels and (j) GLI1 expression levels and percentage of proliferating cells in human pituitary lesions listed in Table 2. ACTH, adreno corticotrophic hormone; GH, growth hormone; PRL, prolactin; FSH, follicle stimulating hormone; LH, luteinizing hormone; TSH, thyroid stimulating hormone; CARC, carcinoma. Mean+/−SEM, *p < 0.05, **p < 0.01. Scale bar: 20 μm.
Subsequently we analyzed the SHH and GLI1 expression levels in a large cohort of 92 PA and 4 pituitary carcinomas by GLI1 in situ hybridization and SHH immunohistology (Table 2, Fig. 5d–g, Suppl. Figs S5 and S6). Throughout the tumor collection a strong and significant correlation of GLI1 and SHH expression was observed (Fig. 5h; Spearman r-coefficient 0.8062; p = 0.0065). Very intense signals were detected in ACTH- and GH-producing tumors (Fig. 5d–g, Suppl. Figs S5 and S6). Prolactinomas as well as mixed GH/PRL-producing adenomas showed high GLI1 expression levels and moderate immunoreactivity for SHH (Fig. 5d–g, Suppl. Figs S5 and S6). TSH- and FSH-expressing tumors and null cell adenomas were moderately positive for both (Fig. 5d–g, Suppl. Figs S5 and S6). LH- and FSH/LH- adenomas expressed low to moderate GLI1 and only low SHH levels (Fig. 5d–g, Suppl. Figs S5 and S6). In pituitary carcinomas GLI1 and SHH expression could only be hardly detected (Fig. 5d–g, Suppl. Figs S5 and S6). There was no association between HH signaling activation and proliferation rates within the tumor specimens (Fig. 5i,j).
Table 2. Expression of GLI1 and SHH in human pituitary adenomas.
| patient no. | age | sex | IHC | GLI1 | SHH | % proliferation |
|---|---|---|---|---|---|---|
| 61 | 39 | m | ACTH | 2.00 | 2.00 | 1.5 |
| 62 | 34 | f | ACTH | 2.00 | 2.00 | 2.3 |
| 63 | 39 | f | ACTH | 1.75 | 3.00 | 6.6 |
| 64 | 47 | f | ACTH | 1.75 | 3.00 | 0.8 |
| 65 | 20 | f | ACTH | 2.25 | 2.00 | 7.0 |
| 66 | 27 | f | ACTH# | 2.00 | 2.00 | 1.3 |
| 66 | 21 | f | ACTH# | 2.50 | 1.00 | 5.4 |
| 67 | 29 | f | ACTH | 2.00 | 4.00 | 3.0 |
| 68 | 21 | f | ACTH | 1.75 | n.d. | n.d. |
| 69 | 46 | m | ACTH | 0.75 | 2.00 | 7.5 |
| 70 | 63 | f | ACTH* | 2.25 | 3.00 | 11.3 |
| 71 | 49 | f | ACTH | 2.00 | 2.00 | 15.9 |
| 72 | 69 | f | ACTH | 1.63 | 4.00 | 8.2 |
| 73 | 38 | f | ACTH | 2.00 | 2.00 | 7.4 |
| 74 | 58 | f | ACTH | 1.75 | 2.00 | 1.4 |
| 75 | 55 | m | ACTH | 2.00 | 3.00 | 1.5 |
| 76 | 25 | m | ACTH | 1.00 | n.d. | 1.4 |
| 77 | 37 | m | ACTH | 2.25 | 2.00 | 0.5 |
| 78 | 32 | f | ACTH | 1.75 | 2.00 | 2.5 |
| 79 | 31 | f | ACTH | 2.00 | 3.00 | 14.0 |
| 80 | 43 | m | ACTH | 1.75 | 2.00 | 1.4 |
| 81 | 68 | m | ACTH | 2.00 | 2.00 | 0.9 |
| 82 | 51 | m | ACTH | 2.75 | 4.00 | 1.0 |
| 83 | 36 | m | GH | 2.25 | 3.00 | 1.1 |
| 84 | 50 | f | GH | 2.25 | 3.00 | 6.5 |
| 85 | 34 | m | GH | 1.50 | 1.00 | 2.4 |
| 86 | 34 | f | GH | 2.50 | 1.00 | 5.8 |
| 87 | 52 | f | GH | 2.00 | 2.00 | 0.3 |
| 88 | 70 | m | GH | 2.25 | 2.00 | 1.9 |
| 89 | 30 | f | GH | 2.25 | 2.00 | 1.3 |
| 90 | 44 | f | GH | 2.25 | 3.00 | 1.4 |
| 91 | 46 | m | GH | n.d. | 2.00 | 0.7 |
| 92 | 37 | f | GH | 2.50 | 3.00 | 0.3 |
| 93 | 72 | f | GH | 2.00 | 3.00 | 0.8 |
| 94 | 37 | f | GH | 2.00 | 3.00 | 1.2 |
| 95 | 46 | m | GH | 1.00 | 3.00 | 0.5 |
| 96 | 45 | m | GH | 2.25 | 3.00 | 0.6 |
| 97 | 33 | f | GH/PRL | 2.75 | 2.00 | 2.6 |
| 98 | 33 | m | GH/PRL | 2.50 | 2.00 | 2.7 |
| 99 | 11 | f | GH/PRL | 2.00 | 1.00 | 3.0 |
| 100 | 15 | f | GH/PRL | 2.00 | 3.00 | 5.8 |
