Abstract
Background
Understanding the potential for vaccination to change cytomegalovirus (CMV) epidemiology is important for developing CMV vaccines and designing clinical trials.
Methods
We constructed a deterministic, age-specific and time-dependent mathematical model of pathogen transmission, parameterized using CMV seroprevalence from the United States and Brazil, to predict the impact of vaccination on congenital CMV infection.
Findings
Concurrent vaccination of young children and adolescents would result in the greatest reductions in congenital CMV infections in populations with moderate and high baseline maternal seroprevalence. Such a vaccination strategy, assuming 70% vaccine efficacy, 90% coverage and 5-year duration of protection, could ultimately prevent 30%-50% of congenital CMV infections. At equilibrium, this strategy could result in a 30% reduction in congenital CMV infections due to primary maternal infection in the United States but a 3% increase in Brazil. The potential for an increase in congenital CMV infections due to primary maternal infections in Brazil was not predicted with use of a vaccine that confers protection for greater than 5 years.
Interpretation
Modeling suggests that vaccination strategies that include young children will result in greater declines in congenital CMV infection than those restricted to adolescents or women of reproductive age. Our study highlights the critical need for better understanding of the relative contribution of type of maternal infection to congenital CMV infection and disease, the main focus of vaccine prevention.
Keywords: cytomegalovirus, congenital infection, vaccination impact, mathematical model
INTRODUCTION
Congenital cytomegalovirus (cCMV) infection occurs when virus from the mother crosses the placenta and infects the immunologically immature fetus, as a result of primary maternal infection, reinfection or reactivation. The consequences of cCMV infection include fetal or infant death or neurological and sensory impairments [1, 2]. Children with cCMV-related disabilities may require extensive medical care, special education services, and interventions. Costs associated with cCMV infections in the United States were estimated in the 1990’s to be at least $1.9 billion annually [3]. Population-based epidemiological data are needed to update and provide more complete estimates of the full spectrum of disease and related disabilities caused by cCMV [4]. Because of the burden associated with cCMV disease, a CMV vaccine was rated as a “highest priority” for vaccine development by the Institute of Medicine in the United States [3, 5]. Several CMV vaccines have been evaluated in clinical trials, although none is yet close to licensure [6].
Mathematical modeling has become increasingly useful for investigating the dynamics of infection and potential impact of vaccination and identifying critical knowledge gaps for study [7]. Identifying which populations to target for CMV vaccination that would result in greatest reductions in the burden of cCMV disease may provide additional insight for the development and design of future CMV vaccines and clinical trials globally. Understanding how vaccination strategies might need to be tailored to underlying population epidemiology is important because of substantial differences in CMV seroprevalence and proportion of cCMV infections due to primary maternal infection within and between countries. We used mathematical modeling to explore the potential impact of CMV vaccination in the United States, a population with moderate seroprevalence, and in Brazil, a population with high seroprevalence. We estimated the potential impact of vaccination on cCMV infections, overall and by type of maternal infection, both at equilibrium and with respect to time after vaccine introduction.
METHODS
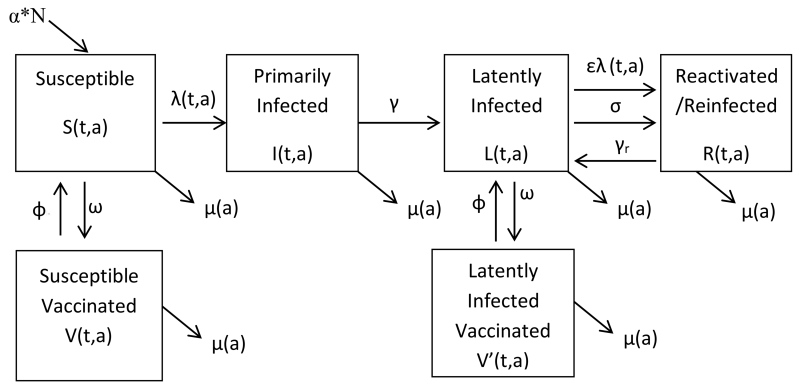
We constructed a deterministic, age-specific and time-dependent mathematical model of pathogen transmission, with six groups in our human population: susceptible, primarily infected, latently infected, reactivated/reinfected, susceptible vaccinated (before primary infection), and latently infected vaccinated (after primary infection) (Figure 1). The system of differential equations describing the model is provided in the supplementary material (Appendix 1).
