G&H When was lymphocytic esophagitis first reported?
RG In 2006, Carlos Rubio, a pathologist at the Karolinska Institutet in Stockholm, Sweden, and colleagues described the esophageal biopsy specimens of 20 patients demonstrating a histologic phenotype of chronic esophagitis, in which the esophageal epithelium was infiltrated by a large number of lymphocytes but not by granulocytes. Eight patients in that study had Crohn’s disease (CD), so Rubio and colleagues suggested that lymphocytic esophagitis (LyE) was a possible associated feature of CD.
G&H What are the clinical symptoms and characteristics of LyE at presentation?
RG LyE affects predominantly older women, with a median age of 63 years. Most patients present with complaints of dysphagia or odynophagia, GERD, nausea, heartburn, and vomiting, and a few patients present with impaction.
G&H What differentiates LyE from other esophageal disorders, such as GERD or eosinophilic esophagitis?
RG Most patients with GERD have very little, if any, pathologic findings in their squamous epithelium. Whereas biopsies from patients with LyE show lymphocytic infiltration in the basal and peripapillary regions and significant spongiosis, approximately 70% to 80% of patients biopsied for GERD have either a normal esophageal epithelium or only minor reactive changes. Massive lymphocytic infiltrations are a feature of LyE, not of reflux-induced damage (Figure 2). What differentiates LyE from eosinophilic esophagitis (EoE) is the absence of eosinophils.
G&H Does LyE have any clinical correlations?
RG Not many studies have been published in this area, but from what is available, it seems that LyE has little relationship to any other conditions, including celiac disease, GERD, ulcerative colitis, allergies, or irritants. Even CD, which was found to be quite common in the series reported by Rubio and colleagues, was not significantly associated with LyE in the study from the University of Michigan.
G&H What were the findings of this study?
RG From a database of more than 100,000 patients with esophageal biopsies, we extracted all cases that either had the words “lymphocytic esophagitis” in the diagnosis or contained comments in the description saying that there were many lymphocytes. After reviewing all of the slides, we identified approximately 100 patients who met our criteria (large amounts of lymphocytes, marked spongiosis, and no eosinophils) and followed up with clinicians to record medical history, symptoms, endoscopic findings, and demographics. We concluded that patients with LyE tended to present with clinical manifestations similar to those of patients with EoE, and, in fact, most of the patients with LyE had an upper endoscopy specifically to investigate the possibility of EoE. Endoscopically, LyE patients lacked the distinctive rings and furrows often found in EoE. Demographically, in contrast to EoE, which more commonly affects younger males, LyE showed a preponderance of older women.
G&H What steps are taken to diagnose LyE?
RG Many cases of LyE are incorrectly diagnosed because the condition is not widely known. Motility studies and scans are not helpful because there is no standard interpretation of those results. The simplest way to diagnose LyE is to obtain a biopsy. Because lymphocytic infiltrations may be absent in one biopsy and present in another, clinicians should acquire multiple sets of biopsies, and the samples should be seen by a pathologist who is aware of this entity and does not dismiss the lymphocytic infiltrates with the all-too-common and unhelpful diagnosis of “chronic inflammation.” The main criteria to look for are a high number of intraepithelial lymphocytes in peri-papillary areas, severe spongiosis, and the absence of both granulocytes and eosinophils.
G&H Are there any treatment options available?
RG The clinical course of LyE—whether the condition resolves, remains, or evolves if left untreated—is unknown. Many patients with LyE are already on proton pump inhibitors (PPIs) with no effect, so PPIs cannot be advocated as a therapy. There are patients who have dysphagia and are taking PPIs and trying to adjust their feeding habits accordingly, but their symptoms do not improve. There are too few patients with LyE to know what to do. We need follow-up studies of patients and studies comparing patients on PPIs to patients not on PPIs to see what, if anything, changes.
Two years later, a group of gastroenterologists and pathologists at the University of Michigan looked at 42 cases of LyE and did not find an association with other conditions, including CD. The researchers concluded that LyE is not a disease, but a histopathologic feature. It is uncertain whether LyE is an inflammatory response to esophageal injury from other conditions, such as gastroesophageal reflux disease (GERD). LyE is currently described as a histologic pattern of injury, and the underlying etiology remains unclear.
The histologic features that Rubio and colleagues described in their initial findings remain accurate: an absence of eosinophils; a large number of lymphocytes in the peripapillary region; intercellular edema, or spongiosis, in the affected areas; and esophageal rings and nodules (Figure 1).
Because of the paucity of clinical information and the lack of guidelines for the diagnosis of LyE, Dr Salima Haque and I at Miraca Life Sciences conducted a review of biopsies to propose some criteria for the histopathologic diagnosis of LyE and to explore its associations.
From this study, we have come to believe that LyE may be either a phase of EoE (preceding or following the condition) or a type of parallel condition. We are currently looking at patients who had esophageal biopsies before they were diagnosed with LyE and are trying to determine the course of LyE by studying patients with follow-up biopsies. We are still studying this disorder and are particularly intrigued by the finding that some patients with LyE eventually develop EoE.
