Abstract
Bariatric surgery is increasingly being performed in the medically complicated obese population as convincing data continue to mount, documenting the success of surgery not only in achieving meaningful weight loss but also in correcting obesity-related illnesses. Several surgical procedures with varying degrees of success and complications are currently being performed. This article discusses the short- and long-term gastrointestinal complications for the 4 most common bariatric surgical procedures: laparoscopic adjustable gastric banding, vertical sleeve gastrectomy, Roux-en-Y gastric bypass, and biliopancreatic diversion with duodenal switch.
Keywords: Bariatric surgery, gastric bypass, biliopancreatic diversion, duodenal switch, vertical sleeve gastrectomy, gastrointestinal complications
Bariatric surgery is increasingly being accepted as a viable treatment for managing the growing obesity epidemic. Surgery can provide a sustainable, long-term option for weight loss.1-3 The National Health and Nutrition Examination Survey from the Centers for Disease Control and Prevention revealed that the prevalence of obesity in adults (20-74 years) had more than doubled from 13.3% to 31.1% of the population from 1960 to 2002.4 The prevalence of obesity (body mass index [BMI] ≥30 kg/m2) has since stabilized at 35% in the United States starting in 2003.5 However, it is estimated that only 1% of eligible patients are undergoing surgical intervention. One barrier to accepting surgery may be the false notion of unacceptable risks and high rate of complications associated with surgery.3
Obesity is associated with multiple medical comorbidities, including type 2 diabetes mellitus, cardiovascular disease, dyslipidemia, hypertension, cholelithiasis, gastroesophageal reflux disease, obstructive sleep apnea, degenerative joint disease, lower back pain, and cancer.2,6-9 In addition, obesity is associated with an increase in early mortality.7 The estimated number of annual deaths attributed to obesity in US adults is 280,000.10
In 1991, the National Institutes of Health consensus panel developed a set of recommendations regarding which patients should be considered for bariatric surgery.2,8 These recommendations included the criteria that patients have a calculated BMI of at least 40 kg/m2 or a BMI of at least 35 kg/m2 with significant obesity-related comorbidities. Since these guidelines have been released, the number of bariatric surgeries has increased 6-fold to more than 100,000 per year in 2003,11,12 but has subsequently reached a steady state of almost 350,000 operations each year.1,13 Along with the increased volume of surgical procedures, a dramatic decrease in mortality and complications related to surgical intervention has been achieved, as demonstrated in a recent meta-analysis showing a mortality rate of 0.08% within 30 days and 0.31% after 30 days.14 Complication rates from bariatric operations have progressively fallen from 10.5% of cases in 1993 to 7.6% of cases in 2006, with the majority of complications now being minor.1,3 Bariatric surgery has now been shown to provide long-term weight loss and to decrease overall mortality in obese patients compared with matched controls.15,16
The surgical weight loss mechanism is generally considered to involve restriction, malabsorption, or a combination of these mechanisms. Restrictive procedures decrease the size of the stomach, resulting in early satiety and decreased caloric intake. The operations performed include laparoscopic adjustable gastric banding (LAGB) and vertical sleeve gastrectomy (VSG; Figure 1). In contrast, malabsorptive procedures decrease the degree of small intestinal absorption of nutrients by bypassing a large portion of the small intestine. These procedures include biliopancreatic diversion with or without a duodenal switch (Figure 2). A bariatric procedure with both components, malabsorption and restriction, is Roux-en-Y gastric bypass (RNYGBP; Figure 3), which has been the most commonly performed bariatric procedure in the United States.3,11 Although all 4 procedures can be beneficial to patients, the operations have varying degrees of success and complication profiles that are unique to each procedure. This article will discuss the common gastrointestinal (GI) complications seen with each technique.
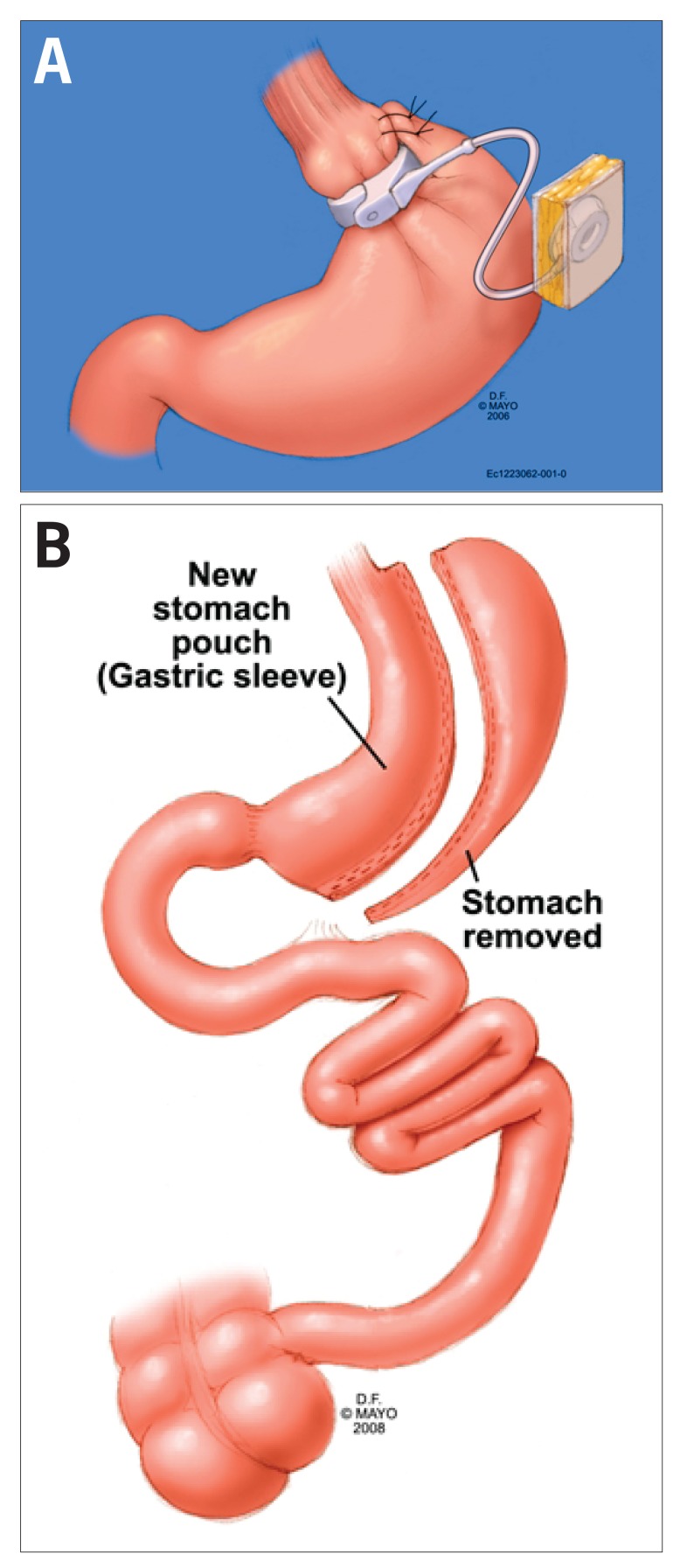
Figure 1.
Laparoscopie adjustable gastric banding (A) and vertical sleeve gastrectomy (B).
Reproduced with permission from the Mayo Clinic.
