Abstract
Klotho, a protein mainly expressed in kidney and cerebral choroid plexus, is a powerful regulator of 1,25(OH)2D3 formation. Klotho-deficient mice (kl/kl) suffer from excessive plasma 1,25(OH)2D3-, Ca2+- and phosphate-concentrations, leading to severe soft tissue calcification and accelerated aging. NH4Cl treatment prevents tissue calcification and premature ageing without affecting 1,25(OH)2D3-formation. The present study explored the impact of excessive 1,25(OH)2D3 formation in NH4Cl-treated kl/kl-mice on behavior. To this end kl/kl-mice and wild-type mice were treated with NH4Cl and either control diet or vitamin D deficient diet (LVD). As a result, plasma 1,25(OH)2D3-, Ca2+- and phosphate-concentrations were significantly higher in untreated and in NH4Cl-treated kl/kl-mice than in wild-type mice, a difference abrogated by LVD. In each, open field, dark-light box, and O-maze NH4Cl-treated kl/kl-mice showed significantly higher exploratory behavior than untreated wild-type mice, a difference abrogated by LVD. The time of floating in the forced swimming test was significantly shorter in NH4Cl treated kl/kl-mice compared to untreated wild-type mice and to kl/kl-mice on LVD. In wild-type animals, NH4Cl treatment did not significantly alter 1,25(OH)2D3, calcium and phosphate concentrations or exploratory behavior. In conclusion, the excessive 1,25(OH)2D3 formation in klotho-hypomorphic mice has a profound effect on murine behavior.
Klotho is expressed mainly in the kidney, but is highly expressed as well in choroid plexus of the brain1. The extracellular domain of the transmembrane protein may be cleaved off and enter blood or cerebrospinal fluid1. Klotho is a powerful inhibitor of 1α-25-hydroxyvitamin D hydroxylase (1 α hydroxylase) thus preventing 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) formation1. Klotho influences mineral metabolism in addition by up-regulation of Ca2+ channels2 and down-regulation of phosphate transport3,4. Klotho affects further channels and transport proteins including Na+/K+-ATPase5,6, Na+/Ca2+-exchanger7, Ca2+ channels8, K+ channels9,10,11,12,13 and excitatory amino acid transporters14,15. Moreover, klotho counteracts inflammation16,17. Klotho-hypomorphic mice (kl/kl) with defective promoter of the klotho gene suffer from severe tissue calcification, a wide variety of age related disorders and a severely decreased life span1,18. Conversely, the life span is substantially increased in klotho overexpressing mice19. Klotho may similarly influence tissue calcification, ageing and life span of humans20,21,22.Klotho has been implicated in the regulation of depression and cognitive function23,24,25,26. Evidence has been presented pointing to an effect of klotho on oligodendrocyte maturation and myelination27 and klotho has been postulated to counteract neurodegeneration28. Overexpression of klotho has been shown to enhance cognition23. Conversely, klotho deficient mice suffer from deterioration of cognitive function25,26,29. The alterations of neuronal function in klotho deficient mice may, however, be due to the severe vascular calcification and may not reflect the effect of klotho or 1,25(OH)2D3 on cerebral function. 1,25(OH)2D3 has previously been shown to affect behavior30,31, emotions and anxiety32. In animals, vitamin D deficiency has been shown to decrease explorative behavior and enhance anxiety, aberrant grooming, submissive social behavior, social neglect and maternal cannibalism33,34,35. Prenatal vitamin D deficiency influences murine self-grooming behavior36. Deletion of the vitamin D receptor (VDR) has similarly been shown to affect murine behavior34,37,38,39,40,41,42. In humans vitamin D deficiency predisposes to several psychiatric disorders, such as depression, bipolar disorder and schizophrenia32,43,44,45. The vitamin D receptor (VDR) and vitamin D metabolizing enzymes are expressed widely in cerebral structures including prefrontal cortex, hippocampus, cingulate gyrus, thalamus, hypothalamus, and substantia nigra46. VDR gene variants are associated with altered behavior47,48 as well as susceptibility to age-related changes in cognitive function and depressive symptoms47. 1,25(OH)2D3 serum concentration correlates with extraversion49, which is negatively correlated with social phobia, cluster C personality disorders and suicide risk45,50. Along those lines, the seasonal variations of sun exposure and thus 1,25(OH)2D3 formation have been associated with seasonal affective disorders51,52,53.
The excessive formation of 1,25(OH)2D3 in kl/kl mice were expected to exert profound effects on behavior. However, due to the severe vascular calcification the kl/kl mice are severely ill and not amenable to behavioral studies. Most recent observations revealed that addition of NH4Cl into the drinking water fully prevents the severe vascular calcification and rapid ageing of kl/kl mice without affecting the excessive formation of 1,25(OH)2D3 and the increase of plasma phosphate and calcium concentrations54. NH4Cl is apparently effective by alkalinizing acidic cellular compartments which compromizes the maturation of TGFß, a critical mediator of osteogenic signaling54. Aging and life span are almost identical in NH4Cl treated kl/kl-mice and wild type mice54. The NH4Cl treated kl/kl mice would thus be an ideal model to study the effect of excessive 1,25(OH)2D3 on behavior. Thus, kl/kl mice and wild-type mice were treated with NH4Cl (280 mM in drinking water) and with either control diet or vitamin D deficient diet, which has previously been shown to normalize plasma 1,25(OH)2D3 levels in kl/kl mice55. The behavior of those mice was explored utilizing open field, dark-light box, O-maze, and forced swimming test.
Results
Without NH4Cl treatment, klotho-hypomorphic mice (kl/kl) suffer from a severe growth deficit (Fig. 1A). Accordingly, the body weight of kl/kl mice was significantly lower than the body weight of wild-type mice (Fig. 1B). NH4Cl treatment increased significantly the body weight of kl/kl mice to similar values as the body weight of wild-type mice (Fig. 1B).
Figure 1. Effect of NH4Cl treatment and low vitamin D diet on body weight of wild-type mice and of kl/kl mice.
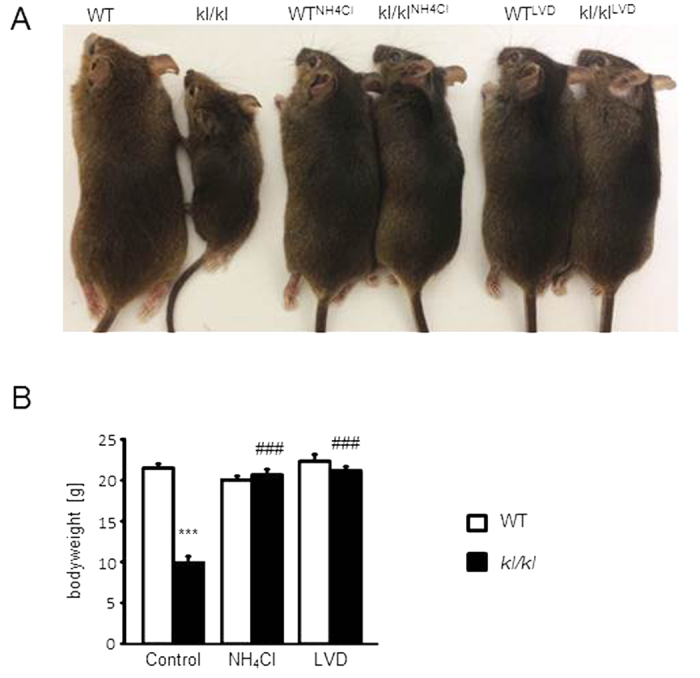
(A) Photograph of male wild-type mice (WT) as well as male klotho-hypomorphic mice (kl/kl) without (left) or with NH4Cl treatment (15 g/l in drinking water) without (NH4Cl, middle) and with (LVD, right) additional low vitamin D diet. (B) Arithmetic means ± SEM of body weight (n = 12–30) of wild-type (WT, white bars) and kl/kl mice (kl/kl, black bars) either untreated (left bars, Control), treated with NH4Cl solution (280 mM in drinking water) (NH4Cl, middle bars) or treated with NH4Cl and a vitamin D deficient diet (LVD, right bars). ***(p < 0.001) indicates statistically significant differences from respective wild-type mice; ###(p < 0.001) indicates statistically significant differences from untreated kl/kl mice. (ANOVA).