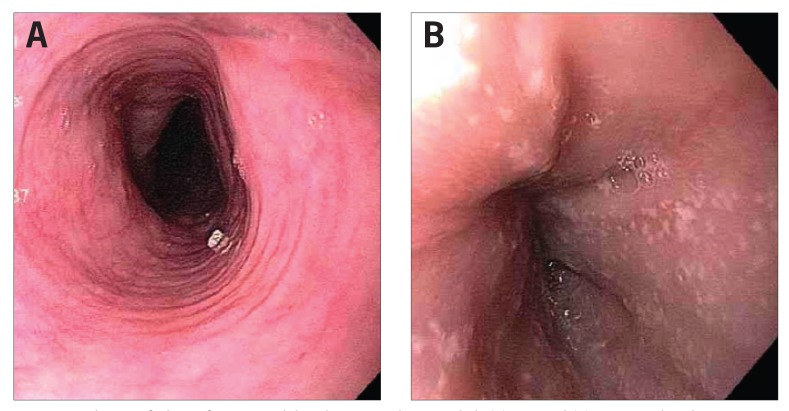
Figure 1.
Endoscopic findings of patients with lymphocytic esophagitis include (A) rings and (B) punctate white dots.
Reproduced from Dunbar KB, Ayyar B, Spechler SJ, Genta R, Melton S. Clinical, endoscopic and histological features of patients with lymphocytic esophagitis compared to patients with GERD. Poster presented at: 45th Annual Digestive Disease Week; May 3-6, 2014; Chicago, IL. Abstract Mo1846.
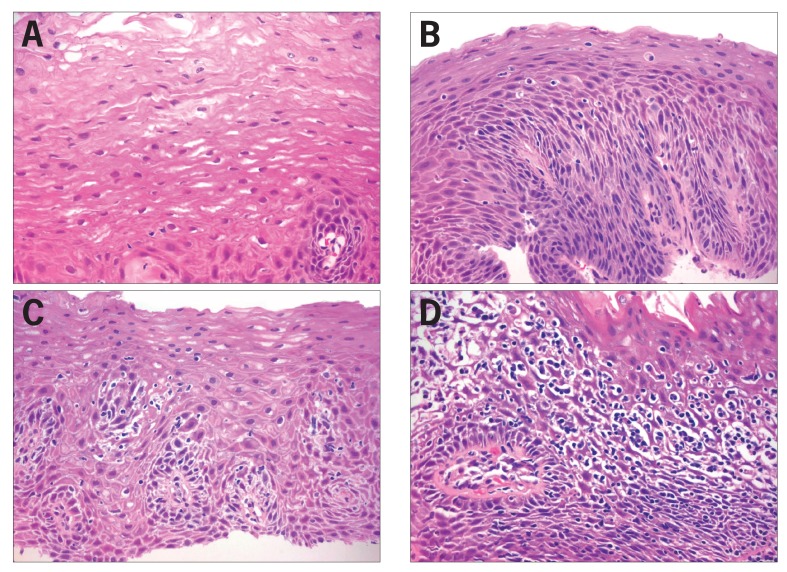
Figure 2.
Histopathologic findings.
(A) Normal esophageal mucosa. Nonkeratinizing stratified squamous epithelium is comprised of large polygonal eosinophilic cells, arising from a basal cell layer of cuboidal basophilic cells. The lower border of the squamous epithelium is irregular due to folds of the lamina propria housing fibrovascular cores (papillae). The basal zone normally occupies less than 15% of the thickness of the epithelium, whereas the lamina propria papillae normally project to no more than half of the thickness. The cell-to-cell borders are indistinct above the basal layer, and there are no inflammatory cells present. (400× magnification, hematoxylin and eosin stain.)
(B) Gastroesophageal reflux disease. Active esophagitis is shown with rare neutrophils and scattered intraepithelial lymphocytes, basal cell hyperplasia (>15% mucosal thickness), elongation of the lamina propria papillae, impaired surface maturation, and minimal intercellular edema. (400× magnification, hematoxylin and eosin stain.)
(C) Mild lymphocytic esophagitis. Increased intraepithelial lymphocytes, predominately peripapillary, and spongiosis of the peripapillary and basal zone intercellular spaces can be seen. Neither neutrophils nor basal cell hyperplasia are present. (400× magnification, hematoxylin and eosin stain.)
(D) Marked lymphocytic esophagitis. The mucosal architecture is almost completely obscured by marked spongiosis and basal and peripapillary intraepithelial lymphocytosis. No neutrophils are seen. (400× magnification, hematoxylin and eosin stain.)
Reproduced from Dunbar KB, Ayyar B, Spechler SJ, Genta R, Melton S. Clinical, endoscopic and histological features of patients with lymphocytic esophagitis compared to patients with GERD. Poster presented at: 45th Annual Digestive Disease Week; May 3-6, 2014; Chicago, IL. Abstract Mo1846.
Biography

Footnotes
Dr Genta is an employee of Miraca Life Sciences.
Suggested Reading
- Cohen S, Saxena A, Waljee AK, et al. Lymphocytic esophagitis: a diagnosis of increasing frequency. J Clin Gastroenterol. 2012;46(10):828–832. doi: 10.1097/MCG.0b013e3182500de8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque S, Genta RM. Lymphocytic oesophagitis: clinicopathological aspects of an emerging condition. Gut. 2012;61(8):1108–1114. doi: 10.1136/gutjnl-2011-301014. [DOI] [PubMed] [Google Scholar]
- Purdy JK, Appelman HD, Golembeski CP, McKenna BJ. Lymphocytic esophagitis: a chronic or recurring pattern of esophagitis resembling allergic contact dermatitis. Am J Clin Pathol. 2008;130(4):508–513. doi: 10.1309/D3PCF6D6YYMQRX9A. [DOI] [PubMed] [Google Scholar]
- Rubio CA, Sjödahl K, Lagergren J. Lymphocytic esophagitis: a histologic subset of chronic esophagitis. Am J Clin Pathol. 2006;125(3):432–437. [PubMed] [Google Scholar]