| 101 | 61 | f | GH/PRL | 2.00 | 1.00 | 0.9 |
| 102 | 54 | f | GH/PRL | 2.00 | 2.00 | 1.6 |
| 103 | 23 | m | GH/PRL | 2.25 | 2.00 | 2.3 |
| 104 | 29 | m | GH/PRL* | 2.25 | 3.00 | 5.8 |
| 105 | 33 | m | GH/PRL* | 1.38 | 1.00 | 4.2 |
| 106 | 26 | f | GH/PRL* | 2.50 | 1.00 | 4.8 |
| 107 | 66 | m | FSH | 1.00 | 2.00 | 1.2 |
| 108 | 41 | m | FSH | 1.00 | 1.00 | 2.8 |
| 109 | 46 | m | FSH | 1.75 | 2.00 | 1.2 |
| 110 | 46 | f | FSH | 2.00 | 2.00 | 0.4 |
| 111 | 38 | f | FSH | 2.25 | 1.00 | 0.5 |
| 112 | 57 | m | FSH | 1.00 | 2.00 | 1.0 |
| 113 | 68 | m | FSH | 1.75 | 2.00 | 0.9 |
| 114 | 67 | f | LH | 0.00 | 1.00 | 1.8 |
| 115 | 74 | m | LH | 2.50 | 1.00 | 0.6 |
| 116 | 51 | m | LH | 1.50 | 1.00 | 2.5 |
| 117 | 65 | m | LH | 2.00 | 1.00 | 3.3 |
| 118 | 65 | f | LH | 1.00 | 1.00 | 2.4 |
| 119 | 47 | f | LH | 2.00 | 1.00 | 2.8 |
| 120 | 61 | m | LH | 1.75 | 1.00 | 0.8 |
| 121 | 66 | f | FSH/LH | 1.75 | 1.00 | 1.7 |
| 122 | 43 | m | FSH/LH | 2.00 | 1.00 | 1.0 |
| 123 | 59 | m | FSH/LH | 1.00 | 1.00 | 1.4 |
| 124 | 57 | m | FSH/LH | 1.00 | 1.00 | 2.3 |
| 125 | 75 | m | FSH/LH | 1.50 | 1.00 | 3.2 |
| 126 | 48 | m | FSH/LH | 2.00 | 2.00 | 3.9 |
| 127 | 60 | m | FSH/LH | 1.38 | 1.00 | 2.7 |
| 128 | 50 | m | none | 2.00 | 2.00 | 5.0 |
| 129 | 67 | m | none | 2.50 | 1.00 | 1.3 |
| 130 | 19 | m | none | 1.00 | 2.00 | 3.6 |
| 131 | 65 | f | none | 1.00 | 1.00 | 0.8 |
| 132 | 57 | f | none | 1.00 | 1.00 | 0.6 |
| 133 | 41 | f | none | 0.75 | 1.00 | 1.0 |
| 134 | 23 | m | none* | 1.38 | 3.00 | 6.5 |
| 135 | 52 | m | none* | 1.25 | 2.00 | 3.8 |
| 136 | 69 | f | TSH | 2.50 | 2.00 | 2.8 |
| 137 | 19 | m | TSH | 1.00 | 3.00 | 9.0 |
| 138 | 31 | f | TSH | 1.00 | 2.00 | 3.7 |
| 139 | 30 | f | TSH | 2.75 | 1.00 | 0.7 |
| 140 | 35 | m | TSH | 1.50 | 0.00 | 0.8 |
| 141 | 51 | f | TSH | 1.50 | 3.00 | 2.0 |
| 142 | 31 | f | TSH | 0.50 | 1.00 | 1.4 |
| 143 | 32 | m | PRL | 2.50 | 1.00 | 0.7 |
| 144 | 27 | m | PRL | 3.25 | 1.00 | 1.6 |
| 145 | 43 | m | PRL | 2.00 | 2.00 | 3.2 |
| 146 | 26 | f | PRL | 2.50 | 2.00 | 1.1 |
| 147 | 39 | f | PRL | 2.50 | 3.00 | 1.6 |
| 148 | 18 | f | PRL | 0.75 | 2.00 | 6.5 |
| 149 | 52 | m | PRL | 2.75 | 2.00 | 3.4 |
| 150 | 40 | m | PRL* | 2.38 | 1.00 | 11.6 |
| 151 | 78 | m | PRL* | 2.00 | 3.00 | 27.8 |
| 152 | 23 | m | ACTH$,§ | 1.25 | 0.00 | 5.5 |
| 152 | 24 | m | ACTH$,§ | 1.00 | 1.00 | 2.1 |
| 153 | 49 | m | PRL$ | 1.25 | 0.00 | 20.5 |
| 154 | 60 | f | PRL$ | 1.25 | 1.00 | 2.0 |
Given are age and sex of patients with hormone-active or -inactive pituitary tumors. The GLI1 and SHH expression levels and the percentage of proliferative tumor cells were scored as described in the Material and Methods section. m, male; f, female; ACTH, adrenocorticotrophic hormone; GH, growth hormone; PRL, prolactin; FSH, follicle stimulating hormone; LH, luteinizing hormone; TSH, thyroid stimulating hormone. *atypic adenoma; $carcinoma; #same patient; §same patient; n.d. not determined.
Taken together, these data show that, in contrast to a former study21, not only corticotrophs but at least lactotrophs and somatotrophs of the normal human adenopituitary express SHH. Moreover, our results suggest that HH signaling activity may play a role in formation and/or maintenance of PA subtypes, especially in ACTH-, GH- or PRL-immunopositive tumors that show the highest GLI1 and SHH expression levels. However, this is pure speculation and remains to be investigated in the future.