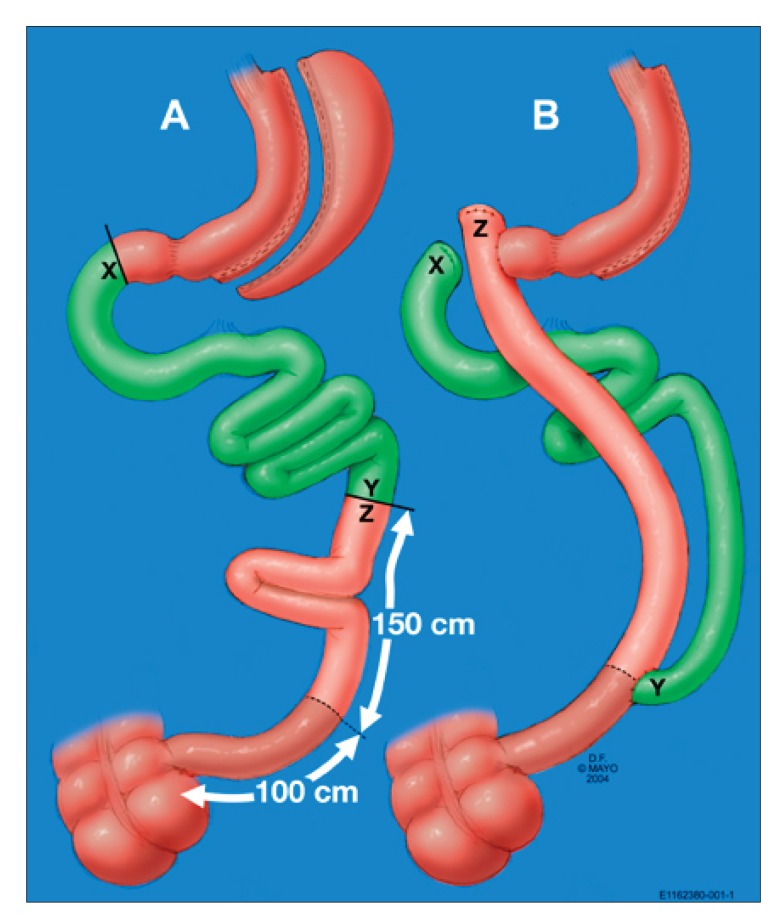
Figure 2.
Before (A) and after (B) biliopancreatic diversion with duodenal switch.
Reproduced with permission from the Mayo Clinic.
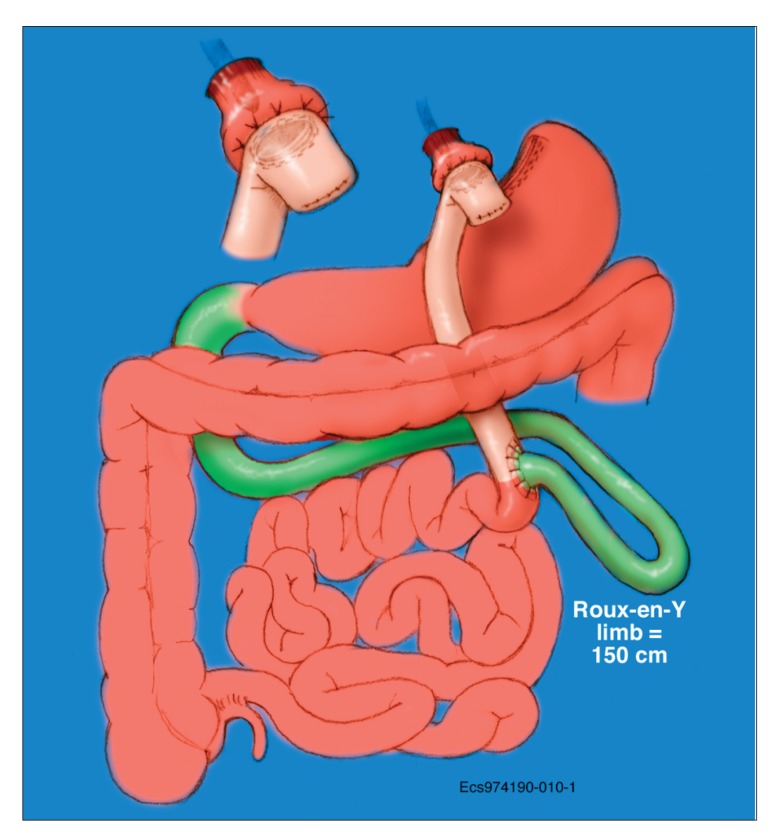
Figure 3.
Roux-en-Y gastric bypass.
Reproduced with permission from the Mayo Clinic.
Laparoscopic Adjustable Gastric Banding
LAGB is billed as one of the least invasive surgical procedures currently available for weight loss. It has the lowest perioperative and postoperative mortality rates (0.07% and 0.21%, respectively) compared with those of the other operative weight-loss techniques.14 Although the complication rate of LAGB was 13% in a meta-analysis of randomized controlled trials, this procedure also resulted in the highest long-term reoperation rate.14 Early complications associated with LAGB are uncommon, as the operation is short and the integrity of the digestive tract is maintained.9 Complications that do occur, however, can include gastroesophageal perforation, improper band positioning, early postoperative band slippage requiring repositioning, acute stomal obstruction, band infection, and bleeding.9,17,18 Intractable postoperative vomiting can develop depending on band positioning, gastric prolapse, or excessive incorporation of fat into the band device.18 A gastrografin swallow study can delineate the majority of early complications with a subsequent barium study to identify subtle findings. Although early complications requiring surgical intervention are rare, they often require reoperation for the adjustment of the band’s position, removal of the gastric band, or conversion to another bariatric operation (Figure 4).
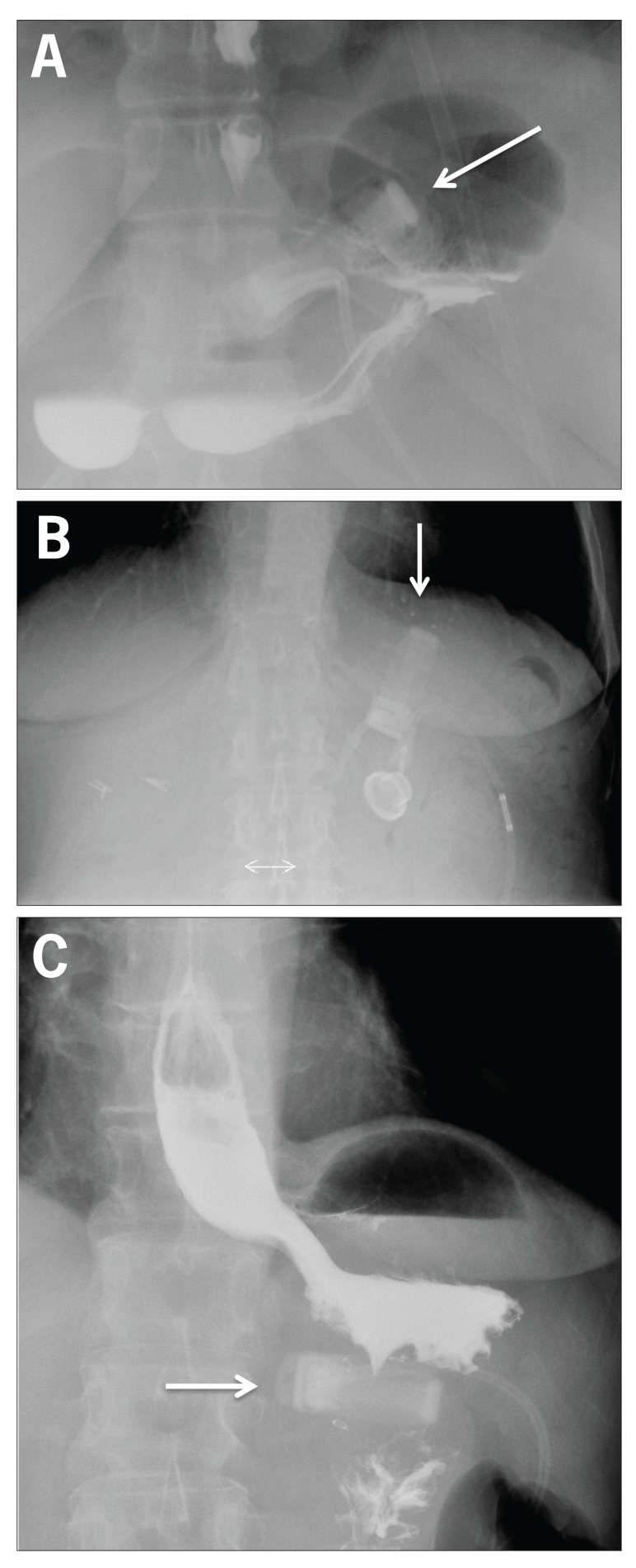
Figure 4.