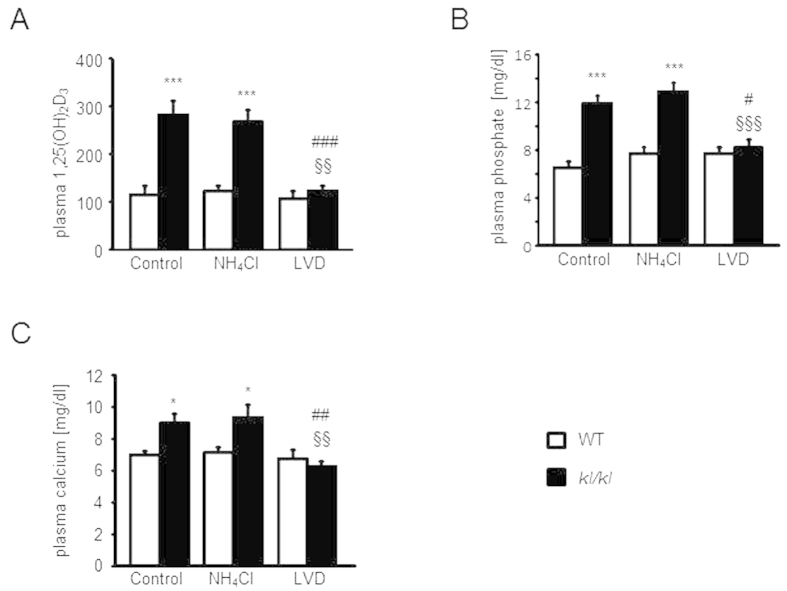
Plasma 1,25(OH)2D3 (Fig. 2A), phosphate (Fig. 2B) and Ca2+ (Fig. 2C) concentrations were significantly higher in untreated kl/kl mice than in wild-type mice, differences not significantly affected by NH4Cl treatment. However, vitamin D deficient diet decreased the values of all three parameters in plasma of kl/kl mice to values similar as those in wild-type mice.
Figure 2. Effect of NH4Cl treatment and low vitamin D diet on plasma 1,25(OH)2D3, phosphate, and Ca2+ concentrations of wild-type mice and kl/kl mice.
(A–C) Arithmetic means ± SEM of (A) plasma 1,25(OH)2D3 (n = 6), (B) phosphate (n = 12), and (C) Ca2+ (n = 12) concentrations of wild-type mice (WT, white bars) and kl/kl mice (black bars) either untreated, treated with NH4Cl solution (280 mM in drinking water) or treated with NH4Cl and vitamin D deficient diet (LVD, right bars). ***(p < 0.001) indicates statistically significant differences from respective wild-type mice (WT); #(p < 0.05), ##(p < 0.01), ###(p < 0.001) indicates statistically significant differences from untreated kl/kl mice; §§(p < 0.01), §§§(p < 0.001) indicates statistically significant differences from respective NH4Cl treated mice on control diet. (ANOVA).
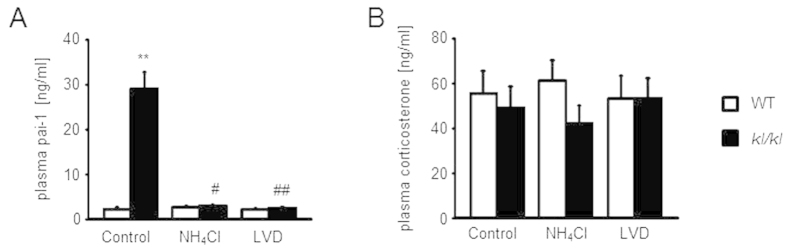
Plasma Pai-1 levels were assessed as an indicator of aging in all groups. Pai-1 levels in plasma were increased in kl/kl mice. NH4Cl treatment and the vitamin D deficient diet normalized the plasma Pai-1 levels (Fig. 3A). As an indicator of stress, corticosterone plasma levels were determined. As a result, the plasma corticosterone levels tended to be lower in untreated and NH4Cl treated kl/kl mice than in the respective wild type mice, differences, however, not reaching statistical significance (Fig. 3B)
Figure 3. Effect of NH4Cl treatment and low vitamin D diet on plasma pai-1and corticosterone levels.
(A) Arithmetic means ± SEM (n = 8, ♂ = 4, ♀ = 4) of plasma pai-1 concentrations in wild-type mice (WT, white bars) and kl/kl mice (black bars) either untreated, treated with NH4Cl solution (280 mM in drinking water) or treated with NH4Cl and a vitamin D deficient diet (LVD, right bars). *(p < 0.05) indicates statistically significant differences from untreated wild-type mice (Control); ##(p < 0.01) indicates statistically significant differences from NH4Cl treated kl/kl mice on control diet. (ANOVA).(B) Arithmetic means ± SEM (n = 12, ♂ = 6, ♀ = 6) of plasma corticosterone concentrations of wild-type mice (WT, white bars) and kl/kl mice (black bars) either untreated, treated with NH4Cl solution (280 mM in drinking water) or treated with NH4Cl and a vitamin D deficient diet (LVD, right bars). Blood was drawn between 4 p.m. and 6 p.m.
Behavioral studies were performed with untreated control wild-type mice (Control), NH4Cl treated WT mice and NH4Cl treated kl/kl mice (NH4Cl) under regular diet as well as WT mice and kl/kl mice under a vitamin D deficient diet (LVD).
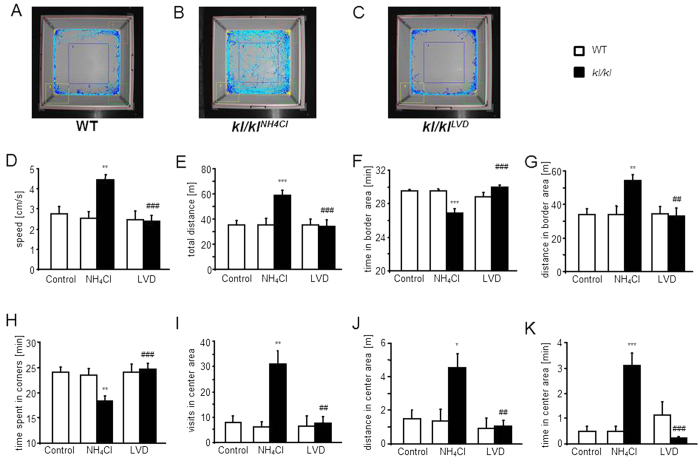
In the open-field, NH4Cl treated kl/kl mice seemed hyperactive which was already obvious from the recorded tracings (Fig. 4A–C). Computer analysis confirmed the visual impressions revealing significant increases in speed (Fig. 4D) and global distance travelled (Fig. 4E). The NH4Cl treated kl/kl mice also spent significantly less time in the border area (Fig. 4F) but still travelled larger distances there (Fig. 4G) than wild-type mice. NH4Cl treated kl/kl mice spent significantly less time in corners (Fig. 4H) and visited the center area more often (Fig. 4I) than wild-type mice. They also travelled larger distances in the center area (Fig. 4J) and spent significantly more time in that section (Fig. 4K). Interestingly, all those behavioral abnormalities were abrogated when kl/kl mice were fed a vitamin D deficient diet. There were no differences between untreated wild-type mice and wild-type mice treated with either NH4Cl drinking solution or LVD. Rearing behavior is shown in Table 1.
Figure 4. Effect of NH4Cl treatment and low vitamin D diet on performance in Open Field Test.
(A–C) Photographs of the Open Field arena with representative tracings of an untreated, male wild-type mouse (WT) (A), a male, klotho-hypomorphic mouse (kl/kl) treated with 280 mM NH4Cl solution (B) and a male, NH4Cl treated kl/kl mouse under vitamin D deficient diet (C). (D–K) Arithmetic means ± SEM (n = 12–30) of (D) average speed measured in the whole observation area, (E) total distance travelled during the observation time, (F) time spent in the border area of the Open Field arena, (G), distance travelled in the border area, (H), time spent in the corners of the Open Field arena, (I) number of visits in the center area, (J) distance travelled in the center area, (K) time spent in the center area of wild-type mice (WT, white bars) and kl/kl mice (kl/kl black bars) either untreated (Control, left bars), treated with 280 mM NH4Cl solution (NH4Cl, middle bars) or treated with NH4Cl and a vitamin D deficient diet (LVD, right bars). *(p < 0.05), **(p < 0.01), ***(p < 0.001) indicates statistically significant differences from untreated wild-type mice (Control); ##(p < 0.01). ###(p < 0.001) indicates statistically significant differences from NH4Cl treated kl/kl mice on control diet. (ANOVA).
