Abstract
Current diagnosis of depression is based solely on behavioral symptomatology. The available FDA approved treatments for depression have come from serendipitous discovery and are ineffective in nearly 30–50% of patients, which is thought to reflect a lack of specificity in targeting underlying pathophysiological mechanisms. Recent evidence has identified depression-related disruptions in a neuroimmune axis that interfaces the immune system and central nervous system to control behavior. This perspective examines the current evidence in human patients and animal models of depression that demonstrates how the peripheral immune system acts upon the brain to alter an individual’s response to stress, ultimately contributing to their vulnerability to mood disorders.
Depression alters the brain of an individual and has a physical impact on the body. Subsets of patients with Major Depressive Disorder (MDD) have higher levels of multiple inflammatory markers, including the cytokine Interleukin 6 (IL-6)1–3 along with a greater number of circulating leukocytes4. Patients with depression have a higher risk of inflammatory illness such as diabetes, metabolic syndrome and heart disease5, 6. Although biological criteria are not currently used to diagnose depression, it’s noteworthy that the newest diagnostic criteria for MDD identify inflammation as a possible cause7. It is estimated that approximately 30–50% of patients with depression are not responsive to approved antidepressant treatments8 and this may reflect disease mechanisms of depression— such as increased inflammation— that are not ubiquitously treated with standard antidepressants. It is also unclear whether inflammation plays a causal role in the development of depression. Here we discuss recent research examining the role of peripheral and central inflammation in depression. We take the perspective that inflammation is a contributing factor to the development of mood disorders and discuss data from human studies and animal models supporting this theory.
The immune system and major depressive disorder
The original link between inflammation and depression was a result of studies that examined psychiatric complications following long term treatment with interferon alpha for hepatitis C9. A subset of patients with hepatitis C who received chronic interferon treatment went on to develop depression9, 10 and some of these patients reported greater physical pain prior to the start of treatment10. There is a great deal of correlational evidence that patients who suffer from depression have elevations in circulating levels of cytokines that are pro-inflammatory in nature, such as Tumor necrosis factor alpha (TNF-a)2, Interleukin 1 beta (IL-1b)11 and IL-61–3. Furthermore these patients have elevated levels of leukocytes4, which may be the source of these increased inflammatory cytokines12. A recent study in humans provided the first evidence that peripheral inflammation predates the occurrence of depression as children with higher circulating levels of IL-6 at age 9 were at a 10% greater risk of developing MDD by age 18 than the general population or children with low levels of IL-613.
These studies and others have led clinical investigations to treat depressed patients with antiinflammatory agents. A recent trial with Infliximab, a monoclonal antibody against TNF-a, was found to be effective to alter mood in depressed patients with high basal levels of inflammation prior to treatment as indicated by circulating levels of C-reactive protein14. Other humanized antibody treatments are in development for a variety of inflammatory illnesses that may also be effective in treating mood disorders. For example, Tocilizumab, a humanized anti-IL-6 receptor antibody, which is FDA approved as a treatment for Castleman’s disease and arthritis15 is currently being considered for the treatment of unipolar and bipolar depression16. Usteknumab, an antibody against interleukin 12/23, has also been shown to decrease symptoms of depression and anxiety in humans treated for psoriasis17.
Additional studies have examined the effects of other anti-inflammatory agents such as non steroidal anti-inflammatory drugs (NSAIDs) on depression18 and a recent meta analysis indicated that these treatments decrease symptoms of depression compared to placebo19, with the cyclooxygenase 2 (COX-2) inhibitor celecoxib found to be particularly effective with few side effects19. However, overall studies of other selective and non selective NSAIDs have been mixed making it difficult to fully evaluate their efficacy in treating depression20. Moreover, the NSAID ibuprofen can even interfere with the bioavailability of selective serotonin reuptake inhibitors21, which may further complicate their use in depressed populations.
Much like the data from studies of NSAID efficacy, the evidence regarding anti-inflammatory properties of traditional antidepressants is mixed with some studies reporting that antidepressants reduce systemic inflammation22, some reporting no effect1, 23 and others demonstrating that antidepressants actually increase inflammatory load24. There is a growing literature that suggests Ketamine, a novel antidepressant therapeutic that mitigates treatment-refractory depression with fast acting effects25, decreases pro inflammatory cytokines such as IL-6 and TNFα in part via the Toll-like receptor 4 family (TLR4)26. Together, studies in humans present strong evidence that inflammation is altered in a subset of depressed patients and has the potential to be used as a novel target for MDD treatment. However, it’s still not clear from these human studies whether peripheral inflammatory mechanisms are causally related to depression or whether more traditional antidepressants exert their therapeutic effects by regulating inflammatory processes. Recent in vivo studies discussed here using animal models of depression (Box 1) provides critical information about the inflammatory mechanisms that are functionally related to the behavioral symptoms of depression and antidepressant responses.
Box 1. Animal models of mood disorders.
The earliest demonstration of a relationship between inflammation and depression associated behavior comes from studies that examined sickness response to systemic administration of the endotoxin, lipopolysaccharide (LPS)95, which causes an induction of pro-inflammatory cytokine release. In this model, following injection with LPS rodents exhibit decreased self care, social interaction, locomotor activity and feeding over the subsequent 24 hour period108. However as the expression of the behavior is directly tied to the inflammatory activation by LPS and subsides following a return to baseline this is considered sickness behavior rather than a model of depression. The models listed below use various forms of stress exposure to induce similar behaviors that last beyond the application of the stressor. These animal models of depression can result in both acute and lasting changes in immune function.
Chronic Mild Stress (CMS)
Experimental animals are exposed to a series of variable stressors for at least two-weeks109. The concept is that mild stressors presented in an unpredictable manner over time induce a depression-like state. Following CMS, animals show anhedonic behaviors, such as decreased sucrose consumption, impairments in natural reward associations (conditioned place preference) and reduced brain reward function110. They also show deficits in grooming, sexual behavior, and a pro-inflammatory immune profile111 (Table 1). Many of these effects can be reversed with chronic but not acute antidepressant exposure, making the model relevant to human antidepressant responses.