Abdominal radiographs of laparoscopic adjustable gastric banding showing appropriate positioning at an approximately 45-degree angle (A), a slipped band with an overly vertical position (B), and a slipped band with an overly horizontal position (C).
Late complications are more common in patients who undergo LAGB, involving 6% to 25%.9 These complications include band slippage with or without pouch dilation (4%-12.5%); band erosion (2.8%); esophageal dilation (5%-71%); obstruction; device-related complications, including failure to lose weight or to maintain weight loss (2%-5%); and gastric necrosis.9,17-20 Esophageal dilation reliably develops in patients with overly inflated LAGB but also may develop with an empty band and is associated with delayed esophageal emptying, dysphagia, vomiting, and reflux. When these scenarios are encountered, the device should be emptied of all fluid as soon as possible, with removal of the device and consideration for conversion to another bariatric procedure in select individuals.18 Subjective reflux reportedly triples from 13% to 39% after gastric banding, with objective 24-hour reflux time increasing 5-fold from 6.4% to 30.9%.21 In addition, esophagitis has been noted in the majority of patients studied (75%). The major reoperation rate in patients who have undergone LAGB approaches 10% to 50%.9,18 Such operations include port or band removal, band replacement, port repositioning, placement of a new port, correction of a leak, or conversion to another bariatric procedure. Long-term ineffectiveness of LAGB, combined with its disproportionate, ongoing reoperation rate, has resulted in a tremendous decrease in LAGB placement from approximately 42% to 18% of all bariatric operations as of 2010.22
Vertical Sleeve Gastrectomy
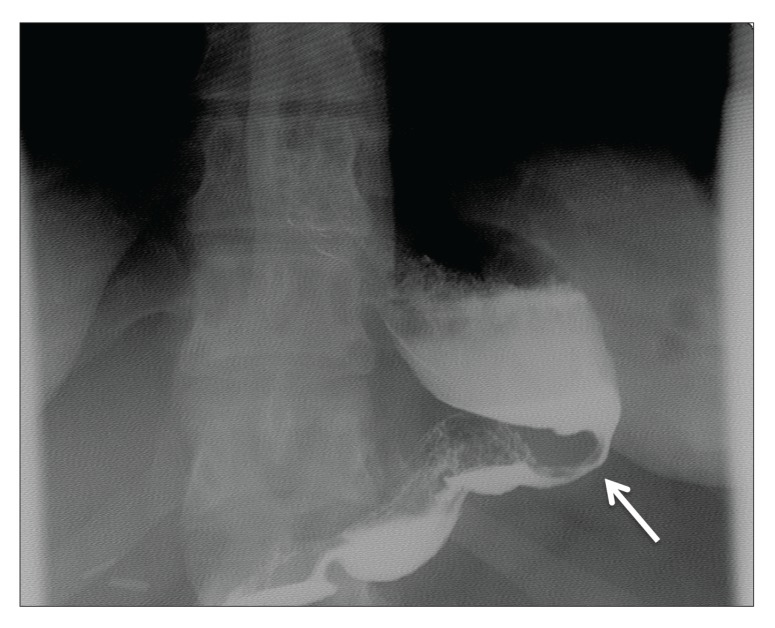
VSG is primarily a restrictive procedure for weight loss. The perioperative and postoperative mortality rates are 0.29% and 0.34%, respectively, with complication rates of 13%.14,23 Rare complications occur in the early postoperative period after VSG; however, serious complications include difficult-to-remedy proximal leaks (4.9%) and bleeding from the long gastric staple line (2.4%).24,25 The majority of complications associated with VSG occur in the late postoperative period. These include gastroesophageal reflux (23%), vomiting (18%), gastric tube stricture (2.3%; Figure 5), stenosis (2.4%), leak (2.4%), incisional hernia (2.4%), gastrocutaneous fistula, and weight regain.19,23,24 Although the main mechanism of VSG is restriction, it has been reported that the gastric tube may dilate over time, or it may simply dilate as a result of being overly large. A neofundus (proximal dilation of the stomach) may form if too much fundus is left at the time of the original operation.24,26,27 The dilation of the gastric tube or stomach may be an anatomic reason for the lack of weight loss or weight regain, as patients are able to consume larger volumes of food. In addition, the neofundus can cause, or be the result of, relative midstomach stenosis with subsequent food stasis that contributes to symptoms of gastroesophageal reflux.24
Figure 5.
An abdominal contrast radiograph showing a gastric tube stricture after vertical sleeve gastrectomy.
Dumping syndrome is the result of mechanical and hormonal changes that are commonly seen after any gastric drainage procedure and expected after RNYGBP.28 Patients usually complain of sweating, dizziness, palpitations, abdominal pain, nausea, vomiting, and/or diarrhea.28-30 Symptoms are often associated with the ingestion of carbohydrates or other hyperosmotic loads. A recent prospective clinical study, however, suggested that dumping syndrome also occurs after VSG.30 It is hypothesized that VSG increases gastric motility due to increased intraluminal pressure within the remnant stomach, ultimately causing rapid emptying to occur in addition to the known restrictive properties of this procedure.30 The primary management of dumping syndrome is diet modification for the prevention of symptoms. Our personal experience is that dumping syndrome symptoms that occur after VSG do not limit patients’ food selection to the degree that they do after RNYGBP.
Nutritional deficiencies are less common after sleeve gastrectomy compared with operations that cause more drastic diet alterations or involve intestinal bypass; however, these deficiencies do occur with enough frequency that postoperative monitoring is mandatory. Despite the lack of a malabsorptive component, deficiencies in iron (43%), vitamin D (39%), folic acid (15%), vitamin B1 (11%), and vitamin B12 (9%) have been observed after VSG. Interestingly, some patients were noted to have an excess of vitamin A and vitamin B6.31
One main concern regarding the increased popularity of VSG is the general paucity of long-term weight loss and weight regain data. Many other restrictive bariatric operations have failed to match the success of RNYGBP over time.
