Abstract
Background
Pancreatic ductal adenocarcinoma (PDAC), which continues to have a dismal prognosis, is associated with a pronounced fibro-inflammatory response. Inflammation in vivo can be mediated by 5-lipoxygenase (5LO), an enzyme that converts omega-6 fatty acids to eicosanoids, including leukotriene B4 (LTB4). We have previously shown that diets rich in omega-6 fatty acids (FA) increase pancreatic lesions and mast cell infiltration in EL-Kras mice. In this study, we evaluated the role of 5LO in generating higher levels of LTB4 from human cells and in mediating lesion development and mast cell infiltration in EL-Kras mice.
Materials and Methods
Human pancreatic ductal epithelial (HPDE) and cancer cells were treated with omega-6 FA in vitro. EL-Kras mice lacking 5LO (EL-Kras/5LO−/−) mice were generated and fed standard chow or omega-6 FA diets. Pancreatic lesion frequency and mast cell infiltration were compared to EL-Kras/5LO+/+ mice. Human PDAC tumors were evaluated for 5LO expression and mast cells.
Results
HPDE and cancer cells treated with omega-6 FA generated increased LTB4 levels in vitro. EL-Kras/5LO−/− developed fewer pancreatic lesions and had decreased mast cell infiltration when compared to EL-Kras/5LO+/+ mice. Human PDAC tumors with increased 5LO expression demonstrate increased mast cell infiltration. Additionally, diets rich in omega-6 FA failed to increase pancreatic lesion development and mast cell infiltration in EL-Kras/5LO−/− mice.
Conclusions
The expansion of mutant Kras-induced lesions via omega-6 FA is dependent on 5LO, and 5LO functions downstream of mutant Kras to mediate inflammation, suggesting that 5LO may be a potential chemo-preventive and therapeutic target in pancreatic cancer.
Keywords: pancreatic cancer, inflammation, 5-lipoxygenase, omega-6 fatty acids
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC), the fourth leading cause of cancer-related deaths in the US, is associated with a pronounced fibro-inflammatory response, which can contribute to cancer progression at all stages of tumor development [1, 2]. This is further supported by the fact that chronic pancreatitis is associated with an increased risk of developing PDAC [3]. Obesity, which is also associated with an increased risk of developing PDAC, has been identified as a chronic inflammatory state [4]. It is possible that chronic inflammation associated with obesity may be driven by diets that are rich in fat [5]. Omega-6 fatty acids provide substrate for the production of inflammatory eicosanoids by the enzymes lipoxygenase and cyclooxygenase [6]. Obese patients have been found to have higher levels of serum fatty acids as well as serum and urinary leukotrienes [7]. While cyclooxygenases have been extensively studied [8, 9], the role of lipoxygenases in PDAC remains relatively undefined.
5-lipoxygenase (5LO) is upregulated in multiple cancer types, including human PDAC tumors, and LTB4 receptors (primarily BLT4) are also upregulated in pancreatic cancer [10]. 5LO can metabolize arachidonic acid, generated from omega-6 fatty acids, to produce the leukotriene LTB4 which is a known chemotactic factor for inflammatory cells such as mast cell and neutrophils [11–14]. Notably, we have previously shown that mast cells are increased in human PDAC tumors compared to adjacent normal tissue, and can also contribute to tumor development [15]. We have also shown that diets rich in omega-6 fatty acids increase pancreatic lesions and mast cell infiltration in EL-Kras transgenic mice [16].
In this study we evaluated the role of 5LO in mediating lesion development and mast cell infiltration in EL-Kras mice. Initially, we showed that human pancreatic ductal epithelial (HPDE and HPDE-Kras) and cancer (AsPC1 and Panc1) cells treated with omega-6 fatty acids generate increased LTB4 levels in vitro. We next showed that EL-Kras mice lacking 5LO (EL-Kras/5LO−/−) developed fewer pancreatic lesions and had decreased mast cell infiltration when compared to EL-Kras/5LO+/+ mice. Additionally, we showed that human PDAC tumors with increased 5LO expression demonstrated increased mast cell infiltration. Importantly, diets rich in omega-6 fatty acids failed to increase pancreatic lesion development and mast cell infiltration in EL-Kras/5LO−/− mice. Thus, our findings indicate that 5LO functions downstream of Kras to mediate inflammation and suggests that 5LO may be a potential chemo-preventive and therapeutic target in pancreatic cancer.