Table 1. Synopsis of rearing parameters in the open field test (arithmetic means ± SEM).
| parameter | WT | WTNH4Cl | kl/klNH4Cl | WTLVD | kl/klLVD | statistics |
|---|---|---|---|---|---|---|
| number of rearings in border area | 84.18 ± 14.05 | 68.87 ± 15.82 | 138.53 ± 10.70 | 60.17 ± 13.66 | 78.10 ± 11.13 | P < 0.0001 ANOVA |
| rearing time in border area [min] | 3.03 ± 0.60 | 2.74 ± 0.68 | 6.37 ± 0.63 | 2.56 ± 0.76 | 2.82 ± 0.50 | P < 0.0001 ANOVA |
| number of rearings in center area | 1.36 ± 0.50 | 1.07 ± 0.93 | 7.67 ± 1.65 | 1.42 ± 0.74 | 0.81 ± 0.45 | P < 0.0001 nonparametric ANOVA |
| Rearing time in center area [s] | 1.23 ± 0.48 | 0.36 ± 0.25 | 10.88 ± 3.01 | 0.18 ± 0.12 | 0.65 ± 0.42 | P < 0.0001 nonparametric ANOVA |
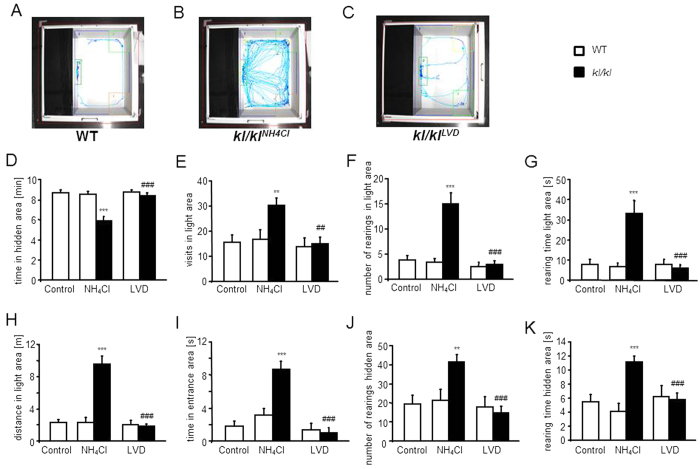
The increased activity of NH4Cl treated kl/kl mice was also apparent in the light dark transition test (Fig. 5A–C). NH4Cl treated kl/kl mice spent less time in the hidden area (Fig. 5D), visited the light area more often (Fig. 5E), showed more rearings in the light area (Fig. 5F), spent more time rearing in the light area (Fig. 5G), spent more time in the entrance area of the box (Fig. 6H) and travelled larger distances in the light compartment (Fig. 5I). Although NH4Cl treated kl/kl mice spent less time in the hidden area the number of rearings in the box (Fig. 5J) and the rearing time in the box (Fig. 5K) were significantly increased. Under LVD, kl/kl mice performed like wild-type mice. Again neither NH4Cl treatment nor LVD had an influence on the behavior of wild-type mice in the light dark transition test. Further parameters are shown in Table 2.
Figure 5. Effect of NH4Cl treatment and low vitamin D diet on performance in Light Dark Box.
(A–C) Photograph of the Light Dark Box with representative tracings of an untreated male wild-type mouse (WT) (A), a male, klotho-hypomorphic mouse (kl/kl) treated with 280 mM NH4Cl solution (B) and a male, NH4Cl treated kl/kl mouse under vitamin D deficient diet (C). (D–K) Arithmetic means ± SEM (n = 12–30) of (D) time spent in the hidden area of the Light Dark Box arena, (E) number of visits in the light area, (F) number of rearings in the light area, (G) average rearing time in the light area, (H) distance travelled in the light area, (I) time spent in the entrance area, (J) number of rearings in the hidden area, (K) average rearing time in the hidden area of wild-type mice (WT, white bars) and kl/kl mice (kl/kl black bars) either untreated (Control, left bars), treated with 280 mM NH4Cl solution (NH4Cl, middle bars) or treated with NH4Cl and a vitamin D deficient diet (LVD, right bars). **(p < 0.01), ***(p < 0.001) indicates statistically significant differences from untreated wild-type mice (Control); ##(p < 0.01), ###(p < 0.001) indicates statistically significant differences from NH4Cl treated kl/kl mice. (ANOVA).
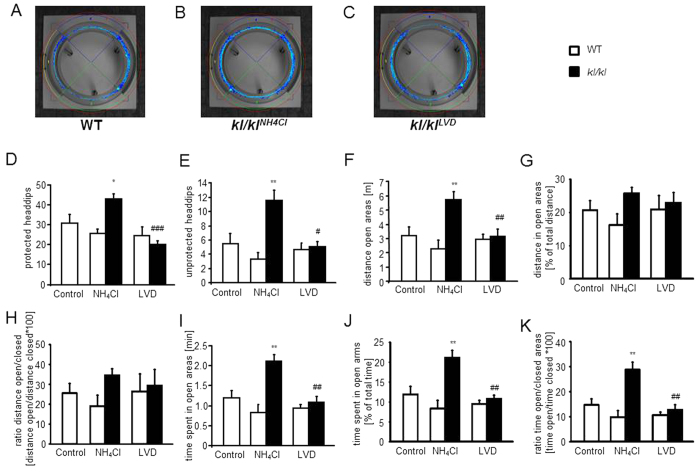
Figure 6. Effect of NH4Cl treatment and low vitamin D diet on performance in O-Maze.
(A–C) Photograph of the O-Maze with representative tracings of an untreated, male wild-type mouse (WT) (A), a male klotho-hypomorphic mouse (kl/kl) treated with 280 mM NH4Cl solution (B) and a male, NH4Cl treated kl/kl mouse under vitamin D deficient diet (C). (D–K) Arithmetic means ± SEM (n = 12–30) of (D) number of protected headdips, (E) number of unprotected headdips, (F) distance travelled in the open areas, (G) distance travelled in the open areas as percentage of total distance, (H) ratio of distance travelled in open areas and distance travelled in closed areas, (I) time spent in open areas, (J) time spent in the open arms as percentage of total time, and (K) ratio of time spent in open arms and time spent in closed arms of wild-type mice (WT, white bars) and kl/kl mice (kl/kl black bars) either untreated (Control, left bar), treated with 280 mM NH4Cl solution (NH4Cl, middle bars) or treated with NH4Cl and a vitamin D deficient diet (LVD, right bars). *(p < 0.05), **(p < 0.01) indicates statistically significant differences from untreated wild-type mice (Control); #(p < 0.05), ##(p < 0.01), ###(p < 0.001) indicates statistically significant differences from NH4Cl treated kl/kl mice. (ANOVA).
Table 2. Synopsis of behavioral parameters in the Light Dark Box test (arithmetic means ± SEM).
| parameter | WT | WTNH4Cl | kl/klNH4Cl | WTLVD | kl/klLVD | statistics |
|---|---|---|---|---|---|---|
| time spent in light area [min] | 1.28 ± 0.26 | 1.45 ± 0.28 | 4.08 ± 0.45 | 1.26 ± 0.25 | 1.57 ± 0.25 | P < 0.0001 nonparametric ANOVA |
| average speed [cm/s] | 2.43 ± 0.29 | 2.45 ± 0.24 | 4.45 ± 0.32 | 2.48 ± 0.27 | 2.37 ± 0.29 | P < 0.0001 nonparametric ANOVA |
The recorded tracings of the O-Maze test also revealed increased activity in the NH4Cl treated kl/kl mice (Fig. 6A–C). They showed significantly more protected and unprotected headdips than wild-type mice (Fig. 6D,E). NH4Cl treated kl/kl mice travelled larger distances in the open areas (Fig. 6F), a differences, however, not reaching statistical significance when normalized to the total distance travelled (Fig. 6G). The ratio between distance travelled in open areas and distance travelled in closed areas tended to be higher in kl/kl mice, a difference, however, again not reaching statistical significance (Fig. 6H). NH4Cl treated kl/kl mice spent more time in the open areas (Fig. 6I), an effect also significant when standardized to the total time spent in the open areas (Fig. 6J). Similarly the ratio of time spent in the open areas and the time spent in closed areas was significantly higher in NH4Cl treated kl/kl mice (Fig. 6K) as compared to wild-type mice. Treatment with LDV abrogated the abnormal behavioral phenotype of kl/kl mice. In the O-Maze test neither NH4Cl treatment nor LVD had an influence on the behavior of wild-type mice. Further parameters are shown in Table 3.
Table 3. Synopsis of behavioral parameters in the O Maze test (arithmetic means ± SEM).
| parameter | WT | WTNH4Cl | kl/klNH4Cl | WTLVD | kl/klLVD | statistics |
|---|---|---|---|---|---|---|
| number of visits in open areas | 24.27 ± 4.25 | 20.27 ± 4.86 | 41.97 ± 5.08 | 19.73 ± 4.09 | 23.95 ± 4.22 | P = 0.0034 ANOVA |
| distance in closed areas [m] | 12.41 ± 0.82 | 11.81 ± 0.77 | 16.58 ± 0.77 | 11.01 ± 1.35 | 10.65 ± 0.79 | P < 0.0001 ANOVA |
| total distance [m] | 15.45 ± 1.22 | 14.08 ± 1.15 | 22.33 ± 1.00 | 14.02 ± 1.33 | 13.81 ± 0.96 | P < 0.0001 ANOVA |
| average speed [cm/s] | 2.74 ± 0.30 | 2.37 ± 0.22 | 3.63 ± 0.18 | 1.99 ± 0.14 | 2.40 ± 0.17 | P < 0.0001 nonparametric ANOVA |
In the Forced Swimming Test the NH4Cl treated kl/kl mice spent significantly less time floating on the surface of the water than wild-type mice (Fig. 7). LVD again abrogated the differences of time floating between kl/kl mice and wild-type mice (Fig. 7). Neither of the treatments had an effect on behavior of wild-type mice in the Forced Swimming Test.
Figure 7. Effect of NH4Cl treatment and low vitamin D diet on performance in Forced Swimming Test.