Roux-en-Y Gastric Bypass
RNYGBP has been the most common weight-loss procedure performed in the United States for the past several decades. Contemporary rates of perioperative and postoperative mortality are 0.38% and 0.72%, respectively.14 This reduction in mortality compared to a previously reported 30-day mortality of 2%32 has been achieved in a time of rapid growth in the volume of the procedure and is comparable to the mortality rate of appendectomy, with pulmonary embolism (not technical surgical issues) being the leading cause of death. In randomized controlled trials, the overall complication rate was 21%.14 Early postoperative serious complications are the minority and include leak, ileus, obstruction, and GI tract hemorrhage.17,33 The most serious complication is an anastomotic leak, which occurs in 0.7% to 5% of patients, with the leak rate significantly lower in contemporary laparoscopic settings.34,35 Leaks occur most commonly at the gastrojejunal anastomosis but have also been noted at the distal esophagus, gastric pouch, remnant stomach, blind jejunal limb, and jejunojejunal anastomosis.34,36 Small bowel obstruction or ileus has been associated with extraluminal leak, with obstruction most often seen at the jejunojejunal anastomosis. Clinical findings of postoperative leaks most commonly include leukocytosis, fever, and/or tachycardia, although typical findings of peritonitis and sepsis may be absent until late in the patient’s clinical course, which can delay diagnosis and treatment.34 Identified risk factors for patients who develop an anastomotic leak include an open operative technique, revision surgery, age older than 50 years, male sex, congestive heart failure, chronic renal failure, and chronic lung disease.35
In regard to early obstruction, the most common culprit is postoperative edema and/or hematoma involving the gastrojejunal or jejunojejunal anastomosis.34,37 If a retrocolic Roux limb is fashioned, the site where the Roux limb crosses the transverse mesocolon is also a location for potential obstruction.34
GI hemorrhage may also develop and is more commonly seen after laparoscopic than open gastric bypass.1,33 Contemporary series suggest that less than 1% of all post-gastric bypass patients experience postoperative bleeding requiring transfusion or intervention, likely the result of improved staple technology and selection.38 The majority (71.4%) of bleeding occurs early from an intraluminal or intra-abdominal source. The potential causes of an intraabdominal bleed include the staple lines (divided gastric remnant, gastrojejunostomy, or jejunojejunostomy), mesenteric vessels, or iatrogenic injury. Almost half of the patients who experience postoperative hemorrhage have undergone prior abdominal surgery requiring adhesiolysis at the time of the bariatric procedure. Patients present with hemodynamic compromise (tachycardia being the most common clinical sign), decreased hemoglobin/hematocrit levels, and/or the need for blood product transfusion.38 However, typical signs of serious bleeding may be delayed in obese patients. Hemostasis may be improved with the use of shorter staple height, oversewn staple-line edges, or staple-line reinforcement materials.33 Less than one-third of patients with intraluminal bleeding will need surgical exploration.38 Late bleeding from ulceration or any other GI source can occur, although it is rare, and should be evaluated and treated in a manner similar to any other GI bleed patient.
Dumping syndrome is expected in the first several months after RNYGBP, with a reported prevalence of severe symptoms as high as 24.3%.29,39 Dumping syndrome results from patients’ inability to regulate gastric emptying of simple carbohydrates or other osmotic loads. Although behavioral modification may initially be beneficial (patients learn to avoid food and dietary habits that initiate dumping syndrome), the symptoms often disappear around 1 year postoperation.39
Long-term complications can result in reoperation in 3% to 20% of patients after RNYGBP.40 Anastomotic stricture is not an infrequent late complication, with the most common site being at the gastrojejunal anastomosis, ranging in occurrence from 4.7% to 16% of patients.33,36,41,42 Patients present with abdominal pain, vomiting, and a progressive decrease in oral intake tolerance often around 8 weeks postoperation; however, strictures can occur at any time after RNYGBP.28,41 The management of anastomotic strictures is usually first approached endoscopically with balloon dilatation. Serial endoscopy with dilation to 15 to 18 mm over several weeks usually results in success. However, recurrence or failure after 3 or 4 endoscopic attempts is usually sufficient evidence that surgical revision will be required. In contrast, jejunojejunal stenosis is a rare complication (0.9%) that is treated with surgical intervention.36
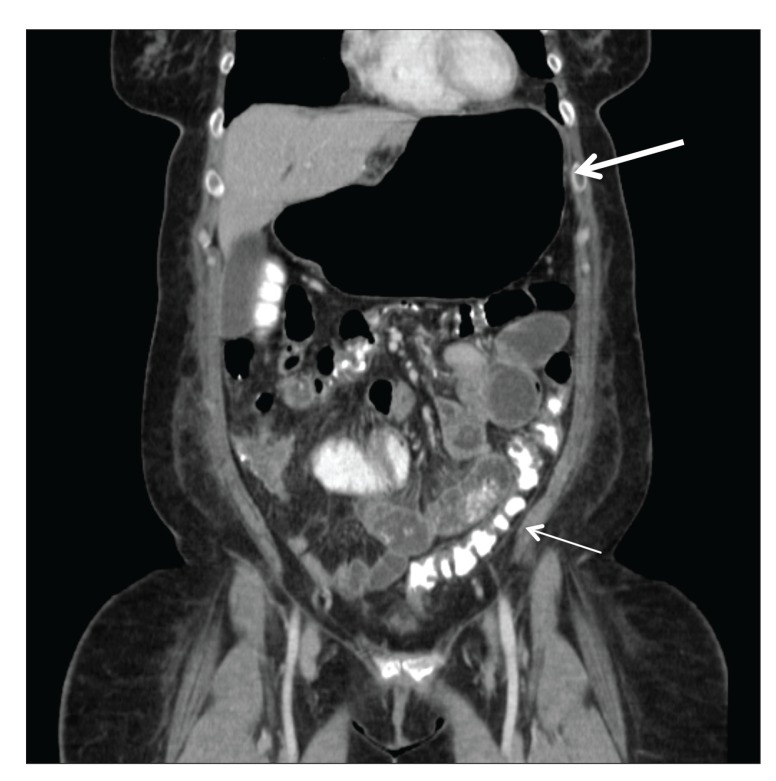
Bowel obstruction has been reported to have an overall incidence of 0% to 5% of patients after laparoscopic RNYGBP, similar to that of other abdominal and pelvic surgeries.28,43 As with other types of bowel obstruction, patients present with abdominal pain, nausea and vomiting, and minimal bowel function after laparoscopic RNYGBP. Etiologies of late small bowel obstruction include adhesions, internal hernias, abdominal wall hernias, and intussusceptions.17,28 Adhesions are reported to be more common after open than laparoscopic surgery, whereas internal hernias are more frequently seen after laparoscopic procedures.1,17,19,36,39 Internal hernias have a reported incidence of 3% to 16% in patients.37,44 The possible locations for internal hernias include the opening of the transverse mesocolon, through which the Roux limb is brought to become connected to the gastric pouch (67%); the small bowel mesenteric defect at the jejunojejunostomy site (21%); and the space between the transverse mesocolon and Roux limb mesentery (known as a Peterson hernia; 7.5%).36,44 Because internal hernias can be elusive from routine radiologic imaging (Figure 6) and devastating in nature, a low threshold for re-exploration is indicated in bariatric patients with unexplained pain or symptoms of bowel obstruction. Incisional hernias were reported to be the most frequent late complication in open gastric bypass, occurring in 8.6% to 20% of patients33,39; however, this complication has decreased dramatically in the laparoscopic era.
Figure 6.
A coronal computed tomography image obtained 1 month after Roux-en-Y gastric bypass demonstrating gastric distention (large arrow) and residual contrast in the colon (small arrow), which is consistent with biliopancreatic limb obstruction. Contrast in the colon is from an earlier upper gastrointestinal barium evaluation that did not demonstrate the obstruction.