Figure 1.
Compartmental model of CMV infection with vaccination
Individuals enter susceptible compartment by birth (αN) and may also leave each compartment by death (µ(a)). The average time individuals in each age group (a) spend in that age group is proportional to the length of the age group (a) (not shown). λ(t,a) is the force of infection (primary infection) among susceptible individuals and ελ(t,a) is the force of infection (reinfection) among individuals with latent infection; γ and γr indicate the rate at which primarily infected develop latency and reactivated/reinfected individuals return to latently infected (1/time to recover from primary or non-primary infection), respectively; σ is the rate at which individuals reactivate a latent infection (1/time to reactivate CMV infection); ω is the effectively vaccinated proportion (vaccine coverage times vaccine efficacy); and ϕ is the rate at which individuals lose vaccine protection (1/time to lose vaccine protection). Vaccination occurs once in any given scenario, with a proportion ω of the susceptible and latently infected moving to their respective effectively vaccinated compartments very rapidly. Primarily infected and reactivated/reinfected individuals are not effectively vaccinated.
We defined susceptible as CMV seronegative individuals who have not been previously infected nor effectively vaccinated. We defined primarily infected as individuals who were infectious after first exposure to wild type CMV strain, latently infected as individuals who were seropositive from wild type infection but not infectious, and reactivated/reinfected as individuals who were infectious during reactivation of the latent virus or after secondary exposure to a new CMV strain. We assumed persons vaccinated while susceptible (susceptible vaccinated) were protected against primary infection, and persons vaccinated while latently infected (latently infected vaccinated) were protected against reactivations or reinfections, and primarily infected or reactivated/reinfected individuals were not effectively vaccinated. We assumed an age-specific duration of infectiousness [8], a lower susceptibility to reinfections among latently infected individuals [9, 10], and latency duration of 20 years [11, 12] (Table 1), although these parameters are not well-understood.
Table 1.
Notation, definition and values of parameters in the mathematical model
| Notation | Definition | Value |
|---|---|---|
| 1/γ | Time to recover from primary infection | Age-specific: |
| ≤ 5 year-olds: 2 years | ||
| 6-19 year-olds: 1 year | ||
| ≥ 20 year-olds: 0.5 year | ||
| 1/γr | Time to recover from non-primary infection | (1/ γ)/2 |
| 1/σ | Time to reactivate CMV infection | 20 years or 5 years |
| ω | Effectively vaccinated proportion (vaccine coverage times vaccine efficacy) |
0-100% |
| 1/ϕ | Time to lose vaccine protection | 2-50 years |
For disease transmission, we used different age group-specific contact mixing matrices (a quantitative description of the average number of contacts between individuals per day) to fit CMV seroprevalence data. The base-case scenario and estimates are based on the contact mixing matrix that best fit the seroprevalence data, a modified version of pattern III of Azevedo’s model [11], in which the child-to-adult transmission route was attenuated. This pattern includes higher transmission probabilities between young children due to their long duration of viral excretion, high viral titers in body fluids [8] and high contact rate, and from adults to children, as a result of transmission through breastfeeding [13, 14].
Our models were parameterized using CMV seroprevalence [15, 16] and population-specific data from the United States and Brazil (Supplementary material – Table 1). We calculated the basic reproduction number (R0), which is the expected number of secondary infections arising from a single individual over the course of its infectious period when introduced into a susceptible population [17], using two different methods: the ‘next generation matrix’ (NGM) method [18], and the ‘constant force of infection’ method [19]. The former allows us to calculate R0 exactly for our specific model structure, while the latter is based only on seroprevalence data to deduce R0 under the assumption that the force of infection is the same for all ages (Supplementary material – Appendix 2).
