Abstract
Noble metals can promote the direct participation of lattice oxygen of very stable oxide materials such as aluminum oxide, to oxidize reactant molecules, while the fundamental mechanism of noble metal catalysis is elusive. Here we report that a single atom of rhodium, a powerful noble metal catalyst, can promote the transfer of five oxygen atoms to oxidize carbon monoxide from a nine-atom rhodium–aluminum oxide cluster. This is a sharp improvement in the field of cluster science where the transfer of at most two oxygen atoms from a doped cluster is more commonly observed. Rhodium functions not only as the preferred trapping site to anchor and oxidize carbon monoxide by the oxygen atoms in direct connection with rhodium but also the primarily oxidative centre to accumulate the large amounts of electrons and the polarity of rhodium is ultimately transformed from positive to negative.
 Noble metals can promote the stable lattice oxygen of metal oxides to take part in oxidation reactions. Here, the authors report the preparation and reactivity of rhodium-aluminum oxide cluster ions, in which the rhodium ion promotes the transfer of five oxygen atoms from a nine-atom parent cluster to oxidize carbon monoxide.
Noble metals can promote the stable lattice oxygen of metal oxides to take part in oxidation reactions. Here, the authors report the preparation and reactivity of rhodium-aluminum oxide cluster ions, in which the rhodium ion promotes the transfer of five oxygen atoms from a nine-atom parent cluster to oxidize carbon monoxide.
Oxide-supported rhodium (Rh) exhibits extraordinary catalytic activity in a large number of reactions1,2,3,4,5,6,7,8,9 such as the oxidation of carbon monoxide (CO)1,2,3,7, carbon dioxide methanation6, partial oxidation of methane to syngas4,5,8,9 and so on. It has been reported that trace amounts of Rh can promote direct participation of lattice oxygen of chemically very inert supports such as aluminum oxide (Al2O3), to oxidize reactant molecules4,5,9, while the fundamental mechanism is elusive. Exploring the function of Rh in invoking the lattice oxygen of oxide support is of great importance to understand heterogeneous catalysis but remains a big challenge because of the structure complexity of bulk material.
Atomic clusters are considered as the intermediate matter to bridge atoms and their bulk counterpart10, and can be ideal models for active sites of condensed-phase system. Cluster reactions11,12,13,14,15,16,17 can be studied under isolated conditions to provide the mechanistic insights of elementary steps in the related condensed-phase systems. Important findings such as spin conservation13 and the complementary active sites15 have been revealed by studying the reactions of aluminum clusters with molecular O2 and water, respectively. The oxygen atom transfer (OAT) from metal oxide clusters to small molecules is one type of extensively studied reactions12,18,19. Noble metal-doped heteronuclear oxide clusters20 are being actively studied to understand the mechanistic nature of supported catalysts in the OAT reactions such as CO oxidation, an important model reaction in heterogeneous processes21 and its wide applications in air purification. Au and Pt atoms have been emphasized to be crucial, to promote significantly the efficiency