MATERIALS AND METHODS
Cell Culture
HPDE and HPDE-Kras cells were obtained from Dr. Ming Sound-Tsao [17], while AsPC1 and Panc1 cells were obtained from the American Type Culture Collection (Manassas, VA). HPDE and HPDE-Kras cells were grown in keratinocyte serum-free media (KSFM) supplemented with bovine pituitary extract (BPE) and epidermal growth factor (EGF), while AsPC1 and Panc1 cells were cultured in Dulbecco's Modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum. Cells were allowed to grow to approximately 50% confluency, serum-starved overnight and then treated with media containing 40 µM of linoleic acid (LA) using BSA (8.3 µM) as a carrier or BSA alone. After 48 hours the conditioned media was collected, filtered and analyzed for LTB4.
Antibodies/Reagents
The LTB4 ELISA kit (KGE006B) was purchased from R&D Systems (Minneapolis, MN). Reagents for chloroacetate (CAE) and toluidine blue (TB) were purchased from Sigma (St. Louis, MO). 5LO antibody (C49G1) was purchased from Cell Signaling (Danvers, MA).
Establishment of transgenic mice and study groups
Previously, EL-Kras transgenic mice were generated by microinjection of FVB/N fertilized mouse embryos and subsequent implantation into pseudopregnant females as described in a prior publication [18]. EL-Kras FVB/N mice were made congenic on a C57/BL6 (B6) background following 12+ generations of crossing onto B6. Cohorts of EL-Kras B6 mice were crossed into 5LO KO B6 mice (B6.129S2-Alox5tm1Fun/J (JAX) [19] to generate EL-Kras B6 mice with wild type and homozygous allelic loss of mouse 5LO (EL-Kras/5LO+/+ and EL-Kras/5LO−/−). Mice were genotyped via PCR as previously described (EL-Kras [18], 5LO−/−(Jackson Lab website)).
To evaluate the differences in the pancreatic neoplastic phenotype between EL-Kras mice in these 5LO genotypes (+/+ and −/−), a group of 10 EL-Kras B6 mice (10–12 months old) were compared to 10 age-matched EL-Kras/5LO−/− mice for incidence (number of mice with lesions per number of mice with respective genotype). Similar phenotypic comparisons were performed on FVB6 F2 EL-Kras/5LO−/− mice (13–16 months of age) fed chow rich in omega-6 fatty acids.
Dietary Studies
All FVB6 F2 mice were fed ad libitum a standard chow of omega-6-rich diet (prepared by Reseach Diets, New Brunswick, NJ) as previously described (Table 1). All mice were weighed at the time of cull. Following euthanasia, mouse pancreatic tissue was fixed in 10% buffered formalin overnight. Tissue blocks were sectioned at 4–6 µm and placed on positively charged slides for subsequent staining.
Table 1.
Diet Composition
| Ingredient | Standard Diet (g/kg) | High Omega-6 Diet (g/kg) |
|---|---|---|
| Safflower Oil | 0 | 205.9 |
| Total Omega-6 Fatty Acids | 27.9 | 154.4 |
| Omega-3:Omega6 FA Ratio | 1:9.6 | 1:125 |
| Calculated Values | ||
| Protein | 19% | 23.7% |
| Fat | 5.6% | 23.6% |
| Fiber | 4.5% | 5.8% |
| Carbohydrate | 40.3% | 41.4% |
| Protein Energy | 0.76 kcal/g | 0.49 kcal/g |
| Fat Energy | 0.51 kcal/g | 1.11 kcal/g |
| Carbohydrate Energy | 1.61 kcal/g | 0.86 kcal/g |
| Total Energy | 2.88 kcal/g | 2.46 kcal/g |
Histology, Pathology, and Immunohistochemistry
Tissues were deparaffinized by xylenes and rehydrated to water. They were then stained with H&E and dehydrated to xylenes prior to mounting with a cover slip. The number of lesions per random section was quantified and represented as number of lesions per cm2.
Staining
Mast cells were identified with CAE and TB stains and quantified to determine the number of mast cells per mm2 [20].
Patient Samples
Informed consent was obtained from all participants. The protocols were approved by the Scientific Review Committee and the Institutional Review Board of Northwestern University.