Discussion
Within this paper, we demonstrate that excess Hh signaling in the pituitary gland elevates the hormone expression and induces proliferative processes of Gli1+/Sox2+ and Sox9+ adult stem cells. However, in contrast to neural stem cells in which Hh signaling activation results in stem cell accumulation32 Ptch-depletion in the pituitary does not impact the absolute cell number but enhances the percentage of proliferating stem cells. Due to the fact that early Sox2+ progenitors can give rise to Sox2+/Sox9+ transit-amplifying cells25 which are able to generate all hormone producing cell subtypes24, our data thus indicate that Hh signaling activation induces proliferation and differentiation processes of Sox2+ cells, most likely by asymmetric divisions. This might result in maintenance of the stem cell pool and simultaneously in generation of more differentiated hormone producing daughter cells. Moreover our findings that Gli1 expression is not solely restricted to Sox2+ cells furthermore indicate that the Hh pathway – similarly to neural fate specification and cell cycle progression in the retina33 – might be involved in the function of different cellular pituitary subtypes. In fact excess Hh signaling did not induce proliferation of differentiated endocrine cells (see Fig. 3) and Acth-producing AtT-20 cells but enhanced the hormone expression in pituitary explants and Pomc/POMC promoter activity in AtT-20 cells. This implies that activation of Hh signaling in endocrine pituitary cells induces hormone production rather than proliferative processes. This is in contrast to Sox2+/Sox9+ pituitary stem cells, in which Hh signaling activates proliferation.
Contrary to excess Hh signaling activation due to Ptch-depletion a moderate pathway activity induced by Shh did not alter the proliferative capacity of primary cultured pituitary cells. In contrast, Shh induced proliferative processes in AtT-20 cells. Since this was not accompanied by elevated Gli1 or Gli2 expression levels we conclude that it is impossible to induce and study the effects of canonical Hh signaling in AtT-20 cells upon Shh treatment. The fact that Gli1 or Gli2 overexpression in AtT-20 cells also did not affect the proliferation rate of these cells supports our suggestion that activation of Hh signaling has no impact on the proliferation rate of hormone-producing pituitary cells. Beyond that these data indicate that exceeding of a certain Hh signaling threshold is necessary to induce proliferation of pituitary stem cells.
In accordance with our murine explant experiments human adenopituitaries also express GLI1 and SHH. Moreover SHH expression pattern overlaps with hormonally active cells (e.g. ACTH-, PRL- and GH- producing cells). Similar to pituitary gland development in zebrafish34 these data suggest that HH signaling may also play a role in these cellular subpopulations in the adult human pituitary. Remarkably, also ACTH-, GH- or PRL-immunopositive human PA express high SHH/SHH and GLI1 levels whereas TSH-, LH-, or FSH-producing tumors showed only moderate or low HH signaling activity. Previously it has been proposed that active HH signaling is restricted to ACTH-producing endocrine cells and that SHH maintains pituitary cells in a non-proliferative state. Moreover it was suggested that a down-regulation of HH signaling may be involved in the pathogenesis of PA21. In contrast to these studies we here quantified the GLI1 transcript level which is the best indicator of HH signaling activity. Furthermore, we used a SHH antibody that detects the active N-terminal SHH fragment and thus visualizes SHH-expressing and SHH-responding cells. Finally, we revealed a significant and strong correlation of GLI1 and SHH/SHH expression levels in the human PA collection (p = 0.0065 and p = 0.0238). Consequently, our results suggest that HH signaling is active in ACTH-, GH- and PRL-expressing cells of the adenohypophysis and in the respective PA subtypes and might indicate a role of HH signaling in the development or maintenance of PA (e.g. ACTH-, PRL-, GH-immunopositive PA). It is furthermore tempting to speculate that HH signaling activation (e.g. by PTCH mutations) itself drives tumor formation (e.g. in a paracrine, ligand dependent manner) and can drive hormone secretion in pituitary adenomas. However the fact that GLI1 or SHH/SHH expression did not correlate with the proliferation status of PA may be explained by intratumor heterogeneity. Furthermore, Sox2 and Sox9 expression have been associated with tumor growth35,36, self-renewal of oncogene target cells, tumor initiation and invasion37. Moreover Sox2+ pituitary cells have tumor-inducing potential36 indicating that Sox2+/Sox9+ cells indeed play a role in PA formation. Beyond that it is interesting that pituitary carcinomas exhibit the lowest levels of HH signaling activity. These tumors are most commonly ACTH- or PRL-secreting, invasive macroadenomas that spread distant metastases. Although the analyzed case number was low, it is tempting to speculate that the conversion of a rather benign pituitary tumor (i.e. adenoma) into a metastatic tumor necessitates and correlates with downregulation of HH signaling.
To our knowledge mutations in components of the HH signaling pathway have not been reported in tumors of the pituitary. PA are the third most common intracranial tumors and the estimated prevalence in the general population is approximately 17%38. Surgical resection of this tumor is the primary therapy for most patients. However in a portion of patients, surgery does not result in cure39. Our findings suggest that the HH pathway plays a role in the pathogenesis of PA and hormone production, which however have to be verified by future experiments. If this comes true, it would be interesting to test HH pathway inhibitors (e.g. the FDA-approved HH-inhibitor vismodegib) alone or in combination with other drugs to target pituitary tumor cells (reviewed in40).
In summary our data demonstrate that activation of Hh signaling (e.g. by enhanced Gli1 expression), similarly to neural fate specification and cell cycle progression in the retina33, induces cell type-specific cellular processes in the pituitary (e.g. hormone-release of endocrine cells and proliferation of adult pituitary stem cells). Finally, we present data which might be indicative for a role of HH signaling in the development or maintenance of PA.
Methods
Ethical approval and informed consent
All experimental protocols using murine or human samples were approved by the Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit (LAVES) and by the Ethical Committee of the University of Erlangen-Nürnberg, respectively. All used methods were carried out in accordance with the approved guidelines of the Niedersächsisches LAVES or the Ethical Committee of the University of Erlangen-Nürnberg. For experiments involving human tissue samples written informed consent from all subjects was obtained.
Compounds
If not otherwise stated all compounds were obtained from Sigma-Aldrich. For in vitro studies 5 μM cyclopamine (Toronto Research Chemicals Inc.) dissolved in ethanol (EtOH), 10 μM tamoxifen in DMSO and 1 μg/ml recombinant murine Shh-N (rShh-N; R&D systems) in HD-buffer were used. Tamoxifen solution for in vivo use was prepared as described (see below)23.