Learned Helplessness (LH)
Subjects are exposed to a controllable or uncontrollable stress, such as foot or tail shock, and the latency to actively escape a subsequent stressor is examined. Failure to escape a subsequent stressor via a shuttle box is considered LH behavior. Animals that are able to control the stressor will learn the new task and escape the shock, whereas the yoked controls exposed to uncontrollable stressors fail to learn the task. Only approximately 20% of animals that undergo the uncontrollable stress are susceptible and develop LH, whereas the remaining responders are resilient and do not develop LH. Susceptible animals display anhedonia and altered cytokine profiles (Table 1), such as higher levels of circulating IL-6112, whereas control animals exposed to controllable stressors or resilient animals do not show these same systemic perturbations. In animals that show helplessness behavior, antidepressant treatment of at least 3–5 days is necessary to reverse the effects113.
Repeated Social Defeat Stress (RSDS)
Experimental mice are placed into the home cage of a novel larger aggressive mouse each day for 10 days. The larger mouse quickly establishes dominance through physical interaction114. Following RSDS, approximately two thirds of mice— termed susceptible— exhibit depression-like phenotypes measured by social avoidance, anhedonia, disruptions of the circadian system, increased activation of pro-inflammatory immune markers such as IL-6 (Table 1) and metabolic changes. The remaining 1/3 of animals do not develop any significant depression-like behavioral or physiological changes and are termed resilient. 28 day chronic antidepressant treatment is required to reverse social avoidance behavior115, however, such treatment does not reduce IL-6 levels3.
Sensing of stress by peripheral immune cells
In this review, we refer to peripheral immune cells as immune cells located outside the central nervous system (CNS). Peripheral immune cells are traditionally assigned to either the innate or the adaptive immune system and they are largely, but not exclusively, derived from hematopoietic stem cells (HSCs) within bone marrow (BM) stores27. The innate immune system includes myeloid cells such as granulocytes, monocytes, macrophages and dendritic cells (DC)28 and innate lymphocytes29, such as natural killer (NK) cells.
Innate immune cells mount rapid and effective responses against microbial or sterile injury using readily available cells equipped with pattern recognition receptors that are activated by pathogen activated molecular patterns (PAMPs) or damage/danger activated molecular patterns (DAMPs)30, 31, respectively. For example, activation of the pattern recognition toll-like receptors (TLRs) results in a cascade that leads to inflammatory cytokine release along with antigen presentation and phagocytosis31. DAMP molecules in particular are powerful danger signals which can be elicited as a response to both physiological and psychological stressors30. The “danger model” of the immune system proposed by Matzinger in 1994 postulates that the role of the immune system is to protect the body from potential exogenous and endogenous injury rather than to differentiate self from non self32. An extension of this idea is that engagement of the innate immune system is one of the first steps in the fight or flight response. It is proposed that DAMPs released from epithelial cells in the body and endothelial cells in the blood brain barrier (BBB)33 along with activation of the sympathetic nervous system, trigger the hypothalamic-pituitary-adrenal (HPA) axis as a feedback loop to modulate peripheral inflammation (Fig. 1). Because immune cells express both adrenergic and glucocorticoid receptors34, 35 they are able to directly respond to sympathetic nerve signals and HPA axis activation, as exemplified by the inflammatory reflex, a polysynaptic reflex arc (Fig. 1), which modulates the inflammatory response and contributes to the resolution of inflammation36, 37. Dysregulation of this feedback loop through changes in sensitivity of the glucocorticoid receptors on innate immune cells38, 39 has been shown to shift the immune system to a stress sensitive response (see40 for an in-depth review).
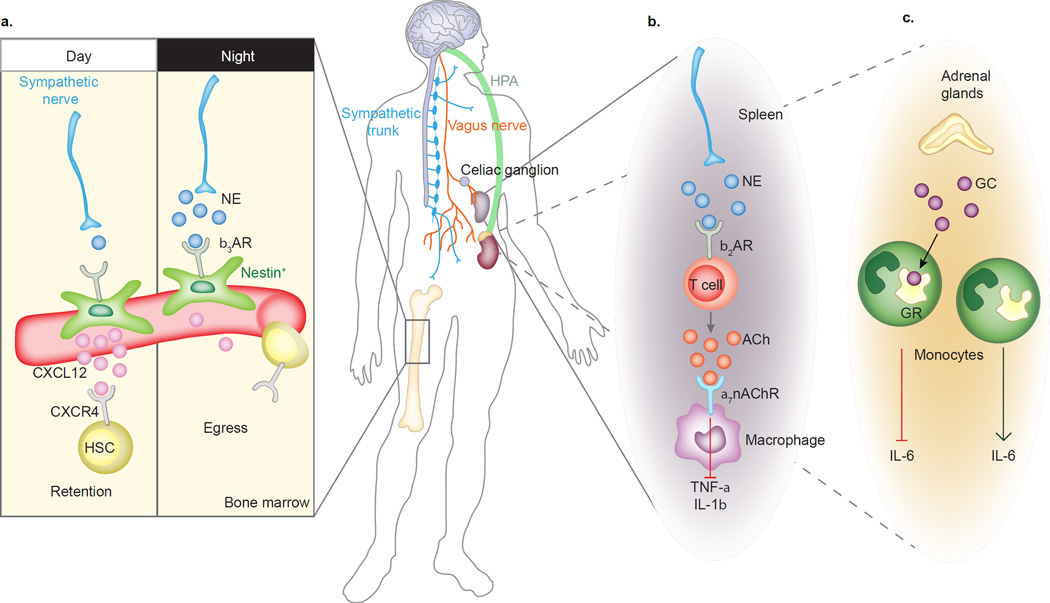
Figure 1. Circuits of the neuro-immune axis.
The autonomic nervous system connects peripheral immune organs with the central nervous system and regulates homeostatic and inflammatory functions. A) Sympathetic nerves innervate Nestin+ bone marrow niche cells. Circadian release of NE activates β3 adrenergic receptors (b3AR) on perivascular Nestin+ niche cells, which leads to rhythmic downregulation of CXCL12 and the subsequent release of hematopoietic stem cells (HSC) into the blood stream during the resting period. B) The inflammatory reflex: nerve endings of the vagus nerve sense inflammatory mediators and transport the signals to the brain stem. From there, the signal travels via efferent cholinergic vagal nerves to the celiac ganglion and through adrenergic fibers of the splenic nerve and delivers NE to β2 adrenergic receptors (b2AR) on a subset of splenic T cells, which in turn secrete acetylcholine (ACh) that binds to α7 nicotinic acetylcholine receptors (a7nAChR) on marginal zone macrophages and suppresses the production of inflammatory cytokines, such as TNF-α and IL-1β36, 37. C) Activation of the hypothalamic-pituitary-adrenocortical (HPA) axis leads to glucocorticoid (GC) release from the adrenal glands that exerts anti-inflammatory effects through binding of GC to cytosolic glucocorticoid receptor (GR) and inhibition of IL-6 release by monocytes.