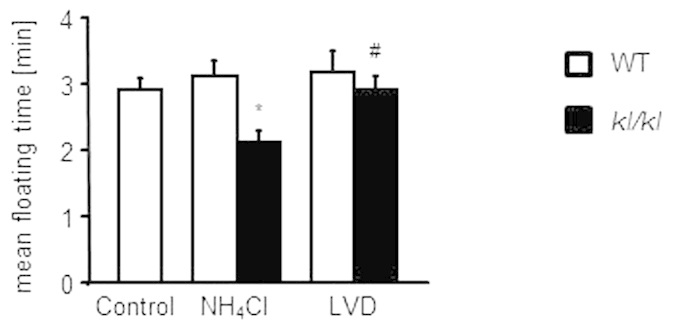
Arithmetic means ± SEM (n = 12–30) of floating time of wild-type mice (WT, white bars) and kl/kl mice (kl/kl black bars) either untreated (Control, left bar), treated with 280 mM NH4Cl solution (NH4Cl, middle bars) or treated with NH4Cl and a vitamin D deficient diet (LVD, right bars). *(p < 0.05) indicates statistically significant differences from untreated wild-type mice (Control); #(p < 0.05) indicates statistically significant differences from NH4Cl treated kl/kl mice. (ANOVA).
Gender differences in the behavioral tests are apparent from Tables 4, 5, 6, 7.
Table 4. Differences between male and female mice in the open field test (arithmetic means ± SEM).
| parameter | WT | WTNH4Cl | kl/klNH4Cl | WTLVD | kl/klLVD | |
|---|---|---|---|---|---|---|
| speed [cm/s] | ♂ | 3.00 ± 0.58 | 2.75 ± 0.39 | 4.86 ± 0.47 | 2.37 ± 0.68 | 2.51 ± 0.40 |
| ♀ | 2.55 ± 0.41 | 2.47 ± 0.58 | 4.11 ± 0.29 | 2.53 ± 0.64 | 2.24 ± 0.45 | |
| ttest | 0.5266 | 0.5381 | 0.1651 | 0.8619 | 0.6612 | |
| total distance [m] | ♂ | 37.71 ± 6.95 | 36.03 ± 5.58 | 61.13 ± 6.53 | 35.91 ± 7.44 | 33.81 ± 6.45 |
| ♀ | 33.09 ± 3.30 | 32.27 ± 9.82 | 56.84 ± 5.37 | 34.74 ± 6.83 | 35.34 ± 7.33 | |
| ttest | 0.5553 | 0.9000 | 0.6128 | 0.9103 | 0.8762 | |
| time in border area [min] | ♂ | 29.66 ± 0.10 | 29.48 ± 0.40 | 27.47 ± 0.75 | 29.28 ± 0.32 | 29.86 ± 0.05 |
| ♀ | 29.35 ± 0.39 | 29.62 ± 0.20 | 26.43 ± 0.66 | 28.45 ± 0.99 | 29.71 ± 0.15 | |
| ttest | 0.4611 | 0.8112 | 0.3062 | 0.4423 | 0.3597 | |
| distance in border area [m] | ♂ | 36.31 ± 6.39 | 34.29 ± 5.93 | 58.04 ± 5.78 | 34.40 ± 4.56 | 33.03 ± 6.28 |
| ♀ | 34.50 ± 3.45 | 31.39 ± 9.61 | 51.21 ± 4.70 | 34.40 ± 6.77 | 34.04 ± 6.77 | |
| ttest | 0.5153 | 0.9585 | 0.3627 | 1 | 0.9141 | |
| time spent in corners [min] | ♂ | 24.03 ± 1.62 | 22.33 ± 2.05 | 18.96 ± 0.88 | 23.24 ± 2.32 | 25.41 ± 1.12 |
| ♀ | 23.97 ± 1.58 | 24.16 ± 1.61 | 17.94 ± 1.62 | 24.91 ± 2.39 | 23.95 ± 2.13 | |
| ttest | 0.9805 | 0.3798 | 0.6167 | 0.6276 | 0.5417 | |
| distance in center area [m] | ♂ | 1.48 ± 0.69 | 1.74 ± 1.28 | 5.63 ± 1.32 | 1.35 ± 1.26 | 0.77 ± 0.39 |
| ♀ | 1.60 ± 0.86 | 0.87 ± 0.48 | 3.09 ± 0.85 | 0.49 ± 0.17 | 1.30 ± 0.68 | |
| ttest | 0.8627 | 0.5802 | 0.1446 | 0.5154 | 0.4969 | |
| visits in center area | ♂ | 5.73 ± 1.75 | 5.13 ± 2.75 | 23.39 ± 6.39 | 9.67 ± 7.97 | 5.82 ± 2.68 |
| ♀ | 10.18 ± 4.68 | 7.25 ± 3.44 | 36.53 ± 8.42 | 3.00 ± 1.75 | 9.60 ± 4.74 | |
| ttest | 0.3837 | 0.6284 | 0.2482 | 0.4329 | 0.4854 | |
| time in center area [min] | ♂ | 0.35 ± 0.10 | 0.53 ± 0.40 | 2.53 ± 0.75 | 0.72 ± 0.32 | 0.14 ± 0.05 |
| ♀ | 0.65 ± 0.39 | 0.39 ± 0.20 | 3.57 ± 0.66 | 1.56 ± 0.99 | 0.29 ± 0.15 | |
| ttest | 0.4611 | 0.8112 | 0.3062 | 0.4423 | 0.3597 | |
| number of rearings in border area | ♂ | 96.73 ± 19.95 | 68.50 ± 15.56 | 154.69 ± 15.97 | 68.50 ± 24.14 | 86.82 ± 15.65 |
| ♀ | 71.64 ± 20.00 | 69.75 ± 30.48 | 126.18 ± 14.07 | 51.83 ± 14.50 | 68.50 ± 16.09 | |
| ttest | 0.3850 | 0.9813 | 0.1916 | 0.5671 | 0.4251 | |
| rearing time in border area [min] | ♂ | 3.22 ± 0.81 | 3.18 ± 1.00 | 7.56 ± 0.91 | 2.59 ± 1.14 | 3.10 ± 0.67 |
| ♀ | 2.83 ± 0.91 | 2.33 ± 0.96 | 6.37 ± 0.63 | 2.56 ± 1.13 | 2.52 ± 0.79 | |
| ttest | 0.7506 | 0.5128 | 0.1016 | 0.9865 | 0.5800 | |
| number of rearings in center area | ♂ | 1.46 ± 0.78 | 1.75 ± 1.05 | 5.69 ± 1.67 | 1.67 ± 1.31 | 0.27 ± 0.20 |
| ♀ | 1.27 ± 0.68 | 0.25 ± 0.29 | 9.18 ± 2.59 | 1.17 ± 0.83 | 1.4 ± 0.95 | |
| ttest | 0.8618 | 0.4543 | 0.3019 | 0.7538 | 0.2364 | |
| rearing time in center area [min] | ♂ | 1.36 ± 0.63 | 0.28 ± 0.20 | 6.92 ± 2.05 | 0.18 ± 0.16 | 0.34 ± 0.23 |
| ♀ | 1.10 ± 0.76 | 0.40 ± 0.36 | 13.91 ± 5.03 | 0.18 ± 0.17 | 0.99 ± 0.86 | |
| ttest | 0.7945 | 0.7266 | 0.2573 | 1 | 0.4501 |
Table 5. Differences between male and female mice in the Light Dark Box test (arithmetic means ± SEM).