of OAT in CO oxidation22,23,24,25,26. However, each of the reported Au or Pt-doped clusters such as AuAl3O5+ and PtAl3O7− can transfer at most two oxygen atoms to oxidize CO (refs 22, 23, 24, 25, 26). Here we report that a single Rh atom can unexpectedly promote the transfer of five oxygen atoms to oxidize CO from a nine-atom cluster RhAl2O6+, which produces the oxygen very deficient species RhAl2O+. In contrast, reported homonuclear aluminum oxide clusters (AlxOy±)26,27 can deliver only one oxygen atom to CO and these reactive clusters such as Al2O3+ and Al4O7− are all oxygen-rich species. Identification of multiple OAT from a single Rh-atom-doped cluster to reactant molecules is an important step to understand the participation of lattice oxygen promoted by noble metals. This gas-phase study that a nine-atom rhodium–aluminum oxide cluster oxidizes five CO molecules is a sharp improvement in the field of cluster science and provides a strictly molecular level understanding of the fundamental mechanism of noble metal catalysis in the related condensed phase.
Results
Reactivity of rhodium–aluminum oxide clusters with CO
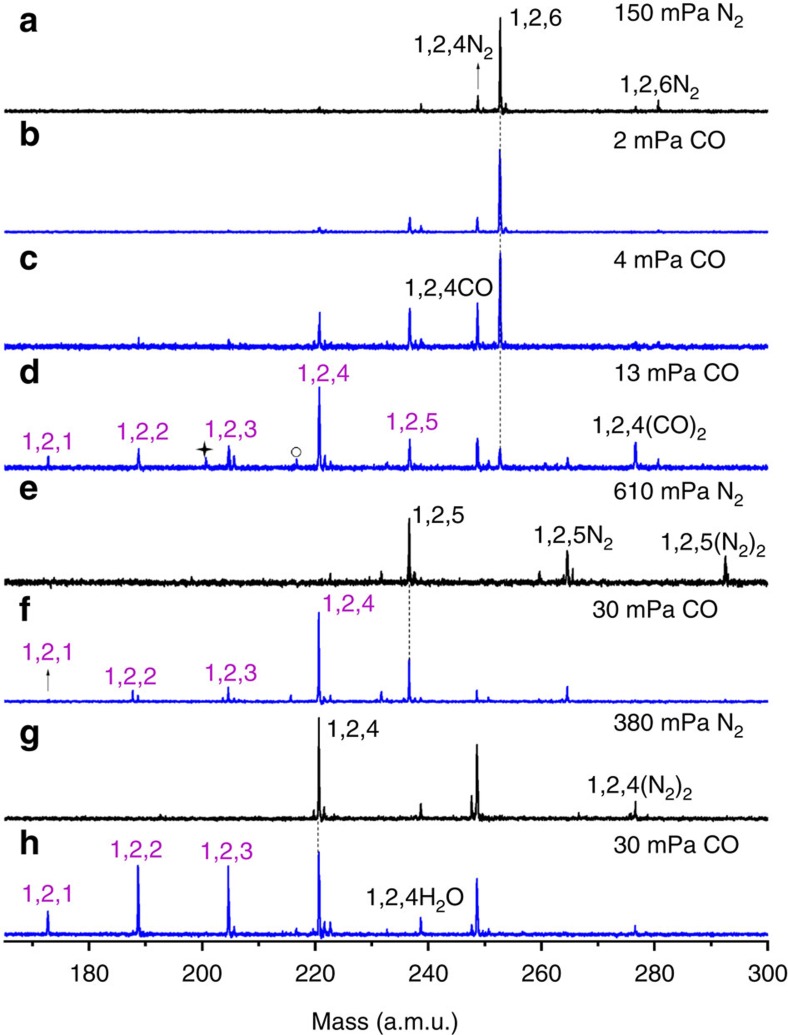
The RhAl2Om+ (m=2–6) cluster ions were generated by laser ablation of a mixed-metal disk compressed with Rh and Al powders. The generated RhAl2Om+ cluster ions were mass-selected, cooled and then interacted with N2 and CO in an ion trap reactor, as shown in Figs 1 and 2. On the interaction of RhAl2O6+ with 150 mPa N2 (Fig. 1a), weak N2 adsorption (RhAl2O6N2+) and N2/O2 exchange (RhAl2O4N2+) products were generated. Generation of RhAl2O4N2+ suggests the possible presence of superoxide (O2−·) or peroxide (O22−) unit in RhAl2O6+ (RhAl2O6+ + N2 → RhAl2O4N2+ + O2). In sharp contrast, on the interaction of RhAl2O6+ with CO (Fig. 1b–d), a series of products, from RhAl2O5+ to RhAl2O+, were generated gradually with the increase of CO partial pressure from 2 to 13 mPa. Signals RhAl2O1–5+ did not appear on the interaction of RhAl2O6+ with even high pressure N2 (Fig. 1a). Additional experimental techniques such as multiphoton ionization28,29 employing pulsed lasers are required to observe the neutral CO2 molecules. However, N2 experiment in Fig. 1a also indicates that products RhAl2O1–5+ are due to the chemical reactions of RhAl2O6+ with CO rather than collision-induced dissociation and RhAl2O6+ may oxidize five CO molecules consecutively (equation (1)).
Figure 1. Reactivity of RhAl2O4–6+ clusters with CO.
Time-of-flight mass spectra for reactions of mass selected RhAl2O4–6+ with N2 (a,e,g) and CO (b–d,f,h) are shown. Peaks marked with asterisk and hollow circle in d are CO adsorption products of RhAl2OCO+ and RhAl2O2CO+, respectively. RhxAlyOz+ and RhxAlyOzX+ (X=N2, CO and H2O) species are labeled as x,y,z and x,y,zX, respectively. Signal 1,2,4H2O in h is due to the residual water in the gas handling system. The time periods for reactions RhAl2O6+ + CO, RhAl2O5+ + CO and RhAl2O4+ + CO were about 1.1, 0.7 and 0.6 ms, respectively. The reactant gas pressures are shown in mPa.
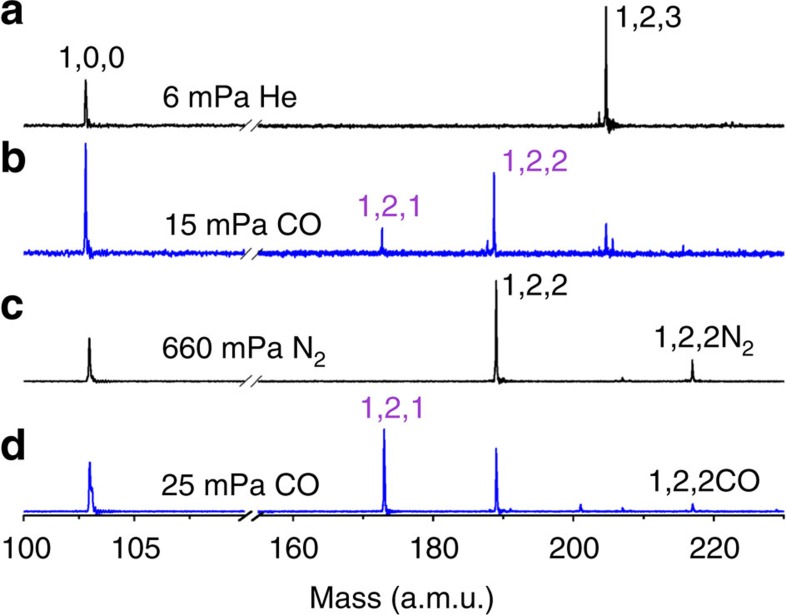
Figure 2. Reactivity of RhAl2O2–3+ clusters with CO.
Time-of-flight mass spectra for reactions of mass selected RhAl2O2–3+ with He (a), N2 (c) and CO (b,d) are shown. The time period was ∼0.6 ms for both reactions.