In response to both psychological stress and trauma-related injury, neutrophils rapidly increase in number – a process termed reactive granulopoiesis41 – followed by an increase of monocytes. Immature Ly6Chi monocytes and neutrophils circulate in blood and are recruited to tissues when inflammatory signals are present42. In blood, Ly6Chi monocytes also mature into Ly6Clo monocytes, a functionally distinct subset that is thought mainly to survey the integrity of the blood endothelium42. Injury signals lead to the release of inflammatory chemokines and cytokines by tissue resident DCs and macrophages that recruit neutrophils and monocytes to the damaged tissues. Infiltrating monocytes can differentiate into macrophages to supplement tissue-resident macrophages and DCs contributing to the inflammatory process or the resolution of inflammation42. When inflammatory cues are presented continuously, their presence creates an important part of a vicious cycle. Hence, inflammatory monocytosis is a hallmark of chronic stress-related pathologies, which are characterized by an exaggerated and sustained inflammatory state within the body that may impact brain circuits to control behaviors relevant to depression and anxiety.
Adaptive immune cells comprise T and B lymphocytes and mount targeted and enhanced responses within secondary lymphoid organs, such as lymph nodes and spleen. Adaptive immune responses are stored as immunological memory in the form of specialized memory cells. Prolonged sympathetic activation restricts T cell mobility by blocking lymphocyte egress from secondary lymphoid organs and suppresses inflammation43. Although speculative, in the case of chronic stress, the adaptive immune system may also store immunological memory through these specialized memory cells thereby inoculating an individual to protect against future stress exposure. Thus, the neuroimmune axis exerts important homeostatic functions and regulates both innate and adaptive immunity in response to stress signals.
Sensing of stress by CNS resident immune cells
Microglia are the tissue resident macrophages of the brain44. They colonize the rudimentary brain early during embryonic development and self-renew locally throughout the life of the animal in the steady state45, 46. Microglia are dependent on two major cytokines to survive, colony stimulating factor 1 and IL-34, which are produced by distinct neuronal populations in the brain47, 48. There is also evidence that DCs along with other leukocytes and lymphocytes may enter the healthy brain in small numbers via the circumventricular organs49, the choroid plexus50, 51 and a brain lymphatic system52. It has been proposed that the endothelial cells that comprise these areas act as a permissive immunomodulatory “gate” actively promoting homeostasis49 and that this permissibility, along with an increase in infiltration, may increase with aging51. Once within the brain these peripheral cells act to support brain plasticity and may even contribute to cognitive function51. While beyond the scope of this review, these findings have important implications for cognitive decline associated with normal aging, Alzheimer’s disease and depression.
While the role of microglia in the healthy adult brain is still under investigation, recent studies have demonstrated that microglia dynamically survey their environment53, promote synaptic pruning54, 55, and can use the chemokine receptor CX3CR1 to detect neuronal damage by sensing the neuron’s downregulation of CX3CL156. Upon exposure to tissue damage microglia, like other tissue resident macrophages, promote the recruitment of blood circulating monocytes for help in mitigating damage57, 58. In mice exposed to social defeat stress, monocytes have been shown to infiltrate brain regions specifically associated with depression and anxiety59, where they differentiate into microglia-like cells and increase local inflammatory processes60 (Fig. 2). Interestingly, recent research using deep sequencing techniques on microglia and peripheral macrophages has indicated that a number of genes have cell type specific expression patterns61. Through this analysis the authors were able to elucidate a microglial specific sensome leading to an increased understanding of how microglia survey their environment. As animals age their microglial profiles shifted away from inflammatory activation to a more neuroprotective state that acted to maintain homeostasis. In the case of stress disorders where the periphery continues to be primed for a pro-inflammatory, potentially neurotoxic state, there may well be cell type specific transcriptional profiles governing the microglial sensome that contributes to depression onset.
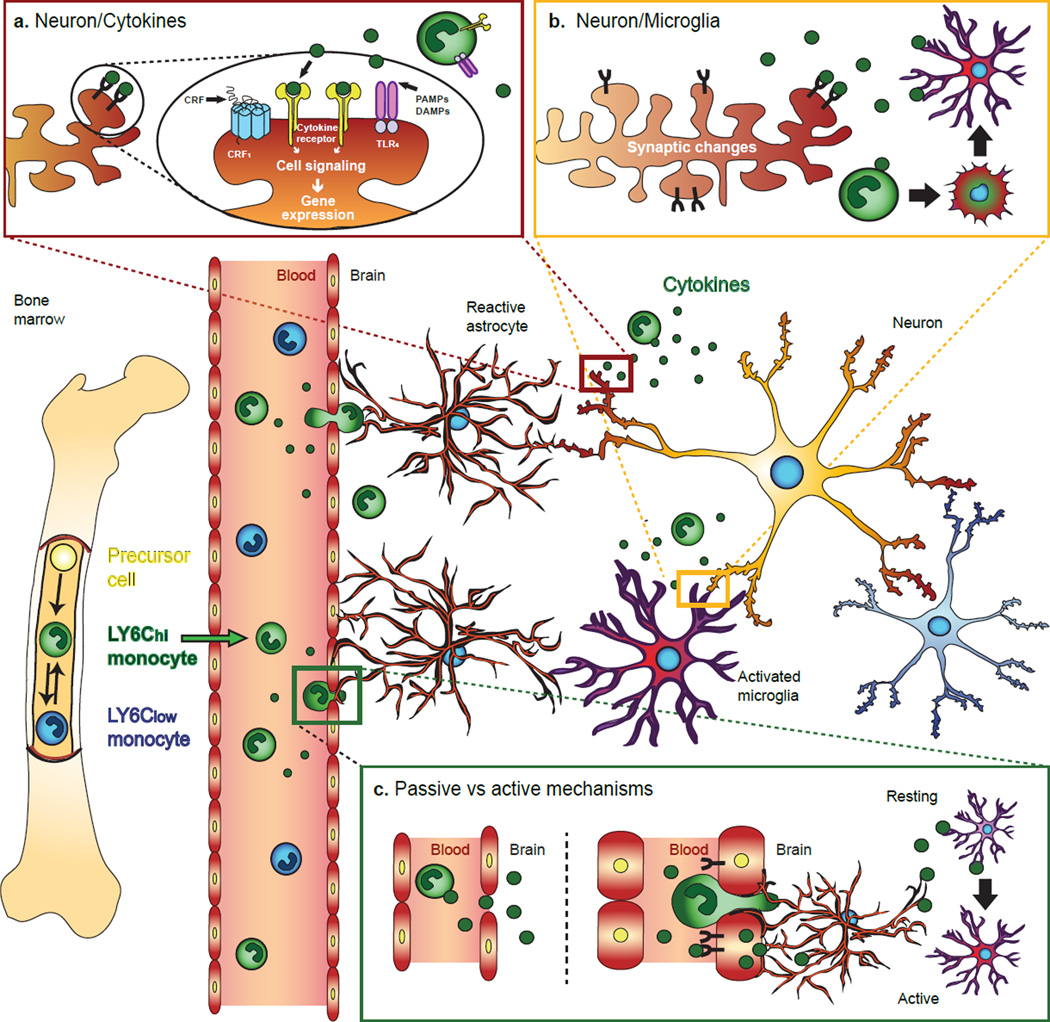
Figure 2. Inflammation and the brain.