| parameter | WT | WTNH4Cl | kl/klNH4Cl | WTLVD | kl/klLVD | |
|---|---|---|---|---|---|---|
| time in hidden area [min] | ♂ | 8.67 ± 0.48 | 8.71 ± 0.29 | 6.26 ± 0.57 | 8.56 ± 0.38 | 8.47 ± 0.40 |
| ♀ | 8.79 ± 0.23 | 8.37 ± 0.53 | 5.66 ± 0.67 | 8.91 ± 0.35 | 8.39 ± 0.32 | |
| ttest | 0.8226 | 0.5645 | 0.5234 | 0.5208 | 0.8785 | |
| visits in light area | ♂ | 13.91 ± 3.69 | 15.75 ± 5.23 | 26.92 ± 3.69 | 11.67 ± 3.48 | 14.36 ± 3.92 |
| ♀ | 17.10 ± 5.23 | 17.57 ± 5.75 | 32.88 ± 4.04 | 15.67 ± 6.56 | 15.40 ± 4.16 | |
| ttest | 0.6217 | 0.8180 | 0.3000 | 0.6017 | 0.8579 | |
| number of rearings in light area | ♂ | 3.55 ± 1.06 | 3.63 ± 1.43 | 16.12 ± 3.32 | 3.50 ± 1.63 | 3.00 ± 0.62 |
| ♀ | 4.27 ± 1.18 | 3.00 ± 1.07 | 13.54 ± 2.85 | 1.67 ± 0.72 | 3.10 ± 1.34 | |
| ttest | 0.6516 | 0.7377 | 0.5747 | 0.3268 | 0.9449 | |
| rearing time light area [s] | ♂ | 8.69 ± 4.40 | 5.37 ± 2.14 | 33.31 ± 6.41 | 5.66 ± 2.86 | 4.34 ± 1.31 |
| ♀ | 7.20 ± 2.33 | 8.51 ± 2.45 | 33.32 ± 11.67 | 6.06 ± 2.41 | 8.13 ± 2.86 | |
| ttest | 0.7673 | 0.3493 | 0.9985 | 0.9166 | 0.2280 | |
| distance in light area [m] | ♂ | 2.51 ± 0.55 | 2.27 ± 0.77 | 8.870 ± 1.05 | 1.77 ± 0.57 | 1.90 ± 0.45 |
| ♀ | 2.17 ± 0.39 | 2.33 ± 1.09 | 10.27 ± 1.58 | 2.33 ± 0.96 | 1.84 ± 0.40 | |
| ttest | 0.6217 | 0.9659 | 0.4444 | 0.6257 | 0.9242 | |
| time in entrance area [min] | ♂ | 1.55 ± 0.93 | 3.46 ± 1.17 | 9.24 ± 1.31 | 1.39 ± 0.83 | 1.05 ± 0.75 |
| ♀ | 1.99 ± 0.03 | 2.71 ± 1.32 | 8.25 ± 1.44 | 1.97 ± 1.66 | 0.57 ± 0.35 | |
| ttest | 0.7532 | 0.6746 | 0.6248 | 0.5077 | 0.6521 | |
| number of rearings in hidden area | ♂ | 21.33 ± 6.29 | 20.38 ± 7.01 | 41.35 ± 5.39 | 16.67 ± 5.10 | 15.46 ± 5.42 |
| ♀ | 19.82 ± 6.27 | 22.86 ± 9.45 | 41.52 ± 6.01 | 18.67 ± 10.27 | 14.00 ± 4.70 | |
| ttest | 0.8665 | 0.8335 | 0.9444 | 0.8650 | 0.8430 | |
| rearing time hidden area [s] | ♂ | 5.51 ± 1.07 | 3.91 ± 1.50 | 11.19 ± 0.85 | 6.34 ± 1.82 | 5.24 ± 1.32 |
| ♀ | 5.79 ± 1.42 | 4.41 ± 1.86 | 11.40 ± 1.07 | 6.11 ± 2.70 | 6.34 ± 1.59 | |
| ttest | 0.7965 | 0.8361 | 0.8345 | 0.9474 | 0.5904 | |
| time in light area [min] | ♂ | 1.34 ± 0.48 | 1.29 ± 0.29 | 3.74 ± 0.57 | 1.44 ± 0.38 | 1.53 ± 0.40 |
| ♀ | 1.22 ± 0.23 | 1.64 ± 0.53 | 4.34 ± 0,67 | 1.09 ± 0.35 | 1.62 ± 0.32 | |
| ttest | 0.8226 | 0.5645 | 0.5234 | 0.5208 | 0.8785 | |
| speed [cm/s] | ♂ | 3.45 ± 0.43 | 2.41 ± 0.36 | 4.53 ± 0.38 | 2.95 ± 0.36 | 2.23 ± 0.36 |
| ♀ | 2.40 ± 0.41 | 2.49 ± 0.33 | 4.39 ± 0.50 | 2.03 ± 0.34 | 2.52 ± 0.48 | |
| ttest | 0.9335 | 0.8677 | 0.8353 | 0.0948 | 0.6244 |
Table 6. Differences between male and female mice in the O Maze test (arithmetic means ± SEM).
| parameter | WT | WTNH4Cl | kl/klNH4Cl | WTLVD | kl/klLVD | |
|---|---|---|---|---|---|---|
| protected headdips | ♂ | 27.00 ± 6.15 | 23.88 ± 2.86 | 45.65 ± 3.65 | 22.83 ± 5.06 | 20.50 ± 2.66 |
| ♀ | 34.27 ± 6.42 | 29.75 ± 3.76 | 41.00 ± 3.77 | 26.50 ± 7.21 | 19.82 ± 2.93 | |
| ttest | 0.4231 | 0.4412 | 0.4735 | 0.6858 | 0.8660 | |
| unprotected headdips | ♂ | 4.36 ± 1.50 | 3.50 ± 1.35 | 11.77 ± 1.71 | 5.67 ± 1.45 | 5.40 ± 1.32 |
| ♀ | 6.64 ± 2.44 | 2.75 ± 1.30 | 11.47 ± 2.14 | 3.67 ± 1.17 | 4.73 ± 0.95 | |
| ttest | 0.4365 | 0.8528 | 0.9350 | 0.3095 | 0.6799 | |
| visits in open arms | ♂ | 27.27 ± 7.36 | 18.63 ± 7.10 | 44.14 ± 5.10 | 19.50 ± 3.23 | 22.00 ± 4.69 |
| ♀ | 21.27 ± 4.46 | 19.88 ± 7.06 | 39.59 ± 6.95 | 20.00 ± 8.74 | 25.73 ± 7.02 | |
| ttest | 0.4937 | 0.7324 | 0.5636 | 0.9552 | 0.6705 | |
| distance in open arms [m] | ♂ | 3.46 ± 1.02 | 2.24 ± 0.97 | 6.03 ± 0.75 | 3.28 ± 0.41 | 2.98 ± 0.82 |
| ♀ | 2.96 ± 0.73 | 2.03 ± 0.82 | 5.53 ± 0.76 | 2.57 ± 0.62 | 3.32 ± 0.66 | |
| ttest | 0.6895 | 0.9564 | 0.6933 | 0.3616 | 0.7462 | |
| time in open arms [min] | ♂ | 1.11 ± 0.30 | 0.73 ± 0.27 | 2.02 ± 0.26 | 0.94 ± 0.12 | 1.12 ± 0.21 |
| ♀ | 1.27 ± 0.25 | 0.82 ± 0.33 | 2.18 ± 0.25 | 0.96 ± 0.15 | 1.03 ± 0.24 | |
| ttest | 0.6783 | 0.6649 | 0.6700 | 0.9447 | 0.7722 | |
| distance in closed arms [m] | ♂ | 12.42 ± 1.00 | 12.07 ± 1.03 | 15.65 ± 0.76 | 11.69 ± 2.32 | 10.45 ± 1.26 |
| ♀ | 12.40 ± 1.34 | 11.26 ± 1.25 | 17.92 ± 1.23 | 10.49 ± 1.56 | 10.84 ± 1.03 | |
| ttest | 0.9898 | 0.7379 | 0.3923 | 0.6770 | 0.8103 | |
| total distance [m] | ♂ | 15.56 ± 1.88 | 14.30 ± 1.62 | 21.68 ± 1.22 | 14.92 ± 2.31 | 13.42 ± 1.64 |
| ♀ | 15.35 ± 1.63 | 13.30 ± 1.76 | 22.82 ± 1.51 | 13.12 ± 1.46 | 14.16 ± 1.13 | |
| ttest | 0.9336 | 0.8454 | 0.6513 | 0.5265 | 0.7114 | |
| speed [cm/s] | ♂ | 2.29 ± 0.30 | 2.22 ± 0.29 | 3.67 ± 0.20 | 2.01 ± 0.29 | 2.47 ± 0.25 |
| ♀ | 3.82 ± 0.49 | 2.43 ± 0.34 | 3.63 ± 0.29 | 1.97 ± 0.09 | 2.33 ± 0.23 | |
| ttest | 0.1353 | 0.4781 | 0.8705 | 0.8882 | 0.6823 | |
| time in open arms [%] | ♂ | 11.06 ± 2.99 | 7.34 ± 2.74 | 20.17 ± 2.55 | 9.41 ± 1.23 | 11.22 ± 2.10 |
| ♀ | 12.70 ± 2.51 | 8.17 ± 3.33 | 21.76 ± 2.47 | 9.55 ± 1.46 | 10.28 ± 2.39 | |
| ttest | 0.6783 | 0.6649 | 0.6696 | 0.9447 | 0.7721 | |
| time open/closed arms *100 | ♂ | 13.75 ± 3.94 | 8.67 ± 3.57 | 26.89 ± 4.27 | 10.50 ± 1.54 | 13.21 ± 2.68 |
| ♀ | 15.56 ± 3.54 | 9.85 ± 4.48 | 30.25 ± 4.88 | 10.70 ± 1.78 | 12.35 ± 3.33 | |
| ttest | 0.7361 | 0.6674 | 0.6428 | 0.9328 | 0.8439 | |
| distance open arms [%] | ♂ | 22.24 ± 4.33 | 15.62 ± 5.12 | 27.81 ± 2.83 | 22.01 ± 2.73 | 22.18 ± 4.81 |
| ♀ | 19.26 ± 3.96 | 15.29 ± 4.79 | 24.21 ± 2.24 | 19.60 ± 8.08 | 23.45 ± 4.34 | |
| ttest | 0.6184 | 0.7854 | 0.5286 | 0.4239 | 0.6645 | |
| distance open/closed arms *100 | ♂ | 27.87 ± 6.49 | 18.52 ± 9.19 | 38.53 ± 5.27 | 28.09 ± 4.35 | 28.50 ± 9.67 |
| ♀ | 23.86 ± 6.95 | 18.05 ± 7.11 | 31.95 ± 4.30 | 24.52 ± 7.26 | 30.64 ± 12.81 | |
| ttest | 0.6544 | 0.9178 | 0.4911 | 0.2703 | 0.6927 |
Table 7. Differences between male and female mice in the Forced Swimming test (arithmetic means ± SEM).