Mice
Experiments using animals were performed in compliance with all German legal and ethical requirements. The Rosa26CreERT2 (CreERT2) knock-in mouse strain (kindly provided by Dr. Anton Berns, The Netherlands Cancer Institute, Amsterdam, The Netherlands) expresses a fusion gene encoding Cre recombinase and a modified ligand-binding domain for the estrogen receptor under control of the endogenous Rosa26 promoter41. Generation, genotyping and CreERT2-activation of Ptchflox/floxCreERT2+/− mice are described in Uhmann et al.23. Briefly, CreERT2 mice were bred to Ptchflox/flox mice to obtain Ptchflox/floxCreERT2+/− mice. Eight-week-old Ptchflox/floxCreERT2+/− mice were injected intraperitoneally with 1 mg tamoxifen dissolved in a 1:10 ethanol-sunflower oil emulsion42 on 5 consecutive days to induce the Ptchdel mutation (named tPtch−/− mice) or with solvent alone. Mice with Ptchflox/floxCreERT−/− genotypes were used to assess any unspecific effects of tamoxifen. After tamoxifen-mediated induction of the Ptchdel mutation in Ptchflox/floxCreERT2+/− mice animals are named tPtch−/− mice23. Solvent-injected Ptchflox/floxCreERT2+/− mice or tamoxifen-injected Ptchflox/flox mice served as controls. Female and male mice were used for in vitro and in vivo assays because the sex of the animals did not impact on the results.
Buffers and media for organ culture and culture of single-cell suspensions of murine pituitaries
HD-Buffer and growth medium for organ culture and culture of single-cell suspensions of murine pituitaries consisted of 25 mM N-2-hydroxyethylpiperazine-N′-2-ethane sulfonic acid (HEPES), 137 mM NaCl, 5 mM KCl, 0.7 mM Na2HPO4, 10 mM Glucose, 1x Partricin (Biochrom) and 1% penicillin/streptomycin (PS) or Dulbeccos’s modified Eagle’s medium++− (DMEM++−), 10% heat-inactivated FCS, 1x L-glutamine (Gibco), 1% PS, 1x Partricin, 1x minimal essential medium-vitamins (Gibco), 5 μg/ml insulin, 5 μg/ml transferrin, 60 pmol 3,3′,5-triiodo-L-thyroinine sodium salt and 20 pg/ml sodium selenite, respectively. Collagenase solution for preparing single-cell suspensions of murine pituitaries consisted of 1000 U/ml collagenase T1 (Worthington Biochem Corp.), 0.1 mg/ml trypsin inhibitor, 1 mg/ml hyaluronidase, 4 mg/ml bovine serum albumin (BSA) and 10 μg/ml DNAse II.
Organ culture and culture of single-cell suspensions of murine pituitaries
Pituitaries of 6–8 week old Ptchflox/floxCreERT2+/− and Ptchflox/flox mice of both sexes were isolated and washed in HD-Buffer. For organ cultures, the glands were transferred into cell culture inserts (Falcon, Corning) in 24-well-plates filled with growth medium (see above) supplemented with tamoxifen, cyclopamine, rShh-N or the respective solvents. Media were exchanged after 2 days and pituitary glands and media were harvested for ribonucleic acid (RNA) isolation and hormone quantification, respectively, after an overall culture period of 5 days. For BrdU incorporation analyses of pituitary explants 10 μM BrdU was added to the culture medium for the last 48 h of the experiments. The vitality of the explants was verified by immunohistological stainings of active Caspase 3 (data not shown), Acth, Gh and Prl (see below) (Fig. 2).
Single pituitary cell suspensions were prepared using a collagenase solution (see above) for 1.5 h. After filtering through a 40 μm nylon cell strainer (BD Falcon) 20,000 cells were seeded for BrdU incorporation assays in 96-well-plates. After 24 h the media were changed to growth media supplemented with tamoxifen, cyclopamine or the respective solvent for 48 h. For the last 22 h of the experiments 10 μM BrdU was added to the culture medium. The experiments were conducted at least 3 times in triplicates. If not stated otherwise data represent the mean of all experiments.
Isolation of genomic DNA and analysis of genomic recombination at the Ptch flox locus
Isolation of genomic deoxyribonucleic acid (DNA) from freshly isolated and cultured pituitary glands and quantification of the recombination efficiency at the Ptchflox locus were performed as previously described23. For polymerase chain reaction (PCR)-based detection of the CreERT2-mediated Ptchflox recombination the primer pair 5′-gcatgtgacctgcctactaattc-3′/5′-cctacttatctgatggtctgcatc-3′ was used.
Plasmids
The Gli-binding site luciferase reporter construct (p9xGliBS) has been described previously43. The plasmid pCR3.1 mGli1 was constructed by cloning mGli1 complementary deoxyribonucleic acid (cDNA) from the pcDNA3.1-His mGli1 vector44 (provided by Hiroshi Saraki) into the pCR3.1 plasmid (Life Technologies). The Gli2 expression vector pCMV Gli2 FLAG was provided by Chi-Chung Hui.
POMC-Prom plasmid was cloned by amplifying a 4734 bp fragment upstream of the first ATG of the human POMC gene sequence from genomic DNA and insertion into pGL3-basic (Promega GmbH). Primer sequences are available upon request. The plasmid pRL-TK (Promega GmbH) was used as endogenous control for the normalization of firefly luciferase activity in dual luciferase-based reporter assays.