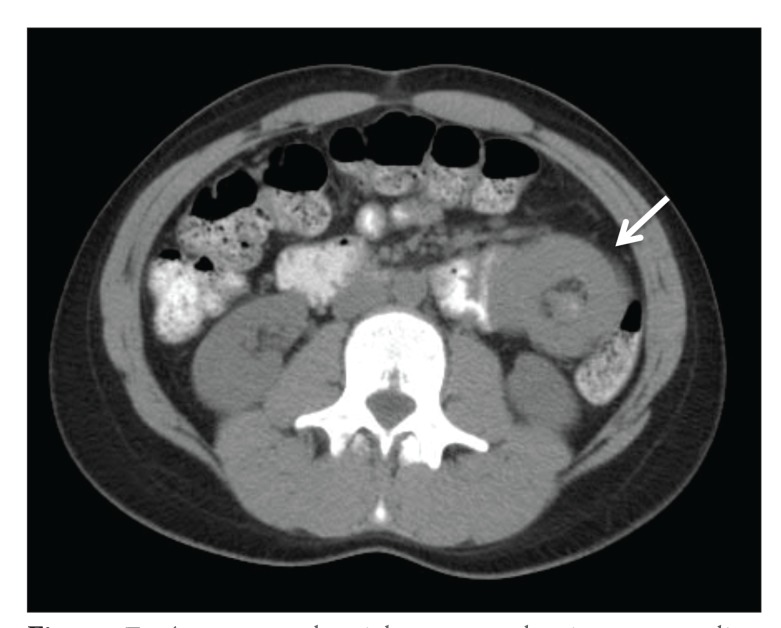
Intussusception is a rare and somewhat unique late complication after gastric bypass that typically occurs after significant weight loss.19,34,40,45 Reported incidence rates range from 0.1% to 1%; however, they may be higher, as there is increased awareness associated with the complication.46-48 It is most commonly seen at the jejunojejunal anastomosis, where the common limb telescopes into the jejunal anastomosis in a retrograde fashion (distal to proximal).19,46,49 Post-bariatric surgery patients do not present with the classic signs of bloody stool, abdominal pain, and palpable mass; however, early detection is possible with imaging (computed tomography with contrast), where the classic target sign can be seen (Figure 7).47,49 This diagnosis is also seen more commonly in women with significant weight loss.48,49 Patients may present several years after surgery.40 The etiology is not well understood but appears to be multifactorial, involving a lead point (suture lines, adhesions, food boluses) or motility disturbances from the ectopic pacemaker development that occurs with the change in anatomy.19,40,45,50 Another possible reason may be the thinning of intestinal mesentery that occurs after surgery due to weight loss that allows increased mobility of the bowel and an unstable region around the site of the Roux limb.40,47 Management can be laparoscopic or open to reduce the intussusception with or without an enteropexy, or resection and revision of the jejunojejunostomy site.49 There is conflicting evidence about whether nonoperative management should be attempted, as a retrospective study reported that 43% of patients who underwent operative exploration for a radiologic diagnosis of retrograde intussusception did not have intraoperative evidence of this complication; however, the consequences for delaying a diagnosis of intussusception can be significant.40,49
Figure 7.
A computed axial tomography image revealing intussusception in the left abdomen 1 year after Roux-en-Y gastric bypass.
A common late postoperative GI complication noted to occur with rapid weight loss from bariatric surgery is gallstone formation.51,52 The incidence of developing gallstones after bariatric surgery ranges from 22% to 71%.6,52,53 In assessing the efficacy of ursodeoxycholic acid in reducing gallstone formation in the setting of gastric banding and RNYGBP, studies have shown significantly lower rates of gallstone formation in these groups compared with patients taking placebo or ibuprofen, respectively.51,52 Therefore, regular use of ursodeoxycholic acid during rapid weight loss (6 months after RNYGBP) is recommended and reported to reduce the rate of gallstone formation to less than 5%.28,54,55
Late postoperative bleeding from ulceration or any GI source occurs at a mean time of 553 days postoperation, although it may occur at any time after surgery.38 Patients usually present with hematemesis, hematochezia, or melena. Bleeding is most often the result of marginal, anastomotic, or gastric pouch ulceration; however, treatment primarily involves supportive measures. Diagnostic and treatment approaches should be similar to those used for any GI bleed patient, with endoscopic evaluation and treatment being the primary approaches. For marginal ulcers, the incidence is 4%, with a mean time to diagnosis from surgery between 22 and 36 months.56-58 Identified risk factors include diabetes, smoking, and gastric pouch length.57 The majority of patients present with epigastric pain, bleeding, and emesis, with the most common site for marginal ulcer formation found on upper endoscopy being (in descending frequency) the gastrojejunal anastomosis, proximal jejunal limb adjacent to the anastomosis, and gastric pouch.57 Management is medically oriented and typically effective with the elimination of offending ulcerogenic medications and the use of a proton pump inhibitor as a single agent or combined with sucralfate; however, some patients require endoscopic removal of a suture material when identified or surgical revision when refractory.57 Routine use of acid inhibitors postoperatively may lower the short-term incidence of marginal ulcers.56
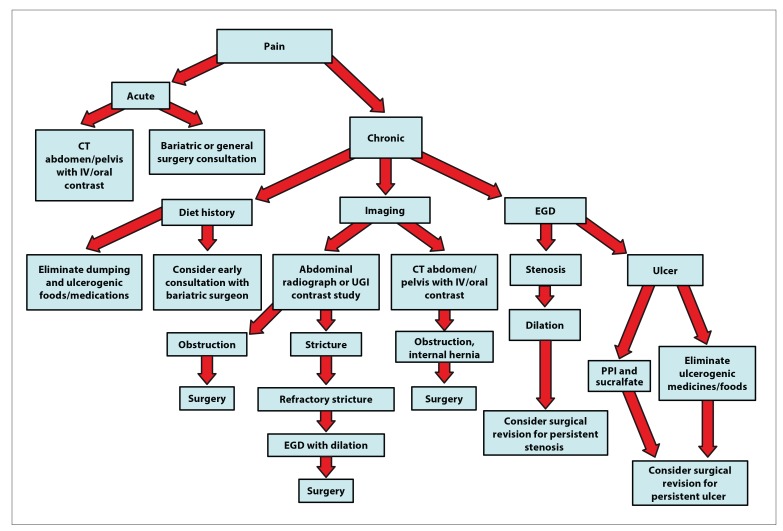
Pain after gastric bypass surgery is a frequent complaint and often simply the result of dietary issues. However, life-threatening conditions can also occur. A thoughtful approach considering the acuity and severity of pain should be a collaborative effort with early discussion with, or referral to, a bariatric surgeon (Figure 8).
Figure 8.
An algorithm for the evaluation of abdominal pain after gastric bypass. Surgical exploration should be considered when pain persists even without findings from the studies listed in the algorithm.
CT, computed tomography; EGD, esophagogastroduodenoscopy; IV, intravenous; PPI, proton pump inhibitor; UGI, upper gastrointestinal.
Nutritional deficiencies that occur with gastric bypass are the result of a partial malabsorptive anatomy, bypass of the stomach, and a prolonged starvation state. Vitamin B12 deficiency is common in the absence of supplementation, as well as deficiencies in thiamine (vitamin B1), iron, calcium, copper, and vitamin D.59,60 One-quarter of patients in a retrospective study were found to have low serum zinc, selenium, and vitamin A levels; however, this resolved in the majority of patients receiving supplementation when evaluated at 2 years after surgery.61 In a recent retrospective study of patients who underwent laparoscopic RNYGBP, 51.3% of patients developed iron deficiency within 1 year after surgery and 22.7% developed iron deficiency anemia.62 Iron deficiency is associated with inadequate dietary intake, diminished absorption, and physiologic or pathologic losses.62 Long-term deficiencies are not well studied but generally improve with weight and diet stabilization and adherence to supplementation recommendations.