| parameter | WT | WTNH4Cl | kl/klNH4Cl | WTLVD | kl/klLVD | |
|---|---|---|---|---|---|---|
| mean floating time [min] | ♂ | 3.10 ± 0.23 | 3.01 ± 0.33 | 2.20 ± 0.36 | 2.93 ± 0.61 | 2.81 ± 0.30 |
| ♀ | 2.70 ± 0.30 | 3.15 ± 0.35 | 2.02 ± 0.24 | 3.40 ± 0.29 | 3.01 ± 0.26 | |
| ttest | 0.3018 | 0.9084 | 0.6702 | 0.5035 | 0.7693 |
Discussion
The present observations reveal a dramatic difference between NH4Cl treated kl/kl mice and NH4Cl treated wild-type mice in several behavioral tests measuring exploratory behavior and anxiety. The difference is abrogated by vitamin D deficient diet, indicating that the excessive 1,25(OH)2D3 formation in kl/kl mice accounted for the observed differences between NH4Cl treated kl/kl mice and wild-type mice. The observations do not rule out more direct effects of klotho deficiency but indicate that the observed differences are in large part explained by excessive formation of 1,25(OH)2D3. The effects are,however, not necessarily due to a direct effect of 1,25(OH)2D3 on neuronal function and behavior.
NH4Cl treatment had no significant effect in wildtype mice indicating that the NH4Cl treatment does not alter any of the measured parameters on its own. Similar to earlier observations54, NH4Cl treatment did not appreciably influence plasma 1,25(OH)2D3, Ca2+ and phosphate concentrations. NH4Cl interferes with osteogenic signaling thus preventing the disastrous tissue calcification in kl/kl mice54.
The present observations underscore the powerful direct or indirect influence of 1,25(OH)2D3 on the brain, which presumably accounts for the various cerebral effects of vitamin D deficiency. Decreased serum levels of the 1,25(OH)2D3 precursor 25(OH)D3 were found in patients suffering from depression56,57. Conversely, vitamin D supplementation has been reported to counteract depressive symptoms51,52,53. Vitamin D deficiency during brain development is apparently a risk factor for the development of schizophrenia, a condition associated with enhanced neuroticism and decreased extraversion58. Conversely vitamin D supplementation decreases the risk to develop psychotic-like symptoms44.
The present observations did not address the mechanisms underlying the altered behavior of kl/kl mice Several mechanisms have been suggested to participate in the cerebral effects of 1,25(OH)2D3, including antioxidant effects, inhibition of inflammation and vascular injury, stimulation of neurotrophins and improvement of metabolic and cardiovascular function30. Vitamin D deficiency has been suggested to modify cellular development, dopamine metabolism, and brain morphology59. In theory, 1,25(OH)2D3 could affect neuronal function by influencing neuronal or glial cytosolic Ca2+ activity60,61,62. 1,25(OH)2D3 may interfere with the cerebral action of glucocorticoids, which are involved in the development of major depression63. 1,25(OH)2D3 dependent calcium binding protein has been observed in nuclei influencing the pineal gland64 and vitamin D3 deficiency may contribute to the desynchronisation in seasonal affective disorders65.
In wild type animals, dietary vitamin D does not necessarily influence 1,25(OH)2D3 concentration, as 1α-25-hydroxyvitamin D hydroxylase and thus 1,25(OH)2D3 formation is under tight regulation by FGF23 and klotho1. Both, FGF23 and klotho expression are stimulated by 1,25(OH)2D3 and thus 1,25(OH)2D3 formation is limited by negative feedback regulation1,66,67. In the presence of klotho and FGF23, the diet becomes critically important only during vitamin D deficiency. The negative feedback is missing in kl/kl mice and in those mice the formation of 1,25(OH)2D3 is a function of dietary vitamin D even at excessive 1,25(OH)2D3 concentrations. In view of the present observation any regulator of FGF23 and/or klotho expression or any regulator of 1α-25-hydroxyvitamin D hydroxylase may be expected to impact on exploratory behavior. In this respect it is noteworthy that klotho is downregulated and 1,25(OH)2D3 formation up-regulated by dehydration68 and parathyroid hormone69, FGF23 is up-regulated and 1,25(OH)2D3 formation downregulated by lithium70,71 and 1α-25-hydroxyvitamin D hydroxylase inhibited by CO-releasing molecule CORM-272.
In conclusion, the present observations reveal that disruption of klotho dependent inhibition of 1α-25-hydroxyvitamin D hydroxylase and thus excessive 1,25(OH)2D3 formation leads to profound stimulation of exploratory behavior.
Materials and Methods
Mice
All animal experiments were conducted according to the German law for the welfare of animals and were approved by local authorities (Regierungspräsidium Tübingen). The methods were carried out in accordance with the approved guidelines. The original klotho-hypomorphic (kl/kl) mice were generated by Kuro-o et al.19. In an attempt to insert the rabbit type-I Na+/H+ exchanger via a standard microinjection method into the genome of the mice, the promoter region of the klotho gene was disrupted. The mice do not express the expected transgene but cross-breeding of the heterozygous mice resulted in animals homozygous for the insertional mutation and a severe aging-like phenotype. RT-PCR analysis revealed that klotho is still expressed to a low extent and therefore the mice are referred to as klotho-hypomorphic mice. The original kl/kl mice had a mixed background of C57BL/6J and C3H/J. Congenic strains of kl/kl mice were produced by repeated backcrosses (>9 generations) to the 129Sv inbred strain and used in this study. The mice were generated from heterozygous breedings, and male and female kl/kl mice were compared to male and female wild-type (WT) mice54. The animals were housed in groups of 2–6 mice per cage. The temperature was set to 22 ± 2 °C and the humidity was 55 ± 10%. The mice had access to either tap water or a solution of NH4Cl in tap water (280 mM) ad libitum and were fed either a standard chow diet (Altromin C1000) or a vitamin D deficient diet (Altromin C1017). The lifelong NH4Cl treatment started with the mating of the parental generation and was maintained from pregnancy until the end of the experiment. The animals were maintained at a 12:12 h inverted cycle with lights on between 7 p.m. and 7 a.m. Behavioral testing occurred between 7 a.m. and 7 p.m. Only one type of experiment was done on the same day and the home cage rack was brought to the test room at least 30 min before each experiment and dry surfaces of apparatus were thoroughly cleaned with 70% ethanol before releasing the animal. Experiments extended over a total of 4 months, the age was 10–11 weeks at the beginning and 6 months at the end of the experiments. Untreated kl/kl mice could not be used in the behavioral tests because of their poor physical condition (Table 8).
Table 8. Number of animals used in the experiment.
| total number of animals | number of ♂ | number of ♀ | |
|---|---|---|---|
| kl/klNH4Cl | 30 | 13 | 17 |
| kl/klLVD | 21 | 10 | 11 |
| WTNH4Cl | 15 | 8 | 7 |
| WT | 22 | 11 | 11 |
| WTLVD | 12 | 6 | 6 |
Blood chemistry
Blood specimens were obtained the day after the completion of the behavioral studies between 4–6 p.m. by puncturing the retro-orbital plexus. Plasma phosphate and calcium concentrations were determined utilizing a photometric method (FUJI FDC 3500i, Sysmex, Norsted, Germany). The plasma 1,25(OH)2-vitamin D3 (IDS, Boldon, UK), corticosterone (DRG, Marburg, Germany) and Pai 1 (Molecular Innovations, Novi, USA) concentrations were measured by ELISA.
Behavioral studies
For data acquisition, animals were video tracked by the camera 302050-SW-KIT-2-CAM at a resolution of 0.62 to 0.72 pixel (TSE-Systems, Bad Homburg, Germany). Raw data were transferred to Microsoft Excel for further analysis.
Tests were done in the following order: Open-field, light-dark box, O-maze, and forced swimming test. Experiments were performed with diffuse indirect room light produced by dimmable bulbs, adjusted to yield approximately 12 lux in the center of the experimental arena. The only exception was the light-dark-box test where full room light was switched on to obtain approximately 500 lux in the lit chamber. The experiments have been performed as described previously in detail73.
For open-field the quadratic open-field arena had a side length of 50 cm, a white plastic floor, and 40 cm high sidewalls made of white polypropylene. Rearing behavior was assessed by a metallic frame surrounding the arena generating a photoelectric barrier (vertical activity). A border area was considered with a width of 10 cm from the wall dividing the arena in a center and a border area. Each subject was released near the wall and observed for 30 min.