 |
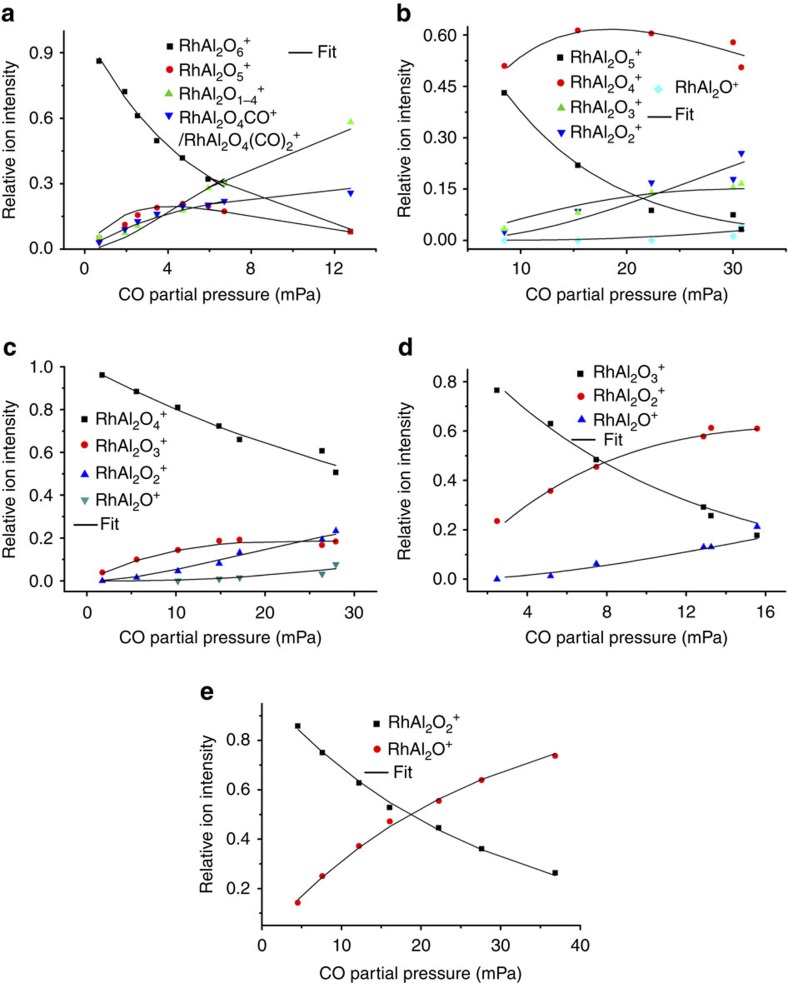
The strong signals that can be assigned as RhAl2O4CO+ and RhAl2O4(CO)2+ (Fig. 1a–d) on the interaction of RhAl2O6+ with CO indicate the displacement of the O–O unit in RhAl2O6+ by CO, which is more facile than N2 displacement (Fig. 1a), and further demonstrates the presence of O2−· or O22− unit in RhAl2O6+. Each of the cluster source generated RhAl2Om+ (m=5−2) clusters could also react with CO to generate products, from RhAl2Om−1+ to RhAl2O+ (Figs 1f,h and 2b,d). This provides convincing evidence that RhAl2O6+ can indeed oxidize five CO molecules consecutively. The pseudo-first-order rate constants (k1, in unit of 10−10 cm3 per molecule per second) on the interaction of RhAl2Om+ (m=6–2) cluster ions with CO can be well fitted (Fig. 3) and the determined rate constants are presented in Supplementary Table 1. The rate constants for the reactions of the cluster source generated RhAl2Om+ (m=6–2) with CO are 4.9±1.5 (m=6), 6.2±1.9 (m=5), 1.6±0.5 (m=4), 6.9±2.0 (m=3) and 2.4±0.7 (m=2), which correspond to the reaction efficiencies30 of about (37±11)%, (47±14)%, (13±4)%, (54±16)% and (19±6)%, respectively. Furthermore, we note that the clusters with odd number of oxygen atoms such as RhAl2O5+ are more reactive towards CO oxidation than clusters with even number of oxygen atoms such as RhAl2O6+.
Figure 3. Reaction kinetics.
Variation of ion intensities with respect to the partial pressures of CO in RhAl2Om+ (m=6−2) + CO are shown (a–e). The solid lines are fitted to the experimental data points by the least-square procedure. The Rh+ (1,0,0) ions (Fig. 2) are mostly generated during cooling of the RhAl2O3+ and RhAl2O2+ cluster ions through collisions with He gas in the ion trap reactor; thus, the ion intensity of Rh+ (nearly independent on the CO partial pressure) is excluded in the fitting. See Supplementary Table 1 for details of the determined rate constant values.
Reaction mechanism
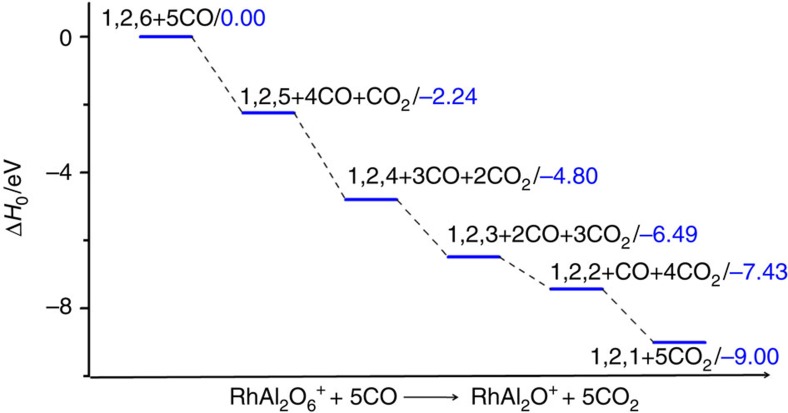
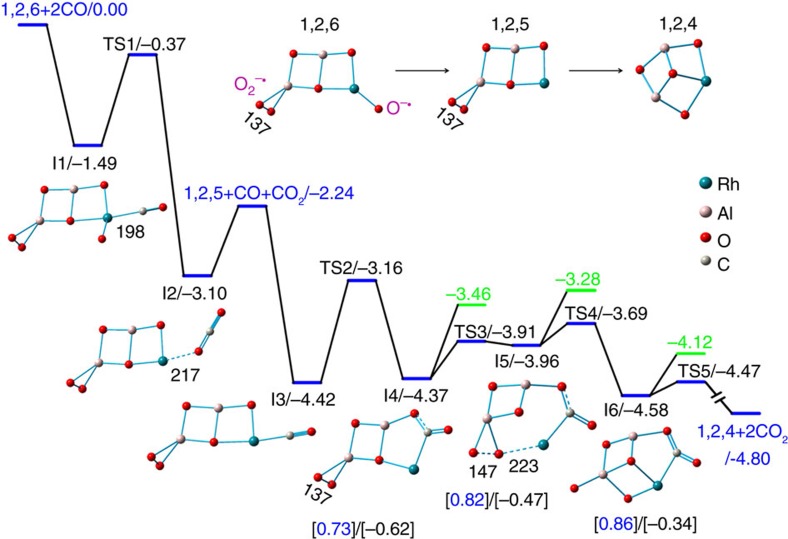
The density functional theory calculated thermodynamic data for CO oxidation by RhAl2Om+ (m=2–6) are shown in Fig. 4. The overall oxidation (RhAl2O6+ + 5CO → RhAl2O+ + 5CO2) is highly exothermic (−9.00 eV). The low-lying energy isomers of clusters RhAl2Om+ (m=6–1) are provided in Supplementary Figs 1–6. The lowest energy isomer of RhAl2O6+ is in the triplet spin state (Supplementary Fig. 1) and contains a superoxide O2−· unit (O–O bond: 137 pm; Fig. 5). The existence of O2−· unit in RhAl2O6+ is consistent with the appearance of RhAl2O4N2+ and RhAl2O4CO+ (or RhAl2O4(CO)2+) on the interaction of RhAl2O6+ with N2 and CO (Fig. 1a–d), respectively. Supplementary Figs 7 and 8 show that the Al-site adsorption contributes to the displacement of the O2−· unit in RhAl2O6+ by N2 or CO and both reactions are calculated to be thermodynamically and kinetically favourable, and CO displacement is more facile than N2 displacement. This is consistent with the relatively higher intensity of RhAl2O4CO+ (or RhAl2O4(CO)2+) than RhAl2O4N2+ observed in the experiment (Fig. 1a–d). The positively charged Rh in RhAl2O6+ (natural charge: +1.14 e) can trap CO tightly at the first step (I1, ΔH0=−1.49 eV; Fig. 5) and then the oxidation of CO (I1→TS1→I2) by the highly reactive atomic oxygen radical anion O−· (ref. 31) takes place. Direct CO oxidation by the O2−· unit has to suffer from a positive barrier of 0.03 eV, which is much less favourable than the oxidation by O−·. The Rh atom in product RhAl2O5+ (denoted as PRhAl2O5+, the structure of which is different from the lowest energy structure; Supplementary Fig. 2) can capture another CO tightly (I3, binding energy of −2.18 