Statistical analysis
in vitro and in vivo experiments were analyzed using t-test analysis. The ELISAs in Figure 1 comparing the conditioned media were analyzed using a paired t-test analysis. The staining and IHC comparisons included in Figure 2(A-C) and Figure 3 were analyzed using an unpaired t-test. All error bars represent standard error of the mean. In Figure 2D a Fisher exact test was used to determine the association between 5LO-positive cells and mast cells. Analyses were performed using Graphpad Prism (San Diego, CA) for Windows PC.
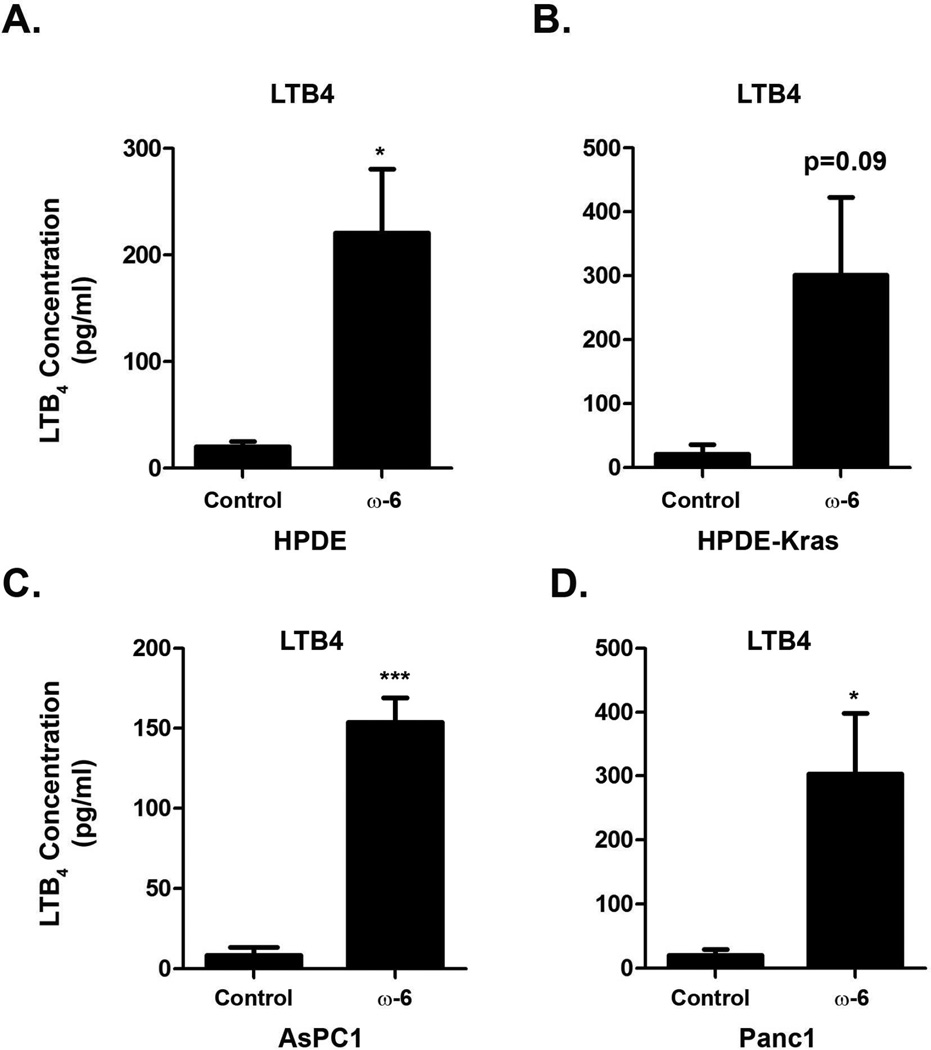
Figure 1. Treatment of pancreatic cells in vitro with omega-6 fatty acids increases LTB4 levels A-D.
Human pancreatic ductal epithelial (HPDE) cells (A), HPDE-Kras (B), AsPC1 (C) and Panc1 cells (D), were treated with BSA carrier or omega-6 fatty acids and conditioned media was collected as detailed in the Material and Methods. LTB4 levels in the conditioned media were determined using a Quantikine ELISA kit. The p-values were calculated using a paired t-test (*, p<0.05; ***, p<0.001).