In response to chronic stress, there is an increase in circulating monocyte levels, notably LY6Chi. These monocytes are then lured by chemotactic cytokines in brain regions associated with anxiety and depression. A) Once in the brain, monocytes and cytokines affect neuronal synaptic plasticity by modifying cell signaling and gene expression through activation of cytokines receptors, corticotropin-releasing hormone receptor 1 (CRF1) or toll-like receptor 4 (TLR4), by either cytokines themselves, corticotropin-releasing factor (CRF), pathogen-associated molecular patterns (PAMPs) and/or damage-associated molecular pattern molecules (DAMPs). B) Synaptic changes such as down-regulation of CX3CL1 are detected by microglia inducing cytokine release and monocytes recruitment. A proportion of recruited monocytes will remain in the brain and adopt microglia-like properties. C) Cytokines can penetrate into the brain via passive or active mechanisms. Alteration of tight junction protein expression can result in the formation of transient holes allowing small circulating molecules such as cytokines to passively diffuse between endothelial cells. Binding of cytokines to specific receptors on endothelial cell can also induce production and subsequent release of inflammatory mediators into the brain. Finally, if the BBB is weakened and become more permeable monocytes can burrow through affecting neighboring astrocytes, which engulf blood vessels with their astrocytic end-feet. Circulating cytokines into the brain activate microglia stress response and may induce persistent synaptic changes.
Peripheral immune cells and cytokines in depression
In the early 90’s Maes, Smith and colleagues examined the relationship between the peripheral immune system and depression4 and put forth a theory suggesting that immune dysregulation contributed to behavioral symptoms of the illness62, 63. This was largely based on the observation of increased levels of neutrophils and Ly6Chi monocytes reported in the blood of MDD patients. Subsequent work from animal models reported elevated neutrophils and Ly6Chi monocytes following prolonged periods of stress4, 64 and causal links were made between peripheral inflammation and the expression of depression- or anxiety-like behaviors3, 59, supporting the theory proposed by Maes and colleagues.
Our group has recently demonstrated that differences in the innate peripheral immune system predict susceptibility or resilience to repeated social defeat stress (RSDS)3 (see Box 1 for an operational definition of susceptibility versus resilience). Prior to stress exposure animals that later become susceptible had higher numbers of circulating monocytes and they released more IL-6 when their leukocytes were stimulated via lipopolysaccharide (LPS) ex vivo or following acute stress in vivo. To examine whether these individual differences in the peripheral immune system were causal to the development of stress susceptibility, HSCs were removed from stresssusceptible mice releasing high IL-6 or from IL-6 knockout (IL-6−/−) mice and transplanted into wildtype mice whose peripheral immune cells were lethally irradiated. Stress-susceptible BM chimeras exhibited increased susceptibility to RSDS whereas IL-6−/− BM chimeric, as well as those treated with a systemic IL-6 monoclonal antibody, were resilient to RSDS. These findings were replicated in a purely emotional stressor with no physical component demonstrating that this is not simply a peripheral response to physical trauma. Altogether these results suggest that dysregulated innate immune responses can increase stress susceptibility and contribute to the development of depression behaviors.
A recent study reported that transplantation of lymph node cell suspensions from chronically stressed mice into Rag2 knockout mice, which lack mature lymphocytes, was associated with subsequent active coping responses in the unstressed host mice65. Thus, it is possible that transplantation and/or differentiation of progenitor cells within the adaptive immune system contributes to opposite behavioral effects relative to those described above for the innate immune system. Despite the fact that donors exhibited a clear pro-inflammatory profile following stress, transfusion of these donor derived lymphocytes in Rag2 knockout mice resulted in a shift toward anti-inflammatory like responses, suggesting that adaptive immune cells may somehow be reprogrammed by stress to promote resilience. However, the question remains whether the developmental effects of lymphocyte knockout in Rag2 mice is truly representative of what might occur after transfusion of lymphocytes into normally developing wild type mice or whether this is a reflection of developmental side effects due to chronic immunosuppressive profiles in Rag2 knockout mice.
Additional studies have shed light on the potential upstream mechanisms behind the inflammatory changes observed in chronic stress. In mice, chronic mild stress (CMS) led to increased adrenergic innervation of the bone marrow stroma. Norepinephrine (NE) release from these adrenergic neurons decreased the expression of HSC quiescence factors produced by BM stromal cells and consequently induced HSC activation and output of neutrophils and monocytes through a β3-adrenergic receptor mechanism64 (Fig. 1). Additionally, stress-induced desensitization of the glucocorticoid receptors directly on splenic monocytes66 can contribute to anxiety-like behavior and increased levels of circulating cytokines. This occurs in part through a cytokine mediated mechanism that fails to separate nuclear factor kappa B from the glucocorticoid receptor thereby blocking translocation to the nucleus67. In addition, glucocorticoids can act directly on HSCs to balance proliferation and function68, however, further studies are needed to determine whether glucocorticoids, like β3-adrenergic receptor mechanisms, regulate HSCs in chronic stress models and depression.
Interface between peripheral immune cells and brain
There are numerous ways that BM derived leukocytes can alter activation of the brain and affect behavior. Some cytokines, like IL-6 and IL-1b can cross the blood brain barrier via saturable transport69, 70 to act directly on astrocytes, microglia and neurons throughout the CNS (Fig. 2) where they have a role in normal physiological processes such as temperature regulation, neuronal differentiation/survival, astrocyte proliferation and modulation of pain71. Additionally, monocytes can traffic to the brain by traversing through gaps in the epithelial lining that makes up the BBB59, 72. This is particularly interesting in light of recent evidence that stress itself can disrupt the BBB allowing greater access of peripheral cells to traffic directly into brain73. This is partly supported by evidence that in elderly patients with mood disorders, the ratio of cerebrospinal fluid to albumin is indicative of BBB disruption74. A recent study conducted on post mortem brains has suggested that more peripheral monocytes are indeed being recruited into the brain of depressed patients compared to non-psychiatric controls75.