eV). Formation of the bent CO2 is the bottleneck (I3→TS2→I4) for CO oxidation by PRhAl2O5+. This step is to subject to a barrier of 1.26 eV. The subsequent steps follow a nearly downhill pathway characterized by small barriers to yield RhAl2O4+ and CO2 (Supplementary Fig. 9). Furthermore, theoretical calculations show that the energy of the critical transition state for reaction RhAl2O5+ + CO (−0.47 eV; Supplementary Fig. 10) is lower with respect to that for reaction RhAl2O6+ + CO (TS1, −0.37 eV; Fig. 5). This can well interpret the more reactive behaviour of the cluster source-generated RhAl2O5+ than RhAl2O6+ in the experiment.
Figure 4. Reaction thermodynamics.
Density functional theory (DFT)-calculated thermodynamic data for CO oxidation by RhAl2O2–6+. The energies are zero-point vibration-corrected in unit of eV.
Figure 5. Structures and reaction mechanisms.
Density functional theory (DFT)-calculated potential energy profiles for the oxidation of the first two CO molecules by RhAl2O6+. The lowest energy structure of RhAl2O6+ (1,2,6) and the products PRhAl2O5+ (1,2,5) and RhAl2O4+ (1,2,4) are provided. Symbols O2−· and O−· denote superoxide and atomic oxygen radical species, respectively. The relative energies for intermediates (I1–I6) and transition states (TS1–TS5) are in unit of eV. Structures of I1–I6 are shown. Bond lengths are given in pm. The values in green show the relative energies for direct CO2 desorption from I4 to I6. The values in the square brackets show the natural charges on Rh (blue) and CO2 unit (black). See also Supplementary Figs 9–13 for more information.
The key step for the transfer of five oxygen atoms from RhAl2O6+ to CO lies in the facile dissociation of the O2−· unit in PRhAl2O5+. Dissociation of the chemically adsorbed molecular O2 (superoxide O2−· or peroxide O2 2−) is often considered to be the crucial step in oxidation reactions32. Recent gas-phase studies indicated that a single Au atom in AuTi3O8−22 is not enough to promote the dissociation of the O2 2− unit, while the Au dimer in Au2VO4− can promote O2 2− unit dissociation or direct participation in CO oxidation25. In this study, a single Rh atom in PRhAl2O5+ can promote the dissociation of the O2−· unit and the process is much more favourable than CO2 desorption (Fig. 5). This is rationalized by the strong Rh–C multiple bonds (5.97 eV)33 and the strong Rh–O bond (4.16 eV)34. Thus, the Al–O2−· unit in I4 can approach the Rh atom favourably to form structure Al–O2 2−˙̇̇Rh–CO2 in I5. The elongation of the O–O bond from 137 pm in I4 to 147 pm in I5 is a good indicator for the activation of the superoxide O2−· to peroxide O2 2− unit. The structure of I5 is crucial to induce further electron flowing into the O2 2− unit from both of the Rh atom and the CO2 unit (Fig. 5), and then the O2 2− unit can be dissociated favourably to produce O2−–Al–O2−–Rh–CO2 (I5→TS4→I6). Direct oxidation of CO by the O2−· unit in PRhAl2O5+ (Supplementary Fig. 9) is less favourable than the pathway in Fig. 5. This is consistent with previous study that instead of the direct participation in CO oxidation, molecular oxygen adsorbs at the interface between the oxygen vacancy and the single Rh site and then is followed by facile dissociation35. Release of three additional oxygen atoms from the resulting RhAl2O4+ to CO are calculated to be thermodynamically and kinetically favourable (Supplementary Figs 11–13). In each of these OAT steps, Rh atom functions as the preferred trapping site to anchor CO and then delivers CO for oxidation by the oxygen atoms in direct connection with Rh. The theoretical calculations well interpret the unique reactivity of RhAl2O6+ observed in the experiment.