Cell lines and cell culture experiments
The murine PA cell line AtT-20 (ATCC; CCL-89TM, obtained from ATCC in July 2014) was cultured in accordance with the ATCC protocol. The Shh-N-conditioned medium (Shh-N-CM) and the respective control medium (CoM) were obtained from HEK293-Shh that express the N-terminal active fragment of Shh or HEK293 cells, respectively45. For gene expression analysis or BrdU incorporation assays (Roche life science) AtT-20 cells were seeded in F-12K/1% PS/2.5% FCS/15% HS at densities of 200,000 or 20,000 cells/well into 24-well- or 96-well-plates, respectively. Afterwards the cells were transfected with 2 μg plasmid DNA and RotiFect transfection reagent (Carl Roth GmbH) as indicated and/or incubated for 48 h with Shh-N-CM or CoM and cyclopamine or EtOH as indicated in the respective experiments. For the last 22 h of the experiments 10 μM BrdU was added to the culture medium. BrdU incorporation assays were performed in accordance with the manufacturer’s instructions and analyzed using a microplate reader (SynergyMX, BioTek Instruments, Inc.). For POMC promoter analyses 20,000 AtT-20 cells were seeded in F-12K/2.5% FCS/1.5% HS/1% PS or Shh-N-CM or CoM in 96-well-plates. Cells were transfected with 500 ng plasmid DNA as indicated in the respective experiments and 10 ng pRL-TK using RotiFect transfection reagent. After 48 h the activity of the POMC promoter reporter constructs were measured using the Dual Luciferase Assay Kit (Promega GmbH) and a microplate reader (SynergyMX). The experiments were conducted at least 3 times in triplicates. As not stated otherwise the shown data represent the mean of all experiments.
Reverse transcription and quantitative real time-PCR-analyses (qRT-PCR)
Total RNA was extracted using TRIzol reagent (Life Technologies GmbH). cDNA synthesis, quantification of 18S ribosomal RNA (rRNA), Gli1 and wt Ptch transcripts and the standard curve method for qRT-PCR analyses were recently described46,47. Primer sequences of intron-flanking primer pairs used for relative quantification of the expression of Ptchdel, proopiomelanocortin (Pomc), Gh, Cga, Prl, Oxytocin (Oxt), luteinizing hormone (subunit β, Lhβ), thyroid-stimulating hormone (subunit β; Tshβ), follicle-stimulating hormone (subunit β; Fshβ), human GLI1 and human SHH are listed in Supplemental Table S1. Each sample was measured in triplicates. Graphs represent the mean value of all measurements.
Hormone determination
Acth blood serum levels were quantified using an enzyme-linked immunosorbent assay (ELISA) (Uscn Life Science Inc., BIOZOL Diagnostica). Acth concentrations of supernatants of cultured pituitary were quantified using the IMMULITE® 2000 Immunoassay System (Siemens AG).
In situ hybridization, immunohistological and immunofluorescent stainings of cultured murine pituitary gland
Cultured murine pituitary glands were fixed in 4% PFA, embedded in paraffin and sectioned. The sections were stained with hematoxylin and eosin (H&E) for histopathological analyses or were analyzed by in situ hybridization, immunohistological or immunofluorescent stainings. The in situ hybridization procedure and the probes for detecting wt Ptch and whole Ptch transcripts have been described previously48. Immunohistological and immunofluorescent stainings were conducted by boiling the sections in 10 mM citric acid, pH 6.0 (which also allows for specific staining with anti-BrdU antibodies)49 and staining with the antibodies listed in Supplemental Table S2. The specificity of the antibodies was verified by showing that antibodies against Pomc, Acth, Prl and Gh detected selective populations of cells in the anterior pituitary gland, antibodies against Sox2 and Sox9 detected selective populations of cells in the marginal zone and in the anterior pituitary gland. None of the antibodies detected antigens in the posterior lobe of the pituitary. The specificity of the antibody against Gli1 and Caspase 3 was verified by specific staining of murine basal cell carcinoma. The specificity of the antibody against BrdU was verified by showing that the antibody did not detect antigens in pituitary explants without BrdU-treatment.
Negative controls were carried out by incubation in the absence of the primary antibody and always yielded negative results.
Human pituitary tissue samples
Surgical specimens from 142 different patients with sellar lesions (n = 145) were retrieved from the Department of Neuropathology of the University Hospital of Erlangen. Each tumor specimen was classified and graded according to the currently valid version of the World Health Organization classification system of tumors of endocrine organs22. Twelve normal pituitary tissues and five different tumor subtypes (n = 48) were analyzed by qRT-PCR for relative GLI1 and SHH expression level (data for each case are summarized in Table 1). Fifteen normal pituitary tissues and ten different tumor subtypes (n = 96; data for each case are summarized in Table 2) were analyzed by specific histological stainings (GLI1 in situ hybridization, SHH immunohistological stainings). Tumors were grouped according to clinical symptoms and hormone release as following: 1) ACTH producing adenomas with and without clinical signs of Cushing’s disease (qRT-PCR: 7 male, 9 female, median age 49.7 years; specific histological stainings: 23 tumors from 22 different patients; 8 male,14 female, median age 42.8 years); 2) adenomas from patients with clinical signs of acromegaly and immunohistochemical expression of GH (qRT-PCR: 6 male, 6 female, median age 39.4 years; specific histological stainings: 6 male, 8 female, median age 45.2 years); 3) mixed adenomas from patients with clinical signs of acromegaly and combined immunohistochemical expression of GH and PRL (specific histological stainings: 4 male, 6 female, median age 31.8 years); 4) clinically nonfunctioning tumors with expression of FSH (qRT-PCR: 4 male, 2 female, median age 54.1 years; specific histological stainings: 5 male, 2 female, median age 51.7 years); 5) clinically nonfunctioning adenomas with expression of LH (specific histological stainings: 4 male, 3 female, median age 61.4 years); 6) clinically nonfunctioning tumors with combined expression of FSH and LH (specific histological stainings: 6 male, 1 female, median age 58.3 years); 7) nonfunctioning adenomas without detectable hormone expression (qRT-PCR: 2 male, 1 female, median age 49.0 years; null cell adenomas; specific histological stainings: 5 male, 3 female, median age 46.8 years); 8) TSH producing adenomas (specific histological stainings: 2 male, 5 female, median age 38 years); 9) PRL producing adenomas (qRT-PCR: 3 male, 8 female, median age 46.7 years; specific histological stainings: 6 male, 3 female, median age 39.4 years); 10) pituitary carcinomas (specific histological stainings: 4 tumors and/or metastases from 3 different patients; 2 male, 1 female, median age 39 years). Data for each case included in the study are shown in Table 1 (qRT-PCR) and Table 2 (specific histological stainings).