In the rodent social defeat model, pro-inflammatory Ly6Chi monocytes infiltrate brain regions specifically associated with depression and anxiety59, after having been lured to these sites by local increases of chemotactic cytokines and adhesion molecules on endothelium75 (Fig. 2). Once inside the brain, infiltrating monocytes differentiate into monocyte-derived microglia and produce a local inflammatory response directly contributing to anxiety-like behavior72, 76. In contrast, BM transplantation of wild type mice with CCR2-deficient hematopoietic progenitors prevents monocyte trafficking and promotes resilience to social stress59. Interestingly, CCR2 has also been implicated in Alzheimer’s disease, a neurological condition for which depression and anxiety is highly comorbid77. Knockout of CCR2 in an animal model of Alzheimer’s disease blocks the accumulation of monocyte-derived brain macrophages, which reduces clearance of amyloid plaques resulting in early onset of cognitive symptoms and death78. Because these studies were performed using whole body CCR2 knockouts, it’s unclear if the effects were due to reduced trafficking or other processes. It is also unclear how long infiltrated monocytes actually take up residence in the brain. Studies in a rodent multiple sclerosis model found that they were cleared within a 3 month time period once inflammation was resolved57, whereas other methods of introducing them into the brain have found longer periods of residency76. In the context of depression, we speculate that neurons and resident microglia in brain regions affected by chronic stress will provide continuous stimuli for surrounding glia to allow inflammatory monocytes to enter the affected brain regions.
Blood circulating cytokines/chemokines can also activate the CNS by modulating cell surface receptors on astrocytes and on brain endothelial cells that form the BBB. Astrocyte end-feet line the BBB preserving its integrity and acting as a filter79. Increases in peripheral cytokines lead to changes in transcriptome profiles of astrocytes that include the upregulation of chemokines, cytokines and growth factors80, 81, which can then be released directly into the CNS. There is evidence from human post-mortem tissue in depressed subjects, as well as rodent stress models, that astrocytes are reduced within brain regions controlling mood and emotion82, 83. It is thought that chronic stress may act to decrease astrocytic volume and branching of processes by altering structural proteins, such as glial fibrillary acidic protein (GFAP), rather than by decreasing the overall number of astrocytes84. This loss of complexity within the processes of astrocytes could then lead to decreased coverage of the BBB and increased penetration of peripheral substances directly into the brain.
Peripheral cytokines and hormones can interface on resident microglia in the CNS to affect depression. Post-mortem analysis of brain tissue from subjects with depression85 and those that commit suicide86 exhibit increased activation of microglia. A recent positron emission tomography (PET) study in humans shows that there is greater microglia activation in cortical areas that directly correlate with depression severity87. In rodent models of depression, microglia isolated from stressed mice release higher levels of IL-1b and IL-6 following ex-vivo exposure to LPS88, 89. Furthermore, glucocorticoids may prime microglia during times of stress as glucocorticoid administration 2–24 hours before an acute inflammatory challenge increases release of pro-inflammatory cytokines including TNF-α, IL-1β and IL-6 from microglia90.
Cytokines/chemokines, as well as other danger signals can also act directly on neurons to alter plasticity and promote depression-like behaviors. Intracranial infusions of IL-691 or administration of IL-1b92, 93 into the hippocampus increase depression associated behavior and reduces neurogenesis, whereas infusion of IL-1b93 and IL-691 antibodies, or genetic deletion of IL-694, blocks the depressive-like effects induced by CMS. Cytokines can act on serotonin neurons through the kynurenine pathway and tryptophan catabolites95, 96 as well as directly on glutamatergic neurons in the frontal cortex97 and hippocampus98 to alter synaptic plasticity. Danger signals also act directly on nerve cells that express the TLR4 receptor to alter neurogenesis in the hippocampus99, a process important for depression and antidepressant responses100 Lastly, cytokines/chemokines can activate a microglia-dependent phagocytic process that engulfs and clears excitatory synapses altering synaptic transmission and behavior101. Additional studies are needed to determine exactly how stress activated cytokines or danger signals alter plasticity in astrocytes, microglia and neurons across a host of stress responsive brain regions important to depression.
Collectively, these studies suggest that dysregulated immune responses to stress can lead to exaggerated danger signals in the periphery/circulation. These signals access the CNS via active or passive transport and then amplify the initial inflammatory signal that can act directly or indirectly on plasticity mechanisms known to contribute to stress susceptibility and depression-like behavioral phenotypes.
Future directions
It is imperative that we move beyond characterization of correlational relationships between the immune system and mood disorders. Understanding how the immune system functionally contributes to depression is necessary to develop sensitive bioassays and effective therapeutics. We need to explore how cytokines/chemokines as well as other danger signals are acting within the brain to move towards reversing or repairing the damage in patients that already experience depression. We also need to investigate the dynamics of the neuro-immune system across the lifespan. The juxtaposition between greater central infiltration by the periphery and the central shift to an anti-inflammatory state that attempts to mitigate damage has important implications for depression and other neurological illnesses such as Alzheimer’s disease. Activation of perivascular macrophages has been implicated as a possible treatment for Alzheimer’s102 and there is growing evidence that a brief disruption of the BBB via repeated scanning ultrasound in a mouse model of Alzheimer’s disease leads to plaque clearance by microglia resulting in improved cognition103. Therefore, therapies for depression that decrease permeability of the BBB may be beneficial at one age but detrimental to older patients. By studying the immune system within the context of lifespan, we may be able to better tailor treatments to the individual.
Inflammation is also important to a host of other mental disorders and understanding which aspects of it are specific to depression will be a key question for bioassay development. For example, homeobox protein 8 (Hoxb8), expressed exclusively on BM derived brain macrophages has been implicated in obsessive compulsive disorder and the excessive grooming behavior in a Hoxb8 mutant can be rescued by BM transplantation from wild type mice104. By combining these areas of investigation we may be able to augment current antidepressant treatment or even build personalized therapeutics that address a more heterogeneous understanding of mental disorders.