Discussion
Metal-mediated OAT reaction is usually accompanied with the reduction of central metal by electrons that are stored originally in the removed oxygen atoms36 (equation (2)).
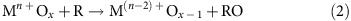 |
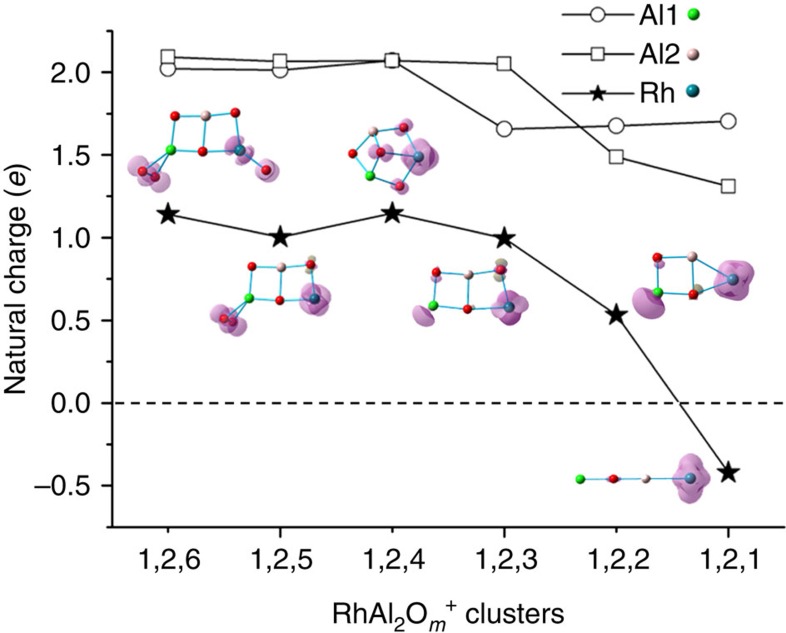
The positively charged metal centre is crucial to provide not only the characteristic site for the adsorption of CO (ref. 37) but also the oxidative centre to accept electrons. Recent gas-phase studies highlighted that the cleavage of Au–O bond and the formation of Au–M bond (M=Al, V, Ti, and Fe) is of great importance in CO oxidation by Au-doped clusters22,23,24,25. However, each of the Au-doped clusters can oxidize only one or at most two CO molecules and then the polarity conversion of Au atom from positive to negative takes place because of the formation of the reductive Au–M bond. In sharp contrast, natural charge analysis demonstrates that after the transfer of four oxygen atoms from RhAl2O6+ to CO, the Rh atom is still positively charged (+0.53e, Fig. 6) in product RhAl2O2+, which can also oxidize a CO molecule. The natural charge on Rh atom is decreased from +1.14e in RhAl2O6+ to +1.00e in PRhAl2O5+ after the oxidation of the first CO. In this step, Rh acts as the primary centre to accumulate the electron that is localized originally on O−· radical, as shown from the change of spin density distribution, RhAl2O6+ versus PRhAl2O5+. However, the Rh atom is re-oxidized in product RhAl2O4+ (+1.15e) after the oxidation of the second CO due to the dissociation of the O2−· unit (I4→I5→I6; Fig. 5). This step is crucial to recover the oxidative reactivity of Rh. In situ Raman spectroscopic study also demonstrated that supported Rh oxide can oxidize CO and then the Rh oxide is subsequently re-oxidized by the oxygen atoms from oxide support7. This phenomenon can be traced back to the well-fitting strength of Rh–O bond (4.16 eV)34, which is strong enough to promote the dissociation of the O2−· unit inPRhAl2O5+ and prevent the formation of the reductive Rh–Al bond (3.26 eV, by theoretical calculation) in RhAl2O2–6+ but at the same time is relatively weak to deliver oxygen atoms to oxidize CO (O–CO: 5.52 eV)38. Previous studies show that Rh prefers to coordinate not only with the surface oxygen atoms but also the subsurface oxygen in oxide support35. The strong Rh–O bond inhibits the migration of Rh atom to the oxygen vacancy and remains positively charged. Using high-resolution in situ X-ray diffraction and transmission electron microscopy, Stierle and colleagues1 reported the reversible and oxygen-induced shape transformation of Rh nanoparticles by the formation of O–Rh–O surface oxide during the cycle of catalytic CO oxidation. In contrast, the stronger Au–M (Au–Al=3.37 eV (ref. 39), Au–V=2.49 eV (ref. 38) and Au–Ti=2.56 eV (by theoretical calculation)) than Au–O bond (2.27 eV)34 facilitates the formation of the reductive Au–M bond after the oxidation of only one or two CO molecules.
Figure 6. Natural charge.
Density functional theory (DFT)-calculated natural charges (e) on the Rh atom and Al atoms in RhAl2O1–6+. The spin density distributions on individual atoms are shown in the purple isosurfaces.
The negatively charged Rh has been theoretically predicted40 and experimentally postulated41. The RhAl2O+ cluster is a linear structure (Al–O–Al–Rh) and the single oxygen atom is sandwiched between two Al atoms. This structure with triplet spin state has been confirmed to be the lowest energy isomer of RhAl2O+ by more accurate CCSD(T) calculation (Supplementary Fig. 6). The two unpaired electrons are mainly localized on the Rh atom (∼1.83 μB, Fig. 6), indicating that such Rh atom can be considered to be Rh−1 (4d95s1). The electron configuration calculations (4d8.755s0.645p0.03) provide solid evidence for the negatively charged Rh in RhAl2O+. Thus, the oxidation state of Rh changes from +2.5 in RhAl2O6+ to −1 in RhAl2O+ (calculated based on the distribution of spin density; Fig. 6) during the transfer of five oxygen atoms from RhAl2O6+ to CO. This rather large range of Rh oxidation state changes in chemical reactions has rarely been reported (the Au oxidation state changes from +1 to −1), covering from cationic to anionic, and it is the driving force to accumulate the electrons that are stored originally in the released oxygen atoms and promotes the unique oxidative reactions to proceed.