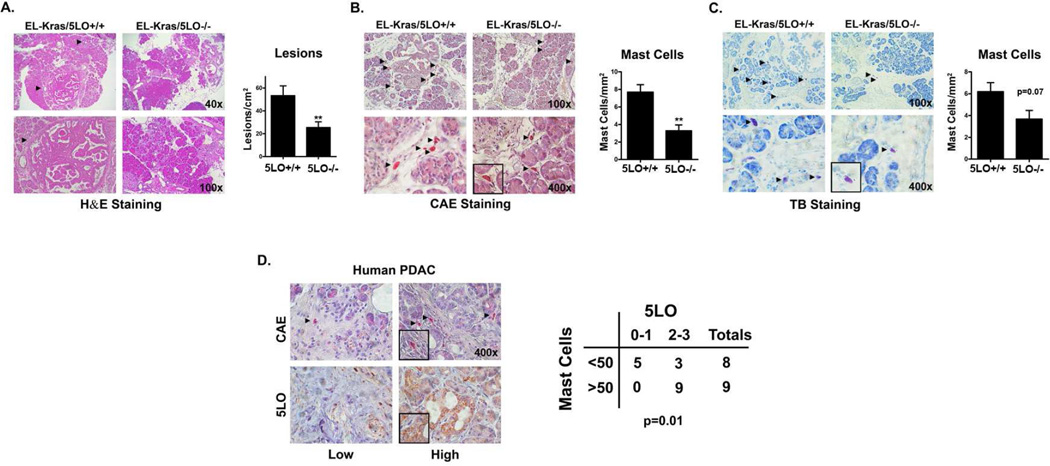
Figure 2. Deletion of 5LO in EL-Kras mice decreases pancreatic lesions and mast cell infiltration.
A. Pancreatic tissue from El-Kras/5LO+/+ and 5LO−/− mice (10 mice in each group) fed standard chow were stained with hematoxylin and eosin to evaluate lesion frequency. Lesions in one section were counted and quantified as lesions/cm2. The p values were calculated using an unpaired t-test. B,C. Pancreatic tissue from El-Kras/5LO+/+ and 5LO−/− mice fed standard chow were stained with chloracetate esterase (CAE) (5 mice in each group) (B) and toluidine blue (4 mice in each group)(C) for mast cells. The total number of mast cells in one section were counted for each stain and quantified as mast cells/mm2. The p-values were calculated using an unpaired t-test. D. De-identified human PDAC samples (17 samples) were procured through an IRB-approved protocol. Formalin-fixed, paraffin-embedded tumors were stained with CAE to identify mast cells and immunostained with 5LO antibody to identify 5LO positive cells. Sections immunostained with 5LO antibody were divided into two groups based on grade of staining: low (0–1) or high (2–3). Likewise, sections stained with CAE were divided into two groups: less than 50 mast cells and those with greater than 50 mast cells (all sections were from a tumor microarray with each section being approximately 3mm2). A Fisher exact test was used to determine the association between 5LO-positive cells and mast cells. (**, p<0.01). All counting above was performed blinded.