Conclusions
Although there is evidence of differences between the mouse and human immune system transcriptionally105 and structurally106, rodent studies have aided in our understanding of the role of the immune system in depression. It is promising that many of the effects found in stress models recapitulate the dysregulated immune responses observed in stressed humans107 or patients with MDD1, 4. In order to translate results obtained in mouse models into effective clinical treatments, it is critical to dissect the molecular determinants of immune dysregulation following stress. Furthermore, for diagnostic and therapeutic purposes, we need to build a better representation of the pro- and anti-inflammatory patterns of cytokine production and release related to stress vulnerability and resilience. By understanding cytokine responses along with their downstream effects on neuronal plasticity we can begin to determine the feed-forward loop of the sympathetic nervous system and HPA axis/immune system/CNS that contributes to the onset and occurrence of depressive episodes across an individual’s lifespan. By determining the mechanisms that allow inflammatory cells, cytokines and other danger signals to enter the brain, we can develop new therapeutics to act on specific cell types in the body to block access of peripherally derived inflammatory signals to the brain.
Table 1.
Cytokine profiles for animal models of depression
| Animal model |
Behavior | Cytokines (peripheral) |
|---|---|---|
| Chronic Mild Stress |
↑ Anhedonia, ↑ Latency to eat in a novel environment, ↑ Sleep disturbance, ↑ Immobility (FST/TST), ↓ Libido, ↓ Conditioned place preference, ↓ Grooming, ↓ Weight |
↑ IL1β, ↑ IL-6, ↑ TNFα. |
| Learned Helplessness |
↓ Active avoidance, ↓ Libido, ↓ Weight, ↑ Sleep disturbance |
↑ IL1α, ↑ IL1β, ↑ IL-6, ↑ TNFα, ↑ IL-3, ↑ IL-10, ↑ IL-13, ↑ IL-17A, ↑ IL-5, ↑ GM-CSF, ↑ G-CSF, ↑ INF-γ, ↑ KC, ↑ RANTES, ↑ IL-2, ↑ MIP-1α ↑ MIP-1β. |
| Repeated Social Defeat Stress |
↑ Anhedonia, ↑ Sleep disturbance. ↑ Insulin insensitivity, ↑ Cocaine conditioned place preference ↓ Exploratory anxiety (EPM), ↓ Libido, ↓ Weight |
↑ IL1β, ↑ IL-6, ↑ IL-10, ↑ KC, ↑ MCP1, ↑ IL-7, ↑ Vegf. |
Acknowledgments
This research was supported by US National Institute of Mental Health Grants RO1 MH090264 (to S.J.R.) and RO1 MH104559 (to S.J.R. and M.M.), the Johnson and Johnson International Mental Health Research Organization Rising Star Award (to S.J.R.), Irma T. Hirschl/Monique Weill-Caulier Trust Research Award (to S.J.R.), the Brain and Behavior Research Foundation (G.E.H.), and the Swiss National Science Foundation Early Postdoc Mobility fellowship (to V.K.).
Conflict of interest statement
Our previous work on IL-6 and depression has been supported in part by a research grant from Janssen Pharmaceuticals.
References
- 1.Maes M, et al. Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J Affect Disord. 1995;34:301–309. doi: 10.1016/0165-0327(95)00028-l. [DOI] [PubMed] [Google Scholar]
- 2.Dowlati Y, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 3.Hodes GE, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maes M, et al. Leukocytosis, monocytosis and neutrophilia: hallmarks of severe depression. J Psychiatr Res. 1992;26:125–134. doi: 10.1016/0022-3956(92)90004-8. [DOI] [PubMed] [Google Scholar]
- 5.Fenton WS, Stover ES. Mood disorders: cardiovascular and diabetes comorbidity. Curr Opin Psychiatry. 2006;19:421–427. doi: 10.1097/01.yco.0000228765.33356.9f. [DOI] [PubMed] [Google Scholar]
- 6.Maes M, Kubera M, Obuchowiczwa E, Goehler L, Brzeszcz J. Depression's multiple comorbidities explained by (neuro)inflammatory and oxidative & nitrosative stress pathways. Neuro Endocrinol Lett. 2011;32:7–24. [PubMed] [Google Scholar]
- 7.Association, A.P. Diagnostic and Statistical Manual of Mental Disorders. 5th. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 8.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renault PF, et al. Psychiatric complications of long-term interferon alfa therapy. Arch Intern Med. 1987;147:1577–1580. [PubMed] [Google Scholar]
- 10.Conversano C, et al. Interferon alpha Therapy in Patients with Chronic Hepatitis C Infection: Quality of Life and Depression. Hematol Rep. 2015;7:5632. doi: 10.4081/hr.2015.5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maes M, et al. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 12.Miller GE, et al. Greater inflammatory activity and blunted glucocorticoid signaling in monocytes of chronically stressed caregivers. Brain Behav Immun. 2014;41:191–199. doi: 10.1016/j.bbi.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry. 2014;71:1121–1128. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raison CL, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkiteshwaran A. Tocilizumab. MAbs. 2009;1:432–438. doi: 10.4161/mabs.1.5.9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brietzke E, Scheinberg M, Lafer B. Therapeutic potential of interleukin-6 antagonism in bipolar disorder. Med Hypotheses. 2011;76:21–23. doi: 10.1016/j.mehy.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Langley RG, et al. Ustekinumab significantly improves symptoms of anxiety, depression, and skin-related quality of life in patients with moderate-to-severe psoriasis: Results from a randomized, double-blind, placebo-controlled phase III trial. J Am Acad Dermatol. 2010;63:457–465. doi: 10.1016/j.jaad.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Iyengar RL, et al. NSAIDs are associated with lower depression scores in patients with osteoarthritis. Am J Med. 2013;126:1017, e1011–e1018. doi: 10.1016/j.amjmed.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 19.Kohler O, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71:1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- 20.Eyre HA, Air T, Proctor S, Rositano S, Baune BT. A critical review of the efficacy of non-steroidal anti-inflammatory drugs in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2015;57:11–16. doi: 10.1016/j.pnpbp.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proc Natl Acad Sci U S A. 2011;108:9262–9267. doi: 10.1073/pnas.1104836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sluzewska A, et al. Interleukin-6 serum levels in depressed patients before and after treatment with fluoxetine. Ann N Y Acad Sci. 1995;762:474–476. doi: 10.1111/j.1749-6632.1995.tb32372.x. [DOI] [PubMed] [Google Scholar]