Biliopancreatic Diversion With Duodenal Switch
Biliopancreatic diversion with duodenal switch (BPDDS) is expected to result in the greatest weight loss compared with other contemporary bariatric operations due to the malabsorptive nature of the operation (79% estimated weight loss [EWL] for BPDDS vs 67% EWL for RNYGBP); however, this procedure is also associated with the highest rates of complications for both technical and malabsorptive reasons.48,63,64 A meta-analysis of patients who underwent BPDDS suggests that the procedure has the highest early mortality rate compared with other bariatric surgeries at 0.76% for open procedures and 1.11% for laparoscopic procedures.65 Within 30 days, mortality was 1.1%, whereas 90-day mortality was 1.3%, which is higher but comparable to that reported for RNYGBP.66,67 When comparing the results from a randomized, parallel-group trial between RNYGBP and BPDDS, significantly more patients in the BPDDS group had adverse events (62% vs 32%).64 A consecutive series of 1000 patients who underwent BPDDS found that perioperative serious complications occurred in 7% of the patients, with the most common major complications being anastomotic leak, anastomotic stenosis/small bowel obstruction, and GI abdominal hemorrhage.68 Late complications (>30 days) most commonly involved small bowel obstruction, malnutrition, and incisional hernias.66,68 The majority of patients had long-term complaints of diarrhea, abdominal bloating, and malodorous flatus/stool.66,69 Given the significant malabsorption associated with BPDDS, one of the most common complications is derangement in vitamin lev-els.59 Levels of fat-soluble vitamins (A, D, E, K), zinc, iron, selenium, magnesium, and calcium are lower in patients who have undergone BPDDS.59,64,69 However, severe protein deficiency (albumin <30 g/L) was noted in only 1% of patients.70 To minimize nutrient deficiencies, patients require more intensive supplementation postoperatively, and comprehensive monitoring of their nutritional status is mandatory for life.66,70
Revisional Procedures
The primary indication for revisional surgery is treatment of severe side effects (persistent nausea and vomiting, intolerance to solid food, severe dumping syndrome) or complications from prior bariatric procedures (strictures, nonhealing ulcers); however, an increasing number of revisional surgeries are being performed due to inadequate weight loss from the primary procedure.71,72 A revisional procedure can be defined as a conversion, correction, or reversal.73 A meta-analysis of revisional surgeries found mortality rates up to 1.65%, which is higher than those of primary interventions.65 A prospective, multicenter study compared the demographics and outcomes between initial and revisional bariatric surgeries.71 Among heavier patients with more comorbidities who were identified as undergoing subsequent revision, 65% underwent a RNYGBP for revision.71 The mortality in this study was 0.4%, with the revision group (15.1%) having a greater incidence of adverse outcomes compared with the primary group (5.3%).71
Complication or weight loss failure after LAGB is likely the most common reason for revisional operations, comprising up to 73% of all patients undergoing revision.74 Patients are expected to achieve relief of symptoms and appropriate weight loss with conversion to RNYGBP or VSG, most often accomplished laparoscopically with qualified surgeons.72,74,75 The complication rate after revision of failed adjustable banding to gastric bypass is 7%, with the most common complication being wound infection.75
In the 10% to 15% of patients who fail to achieve adequate weight loss or who regain weight after RNYGBP, an anatomic or technical reason is rarely identified. However, surgical intervention can be considered in patients who are identified as having a large gastric pouch or gastrogastric fistula. Additional weight loss can also be achieved with limb lengthening procedures or conversion to BPDDS, although the attendant risks of those primary malabsorptive procedures should be taken into account.75,76
The role of banding after bypass is controversial, although there may be some potential to improve weight loss.76 Major complications have been reported in this setting, including partial small bowel obstruction related to the band, band slippage, and port infection requiring reoperation, with the long-term concern for band erosion likely higher than that of primary LAGB.76
Summary
Bariatric surgery is safe and has become more so as surgeons gain experience in the evaluation and treatment of the obese patient. Although there are several surgical options, each has its own technical and metabolic issues that should be considered when choosing a weight-loss surgery for each individual patient. Complications and mortality have decreased to the extent that the risk-benefit ratio clearly favors a broader application in the medically complicated obese population.
Footnotes
The authors have no relevant conflicts of interest to disclose.
References
- 1.Livingston EH. The incidence of bariatric surgery has plateaued in the U.S. Am J Surg. 2010;200(3):378–385. doi: 10.1016/j.amjsurg.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am J Clin Nutr. 1992;55(2 suppl):615S–619S. doi: 10.1093/ajcn/55.2.615s. [DOI] [PubMed] [Google Scholar]
- 3. Flum DR, Belle SH, King WC, et al. Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Perioperative safety in the longitudinal assessment of bariatric surgery N Engl J Med. 2009. 361 5 445 454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flegal KM. Epidemiologic aspects of overweight and obesity in the United States. Physiol Behav. 2005;86(5):599–602. doi: 10.1016/j.physbeh.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 5.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 6.Bult MJ, van Dalen T, Muller AF. Surgical treatment of obesity. Eur J Endocrinol. 2008;158(2):135–145. doi: 10.1530/EJE-07-0145. [DOI] [PubMed] [Google Scholar]
- 7.Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L NEDCOM, the Netherlands Epidemiology and Demography Compression of Morbidity Research Group. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med. 2003;138(1):24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- 8.Schneider BE, Mun EC. Surgical management of morbid obesity. Diabetes Care. 2005;28(2):475–480. doi: 10.2337/diacare.28.2.475. [DOI] [PubMed] [Google Scholar]
- 9.Suter M, Giusti V, Worreth M, Héraief E, Calmes JM. Laparoscopic gastric banding: a prospective, randomized study comparing the Lapband and the SAGB: early results. Ann Surg. 2005;241(1):55–62. doi: 10.1097/01.sla.0000150071.86934.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB. Annual deaths attributable to obesity in the United States. JAMA. 1999;282(16):1530–1538. doi: 10.1001/jama.282.16.1530. [DOI] [PubMed] [Google Scholar]
- 11.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005;294(15):1909–1917. doi: 10.1001/jama.294.15.1909. [DOI] [PubMed] [Google Scholar]
- 12.Steinbrook R. Surgery for severe obesity. N Engl J Med. 2004;350(11):1075–1079. doi: 10.1056/NEJMp048029. [DOI] [PubMed] [Google Scholar]
- 13.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2008. Obes Surg. 2009;19(12):1605–1611. doi: 10.1007/s11695-009-0014-5. [DOI] [PubMed] [Google Scholar]