The number of cCMV infections by type of maternal infection in the pre- and post-vaccination equilibrium was estimated using age group-specific birth rates among women 15-49 years-old for each country (Supplementary Material - Table 1) and a 1:1 male-female ratio in the population applied to the annual number of individuals with primary infection, reinfection and reactivation. The impact of vaccination was estimated as the percent reduction in the number of cCMV infections post-vaccination (1 minus the ratio between the post- and pre-vaccination annual number of cCMV infections times 100).
We assessed the effect of age at vaccination, effectively vaccinated proportion (vaccine coverage times vaccine efficacy), and duration of vaccine protection on cCMV infections, overall and by type of maternal infection both at equilibrium and with respect to time since vaccine introduction. The schedules considered for vaccine administration were based on ages when vaccines are typically recommended to children (0-12 months, 12-18 months, 10-11 years) or ages of childbearing potential before first pregnancy (15-19 years, and 20-29 years) [20]. We varied effectively vaccinated proportion from 0 to 100%, with vaccine coverage starting at desired coverage levels, and vaccine efficacy based on ‘all-or-nothing’ mechanism of vaccine action, i.e. complete protection to a subset of the individuals who are given the vaccine but no protection in the other subset [21]. In the model simulations, we performed ‘vaccination’ once at each of the schedules, with a proportion ω of the susceptible and latently infected moving to their respective effectively vaccinated states very rapidly. We varied duration of vaccine protection from 0 to 50 years, after which individuals would return to their original susceptible or latently infected states.
We conducted sensitivity analyses to evaluate the model-generated distribution of cCMV infections by type of maternal infection in the pre and post-vaccination equilibrium and the impact of vaccination. In these analyses, we assumed two different contact mixing matrices, pattern I from Azevedo’s study [11], in which the peak of transmission occurs between children-children only, and the UK ‘Polymod’ matrix [22]; 5-year latency duration; and a scenario in which the vaccine would provide no protection against reactivation or reinfection in latently infected individuals. All simulations were conducted using the software package Berkeley-Madonna version 8.3.18 (http://www.berkeleymadonna.com).
RESULTS
Ro and estimated distribution of cCMV infections by type of maternal infection in the pre-vaccine state
Using the NGM method, we estimated an R0 of 1.94 in the United States, and 5.17 in Brazil, similar to those estimated using the ‘constant force of infection’ method (Supplementary material). Assuming the modified contact mixing matrix pattern III and 20-year latency duration, the model-generated distribution of cCMV infections by type of maternal infection in a pre-vaccine state was 16% from primary maternal infection, 12% from reinfection and 72% from reactivation for the United States and 15%, 38% and 47% respectively for Brazil (Table 2). In the sensitivity analyses, the proportion of cCMV infections from primary maternal infections ranged from 5% to 30% in the United States and from 6% to 15% in Brazil (Supplementary material - Table 2). The proportion of cCMV infections from maternal reinfections ranged from 3% to 12% in the United States and from 13% to 48% in Brazil (Supplementary material - Table 2).
Table 2.
Change in the distribution and reductions in cCMV infection by type of maternal infection, 10, 20 and 50 years after introduction of vaccine, assuming mixing pattern III, age-specific duration of infectiousness, 20 year duration of latency, 90% vaccine coverage, 70% vaccine efficacy, 5-year duration of vaccine protection, and different ages at vaccination, United States and Brazil.