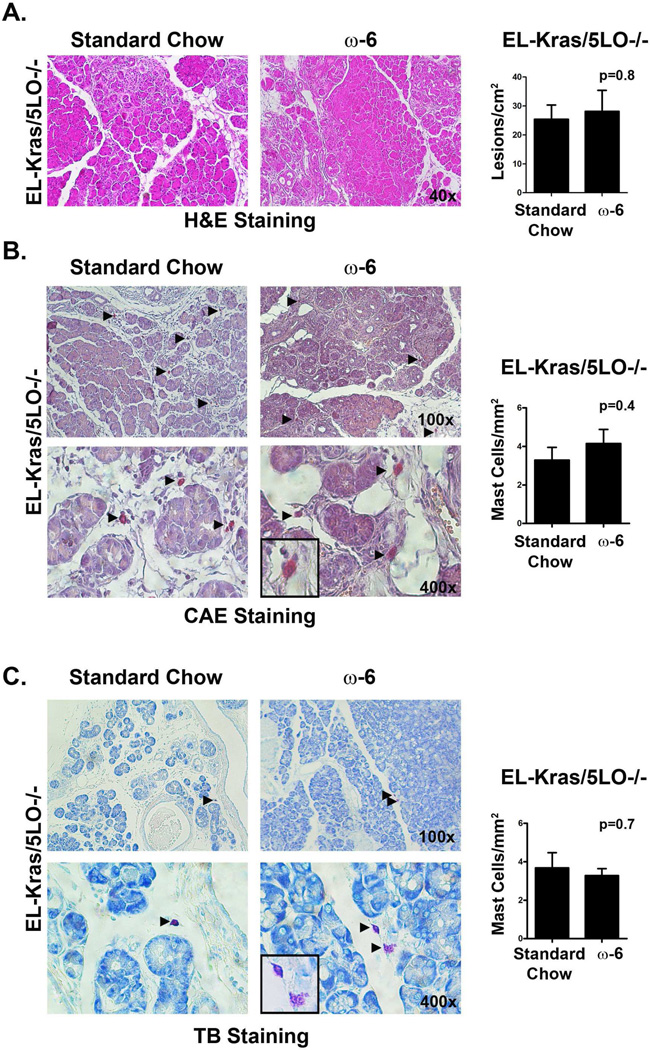
Figure 3. Deletion of 5LO abrogates the effect of omega-6 fatty acid diets on pancreatic lesions and pancreatic mast cell infiltration in EL-Kras mice.
A. Pancreatic tissue from EL-Kras/5LO−/− mice fed standard chow or high omega-6 chow (4 mice in each group) were stained with hematoxylin and eosin to evaluate lesion frequency. Lesions in one section were counted and quantified as lesions/cm2. B,C. Pancreatic tissue from 5LO−/− mice fed a standard chow or high-omega-6 chow were stained with with chloracetate esterase (CAE) (4 mice in each group) (B) and toluidine blue (TB) (4 mice in the standard group and 3 mice in the high-fat group) (C) for mast cells. The total number of mast cells in one section were counted and quantified as mast cells/mm2.
RESULTS
Treatment of pancreatic cells in vitro with omega-6 fatty acids increases LTB4 levels
We have previously demonstrated that EL-Kras mice fed a diet rich in omega-6 fatty acids have increased lesion numbers and increased mast cell infiltration [16]. Since LTB4 is a potent chemotactic factor for mast cells [12], we extended these findings into human cells by determining the effect of omega-6 fatty acids on LTB4 production in vitro. As shown in Fig. 1, the LTB4 levels in the conditioned media from immortalized ductal HPDE and HPDE-Kras cells and malignant AsPC1 and Panc1 cells were increased following treatment with the omega-6 fatty acid, linoleic acid (LA).
Deletion of 5LO in EL-Kras mice decreases pancreatic lesions and pancreatic mast cell infiltration
Since LTB4 is generated by 5-lipoxygenase (5LO), we examined the role of 5LO in pancreatic lesion formation by crossing EL-Kras mice with 5LO-null (5LO−/−) mice to generate EL-Kras/5LO+/+ and EL-Kras/5LO−/− mice. As shown in Fig. 2A, the EL-Kras/5LO−/− mice have significantly fewer pancreatic lesions compared to the EL-Kras/5LO+/+ mice. The EL-Kras/5LO−/− mice also have decreased numbers of pancreatic mast cells compared to the control EL-Kras/5LO+/+ mice (Fig. 2B,C). To further determine the relationship between 5LO and mast cells, human PDAC tumors were immunostained with 5LO antibody and stained for mast cells. As shown in Fig. 2D, human PDAC tumors with increased 5LO expression also demonstrate increased mast cell infiltration.
Deletion of 5LO abrogates the effect of omega-6 fatty acid-rich diet on pancreatic lesions and pancreatic mast cell infiltration in EL-Kras mice
We next examined the effect of omega-6 fatty acid-rich diets on pancreatic lesions and mast cell infiltration in EL-Kras/5LO−/− mice. As shown in Fig. 3, there was no significant difference in lesion frequency in EL-Kras/5LO−/− mice fed a omega-6 fatty acid-rich diet compared to mice fed standard chow. Similarly, there was no significant difference in mast cell infiltration in EL-Kras/5LO−/− mice fed omega-6 fatty acid-rich diets compared to standard chow.