In conclusion, we have demonstrated that a single atom of Rh can unexpectedly promote the transfer of five oxygen atoms to oxidize CO from a nine-atom cluster RhAl2O6+. This study leads a leap ahead towards OAT reactions in the field of cluster science and represents an important step to understand the participation of lattice oxygen promoted by noble metals. The preferable Rh–O rather than the reductive Rh–Al bond formation together with the capability of Rh to accumulate the large amounts of electrons are crucial factors to drive the unique reactions. This gas-phase study reveals the molecular-level origin for the puzzling experimental observation that trace amounts of Rh can promote the reactivity of lattice oxygen of Al2O3 (refs 4, 5, 9), a chemically very inert material.
Methods
Cluster generation and reactivity detection
The RhxAlyOz+ cluster ions were generated by laser ablation of a mixed-metal disk compressed with Rh and Al powders (molar radio Rh/Al=1/1) in the presence of O2 (0.4%) seeded in a He carrier gas with a backing pressure of 6.0 standard atmospheres. The cluster ions of interest were mass selected using a quadrupole mass filter and then entered into a linear ion trap (LIT) reactor, where they were cooled by collisions with a pulse of He gas and then interacted with a pulse of 5% (for reactions RhAl2Om+ (m=2–5) + CO) or 2% (for reaction RhAl2O6+ + CO) CO seeded in He for around 0.6∼1.1 ms. The temperature of cooling gas (He), reactant gases (CO or N2) and the LIT reactor was around 298 K. The cluster ions ejected from the LIT were detected by a reflector time-of-flight mass spectrometer. The details of running the time-of-flight mass spectrometer42, quadrupole mass filter43 and the LIT44 can be found in our previous works.
Rate constant fitting
Equation (3) was used to determine the pseudo-first-order rate constants (k1) of cluster reactions in an ion trap reactor44, in which IR is the signal intensity of the reactant cluster ions, IT is the total ion intensity including product ion contribution, kB is the Boltzmann constant, T is the temperature (∼298 K), tR is the reaction time and P is the effective pressure of the reactant gas in the ion trap reactor.
 |
To calculate the reaction efficiencies (the possibilities of reaction on each collision), the collision rate constants were calculated on the basis of the surface charge capture model developed in the literature30. It is noteworthy that for reaction RhAl2O5+ + CO, the relative ion intensity of RhAl2O5+ is the reactive component generated in the experiment and the unreactive component is not included. The unreactive component of RhAl2O5+ could be well-fitted by equation (4)45.
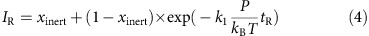 |
in which xinert is the relative intensity of the unreactive component of RhAl2O5+ and k1 is the pseudo-first-order rate constant of the reactive component of RhAl2O5+. The xinert was determined to be about 12%, indicating that the experimentally generated RhAl2O5+ may have isomers that are not or less reactive with CO.
Computational details
Density functional theory calculations using the Gaussian 09 (ref. 46) programme were carried out to investigate the mechanistic details on the oxidation of five CO molecules by a nine-atom rhodium–aluminum oxide cluster (RhAl2O6+). To find an appropriate functional for the Rh–Al–O system, the bond dissociation energies of Rh–O, Rh–C, Al–O, O–O, Rh–Al and O–CO were computed by various functionals and compared with available experimental data (Supplementary Table 2). It turns out that M06L47 was the best overall; thus, the results by M06L were given throughout the work. The TZVP basis set48 for Al, C and O atoms and a D95V basis set49 combined with the Stuttgart/Dresden relativistic effective core potential (denoted as SDD in Gaussian software) for Rh atom were used in all the calculations. A Fortran code based on a genetic algorithm50 was used to generate initial guess structures of RhAl2O1–6+. The reaction mechanisms were studied for RhAl2O6+ + 5CO → RhAl2O+ + 5CO2. The relaxed potential energy surface scan was used extensively to obtain good guess structures for intermediates and transition states along the pathways. The transition states were optimized using the Berny algorithm51. Intrinsic reaction coordinate calculations52,53 were performed so that each transition state connects two appropriate local minima. Vibrational frequency calculations were carried out to check that intermediates and transition state have zero and only one imaginary frequency, respectively.
Additional information
How to cite this article: Li, X.-N. et al. A nine-atom rhodium–aluminum oxide cluster oxidizes five carbon monoxide molecules. Nat. Commun. 7:11404 doi: 10.1038/ncomms11404 (2016).
Supplementary Material
Supplementary Figures 1-13 and Supplementary Tables 1-2
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos 21303215, 21325314, 21273247 and 21573246) and the Major Research Plan of China (No. 2013CB834603).
Footnotes
Author contributions The original manuscript, figures, tables and the Supplementary Materials were prepared by X.-N.L. The theoretical calculations were prepared by H.-M.Z. and X.-N.L. The experimental data were prepared by Z.Y. and H.-M.Z. S.-G.H. provided the original idea, helpful discussions and the contribution in the manuscript revision.