| Setting | Age at vaccination |
Type of maternal infection |
Distribution (%) of cCMV infections by type of maternal infection |
Reduction (%) in cCMV infections by type of maternal infection |
|||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Pre- Vaccination (Baseline) |
Years Post-Vaccination | Years Post-Vaccination | |||||||
|
| |||||||||
| 10 | 20 | 50 | 10 | 20 | 50 | ||||
| United States | 12-18 months |
Primary | 16 | 12 | 14 | 20 | 39 | 35 | 21 |
| Reinfection | 12 | 8 | 8 | 7 | 43 | 50 | 62 | ||
| Reactivation | 72 | 80 | 79 | 72 | 4 | 13 | 35 | ||
| Overall | 100 | 14 | 21 | 36 | |||||
|
| |||||||||
| 15-19 years |
Primary | 16 | 16 | 17 | 17 | 18 | 16 | 14 | |
| Reinfection | 12 | 12 | 12 | 11 | 17 | 19 | 21 | ||
| Reactivation | 72 | 72 | 72 | 71 | 15 | 17 | 19 | ||
| Overall | 100 | 16 | 17 | 18 | |||||
|
| |||||||||
| 12-18 months + |
Primary | 16 | 11 | 14 | 22 | 49 | 44 | 29 | |
| Reinfection | 12 | 8 | 7 | 7 | 53 | 58 | 69 | ||
| 15-19 years |
Reactivation | 72 | 81 | 79 | 72 | 17 | 25 | 45 | |
| Overall | 100 | 27 | 32 | 45 | |||||
|
| |||||||||
| 20-29 years |
Primary | 16 | 18 | 18 | 18 | 27 | 24 | 23 | |
| Reinfection | 12 | 12 | 12 | 12 | 32 | 32 | 32 | ||
| Reactivation | 72 | 71 | 70 | 70 | 32 | 32 | 32 | ||
| Overall | 100 | 31 | 30 | 31 | |||||
|
| |||||||||
| 12-18 months |
Primary | 15 | 13 | 20 | 25 | 32 | 1 | −24 | |
| Brazil | Reinfection | 38 | 28 | 27 | 27 | 46 | 49 | 48 | |
| Reactivation | 47 | 59 | 53 | 48 | 5 | 17 | 24 | ||
| Overall | 100 | 25 | 27 | 26 | |||||
|
| |||||||||
| 15-19 years |
Primary | 15 | 15 | 16 | 16 | 31 | 26 | 24 | |
| Reinfection | 38 | 38 | 37 | 37 | 34 | 34 | 35 | ||
| Reactivation | 47 | 48 | 47 | 47 | 30 | 32 | 32 | ||
| Overall | 100 | 32 | 32 | 32 | |||||
|
|
|||||||||
| 12-18 months + |
Primary | 15 | 13 | 22 | 29 | 53 | 23 | −3 | |
| Reinfection | 38 | 27 | 25 | 25 | 64 | 66 | 66 | ||
| 15-19 years |
Reactivation | 47 | 60 | 53 | 47 | 32 | 41 | 47 | |
| Overall | 100 | 48 | 48 | 47 | |||||
Footnote: Results for age at vaccination of 20-29 years not shown for Brazil because this scenario would lead to the least reduction in cCMV infections.
Estimated reductions in CMV seroprevalence after vaccine introduction
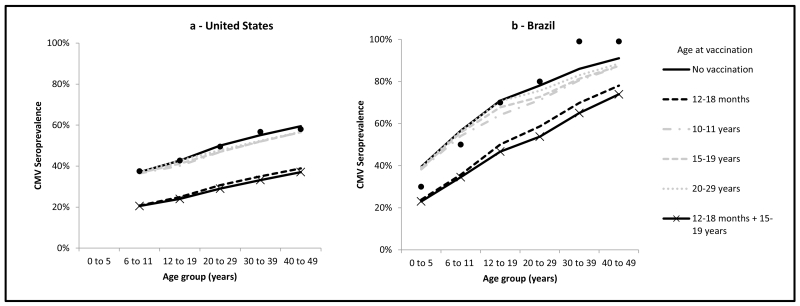
Assuming a vaccine with 70% efficacy, 90% coverage, and 5-year duration of protection, the greatest reduction in CMV seroprevalence from natural infection would be achieved by vaccination at age 0-12 months, potentially leading to CMV elimination both in the United States and in Brazil. This was predicted with shorter duration of vaccine protection (i.e. 2.5 years) as well, due to model assumptions of high infectiousness and contact rates among children ≤ 5 years of age. Considering vaccination of persons beginning at age ≥ 12 months, assuming the same vaccine parameters above, the greatest reduction of CMV seroprevalence would be achieved by a combined schedule of vaccination at ages 12-18 months and 15-19 years, followed by vaccination at age 12-18 months only, in both the United States and Brazil (Figures 2a and 2b). Vaccination at ages 15-19 or 20-29 years would result in limited reduction in CMV seroprevalence.