- 14.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149(3):275–287. doi: 10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sjöström L, Narbro K, Sjöström CD, et al. Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 16.Arterburn DE, Olsen MK, Smith VA, et al. Association between bariatric surgery and long-term survival. JAMA. 2015;313(1):62–70. doi: 10.1001/jama.2014.16968. [DOI] [PubMed] [Google Scholar]
- 17.Carucci LR, Turner MA. Imaging after bariatric surgery for morbid obesity: Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding. Semin Roentgenol. 2009;44(4):283–296. doi: 10.1053/j.ro.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 18.DeMaria EJ. Laparoscopic adjustable silicone gastric banding: complications. J Laparoendosc Adv Surg Tech A. 2003;13(4):271–277. doi: 10.1089/109264203322333601. [DOI] [PubMed] [Google Scholar]
- 19.Hamdan K, Somers S, Chand M. Management of late postoperative complications of bariatric surgery. Br J Surg. 2011;98(10):1345–1355. doi: 10.1002/bjs.7568. [DOI] [PubMed] [Google Scholar]
- 20. Snyder B, Wilson T, Mehta S, et al. Past, present, and future: critical analysis of use of gastric bands in obese patients Diabetes Metab Syndr Obes. 2010;3:55 65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.OvrebØ KK, Hatlebakk JG, Viste A, Bass0e HH, Svanes K. Gastroesophageal reflux in morbidly obese patients treated with gastric banding or vertical banded gastroplasty. Ann Surg. 1998;228(1):51–58. doi: 10.1097/00000658-199807000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23(4):427–436. doi: 10.1007/s11695-012-0864-0. [DOI] [PubMed] [Google Scholar]
- 23.Triantafyllidis G, Lazoura O, Sioka E, et al. Anatomy and complications following laparoscopic sleeve gastrectomy: radiological evaluation and imaging pitfalls. Obes Surg. 2011;21(4):473–478. doi: 10.1007/s11695-010-0236-6. [DOI] [PubMed] [Google Scholar]
- 24.Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252(2):319–324. doi: 10.1097/SLA.0b013e3181e90b31. [DOI] [PubMed] [Google Scholar]
- 25.Topart P, Becouarn G, Ritz P. Comparative early outcomes of three laparoscopic bariatric procedures: sleeve gastrectomy, Roux-en-Y gastric bypass, and biliopancreatic diversion with duodenal switch. Surg Obes Relat Dis. 2012;8(3):250–254. doi: 10.1016/j.soard.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Braghetto I, Csendes A, Lanzarini E, Papapietro K, Cárcamo C, Molina JC. Is laparoscopic sleeve gastrectomy an acceptable primary bariatric procedure in obese patients? Early and 5-year postoperative results. Surg Laparosc Endosc Percutan Tech. 2012;22(6):479–486. doi: 10.1097/SLE.0b013e318262dc29. [DOI] [PubMed] [Google Scholar]
- 27.Baltasar A, Serra C, Pérez N, Bou R, Bengochea M. Re-sleeve gastrectomy. Obes Surg. 2006;16(11):1535–1538. doi: 10.1381/096089206778869924. [DOI] [PubMed] [Google Scholar]
- 28.Swanson CM, Roust LR, Miller K, Madura JA II. What every hospitalist should know about the post-bariatric surgery patient. J Hosp Med. 2012;7(2):156–163. doi: 10.1002/jhm.939. [DOI] [PubMed] [Google Scholar]
- 29.Padoin AV, Galvão Neto M, Moretto M, Barancelli F, Schroer CE, Mottin CC. Obese patients with type 2 diabetes submitted to banded gastric bypass: greater incidence of dumping syndrome. Obes Surg. 2009;19(11):1481–1484. doi: 10.1007/s11695-009-9943-2. [DOI] [PubMed] [Google Scholar]
- 30.Tzovaras G, Papamargaritis D, Sioka E, et al. Symptoms suggestive of dumping syndrome after provocation in patients after laparoscopic sleeve gastrectomy. Obes Surg. 2012;22(1):23–28. doi: 10.1007/s11695-011-0461-7. [DOI] [PubMed] [Google Scholar]
- 31. Aarts EO, Janssen IM, Berends FJ. The gastric sleeve: losing weight as fast as micronutrients? Obes Surg. 2011. 21 2 207 211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flum DR, Salem L, Elrod JA, Dellinger EP, Cheadle A, Chan L. Early mortality among Medicare beneficiaries undergoing bariatric surgical procedures. JAMA. 2005;294(15):1903–1908. doi: 10.1001/jama.294.15.1903. [DOI] [PubMed] [Google Scholar]
- 33.Podnos YD, Jimenez JC, Wilson SE, Stevens CM, Nguyen NT. Complications after laparoscopic gastric bypass: a review of 3464 cases. Arch Surg. 2003;138(9):957–961. doi: 10.1001/archsurg.138.9.957. [DOI] [PubMed] [Google Scholar]
- 34.Carucci LR, Turner MA, Conklin RC, DeMaria EJ, Kellum JM, Sugerman HJ. Roux-en-Y gastric bypass surgery for morbid obesity: evaluation of postoperative extraluminal leaks with upper gastrointestinal series. Radiology. 2006;238(1):119–127. doi: 10.1148/radiol.2381041557. [DOI] [PubMed] [Google Scholar]
- 35.Masoomi H, Kim H, Reavis KM, Mills S, Stamos MJ, Nguyen NT. Analysis of factors predictive of gastrointestinal tract leak in laparoscopic and open gastric bypass. Arch Surg. 2011;146(9):1048–1051. doi: 10.1001/archsurg.2011.203. [DOI] [PubMed] [Google Scholar]
- 36.Blachar A, Federle MP. Gastrointestinal complications of laparoscopic Rouxen-Y gastric bypass surgery in patients who are morbidly obese: findings on radiography and CT. AJR Am J Roentgenol. 2002;179(6):1437–1442. doi: 10.2214/ajr.179.6.1791437. [DOI] [PubMed] [Google Scholar]
- 37.Higa K, Ho T, Tercero F, Yunus T, Boone KB. Laparoscopic Roux-en-Y gastric bypass: 10-year follow-up. Surg Obes Relat Dis. 2011;7(4):516–525. doi: 10.1016/j.soard.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 38.Heneghan HM, Meron-Eldar S, Yenumula P, Rogula T, Brethauer SA, Schauer PR. Incidence and management of bleeding complications after gastric bypass surgery in the morbidly obese. Surg Obes Relat Dis. 2012;8(6):729–735. doi: 10.1016/j.soard.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 39.McMahon MM, Sarr MG, Clark MM, et al. Clinical management after bariatric surgery: value of a multidisciplinary approach. Mayo Clin Proc. 2006;81(10 suppl):S34–S45. doi: 10.1016/s0025-6196(11)61179-8. [DOI] [PubMed] [Google Scholar]
- 40.Daellenbach L, Suter M. Jejunojejunal intussusception after Roux-en-Y gastric bypass: a review. Obes Surg. 2011;21(2):253–263. doi: 10.1007/s11695-010-0298-5. [DOI] [PubMed] [Google Scholar]
- 41.Go MR, Muscarella P, II, Needleman BJ, Cook CH, Melvin WS. Endoscopic management of stomal stenosis after Roux-en-Y gastric bypass. Surg Endosc. 2004;18(1):56–59. doi: 10.1007/s00464-003-8919-x. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen NT, Stevens CM, Wolfe BM. Incidence and outcome of anastomotic stricture after laparoscopic gastric bypass. J Gastrointest Surg. 2003;7(8):997–1003. doi: 10.1016/j.gassur.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 43.Husain S, Ahmed AR, Johnson J, Boss T, O’Malley W. Small-bowel obstruction after laparoscopic Roux-en-Y gastric bypass: etiology, diagnosis, and management. Arch Surg. 2007;142(10):988–993. doi: 10.1001/archsurg.142.10.988. [DOI] [PubMed] [Google Scholar]
- 44.Higa KD, Ho T, Boone KB. Internal hernias after laparoscopic Roux-en-Y gastric bypass: incidence, treatment and prevention. Obes Surg. 2003;13(3):350–354. doi: 10.1381/096089203765887642. [DOI] [PubMed] [Google Scholar]
- 45.Edwards MA, Grinbaum R, Ellsmere J, Jones DB, Schneider BE. Intussusception after Roux-en-Y gastric bypass for morbid obesity: case report and literature review of rare complication. Surg Obes Relat Dis. 2006;2(4):483–489. doi: 10.1016/j.soard.2006.04.232. [DOI] [PubMed] [Google Scholar]
- 46.Simper SC, Erzinger JM, McKinlay RD, Smith SC. Retrograde (reverse) jejunal intussusception might not be such a rare problem: a single group’s experience of 23 cases. Surg Obes Relat Dis. 2008;4(2):77–83. doi: 10.1016/j.soard.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Yin J, Hou X. Complications of laparoscopic versus open bariatric surgical interventions in obesity management. Cell Biochem Biophys. 2014;70(2):721–728. doi: 10.1007/s12013-014-0041-2. [DOI] [PubMed] [Google Scholar]