Normal pituitary tissue samples (n = 27) were acquired from patients with sellar exploration in cases of magnetic resonance imaging negative microadenomas and Rathke’s cleft cysts. The latter specimens showed a regular lobulated reticulin fiber network typically observed in the adenohypophysis and IHC confirmed the regular spectrum of hormone production.
The study concept was approved by the Ethical Committee of the University of Erlangen-Nürnberg.
IHC procedure and evaluation on human samples
IHC was performed as described using a semiautomated benchmark apparatus (Nexes; Ventana, Illkirch, France) and the Ventana DAB staining system50. Positive and negative controls were used to validate the staining of the primary antibodies listed in Supplemental Table S3. The specificity of the antibodies was tested by staining human adenopituitaries showing selective populations of cells only. The specificity of the anti-SHH antibody was verified by staining human pancreas carcinoma and intestine. Negative controls were carried out by incubation in the absence of the primary antibody and always yielded negative results.
The intensity of SHH staining was assessed semi-quantitatively blinded to any diagnosis concerning the hormonal status of patients (D.S. and R.B.) and grouped into five different categories (0 = no SHH expression; 1 = very low intensity, 2 = moderate intensity, 3 = strong intensity, 4 = highest intensity of all). In cases without detectable SHH expression, the IHC was repeated to verify the result. The proliferation rate of each tumor was assessed quantitatively (D.S.) by counting a minimum of 1,000 tumor cell nuclei in a region with the highest amount of distinct positive Ki76+ cell nuclei.
In situ hybridization and evaluation on human samples
In situ hybridization was conducted as described48,51 using antisense and sense (negative control) riboprobes reversely transcribed from a pBS-hGLI1 plasmids containing the hGLI1 cDNA (GenBank: X07384.1) (provided by Mark Wijgerde).
The intensity of GLI1 in situ hybridization was assessed semi-quantitatively blinded to any diagnosis concerning the hormonal status of patients (A.U., D.S. and R.B.) and grouped into 7 different categories (0 = no expression; 0.5 = very low intensity, 1 = low intensity, 2 = intermediate intensity, 2.5 = intermediate to strong intensity, 3 = strong intensity, 3.5 = highest intensity of all).
Statistical analyses
Statistical analyses were conducted using the software GraphPadPrism 6 (GraphPad Software Inc.). Correlation or significance of GLI1 and SHH/SHH expression in human PA was tested by a nonparametric Spearman correlation test or unpaired nonparametric Mann-Whitney test, respectively. Statistical significance of the recombination efficiency at the Ptchflox locus, the gene expression in pituitary glands and human PA, the Acth blood serum levels and Acth concentration of media were tested using Holm-Sidak’s multiple comparison test (unpaired t test). Statistical significance of gene expression levels of ex vivo cultured pituitaries was tested after outliers correction using ROUT method (Q = 1%) and testing the Gaussian distribution by D’Agustino and Pearson omnibus normality test followed by an one-way-ANOVA (analysis of variance; Holm-Sidak’s multiple comparison test). Statistical significance of proliferative Sox2+ or Sox9+ cells, of BrdU incorporation assays of in vitro cultured pituitary cells or AtT-20 cells, of dual-luciferase assays and expression analyses of AtT-20 cells were tested using nonparametric Mann-Whitney test, Dunn’s multiple comparison test or Kruskal-Wallis test, respectively.
Additional Information
How to cite this article: Pyczek, J. et al. Hedgehog signaling activation induces stem cell proliferation and hormone release in the adult pituitary gland. Sci. Rep. 6, 24928; doi: 10.1038/srep24928 (2016).
Supplementary Material
Acknowledgments
We are grateful to Stefan Wolf and Susan Peter for excellent animal care. We thank Anton Berns (The Netherlands Cancer Institute, Amsterdam, The Netherlands) for providing CreERT2 mice, A.F. Parlow (National Hormone & Peptide Program; Torrance, USA) for providing anti-Acth, anti-Gh and anti-Prl antibodies, Mark Wijgerde (Rotterdam, The Netherlands) for the plasmids carrying hGLI1 cDNA sequences, Hiroshi Saraki (Osaka University, Japan) for the pcDNA3.1-His mGli1 vector, Chi-Chung Hui (University of Toronto, Toronto, Kanada) for the pCMV Gli2 FLAG plasmid and Jessica Nolte-Kaitschick (Medical Center of the University of Göttingen, Germany) for the rabbit anti-Sox-2 and the secondary FITC-labeled goat anti-rat antibody. Research of H.H. is supported by the Wilhelm Sander-Stiftung (grant# 2013.058.1) and by the Deutsche Forschungsgemeinschaft (grant# HA2197/7-1).
Footnotes
Author Contributions J.P. performed research, collected and analyzed data. R.B. contributed vital reagents and analytical tools, designed research, collected and analyzed data and wrote the paper. D.S. performed research, collected and analyzed data. A.H. collected and analyzed data. M.B. contributed vital reagents and analytical tools, and collected data. I.H. performed research. H.H. contributed vital reagents and analytical tools, designed research, and wrote the paper. A.U. designed and performed research, collected and analyzed data, and wrote the paper. R.B. and A.U. prepared the figures. All authors reviewed the manuscript.