References
- Nolte P. et al. Shape changes of supported Rh nanoparticles during oxidation and reduction cycles. Science 321, 1654–1658 (2008). [DOI] [PubMed] [Google Scholar]
- Grass M. E. et al. A reactive oxide overlayer on rhodium nanoparticles during CO oxidation and its size dependence studied by in situ ambient-pressure X-ray photoelectron spectroscopy. Angew. Chem. Int. Ed. 47, 8893–8896 (2008). [DOI] [PubMed] [Google Scholar]
- Ligthart D. A. J. M., van Santen R. A. & Hensen E. J. M. Supported rhodium oxide nanoparticles as highly active CO oxidation catalysts. Angew. Chem. Int. Ed. 50, 5306–5310 (2011). [DOI] [PubMed] [Google Scholar]
- Karin C. B., Wohlrab S., Rodemerck U. & Kondratenko E. V. The tremendous effect of trace amounts of Rh on redox and catalytic properties of CeO2-TiO2 and Al2O3 in CH4 partial oxidation. Catal. Commun. 18, 121–125 (2012). [Google Scholar]
- Karin C. B. et al. Tailored noble metal nanoparticles on γ-Al2O3 for high temperature CH4 conversion to syngas. ChemCatChem. 4, 1368–1375 (2012). [Google Scholar]
- Karelovic A. & Ruiz P. Improving the hydrogenation function of Pd/γ-Al2O3 catalyst by Rh/γ-Al2O3 addition in CO2 methanation at low temperature. ACS Catal. 3, 2799–2812 (2013). [Google Scholar]
- Song W., Jansen A. P. J., Degirmenci V., Ligthart D. A. J. M. & Hensen E. J. M. A computational study of the mechanism of CO oxidation by a ceria supported surface rhodium oxide layer. Chem. Commun. 49, 3851–3853 (2013). [DOI] [PubMed] [Google Scholar]
- Duarte R. B., Krumeich F. & van Bokhoven J. A. Structure, activity, and stability of atomically dispersed Rh in methane stream reforming. ACS Catal. 4, 1279–1286 (2014). [Google Scholar]
- Kondratenko V. A., Karin C. B. & Kondratenko E. V. Partial oxidation of methane to syngas over γ-Al2O3-supported Rh nanoparticles: kinetic and mechanistic origins of size effect on selectivity and activity. ACS Catal. 4, 3136–3144 (2014). [Google Scholar]
- Castleman A. W. Jr & Jena P. Clusters: a bridge between disciplines. Proc. Natl Acad. Sci. USA 103, 10552–10553 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hair R. A. J. & Khairallah G. N. Gas phase ion chemistry of transition metal clusters: production, reactivity, and catalysis. J. Clust. Sci. 15, 331–363 (2004). [Google Scholar]
- Böhme D. K. & Schwarz H. Gas-phase catalysis by atomic and cluster metal ions: the ultimate single-site catalysts. Angew. Chem. Int. Ed. 44, 2336–2354 (2005). [DOI] [PubMed] [Google Scholar]
- Burgert R. et al. Spin conservation accounts for aluminum cluster anion reactivity pattern with O2. Science 319, 438–442 (2008). [DOI] [PubMed] [Google Scholar]
- Gong Y. & Zhou M. Spectroscopic and theoretical studies of transition metal oxides and dioxygen complexes. Chem. Rev. 109, 6765–6808 (2009). [DOI] [PubMed] [Google Scholar]
- Roach P. J., Woodward W. H., Castleman A. W. Jr, Reber A. C. & Khanna S. N. Complementary active sites cause size-selective reactivity of aluminum cluster anions with water. Science 323, 492–495 (2009). [DOI] [PubMed] [Google Scholar]
- Lang S. M. & Bernhardt T. M. Gas phase metal cluster model systems for heterogeneous catalysis. Phys. Chem. Chem. Phys. 14, 9255–9269 (2012). [DOI] [PubMed] [Google Scholar]
- Asmis K. R. Structure characterization of metal oxide clusters by vibrational spectroscopy: possibilities and prospects. Phys. Chem. Chem. Phys. 14, 9270–9281 (2012). [DOI] [PubMed] [Google Scholar]
- Blagojevic V., Orlova G. & Bohme D. K. O-atom transport catalysis by atomic cations in the gas phase: reduction of N2O by CO. J. Am. Chem. Soc. 127, 3545–3555 (2005). [DOI] [PubMed] [Google Scholar]
- Liu Q. Y. & He S. G. Oxidation of carbon monoxide on atomic clusters. Chem. J. Chin. Univ. 35, 665–688 (2014). [Google Scholar]
- Schwarz H. Doping effects in cluster-mediated bond activation. Angew. Chem. Int. Ed. 54, 10090–10100 (2015). [DOI] [PubMed] [Google Scholar]
- Freund H. J., Meijer G., Scheffler M., Schlögl R. & Wolf M. CO oxidation as prototypical reaction for heterogeneous processes. Angew. Chem. Int. Ed. 50, 10064–10094 (2011). [DOI] [PubMed] [Google Scholar]
- Li X. N., Yuan Z. & He S. G. CO oxidation promoted by gold atoms supported on titanium oxide cluster anions. J. Am. Chem. Soc. 136, 3617–3623 (2014). [DOI] [PubMed] [Google Scholar]
- Li Z. Y., Yuan Z., Li X. N., Zhao Y. X. & He S. G. CO oxidation catalyzed by single gold atoms supported on aluminum oxide clusters. J. Am. Chem. Soc. 136, 14307–14313 (2014). [DOI] [PubMed] [Google Scholar]
- Yuan Z., Li X. N. & He S. G. CO oxidation promoted by gold atoms loosely attached in AuFeO3− cluster anions. J. Phys. Chem. Lett. 5, 1585–1590 (2014). [DOI] [PubMed] [Google Scholar]
- Wang L. N. et al. CO oxidation promoted by the gold dimer in Au2VO3− and Au2VO4− clusters. Angew. Chem. Int. Ed. 54, 11720–11724 (2015). [DOI] [PubMed] [Google Scholar]
- Li X. N., Yuan Z., Meng J. H., Li Z. Y. & He S. G. Catalytic CO oxidation on single Pt-atom doped aluminum oxide clusters: electronegativity-ladder effect. J. Phys. Chem. C 119, 15414–15420 (2015). [Google Scholar]