- 23.Jazayeri S, et al. Effects of eicosapentaenoic acid and fluoxetine on plasma cortisol, serum interleukin-1beta and interleukin-6 concentrations in patients with major depressive disorder. Psychiatry Res. 2010;178:112–115. doi: 10.1016/j.psychres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Kubera M, et al. Stimulatory effect of antidepressants on the production of IL-6. International immunopharmacology. 2004;4:185–192. doi: 10.1016/j.intimp.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Murrough JW, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Kock M, Loix S, Lavand'homme P. Ketamine and peripheral inflammation. CNS Neurosci Ther. 2013;19:403–410. doi: 10.1111/cns.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheiermann C, Frenette PS, Hidalgo A. Regulation of leucocyte homeostasis in the circulation. Cardiovasc Res. 2015 doi: 10.1093/cvr/cvv099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35:323–335. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spits H, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nature reviews. Immunology. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 30.Frank MG, Weber MD, Watkins LR, Maier SF. Stress sounds the alarm in: The role of the danger-associated molecular pattern HMGB1 in stress-induced neuroinflammatory priming. Brain Behav Immun. 2015 doi: 10.1016/j.bbi.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Portou MJ, Baker D, Abraham D, Tsui J. The innate immune system, toll-like receptors and dermal wound healing: A review. Vascul Pharmacol. 2015 doi: 10.1016/j.vph.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 33.Raz E. Organ-specific regulation of innate immunity. Nat Immunol. 2007;8:3–4. doi: 10.1038/ni0107-3. [DOI] [PubMed] [Google Scholar]
- 34.Marino F, Cosentino M. Adrenergic modulation of immune cells: an update. Amino acids. 2013;45:55–71. doi: 10.1007/s00726-011-1186-6. [DOI] [PubMed] [Google Scholar]
- 35.Amsterdam A, Tajima K, Sasson R. Cell-specific regulation of apoptosis by glucocorticoids: implication to their anti-inflammatory action. Biochem Pharmacol. 2002;64:843–850. doi: 10.1016/s0006-2952(02)01147-4. [DOI] [PubMed] [Google Scholar]
- 36.Rosas-Ballina M, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tracey KJ. Reflex control of immunity. Nature reviews. Immunology. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Avitsur R, Powell N, Padgett DA, Sheridan JF. Social interactions, stress, and immunity. Immunol Allergy Clin North Am. 2009;29:285–293. doi: 10.1016/j.iac.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Engler H, et al. Interleukin-1 receptor type 1-deficient mice fail to develop social stress-associated glucocorticoid resistance in the spleen. Psychoneuroendocrinology. 2008;33:108–117. doi: 10.1016/j.psyneuen.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frank MG, Watkins LR, Maier SF. Stress-induced glucocorticoids as a neuroendocrine alarm signal of danger. Brain Behav Immun. 2013;33:1–6. doi: 10.1016/j.bbi.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manz MG, Boettcher S. Emergency granulopoiesis. Nature reviews. Immunology. 2014;14:302–314. doi: 10.1038/nri3660. [DOI] [PubMed] [Google Scholar]
- 42.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nature reviews. Immunology. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 43.Nakai A, Hayano Y, Furuta F, Noda M, Suzuki K. Control of lymphocyte egress from lymph nodes through beta2-adrenergic receptors. The Journal of experimental medicine. 2014;211:2583–2598. doi: 10.1084/jem.20141132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ransohoff RM, Brown MA. Innate immunity in the central nervous system. The Journal of clinical investigation. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ginhoux F, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nature neuroscience. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 47.Greter M, et al. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity. 2012;37:1050–1060. doi: 10.1016/j.immuni.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nandi S, et al. The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Developmental biology. 2012;367:100–113. doi: 10.1016/j.ydbio.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shechter R, London A, Schwartz M. Orchestrated leukocyte recruitment to immune-privileged sites: absolute barriers versus educational gates. Nat Rev Immunol. 2013;13:206–218. doi: 10.1038/nri3391. [DOI] [PubMed] [Google Scholar]
- 50.Bulloch K, et al. CD11c/EYFP transgene illuminates a discrete network of dendritic cells within the embryonic, neonatal, adult, and injured mouse brain. J Comp Neurol. 2008;508:687–710. doi: 10.1002/cne.21668. [DOI] [PubMed] [Google Scholar]
- 51.Baruch K, Schwartz M. CNS-specific T cells shape brain function via the choroid plexus. Brain Behav Immun. 2013;34:11–16. doi: 10.1016/j.bbi.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Louveau A, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015 doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 54.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 55.Paolicelli RC, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 56.Limatola C, Ransohoff RM. Modulating neurotoxicity through CX3CL1/CX3CR1 signaling. Frontiers in cellular neuroscience. 2014;8:229. doi: 10.3389/fncel.2014.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 58.Yamasaki R, et al. Differential roles of microglia and monocytes in the inflamed central nervous system. The Journal of experimental medicine. 2014;211:1533–1549. doi: 10.1084/jem.20132477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33:13820–13833. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sawicki CM, et al. Social defeat promotes a reactive endothelium in a brain region-dependent manner with increased expression of key adhesion molecules, selectins and chemokines associated with the recruitment of myeloid cells to the brain. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hickman SE, et al. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci. 2013;16:1896–1905. doi: 10.1038/nn.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- 63.Smith RS. The macrophage theory of depression. Med Hypotheses. 1991;35:298–306. doi: 10.1016/0306-9877(91)90272-z. [DOI] [PubMed] [Google Scholar]
- 64.Heidt T, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20:754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brachman RA, Lehmann ML, Maric D, Herkenham M. Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice. J Neurosci. 2015;35:1530–1538. doi: 10.1523/JNEUROSCI.2278-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pariante CM. The glucocorticoid receptor: part of the solution or part of the problem? J Psychopharmacol. 2006;20:79–84. doi: 10.1177/1359786806066063. [DOI] [PubMed] [Google Scholar]
- 67.Quan N, et al. Molecular mechanisms of glucocorticoid resistance in splenocytes of socially stressed male mice. J Neuroimmunol. 2003;137:51–58. doi: 10.1016/s0165-5728(03)00042-0. [DOI] [PubMed] [Google Scholar]
- 68.Kollet O, et al. Physiologic corticosterone oscillations regulate murine hematopoietic stem/progenitor cell proliferation and CXCL12 expression by bone marrow stromal progenitors. Leukemia. 2013;27:2006–2015. doi: 10.1038/leu.2013.154. [DOI] [PubMed] [Google Scholar]
- 69.Banks WA, Kastin AJ, Gutierrez EG. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci Lett. 1994;179:53–56. doi: 10.1016/0304-3940(94)90933-4. [DOI] [PubMed] [Google Scholar]