References
- Hooper J. E. & Scott M. P. Communicating with Hedgehogs. Nat Rev Mol Cell Biol 6, 306–317 (2005). [DOI] [PubMed] [Google Scholar]
- Machold R. et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron 39, 937–950 (2003). [DOI] [PubMed] [Google Scholar]
- Shin K. et al. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature 472, 110–114, 10.1038/nature09851 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler E. et al. Loss-of-function mutations in the human GLI2 gene are associated with pituitary anomalies and holoprosencephaly-like features. Proc Natl Acad Sci USA 100, 13424–13429 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S. et al. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev 10, 60–69 (1996). [DOI] [PubMed] [Google Scholar]
- Pabst O., Herbrand H., Takuma N. & Arnold H. H. NKX2 gene expression in neuroectoderm but not in mesendodermally derived structures depends on sonic hedgehog in mouse embryos. Dev Genes Evol 210, 47–50 (2000). [DOI] [PubMed] [Google Scholar]
- Franca M. M. et al. Novel heterozygous nonsense GLI2 mutations in patients with hypopituitarism and ectopic posterior pituitary lobe without holoprosencephaly. J Clin Endocrinol Metab 95, E384–391 (2010). [DOI] [PubMed] [Google Scholar]
- Flemming G. M. et al. Functional characterization of a heterozygous GLI2 missense mutation in patients with multiple pituitary hormone deficiency. J Clin Endocrinol Metab 98, E567–575, 10.1210/jc.2012-3224 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treier M. et al. Hedgehog signaling is required for pituitary gland development. Development 128, 377–386 (2001). [DOI] [PubMed] [Google Scholar]
- Bale S. J., Amos C. I., Parry D. M. & Bale A. E. Relationship between head circumference and height in normal adults and in the nevoid basal cell carcinoma syndrome and neurofibromatosis type I. Am J Med Genet 40, 206–210, 10.1002/ajmg.1320400217 (1991). [DOI] [PubMed] [Google Scholar]
- Bale A. E., Gailani M. R. & Leffell D. J. Nevoid basal cell carcinoma syndrome. J Invest Dermatol 103, 126S–130S (1994). [DOI] [PubMed] [Google Scholar]
- Wicking C. & Bale A. E. Molecular basis of the nevoid basal cell carcinoma syndrome. Curr Opin Pediatr 9, 630–635 (1997). [DOI] [PubMed] [Google Scholar]
- Lo Muzio L. et al. Nevoid basal cell carcinoma syndrome. Clinical findings in 37 Italian affected individuals. Clin Genet 55, 34–40 (1999). [DOI] [PubMed] [Google Scholar]
- Hahn H. et al. Rhabdomyosarcomas and radiation hypersensitivity in a mouse model of Gorlin syndrome. Nature Med 4, 619–622 (1998). [DOI] [PubMed] [Google Scholar]
- Cramer H. & Niederdellmann H. Cerebral gigantism associated with jaw cyst basal cell naevoid syndrome in two families. Arch Psychiatr Nervenkr (1970) 233, 111–124 (1983). [DOI] [PubMed] [Google Scholar]
- Kahn L. B. & Gordon W. The basal cell naevus syndrome–report of a case. S Afr Med J 41, 832–835 (1967). [PubMed] [Google Scholar]
- Codish S. D., Kraszeski J. & Pratt K. CNS developmental anomaly in the basal cell nevus syndrome: another congenital neurocutaneous syndrome? Neuropadiatrie 4, 338–343, 10.1055/s-0028-1091750 (1973). [DOI] [PubMed] [Google Scholar]
- Marcos M. V., Querol X., Armengol A., Hierro F. R. & Cruz M. [Basal cell nevus syndrome and gigantism]. An Esp Pediatr 16, 513–519 (1982). [PubMed] [Google Scholar]
- Kimonis V. E. et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet 69, 299–308 (1997). [PubMed] [Google Scholar]
- Vila G. et al. Sonic hedgehog regulates CRH signal transduction in the adult pituitary. Faseb J 19, 281–283 (2005). [DOI] [PubMed] [Google Scholar]
- Vila G. et al. Expression and function of sonic hedgehog pathway components in pituitary adenomas: evidence for a direct role in hormone secretion and cell proliferation. J Clin Endocrinol Metab 90, 6687–6694 (2005). [DOI] [PubMed] [Google Scholar]
- The International Agency for Research on Cancer Pathology and Genetics of Tumours of Endocrine Organs (IARC WHO Classification of Tumours). Vol. 1 (eds DeLellis R. A. et al.) (IARC Press, Lyon, France, 2004). [Google Scholar]
- Uhmann A. et al. The Hedgehog receptor Patched controls lymphoid lineage commitment. Blood 110, 1814–1823 (2007). [DOI] [PubMed] [Google Scholar]
- Rizzoti K., Akiyama H. & Lovell-Badge R. Mobilized adult pituitary stem cells contribute to endocrine regeneration in response to physiological demand. Cell Stem Cell 13, 419–432, 10.1016/j.stem.2013.07.006 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauquier T., Rizzoti K., Dattani M., Lovell-Badge R. & Robinson I. C. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci USA 105, 2907–2912, 10.1073/pnas.0707886105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney A. P., Fernando M., Yong W. H. & Melmed S. Functional PPAR-gamma receptor is a novel therapeutic target for ACTH-secreting pituitary adenomas. Nat Med 8, 1281–1287, 10.1038/nm784 (2002). [DOI] [PubMed] [Google Scholar]
- Shan B. et al. Curcumin suppresses HIF1A synthesis and VEGFA release in pituitary adenomas. J Endocrinol 214, 389–398, 10.1530/JOE-12-0207 (2012). [DOI] [PubMed] [Google Scholar]