- 48.Baptista V, Wassef W. Bariatric procedures: an update on techniques, outcomes and complications. Curr Opin Gastroenterol. 2013;29(6):684–693. doi: 10.1097/MOG.0b013e3283651af2. [DOI] [PubMed] [Google Scholar]
- 49.Varban O, Ardestani A, Azagury D, et al. Resection or reduction? The dilemma of managing retrograde intussusception after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2013;9(5):725–730. doi: 10.1016/j.soard.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Hocking MP, McCoy DM, Vogel SB, Kaude JV, Sninsky CA. Antiperistaltic and isoperistaltic intussusception associated with abnormal motility after Roux-en-Y gastric bypass: a case report. Surgery. 1991;110(1):109–112. [PubMed] [Google Scholar]
- 51.Miller K, Hell E, Lang B, Lengauer E. Gallstone formation prophylaxis after gastric restrictive procedures for weight loss: a randomized double-blind placebo-controlled trial. Ann Surg. 2003;238(5):697–702. doi: 10.1097/01.sla.0000094305.77843.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wudel LJ, Jr, Wright JK, Debelak JP, Allos TM, Shyr Y, Chapman WC. Prevention of gallstone formation in morbidly obese patients undergoing rapid weight loss: results of a randomized controlled pilot study. J Surg Res. 2002;102(1):50–56. doi: 10.1006/jsre.2001.6322. [DOI] [PubMed] [Google Scholar]
- 53. Villegas L, Schneider B, Provost D, et al. Is routine cholecystectomy required during laparoscopic gastric bypass? Obes Surg. 2004. 14 2 206 211 [DOI] [PubMed] [Google Scholar]
- 54.Lynch RJ, Eisenberg D, Bell RL. Metabolic consequences of bariatric surgery. J Clin Gastroenterol. 2006;40(8):659–668. doi: 10.1097/00004836-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 55.Sugerman HJ, Brewer WH, Shiffman ML, et al. A multicenter, placebocontrolled, randomized, double-blind, prospective trial of prophylactic ursodiol for the prevention of gallstone formation following gastric-bypass-induced rapid weight loss. Am J Surg. 1995;169(1):91–96. doi: 10.1016/s0002-9610(99)80115-9. [DOI] [PubMed] [Google Scholar]
- 56.Gumbs AA, Duffy AJ, Bell RL. Incidence and management of marginal ulceration after laparoscopic Roux-Y gastric bypass. Surg ObesRelat Dis. 2006;2(4):460–463. doi: 10.1016/j.soard.2006.04.233. [DOI] [PubMed] [Google Scholar]
- 57.Azagury DE, Abu Dayyeh BK, Greenwalt IT, Thompson CC. Marginal ulceration after Roux-en-Y gastric bypass surgery: characteristics, risk factors, treatment, and outcomes. Endoscopy. 2011;43(11):950–954. doi: 10.1055/s-0030-1256951. [DOI] [PubMed] [Google Scholar]
- 58.Csendes A, Torres J, Burgos AM. Late marginal ulcers after gastric bypass for morbid obesity. Clinical and endoscopic findings and response to treatment. Obes Surg. 2011;21(9):1319–1322. doi: 10.1007/s11695-011-0429-7. [DOI] [PubMed] [Google Scholar]
- 59.Aills L, Blankenship J, Buffington C, Furtado M, Parrott J Allied Health Sciences Section Ad Hoc Nutrition Committee. ASMBS Allied Health Nutritional Guidelines for the Surgical Weight Loss Patient. Surg Obes Relat Dis. 2008;4(5 suppl):S73–S108. doi: 10.1016/j.soard.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 60. Saltzman E, Karl JP. Nutrient deficiencies after gastric bypass surgery Annu Rev Nutr. 2013;33:183 203 [DOI] [PubMed] [Google Scholar]
- 61.Gong K, Gagner M, Pomp A, Almahmeed T, Bardaro SJ. Micronutrient deficiencies after laparoscopic gastric bypass: recommendations. Obes Surg. 2008;18(9):1062–1066. doi: 10.1007/s11695-008-9577-9. [DOI] [PubMed] [Google Scholar]
- 62.Obinwanne KM, Fredrickson KA, Mathiason MA, Kallies KJ, Farnen JP, Kothari SN. Incidence, treatment, and outcomes of iron deficiency after laparoscopic Roux-en-Y gastric bypass: a 10-year analysis. J Am Coll Surg. 2014;218(2):246–252. doi: 10.1016/j.jamcollsurg.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 63.Nelson DW, Blair KS, Martin MJ. Analysis of obesity-related outcomes and bariatric failure rates with the duodenal switch vs gastric bypass for morbid obesity. Arch Surg. 2012;147(9):847–854. doi: 10.1001/archsurg.2012.1654. [DOI] [PubMed] [Google Scholar]
- 64.Søvik TT, Aasheim ET, Taha O, et al. Weight loss, cardiovascular risk factors, and quality of life after gastric bypass and duodenal switch: a randomized trial. Ann Intern Med. 2011;155(5):281–291. doi: 10.7326/0003-4819-155-5-201109060-00005. [DOI] [PubMed] [Google Scholar]
- 65.Buchwald H, Estok R, Fahrbach K, Banel D, Sledge I. Trends in mortality in bariatric surgery: a systematic review and meta-analysis. Surgery. 2007;142(4):621632; discussion 632–625. doi: 10.1016/j.surg.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 66.Marceau P, Biron S, Hould FS, et al. Duodenal switch: long-term results. Obes Surg. 2007;17(11):1421–1430. doi: 10.1007/s11695-008-9435-9. [DOI] [PubMed] [Google Scholar]
- 67.DeMaria EJ, Portenier D, Wolfe L. Obesity surgery mortality risk score: proposal for a clinically useful score to predict mortality risk in patients undergoing gastric bypass. Surg Obes Relat Dis. 2007;3(2):134–140. doi: 10.1016/j.soard.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 68.Biertho L, Lebel S, Marceau S, et al. Perioperative complications in a consecutive series of 1000 duodenal switches. Surg Obes Relat Dis. 2013;9(1):63–68. doi: 10.1016/j.soard.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 69.Dolan K, Hatzifotis M, Newbury L, Lowe N, Fielding G. A clinical and nutritional comparison of biliopancreatic diversion with and without duodenal switch. Ann Surg. 2004;240(1):51–56. doi: 10.1097/01.sla.0000129280.68540.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Biertho L, Biron S, Hould FS, Lebel S, Marceau S, Marceau P. Is biliopancreatic diversion with duodenal switch indicated for patients with body mass index <50 kg/m2? Surg Obes Relat Dis. 2010;6(5):508–514. doi: 10.1016/j.soard.2010.03.285. [DOI] [PubMed] [Google Scholar]
- 71.Inabnet WB, III, Belle SH, Bessler M, et al. Comparison of 30-day outcomes after non-LapBand primary and revisional bariatric surgical procedures from the Longitudinal Assessment of Bariatric Surgery study. Surg Obes Relat Dis. 2010;6(1):22–30. doi: 10.1016/j.soard.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spivak H, Beltran OR, Slavchev P, Wilson EB. Laparoscopic revision from LAP-BAND to gastric bypass. Surg Endosc. 2007;21(8):1388–1392. doi: 10.1007/s00464-007-9223-y. [DOI] [PubMed] [Google Scholar]
- 73.Brethauer SA, Kothari S, Sudan R, et al. Systematic review on reoperative bariatric surgery: American Society for Metabolic and Bariatric Surgery Revision Task Force. Surg Obes Relat Dis. 2014;10(5):952–972. doi: 10.1016/j.soard.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 74.Kruger RS, Pricolo VE, Streeter TT, Colacchio DA, Andrade UA. A bariatric surgery center of excellence: operative trends and long-term outcomes. J Am Coll Surg. 2014;218(6):1163–1174. doi: 10.1016/j.jamcollsurg.2014.01.056. [DOI] [PubMed] [Google Scholar]
- 75.Gumbs AA, Pomp A, Gagner M. Revisional bariatric surgery for inadequate weight loss. Obes Surg. 2007;17(9):1137–1145. doi: 10.1007/s11695-007-9209-9. [DOI] [PubMed] [Google Scholar]
- 76.Bessler M, Daud A, DiGiorgi MF, et al. Adjustable gastric banding as revisional bariatric procedure after failed gastric bypass—intermediate results. Surg Obes Relat Dis. 2010;6(1):31–35. doi: 10.1016/j.soard.2009.09.018. [DOI] [PubMed] [Google Scholar]