DISCUSSION
Epidemiological studies have shown that diets rich in omega-6 fatty acids are associated with increased rates of breast, prostate, and pancreas cancers [21–23]. This may be likely mediated by an increased inflammatory response induced by conversion of omega-6 fatty acids to pro-inflammatory metabolites through 5LO and COX2. Significantly, 5LO expression is increased in pancreatic cancer cells and inhibition of 5LO can abolish proliferation of human pancreatic cancer cells [14, 24]. Importantly, LTB4, the downstream metabolite catalyzed by 5LO, has been found to be upregulated in pancreatic tumors compared with normal tissue [12]. In this report we show that diets rich in omega-6 fatty acids increase LTB4 production in HPDE cells as well as pancreatic cancer cells. Furthermore, we show that EL-Kras/5LO−/− mice develop fewer pancreatic lesions and are resistant to the effects of diets rich in omega-6 fatty acid.
Previously, we reported that EL-Kras mice fed diets rich in omega-6 fatty acids demonstrated increased pancreatic mast cell infiltration [16]. Significantly, increased infiltration of mast cells into the tumor microenvironment correlates with a poor prognosis [15]. Soucek demonstrated that mast cells mediate expansion of islet-cell tumors in mice and are essential for tumor maintenance [25]. Ma et al showed that PDAC cells promote mast cell migration and activation in vitro, and when mast cell migration is blocked in vivo in an orthotopic PDAC mouse model, PDAC growth is inhibited [26]. In this report, we demonstrate a significant association between 5LO expression and mast cell infiltration in human PDAC tumors. We also show that EL-Kras/5LO−/− mice not only have reduced pancreatic mast cell infiltration, but were resistant to the effects of omega-6 fatty acid-rich diets to induce pancreatic mast cell infiltration, indicating that 5LO is important in regulating mast cell infiltration in vivo. Our results suggest that the absence of 5LO decreases lesion development and mast cell infiltration in EL-Kras mice, and it abrogates the effect of omega-6 fatty acids on lesion formation.
Overall, our results indicate that 5LO is a potential target in preventing pancreatic cancer progression. Preclinical animal studies have shown that the 5LO inhibitor Zileuton can inhibit tumor growth and reduce tumor mass in an orthotopic mouse model of colon cancer and in chemically-induced pancreatic tumors in hamsters [27, 28]. As a drug that is well tolerated in asthma patients and is approved by the Food and Drug Administration, Zileuton may be an ideal chemopreventive therapy for obese patients at risk for pancreatic cancer[10]. Our future work will explore Zileuton chemoprevention in developing neoplastic lesions in EL-Kras mice.
Highlights.
Pancreatic cancer cells treated with ω-6 fatty acids generate increased LTB4
EL-Kras mice lacking 5LO, EL-Kras/5LO−/−, develop fewer pancreatic lesions
EL-Kras/5LO−/− mice also have decreased mast cell infiltration to the pancreas
ω-6 fatty acid-rich diets do not increase pancreatic lesions in EL-Kras/5LO−/− mice
Acknowledgments
The authors gratefully acknowledge the contribution of Carolyn Pelham and Kevin Adrian for their outstanding care and oversight of mice for the duration of this diet study. The research described herein was generously supported by funds provided by the Barnum Foundation and Zell Family Foundation at Northwestern University, the Nathan and Isabel Miller Family Foundation, the IDP Foundation, NIH R21 CA123041-01 (PJG), and NIH R01 CA161283-01 (PJG).