- Lampichler K. et al. The role of proto-oncogene GLI1 in pituitary adenoma formation and cell survival regulation. Endocr Relat Cancer 22, 793–803, 10.1530/ERC-15-0109 (2015). [DOI] [PubMed] [Google Scholar]
- Incardona J. P. et al. Receptor-mediated endocytosis of soluble and membrane-tethered Sonic hedgehog by Patched-1. Proc Natl Acad Sci USA 97, 12044–12049, 10.1073/pnas.220251997 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue S. et al. Requirement of Smurf-mediated endocytosis of Patched1 in sonic hedgehog signal reception. Elife 3, 10.7554/eLife.02555 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronardi F. G., Dimitroulakos J., Kamel-Reid S. & Manoukian A. S. Co-localization of patched and activated sonic hedgehog to lysosomes in neurons. Neuroreport 11, 581–585 (2000). [DOI] [PubMed] [Google Scholar]
- Ferent J. et al. Genetic activation of Hedgehog signaling unbalances the rate of neural stem cell renewal by increasing symmetric divisions. Stem Cell Reports 3, 312–323, 10.1016/j.stemcr.2014.05.016 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami K., Gan L. & Yang X. J. Distinct effects of Hedgehog signaling on neuronal fate specification and cell cycle progression in the embryonic mouse retina. J Neurosci 29, 6932–6944, 10.1523/JNEUROSCI.0289-09.2009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine C. A. et al. A dynamic Gli code interprets Hh signals to regulate induction, patterning, and endocrine cell specification in the zebrafish pituitary. Dev Biol 326, 143–154, 10.1016/j.ydbio.2008.11.006 (2009). [DOI] [PubMed] [Google Scholar]
- Vanner R. J. et al. Quiescent sox2(+) cells drive hierarchical growth and relapse in sonic hedgehog subgroup medulloblastoma. Cancer Cell 26, 33–47, 10.1016/j.ccr.2014.05.005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoniadou C. L. et al. Sox2(+) stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell 13, 433–445, 10.1016/j.stem.2013.07.004 (2013). [DOI] [PubMed] [Google Scholar]
- Larsimont J. C. et al. Sox9 Controls Self-Renewal of Oncogene Targeted Cells and Links Tumor Initiation and Invasion. Cell Stem Cell 17, 60–73, 10.1016/j.stem.2015.05.008 (2015). [DOI] [PubMed] [Google Scholar]
- Ezzat S. et al. The prevalence of pituitary adenomas: a systematic review. Cancer 101, 613–619, 10.1002/cncr.20412 (2004). [DOI] [PubMed] [Google Scholar]
- Heaney A. Management of aggressive pituitary adenomas and pituitary carcinomas. J Neurooncol 117, 459–468, 10.1007/s11060-014-1413-6 (2014). [DOI] [PubMed] [Google Scholar]
- Gupta S., Takebe N. & Lorusso P. Targeting the Hedgehog pathway in cancer. Ther Adv Med Oncol 2, 237–250, 10.1177/1758834010366430 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameyer D. et al. Toxicity of ligand-dependent Cre recombinases and generation of a conditional Cre deleter mouse allowing mosaic recombination in peripheral tissues. Physiol Genomics 31, 32–41 (2007). [DOI] [PubMed] [Google Scholar]
- Imai T., Jiang M., Chambon P. & Metzger D. Impaired adipogenesis and lipolysis in the mouse upon selective ablation of the retinoid X receptor alpha mediated by a tamoxifen-inducible chimeric Cre recombinase (Cre-ERT2) in adipocytes. Proc Natl Acad Sci USA 98, 224–228 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer C., Buhr P., Hahn H., Laubner D. & Wirth M. Gene expression analysis of murine cells producing amphotropic mouse leukaemia virus at a cultivation temperature of 32 and 37 degrees C. J Gen Virol 84, 1677–1686 (2003). [DOI] [PubMed] [Google Scholar]
- Sasaki H., Nishizaki Y., Hui C., Nakafuku M. & Kondoh H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development 126, 3915–3924 (1999). [DOI] [PubMed] [Google Scholar]
- Chen J. K., Taipale J., Young K. E., Maiti T. & Beachy P. A. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci USA 99, 14071–14076 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecke I. et al. Cyclopamine treatment of full-blown Hh/Ptch-associated RMS partially inhibits Hh/Ptch signaling, but not tumor growth. Mol Carcinog 47, 361–372 (2008). [DOI] [PubMed] [Google Scholar]
- Nitzki F. et al. Tumor stroma-derived Wnt5a induces differentiation of basal cell carcinoma of Ptch mutant mice via CaMKII. Cancer Res 70, 2739–2748 (2010). [DOI] [PubMed] [Google Scholar]
- Zibat A. et al. Time-point and dosage of gene inactivation determine the tumor spectrum in conditional Ptch knockouts. Carcinogenesis 30, 918–926 (2009). [DOI] [PubMed] [Google Scholar]
- Tang X., Falls D. L., Li X., Lane T. & Luskin M. B. Antigen-retrieval procedure for bromodeoxyuridine immunolabeling with concurrent labeling of nuclear DNA and antigens damaged by HCl pretreatment. J Neurosci 27, 5837–5844, 10.1523/JNEUROSCI.5048-06.2007 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buslei R. et al. Activation and regulation of endogenous retroviral genes in the human pituitary gland and related endocrine tumors. Neuropathol Appl Neurobiol, 10.1111/nan.12136 (2014). [DOI] [PubMed] [Google Scholar]
- Wijgerde M., Ooms M., Hoogerbrugge J. W. & Grootegoed J. A. Hedgehog signaling in mouse ovary: Indian hedgehog and desert hedgehog from granulosa cells induce target gene expression in developing theca cells. Endocrinology 146, 3558–3566, 10.1210/en.2005-0311 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.