Figure 2.
Impact of vaccination on CMV seroprevalence from natural infection by age group at equilibrium, assuming different ages at vaccination, age-specific duration of infectiousness, 20 year duration of latency, and a vaccine with 70% efficacy, 90% coverage and 5-year duration of protection, United States and Brazil.
Black dots indicate available CMV serological data; U.S. data are from the 1999-2004 National Health and Nutrition Examination Survey [15], data for the age group 0-5 years were not available; Brazilian data are from CMV serological data from Caieiras, Sao Paulo, 1990-1991 [16].
Estimated impact of vaccination on cCMV infections
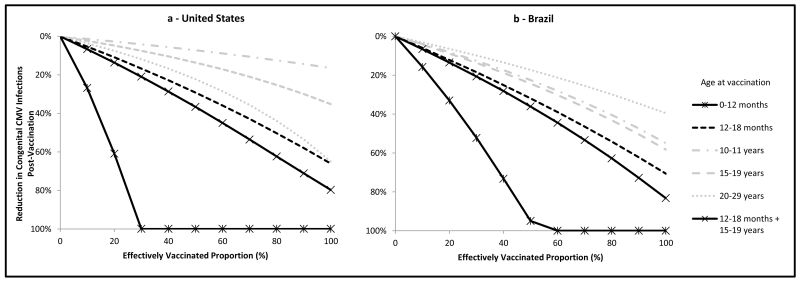
The greatest reduction in the overall number of cCMV infections would result from vaccination at age 0-12 months, potentially leading to elimination of cCMV infection both in the United States and in Brazil (Figures 3a and 3b). Considering other childhood vaccination strategies, a combined schedule of vaccination at ages 12-18 months and 15-19 years in both settings would result in reductions in the overall number of cCMV infections of approximately 40% if 50% of individuals were vaccinated and 80% if 100% were vaccinated. Among the other single age groups > 12 months of age considered for vaccination, the greatest reductions in the overall number of cCMV infections would result from vaccination at age 12-18 months.
Figure 3.
Overall reduction in the annual number of cCMV infections at equilibrium by proportion of individuals effectively vaccinated by age at vaccination, assuming age-specific duration of infectiousness, 20 year duration of latency, and a vaccine with 5-year duration of protection, United States and Brazil.
Regarding vaccination of adolescents or adults, vaccination at age 20-29 years would result in reductions in the overall number of cCMV infections in the United States similar to those predicted for vaccination at age 12-18 months, particularly as the effectively vaccinated proportion approaches 100%, and greater than those predicted with vaccination of adolescents (Figure 3a). In contrast, in Brazil, vaccination targeted at ages 10-11 or 15-19 years would lead to greater reductions in the overall number of cCMV infections than vaccination at age 20-29 years but less than the reductions achievable by childhood vaccination (Figure 3b).
Estimated impact of vaccination and duration of vaccine protection on cCMV infections by type of maternal infection
The changes in the distribution of cCMV infections by type of maternal infection throughout the decades-long period after vaccine introduction leading up to equilibrium are shown in Table 2. In the United States, assuming 90% vaccination coverage, 70% vaccine efficacy and 5-year duration of protection, a combined schedule of vaccination at ages 12-18 months and 15-19 years would have the greatest reduction at equilibrium as well as at 10, 20 and 50 years after vaccine introduction. This strategy would lead to a reduction of approximately 30% in the overall number of cCMV infections 10 years after vaccine introduction, with reductions of approximately 50% in the number of those due to primary maternal infection. Approximately 50 years after vaccine introduction, there would be approximately 30% fewer cCMV infections due to primary maternal infection than pre-vaccination and approximately 70% and 45% fewer cCMV infections due to maternal reinfection and reactivation, respectively, resulting in a reduction of 45% in the overall number of cCMV infections. In the United States, the distribution of cCMV infections by type of maternal infection would change in the post-vaccination equilibrium, with a slight increase in the proportion of cCMV infections due to primary maternal infection.