Abbreviations used
- EL
elastase
- LO
lipoxygenase
- COX
cyclooxygenase
- PDAC
pancreatic ductal adenocarcinoma
- LTB4
leukotriene B4
- MC
mast cells
- HPDE
human pancreatic ductal epithelial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: L Knab, P Grippo, H Munshi, and D Bentrem developed the experimental design. L Knab, M Schultz, D Principe, and E Gounaris performed the experiments and analyzed the data. W Mascarinas and P Grippo carried out the mouse work. L Knab, P Grippo, H Munshi, and D Bentrem prepared the manuscript.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. PMID: 23335087. [DOI] [PubMed] [Google Scholar]
- 2.Chu GC, Kimmelman AC, Hezel AF, DePinho RA. Stromal biology of pancreatic cancer. J Cell Biochem. 2007;101(4):887–907. doi: 10.1002/jcb.21209. PMID: 17266048. [DOI] [PubMed] [Google Scholar]
- 3.Whitcomb DC, Applebaum S, Martin SP. Hereditary pancreatitis and pancreatic carcinoma. Ann N Y Acad Sci. 1999;880:201–209. doi: 10.1111/j.1749-6632.1999.tb09524.x. PMID: 10415865 DOI: [DOI] [PubMed] [Google Scholar]
- 4.Stolzenberg-Solomon RZ, Schairer C, Moore S, Hollenbeck A, Silverman DT. Lifetime adiposity and risk of pancreatic cancer in the NIH-AARP Diet and Health Study cohort. Am J Clin Nutr. 2013 doi: 10.3945/ajcn.113.058123. PMID: 23985810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10(3):181–193. doi: 10.1038/nrc2809. PMID: 20168319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56(8):365–379. doi: 10.1016/s0753-3322(02)00253-6. PMID: 12442909 DOI: [DOI] [PubMed] [Google Scholar]
- 7.Giouleka P, Papatheodorou G, Lyberopoulos P, Karakatsani A, Alchanatis M, Roussos C, Papiris S, Loukides S. Body mass index is associated with leukotriene inflammation in asthmatics. Eur J Clin Invest. 2011;41(1):30–38. doi: 10.1111/j.1365-2362.2010.02371.x. PMID: 20825465. [DOI] [PubMed] [Google Scholar]
- 8.Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, Soslow RA, Masferrer JL, Woerner BM, Koki AT, Fahey TJ., 3rd Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59(5):987–990. PMID: 10070951 DOI: [PubMed] [Google Scholar]
- 9.Molina MA, Sitja-Arnau M, Lemoine MG, Frazier ML, Sinicrope FA. Increased cyclooxygenase-2 expression in human pancreatic carcinomas and cell lines: growth inhibition by nonsteroidal anti-inflammatory drugs. Cancer Res. 1999;59(17):4356–4362. PMID: 10485483 DOI: [PubMed] [Google Scholar]
- 10.Kennedy TJ, Chan CY, Ding XZ, Adrian TE. Lipoxygenase inhibitors for the treatment of pancreatic cancer. Expert Rev Anticancer Ther. 2003;3(4):525–536. doi: 10.1586/14737140.3.4.525. PMID: 12934664. [DOI] [PubMed] [Google Scholar]
- 11.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–1875. doi: 10.1126/science.294.5548.1871. PMID: 11729303. [DOI] [PubMed] [Google Scholar]
- 12.Hennig R, Ding XZ, Tong WG, Schneider MB, Standop J, Friess H, Buchler MW, Pour PM, Adrian TE. 5-Lipoxygenase and leukotriene B(4) receptor are expressed in human pancreatic cancers but not in pancreatic ducts in normal tissue. Am J Pathol. 2002;161(2):421–428. doi: 10.1016/S0002-9440(10)64198-3. PMID: 12163367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tong WG, Ding XZ, Hennig R, Witt RC, Standop J, Pour PM, Adrian TE. Leukotriene B4 receptor antagonist LY293111 inhibits proliferation and induces apoptosis in human pancreatic cancer cells. Clin Cancer Res. 2002;8(10):3232–3242. PMID: 12374694 DOI: [PubMed] [Google Scholar]
- 14.Ding XZ, Iversen P, Cluck MW, Knezetic JA, Adrian TE. Lipoxygenase inhibitors abolish proliferation of human pancreatic cancer cells. Biochem Biophys Res Commun. 1999;261(1):218–223. doi: 10.1006/bbrc.1999.1012. PMID: 10405349. [DOI] [PubMed] [Google Scholar]