- Johnson G. E., Tyo E. C. & Castleman A. W. Jr Oxidation of CO by aluminum oxide cluster ions in the gas phase. J. Phys. Chem. A 112, 4732–4735 (2008). [DOI] [PubMed] [Google Scholar]
- Li S., Mirabal A., Demuth J., Wöste L. & Siebert T. A complete reactant-product analysis of the oxygen transfer reaction in [V4O11˙C3H6]−: a cluster complex for modeling surface activation and reactivity. J. Am. Chem. Soc. 130, 16832–16833 (2008). [DOI] [PubMed] [Google Scholar]
- Li S., Demuth J., Mirabal A., Wöste L. & Siebert T. On the role of thermal activation in selective photochemistry: mechanistic insight into the oxidation of propene on the V4O11− cluster. Phys. Chem. Chem. Phys. 14, 148–156 (2012). [DOI] [PubMed] [Google Scholar]
- Kummerlöwe G. & Beyer M. K. Rate estimates for collisions of ionic clusters with neutral reactant molecules. Int. J. Mass Spectrom. 244, 84–90 (2005). [Google Scholar]
- Ma J. B. et al. Reactivity of atomic oxygen radical anions bound to titania and zirconia nanoparticles in the gas phase: low-temperature oxidation of carbon monoxide. J. Am. Chem. Soc. 136, 2991–2998 (2013). [DOI] [PubMed] [Google Scholar]
- Panov G. I., Dubkov K. A. & Starokon E. V. Active oxygen in selective oxidation catalysis. Catal. Today 117, 148–155 (2006). [Google Scholar]
- Shim I. & Gingerich K. A. Electronic structure and bonding in the RhC molecule by all-electron ab initio HF-CI calculations and mass spectrometric measurements. J. Chem. Phys. 81, 5937–5944 (1984). [Google Scholar]
- Pedley J. B. & Marshall E. M. Thermochemical data for gaseous monoxides. J. Phys. Chem. Ref. Data 12, 967–1031 (1983). [Google Scholar]
- Song W., Jansen A. P. J. & Hensen E. J. M. A computational study of the influence of the ceria surface termination on the mechanism of CO oxidation of isolated Rh atoms. Faraday Discuss 162, 281–292 (2013). [DOI] [PubMed] [Google Scholar]
- Holm R. H. Metal-centered oxygen atom transfer reactions. Chem. Rev. 87, 1401–1449 (1987). [Google Scholar]
- Guzman J. & Gates B. C. Catalysis by supported gold: correlation between catalytic activity for CO oxidation and oxidation states of gold. J. Am. Chem. Soc. 126, 2672–2673 (2004). [DOI] [PubMed] [Google Scholar]
- Luo Y. P. Comprehensive Handbook of Chemical Bond Energies pp. 9–56CRC Press (2007). [Google Scholar]
- Cuthill A. M., Fabian D. J. & Shen S. S. S. Bond dissociation energies of the metallic vapor species aluminum-silver and aluminum-gold measured by Knudsen-Cell mass spectrometry. J. Phys. Chem. 77, 2008–2011 (1973). [Google Scholar]
- Gómez T., Florez E., Rodriguez J. A. & Illas F. Theoretical analysis of the adsorption of late transition-metal atoms on the (001) surface of early transition-meal carbides. J. Phys. Chem. C 114, 1622–1626 (2010). [Google Scholar]
- Zafeiratos S., Nehasil V. & Ladas S. X-ray photoelectron spectroscopy study of rhodium particle growth on different alumina surfaces. Surf. Sci 433-435, 612–616 (1999). [Google Scholar]
- Wu X. N., Xu B., Meng J. H. & He S. G. C-H bond activation by nanosized scandium oxide clusters in gas-phase. Int. J. Mass Spectrom. 310, 57–64 (2012). [Google Scholar]
- Yuan Z., Zhao Y. X., Li X. N. & He S. G. Reactions of V4O10+ cluster ions with simple inorganic and organic molecules. Int. J. Mass Spectrom. 354-355, 105–112 (2013). [Google Scholar]
- Yuan Z. et al. Thermal reactions of (V2O5)nO− (n=1–3) cluster anions with ethylene and propylene: oxygen atom transfer versus molecular association. J. Phys. Chem. C 118, 14967–14976 (2014). [Google Scholar]
- Li H. F. et al. Methane activation by iron-carbide cluster anions FeC6−. J. Phys. Chem. Lett. 6, 2287–2291 (2015). [DOI] [PubMed] [Google Scholar]
- Frisch M. J. et al. Gaussian 09, Revision A. 1 Gaussian, Inc. (2009). [Google Scholar]
- Zhao Y. & Truhlar D. G. A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J. Chem. Phys. 125, 194101 (2006). [DOI] [PubMed] [Google Scholar]
- Schäfer A., Huber C. & Ahlrichs R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 100, 5829–5835 (1994). [Google Scholar]
- Andrae D., Häuβermann U., Dolg M., Stoll H. & Preuβ H. Energy-adjusted ab initio pseudopotentials for the second and trird row transition elements. Theor. Chim. Acta 77, 123–141 (1990). [Google Scholar]
- Ding X. L., Li Z. Y., Meng J. H., Zhao Y. X. & He S. G. Density-functional global optimization of (La2O3)n clusters. J. Chem. Phys. 137, 214311 (2012). [DOI] [PubMed] [Google Scholar]
- Schlegel H. B. Optimization of equilibrium geometries and transition structures. J. Comput. Chem. 3, 214–218 (1982). [Google Scholar]
- Gonzalez C. & Schlegel H. B. An improved algorithm for reaction path following. J. Chem. Phys. 90, 2154–2161 (1989). [Google Scholar]
- Gonzalez C. & Schlegel H. B. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 94, 5523–5527 (1990). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-13 and Supplementary Tables 1-2