- 70.Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood- brain barrier. Neuroimmunomodulation. 1995;2:241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- 71.Gadient RA, Otten UH. Interleukin-6 (IL-6)--a molecule with both beneficial and destructive potentials. Prog Neurobiol. 1997;52:379–390. doi: 10.1016/s0301-0082(97)00021-x. [DOI] [PubMed] [Google Scholar]
- 72.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 73.Reader BF, et al. Peripheral and central effects of repeated social defeat stress: monocyte trafficking, microglial activation, and anxiety. Neuroscience. 2015;289:429–442. doi: 10.1016/j.neuroscience.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gudmundsson P, et al. The relationship between cerebrospinal fluid biomarkers and depression in elderly women. Am J Geriatr Psychiatry. 2007;15:832–838. doi: 10.1097/JGP.0b013e3180547091. [DOI] [PubMed] [Google Scholar]
- 75.Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun. 2014;42:50–59. doi: 10.1016/j.bbi.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 76.Varvel NH, et al. Microglial repopulation model reveals a robust homeostatic process for replacing CNS myeloid cells. Proc Natl Acad Sci U S A. 2012;109:18150–18155. doi: 10.1073/pnas.1210150109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF., 3rd Late-life depression and risk of vascular dementia and Alzheimer's disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202:329–335. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.El Khoury J, et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 79.Wang DD, Bordey A. The astrocyte odyssey. Prog Neurobiol. 2008;86:342–367. doi: 10.1016/j.pneurobio.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meeuwsen S, Persoon-Deen C, Bsibsi M, Ravid R, van Noort JM. Cytokine, chemokine and growth factor gene profiling of cultured human astrocytes after exposure to proinflammatory stimuli. Glia. 2003;43:243–253. doi: 10.1002/glia.10259. [DOI] [PubMed] [Google Scholar]
- 81.Pang Y, Cai Z, Rhodes PG. Analysis of genes differentially expressed in astrocytes stimulated with lipopolysaccharide using cDNA arrays. Brain Res. 2001;914:15–22. doi: 10.1016/s0006-8993(01)02766-4. [DOI] [PubMed] [Google Scholar]
- 82.Nagy C, et al. Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leventopoulos M, et al. Long-term effects of early life deprivation on brain glia in Fischer rats. Brain Res. 2007;1142:119–126. doi: 10.1016/j.brainres.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 84.Tynan RJ, et al. Chronic stress-induced disruption of the astrocyte network is driven by structural atrophy and not loss of astrocytes. Acta Neuropathol. 2013;126:75–91. doi: 10.1007/s00401-013-1102-0. [DOI] [PubMed] [Google Scholar]
- 85.Steiner J, et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflammation. 2011;8:94. doi: 10.1186/1742-2094-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Steiner J, et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 87.Setiawan E, et al. Role of Translocator Protein Density, a Marker of Neuroinflammation, in the Brain During Major Depressive Episodes. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2014.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 89.Wohleb ES, et al. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Frank MG, Miguel ZD, Watkins LR, Maier SF. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun. 2010;24:19–30. doi: 10.1016/j.bbi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 91.Meagher MW, et al. Interleukin-6 as a mechanism for the adverse effects of social stress on acute Theiler's virus infection. Brain Behav Immun. 2007;21:1083–1095. doi: 10.1016/j.bbi.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci U S A. 2010;107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Monje FJ, et al. Constant Darkness Induces IL-6-Dependent Depression-Like Behavior through the NF-{kappa}B Signaling Pathway. J Neurosci. 2011;31:9075–9083. doi: 10.1523/JNEUROSCI.1537-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R. The new '5-HT' hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3- dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:702–721. doi: 10.1016/j.pnpbp.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 97.Garcia-Oscos F, et al. The stress-induced cytokine interleukin-6 decreases the inhibition/excitation ratio in the rat temporal cortex via trans-signaling. Biol Psychiatry. 2012;71:574–582. doi: 10.1016/j.biopsych.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Beattie EC, et al. Control of synaptic strength by glial TNF alpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- 99.Rolls A, et al. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol. 2007;9:1081–1088. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- 100.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 101.Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013;77:10–18. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 102.Hawkes CA, McLaurin J. Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy. Proc Natl Acad Sci U S A. 2009;106:1261–1266. doi: 10.1073/pnas.0805453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leinenga G, Gotz J. Scanning ultrasound removes amyloid-beta and restores memory in an Alzheimer's disease mouse model. Sci Transl Med. 2015;7:278ra233. doi: 10.1126/scitranslmed.aaa2512. [DOI] [PubMed] [Google Scholar]
- 104.Chen SK, et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141:775–785. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lin S, et al. Comparison of the transcriptional landscapes between human and mouse tissues. Proc Natl Acad Sci U S A. 2014;111:17224–17229. doi: 10.1073/pnas.1413624111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 107.Maes M, et al. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- 108.Bluthe RM, Dantzer R, Kelley KW. Effects of interleukin-1 receptor antagonist on the behavioral effects of lipopolysaccharide in rat. Brain Res. 1992;573:318–320. doi: 10.1016/0006-8993(92)90779-9. [DOI] [PubMed] [Google Scholar]
- 109.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 110.Dalla C, Pitychoutis PM, Kokras N, Papadopoulou-Daifoti Z. Sex differences in animal models of depression and antidepressant response. Basic Clin Pharmacol Toxicol. 2010;106:226–233. doi: 10.1111/j.1742-7843.2009.00516.x. [DOI] [PubMed] [Google Scholar]
- 111.Krishnan V, Berton O, Nestler E. The use of animal models in psychiatric research and treatment. Am J Psychiatry. 2008;165:1109. doi: 10.1176/appi.ajp.2008.08071076. [DOI] [PubMed] [Google Scholar]
- 112.Yang C, Shirayama Y, Zhang JC, Ren Q, Hashimoto K. Peripheral interleukin-6 promotes resilience versus susceptibility to inescapable electric stress. Acta Neuropsychiatr. 2015:1–5. doi: 10.1017/neu.2015.36. [DOI] [PubMed] [Google Scholar]
- 113.Slattery DA, Cryan JF. The ups and downs of modelling mood disorders in rodents. ILAR J. 2014;55:297–309. doi: 10.1093/ilar/ilu026. [DOI] [PubMed] [Google Scholar]
- 114.Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Berton O, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]