- 15.Strouch MJ, Cheon EC, Salabat MR, Krantz SB, Gounaris E, Melstrom LG, Dangi-Garimella S, Wang E, Munshi HG, Khazaie, K.Bentrem DJ. Crosstalk between mast cells and pancreatic cancer cells contributes to pancreatic tumor progression. Clin Cancer Res. 2010;16(8):2257–2265. doi: 10.1158/1078-0432.CCR-09-1230. PMID: 20371681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheon EC, Strouch MJ, Barron MR, Ding Y, Melstrom LG, Krantz SB, Mullapudi B, Adrian K, Rao S, Adrian TE, Bentrem DJ, Grippo PJ. Alteration of strain background and a high omega-6 fat diet induces earlier onset of pancreatic neoplasia in EL-Kras transgenic mice. Int J Cancer. 2011;128(12):2783–2792. doi: 10.1002/ijc.25622. PMID: 20725998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouyang H, Mou L, Luk C, Liu N, Karaskova J, Squire J, Tsao MS. Immortal human pancreatic duct epithelial cell lines with near normal genotype and phenotype. Am J Pathol. 2000;157(5):1623–1631. doi: 10.1016/S0002-9440(10)64800-6. PMID: 11073822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grippo PJ, Nowlin PS, Demeure MJ, Longnecker DS, Sandgren EP. Preinvasive pancreatic neoplasia of ductal phenotype induced by acinar cell targeting of mutant Kras in transgenic mice. Cancer Res. 2003;63(9):2016–2019. PMID: 12727811 DOI: [PubMed] [Google Scholar]
- 19.Chen XS, Sheller JR, Johnson EN, Funk CD. Role of leukotrienes revealed by targeted disruption of the 5-lipoxygenase gene. Nature. 1994;372(6502):179–182. doi: 10.1038/372179a0. PMID: 7969451. [DOI] [PubMed] [Google Scholar]
- 20.Knab LM, Ebine K, Chow CR, Raza SS, Sahai V, Patel AP, Kumar K, Bentrem DJ, Grippo PJ, Munshi HG. Snail Cooperates with KrasG12D in vivo to Increase Stem Cell Factor and Enhance Mast Cell Infiltration. Mol Cancer Res. 2014 doi: 10.1158/1541-7786.MCR-14-0111. PMID: 24944064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van't Veer P, Kok FJ, Brants HA, Ockhuizen T, Sturmans F, Hermus RJ. Dietary fat and the risk of breast cancer. Int J Epidemiol. 1990;19(1):12–18. doi: 10.1093/ije/19.1.12. PMID: 2351506 DOI: [DOI] [PubMed] [Google Scholar]
- 22.Howe GR, Jain M, Miller AB. Dietary factors and risk of pancreatic cancer: results of a Canadian population-based case-control study. Int J Cancer. 1990;45(4):604–608. doi: 10.1002/ijc.2910450405. PMID: 2157670 DOI: [DOI] [PubMed] [Google Scholar]
- 23.Woutersen RA, Appel MJ, van Garderen-Hoetmer A, Wijnands MV. Dietary fat and carcinogenesis. Mutat Res. 1999;443(1-2):111–127. doi: 10.1016/s1383-5742(99)00014-9. PMID: 10415435 DOI: [DOI] [PubMed] [Google Scholar]
- 24.Shureiqi I, Lippman SM. Lipoxygenase modulation to reverse carcinogenesis. Cancer Res. 2001;61(17):6307–6312. PMID: 11522616 DOI: [PubMed] [Google Scholar]
- 25.Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13(10):1211–1218. doi: 10.1038/nm1649. PMID: 17906636. [DOI] [PubMed] [Google Scholar]
- 26.Ma Y, Hwang RF, Logsdon CD, Ullrich SE. Dynamic mast cell-stromal cell interactions promote growth of pancreatic cancer. Cancer Res. 2013;73(13):3927–3937. doi: 10.1158/0008-5472.CAN-12-4479. PMID: 23633481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melstrom LG, Bentrem DJ, Salabat MR, Kennedy TJ, Ding XZ, Strouch M, Rao SM, Witt RC, Ternent CA, Talamonti MS, Bell RH, Adrian TA. Overexpression of 5-lipoxygenase in colon polyps and cancer and the effect of 5-LOX inhibitors in vitro and in a murine model. Clin Cancer Res. 2008;14(20):6525–6530. doi: 10.1158/1078-0432.CCR-07-4631. PMID: 18927292. [DOI] [PubMed] [Google Scholar]
- 28.Wenger FA, Kilian M, Achucarro P, Heinicken D, Schimke I, Guski H, Jacobi CA, Muller JM. Effects of Celebrex and Zyflo on BOP-induced pancreatic cancer in Syrian hamsters. Pancreatology. 2002;2(1):54–60. doi: 10.1159/000049449. PMID: 12120008 DOI: [DOI] [PubMed] [Google Scholar]