For the light-dark box a 40 cm black acryl box was inserted in the open-field arena, which covered 33% of the surface area. An aperture of 10 cm length and 11 cm height with rounded down corners led into the dark box. Each subject was released in the the same corner of the illuminated compartment and observed for 10 min74.
For O-maze a 5.5 cm wide annular runway was constructed using grey plastic. It had an outer diameter of 46 cm and was placed inside the above open-field arena 40 cm above the floor73,75. The two opposing 90° closed sectors were protected by 11 cm high inner and outer walls of grey polyvinyl-chloride, while the remaining two open sectors had no walls. Animals were released in one of the closed sectors and observed for 10 min. Over time, the animal’s exploratory drive competes with their natural avoidance of heights. The mice start to explore the cliff by dipping their heads. As an additional parameter the number of headdips was counted. Differentiated were protected headdips, when the headdips occurred with the mice still in the protected zone, and the unprotected headdips, when the mice left the protected zone completely to explore the cliff. The numbers of headdips were counted manually.
In the forced swimming test mice were placed in a container filled with water of temperatures between 24 and 26 °C. The diameter of the container was 20 cm. The mice were placed in the water without being able to touch the ground. Mice were observed during 6 min and the time they spent without movement, called floating, was recorded76.
Statistics
Data are provided as means ± SEM, n represents the number of independent experiments. All data were tested for significance using parametric ANOVA followed by Tukey-Kramer Multiple Comparisons Test in case of equal standard deviations (tested with Bartlett’s) or nonparametric ANOVA (Kruskal-Wallis Test) in case of significant differences in standard deviations followed by Dunn’s Multiple Comparison Test. Only results with p < 0.05 were considered statistically significant. The statistical calculations were performed utilizing the Graph Pad Prism software.
Additional Information
How to cite this article: Leibrock, C. B. et al. 1,25(OH)2D3 dependent overt hyperactivity phenotype in klotho-hypomorphic mice. Sci. Rep. 6, 24879; doi: 10.1038/srep24879 (2016).
Acknowledgments
The authors acknowledge the technical assistance of Elfriede Faber and the meticulous preparation of the manuscript by Tanja Loch. The study was supported by the Deutsche Forschungsgemeinschaft, DFG 315/15-1.
Footnotes
Author Contributions C.B.L., J.V., M.K. and U.E.L. performed experiments and analyzed data. F.L. and U.E.L. wrote the paper. All authors reviewed the manuscript and approved of submission.
References
- Kuro-o M. Klotho, phosphate and FGF-23 in ageing and disturbed mineral metabolism. Nat Rev Nephrol 9, 650–660 (2013). [DOI] [PubMed] [Google Scholar]
- Topala C. N., Bindels R. J. & Hoenderop J. G. Regulation of the epithelial calcium channel TRPV5 by extracellular factors. Curr Opin Nephrol Hypertens 16, 319–324 (2007). [DOI] [PubMed] [Google Scholar]
- Hu M. C. et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 24, 3438–3450 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermaku-Sopjani M. et al. Downregulation of NaPi-IIa and NaPi-IIb Na-coupled phosphate transporters by coexpression of Klotho. Cell Physiol Biochem 28, 251–258 (2011). [DOI] [PubMed] [Google Scholar]
- Imura A. et al. alpha-Klotho as a regulator of calcium homeostasis. Science 316, 1615–1618 (2007). [DOI] [PubMed] [Google Scholar]
- Sopjani M. et al. Regulation of the Na + /K + ATPase by Klotho. FEBS Lett 585, 1759–1764 (2011). [DOI] [PubMed] [Google Scholar]
- Shumilina E. et al. Altered regulation of cytosolic Ca(2)(+) concentration in dendritic cells from klotho hypomorphic mice. Am J Physiol Cell Physiol 305, C70–77 (2013). [DOI] [PubMed] [Google Scholar]
- Borst O. et al. 1,25(OH)2 vitamin D3-dependent inhibition of platelet Ca2 + signaling and thrombus formation in klotho-deficient mice. FASEB J 28, 2108–2119 (2014). [DOI] [PubMed] [Google Scholar]
- Almilaji A. et al. Upregulation of KCNQ1/KCNE1 K + channels by Klotho. Channels (Austin) 8, 222–229 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. Regulation of ion channels by secreted Klotho. Adv Exp Med Biol 728, 100–106 (2012). [DOI] [PubMed] [Google Scholar]
- Munoz C. et al. Klotho sensitivity of the hERG channel. FEBS Lett 587, 1663–1668 (2013). [DOI] [PubMed] [Google Scholar]
- Almilaji A. et al. Regulation of the voltage gated K channel Kv1.3 by recombinant human klotho protein. Kidney Blood Press Res 39, 609–622 (2014). [DOI] [PubMed] [Google Scholar]
- Almilaji A. et al. Upregulation of the creatine transporter Slc6A8 by Klotho. Kidney Blood Press Res 39, 516–525 (2014). [DOI] [PubMed] [Google Scholar]
- Almilaji A. et al. Klotho sensitivity of the neuronal excitatory amino acid transporters EAAT3 and EAAT4. PLos One 8, e70988 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warsi J., Abousaab A. & Lang F. Up-Regulation of Excitatory Amino Acid Transporters EAAT1 and EAAT2 by ß-Klotho. Neurosignals 23, 59–70 (2015). [DOI] [PubMed] [Google Scholar]
- Banerjee S. et al. Klotho ameliorates chemically induced endoplasmic reticulum (ER) stress signaling. Cell Physiol Biochem 31, 659–672 (2013). [DOI] [PubMed] [Google Scholar]
- Izquierdo M. C. et al. Klotho, phosphate and inflammation/ageing in chronic kidney disease. Nephrol Dial Transplant 27 Suppl 4, iv6–10 (2012). [DOI] [PubMed] [Google Scholar]
- Kuro-o M. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51 (1997). [DOI] [PubMed] [Google Scholar]
- Kurosu H. et al. Suppression of aging in mice by the hormone Klotho. Science 309, 1829–1833 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Invidia L. et al. The frequency of Klotho KL-VS polymorphism in a large Italian population, from young subjects to centenarians, suggests the presence of specific time windows for its effect. Biogerontology 11, 67–73 (2010). [DOI] [PubMed] [Google Scholar]
- Nitta K., Okada K., Yanai M. & Takahashi S. Aging and chronic kidney disease. Kidney Blood Press Res 38, 109–120 (2013). [DOI] [PubMed] [Google Scholar]
- Staude H., Jeske S., Schmitz K., Warncke G. & Fischer D. C. Cardiovascular risk and mineral bone disorder in patients with chronic kidney disease. Kidney Blood Press Res 37, 68–83 (2013). [DOI] [PubMed] [Google Scholar]
- Dubal D. B. et al. Life extension factor klotho enhances cognition. Cell Rep 7, 1065–1076 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold P. W., Licinio J. & Pavlatou M. G. Pathological parainflammation and endoplasmic reticulum stress in depression: potential translational targets through the CNS insulin, klotho and PPAR-gamma systems. Mol Psychiatry 18, 154–165 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K. Frontiers of model animals for neuroscience: two prosperous aging model animals for promoting neuroscience research. Exp Anim 62, 275–280 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. J. et al. Inactivation of JAK2/STAT3 signaling axis and downregulation of M1 mAChR cause cognitive impairment in klotho mutant mice, a genetic model of aging. Neuropsychopharmacology 38, 1426–1437 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. D. et al. The antiaging protein Klotho enhances oligodendrocyte maturation and myelination of the CNS. J Neurosci 33, 1927–1939 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham C. R., Chen C., Cuny G. D., Glicksman M. A. & Zeldich E. Small-molecule Klotho enhancers as novel treatment of neurodegeneration. Future Med Chem 4, 1671–1679 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T. et al. Cognition impairment in the genetic model of aging klotho gene mutant mice: a role of oxidative stress. FASEB J 17, 50–52 (2003). [DOI] [PubMed] [Google Scholar]
- Cherniack E. P., Troen B. R., Florez H. J., Roos B. A. & Levis S. Some new food for thought: the role of vitamin D in the mental health of older adults. Curr Psychiatry Rep 11, 12–19 (2009). [DOI] [PubMed] [Google Scholar]
- Schaller M. & Murray D. R. Pathogens, personality, and culture: disease prevalence predicts worldwide variability in sociosexuality, extraversion, and openness to experience. J Pers Soc Psychol 95, 212–221 (2008). [DOI] [PubMed] [Google Scholar]
- Minasyan A., Keisala T., Lou Y. R., Kalueff A. V. & Tuohimaa P. Neophobia, sensory and cognitive functions, and hedonic responses in vitamin D receptor mutant mice. J Steroid Biochem Mol Biol 104, 274–280 (2007). [DOI] [PubMed] [Google Scholar]
- Kalueff A. V., Lou Y. R., Laaksi I. & Tuohimaa P. Increased anxiety in mice lacking vitamin D receptor gene. Neuroreport 15, 1271–1274 (2004). [DOI] [PubMed] [Google Scholar]
- Kalueff A. V., Lou Y. R., Laaksi I. & Tuohimaa P. Abnormal behavioral organization of grooming in mice lacking the vitamin D receptor gene. J Neurogenet 19, 1–24 (2005). [DOI] [PubMed] [Google Scholar]
- Zou J. et al. Progressive hearing loss in mice with a mutated vitamin D receptor gene. Audiol Neurootol 13, 219–230 (2008). [DOI] [PubMed] [Google Scholar]
- Burne T. H. et al. Developmental vitamin D (DVD) deficiency alters pup-retrieval but not isolation-induced pup ultrasonic vocalizations in the rat. Physiol Behav 102, 201–204 (2011). [DOI] [PubMed] [Google Scholar]
- Burne T. H., Johnston A. N., McGrath J. J. & Mackay-Sim A. Swimming behaviour and post-swimming activity in Vitamin D receptor knockout mice. Brain Res Bull 69, 74–78 (2006). [DOI] [PubMed] [Google Scholar]
- Burne T. H., McGrath J. J., Eyles D. W. & Mackay-Sim A. Behavioural characterization of vitamin D receptor knockout mice. Behav Brain Res 157, 299–308 (2005). [DOI] [PubMed] [Google Scholar]
- Kalueff A. V., Lou Y. R., Laaksi I. & Tuohimaa P. Increased grooming behavior in mice lacking vitamin D receptors. Physiol Behav 82, 405–409 (2004). [DOI] [PubMed] [Google Scholar]
- Kalueff A. V., Lou Y. R., Laaksi I. & Tuohimaa P. Impaired motor performance in mice lacking neurosteroid vitamin D receptors. Brain Res Bull 64, 25–29 (2004). [DOI] [PubMed] [Google Scholar]
- Kalueff A. V. et al. Increased severity of chemically induced seizures in mice with partially deleted Vitamin D receptor gene. Neurosci Lett 394, 69–73 (2006). [DOI] [PubMed] [Google Scholar]
- Sakai S. et al. Vitamin D receptor signaling enhances locomotive ability in mice. J Bone Miner Res 30, 128–136 (2015). [DOI] [PubMed] [Google Scholar]
- Glade M. J. Vitamin D: health panacea or false prophet? Nutrition 29, 37–41 (2013). [DOI] [PubMed] [Google Scholar]
- Hedelin M. et al. Dietary intake of fish, omega-3, omega-6 polyunsaturated fatty acids and vitamin D and the prevalence of psychotic-like symptoms in a cohort of 33,000 women from the general population. BMC Psychiatry 10, 38 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jylha P. et al. Differences in neuroticism and extraversion between patients with bipolar I or II and general population subjects or major depressive disorder patients. J Affect Disord 125, 42–52 (2010). [DOI] [PubMed] [Google Scholar]
- Eyles D. W., Smith S., Kinobe R., Hewison M. & McGrath J. J. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat 29, 21–30 (2005). [DOI] [PubMed] [Google Scholar]
- Kuningas M. et al. VDR gene variants associate with cognitive function and depressive symptoms in old age. Neurobiol Aging 30, 466–473 (2009). [DOI] [PubMed] [Google Scholar]
- Wrzosek M. et al. Association between Fok I vitamin D receptor gene (VDR) polymorphism and impulsivity in alcohol-dependent patients. Mol Biol Rep 41, 7223–7228 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubbenhorst A., Striebich S., Lang F. & Lang U. E. Exploring the relationship between vitamin D and basic personality traits. Psychopharmacology (Berl) 215, 733–737 (2011). [DOI] [PubMed] [Google Scholar]
- Voracek M. Big five personality factors and suicide rates in the United States: a state-level analysis. Percept Mot Skills 109, 208–212 (2009). [DOI] [PubMed] [Google Scholar]
- Bertone-Johnson E. R. Vitamin D and the occurrence of depression: causal association or circumstantial evidence? Nutr Rev 67, 481–492 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorde R., Sneve M., Figenschau Y., Svartberg J. & Waterloo K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. J Intern Med 264, 599–609 (2008). [DOI] [PubMed] [Google Scholar]
- Shipowick C. D., Moore C. B., Corbett C. & Bindler R. Vitamin D and depressive symptoms in women during the winter: a pilot study. Appl Nurs Res 22, 221–225 (2009). [DOI] [PubMed] [Google Scholar]
- Leibrock C. B. et al. NH4Cl Treatment Prevents Tissue Calcification in Klotho Deficiency. J Am Soc Nephrol 26, 2423–2433 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S. S. et al. Hyperaldosteronism in Klotho-deficient mice. Am J Physiol Renal Physiol 299, F1171–F1177 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendijk W. J. et al. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry 65, 508–512 (2008). [DOI] [PubMed] [Google Scholar]
- Wilkins C. H., Sheline Y. I., Roe C. M., Birge S. J. & Morris J. C. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry 14, 1032–1040 (2006). [DOI] [PubMed] [Google Scholar]
- Herran A., Sierra-Biddle D., Cuesta M. J., Sandoya M. & Vazquez-Barquero J. L. Can personality traits help us explain disability in chronic schizophrenia? Psychiatry Clin Neurosci 60, 538–545 (2006). [DOI] [PubMed] [Google Scholar]
- Kesby J. P. et al. Developmental vitamin D deficiency alters dopamine-mediated behaviors and dopamine transporter function in adult female rats. Psychopharmacology (Berl) 208, 159–168 (2010). [DOI] [PubMed] [Google Scholar]
- Khanal R. C. & Nemere I. Regulation of intestinal calcium transport. Annu Rev Nutr 28, 179–196 (2008). [DOI] [PubMed] [Google Scholar]
- Ramasamy I. Recent advances in physiological calcium homeostasis. Clin Chem Lab Med 44, 237–273 (2006). [DOI] [PubMed] [Google Scholar]
- Shumilina E. et al. Regulation of calcium signaling in dendritic cells by 1,25-dihydroxyvitamin D3. FASEB J 24, 1989–1996 (2010). [DOI] [PubMed] [Google Scholar]
- Obradovic D., Gronemeyer H., Lutz B. & Rein T. Cross-talk of vitamin D and glucocorticoids in hippocampal cells. J Neurochem 96, 500–509 (2006). [DOI] [PubMed] [Google Scholar]
- Celio M. R. et al. Monoclonal antibodies directed against the calcium binding protein Calbindin D-28k. Cell Calcium 11, 599–602 (1990). [DOI] [PubMed] [Google Scholar]
- Lansdowne A. T. & Provost S. C. Vitamin D3 enhances mood in healthy subjects during winter. Psychopharmacology (Berl) 135, 319–323 (1998). [DOI] [PubMed] [Google Scholar]
- Alesutan I. et al. 25-hydroxyvitamin d3 1-alpha-hydroxylase-dependent stimulation of renal klotho expression by spironolactone. Kidney Blood Press Res 37, 475–487 (2013). [DOI] [PubMed] [Google Scholar]
- Takenaka T. et al. Calcitriol Supplementation Improves Endothelium-Dependent Vasodilation in Rat Hypertensive Renal Injury. Kidney Blood Press Res 39, 17–27 (2014). [DOI] [PubMed] [Google Scholar]
- Abed M. et al. Sensitization of erythrocytes to suicidal erythrocyte death following water deprivation. Kidney Blood Press Res 37, 567–578 (2013). [DOI] [PubMed] [Google Scholar]
- Latus J. et al. Analysis of alpha-klotho, fibroblast growth factor-, vitamin-D and calcium-sensing receptor in 70 patients with secondary hyperparathyroidism. Kidney Blood Press Res 37, 84–94 (2013). [DOI] [PubMed] [Google Scholar]
- Fakhri H. et al. Regulation of mineral metabolism by lithium. Pflugers Arch 466, 467–475 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang B. et al. Lithium- Sensitive Store-Operated Ca2 + Entry in the Regulation of FGF23 Release. Neurosignals 23, 34–48 (2015). [DOI] [PubMed] [Google Scholar]
- Feger M. et al. Effect of carbon monoxide donor CORM-2 on vitamin D3 metabolism. Kidney Blood Press Res 37, 496–505 (2013). [DOI] [PubMed] [Google Scholar]
- Lang U. E. et al. Reduced locomotion in the serum and glucocorticoid inducible kinase 3 knock out mouse. Behav Brain Res 167, 75–86 (2006). [DOI] [PubMed] [Google Scholar]
- Crawley J. & Goodwin F. K. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav 13, 167–170 (1980). [DOI] [PubMed] [Google Scholar]
- Konig M. et al. Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature 383, 535–538 (1996). [DOI] [PubMed] [Google Scholar]
- Leibrock C. et al. Akt2 deficiency is associated with anxiety and depressive behavior in mice. Cell Physiol Biochem 32, 766–777 (2013). [DOI] [PubMed] [Google Scholar]