In Brazil, ten years after introduction of a combined schedule of vaccination at ages 12-18 months and 15-19 years, assuming 90% vaccination coverage, 70% vaccine efficacy and 5-year duration of protection, the overall number of cCMV infections would decrease by approximately 50%, with approximately 50%, 65% and 30% reductions in those due to maternal primary infection, reinfection and reactivation, respectively (Table 2). However, an increase in the number and proportion of cCMV infections due to primary maternal infection would occur after 50 years of vaccination. The strategy with the largest potential for an increase in the number of cCMV infections due to primary maternal infection was vaccination at age 12-18 months only; although the overall number of cCMV infections would still be approximately 30% lower than pre-vaccination levels, there would be an approximately 25% increase in cCMV infections due to primary maternal infection (Table 2).
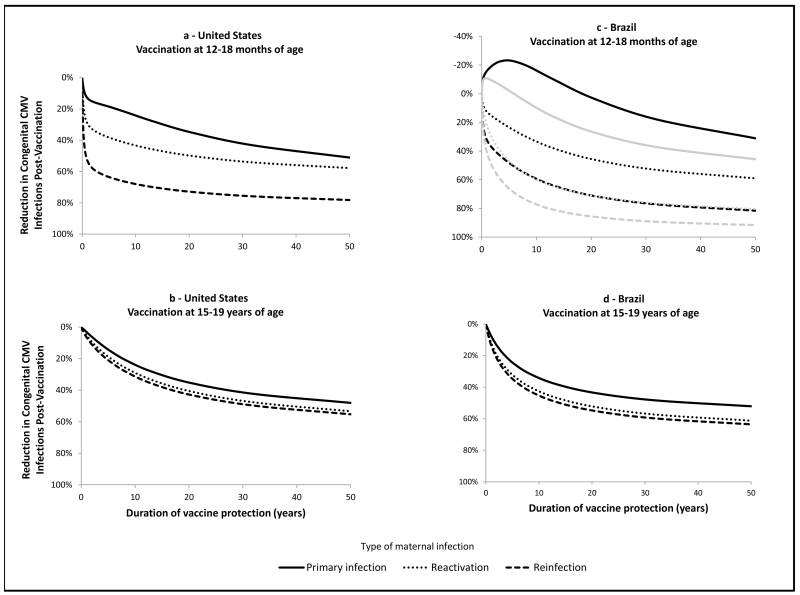
With increases in duration of vaccine protection, vaccination at ages 12-18 months or 15-19 years in the United States would lead to greater reductions in the number of cCMV infections, overall and due to any type of maternal infection (Figure 4a and 4b). Increased duration of vaccine protection would also result in greater reductions in the number of overall cCMV infections in Brazil. The potential for an increase in cCMV infections due to primary maternal infection in Brazil was predicted for vaccination at age 12-18 months when duration of vaccine protection was <20 years (Figure 4c) and, for a combined schedule with vaccination at ages 12-18 months and 15-19 years, when duration of protection was <5 years (Figure 4c). Vaccination at age 15-19 years only in Brazil would not increase cCMV infections due to primary maternal infection, regardless of the duration of vaccine protection (Figure 4d), but reduction in the overall number of cCMV infections and those due to maternal reinfection and reactivation would be lower than with vaccination at age 12-18 months only or combined with vaccination at age 15-19 years.
Figure 4.
Reduction in the annual number of cCMV infections, by type of maternal infection, at equilibrium by duration of vaccine protection, assuming age-specific duration of infectiousness, 20 year duration of latency, 90% vaccine coverage, 70% vaccine efficacy, and vaccination at 12-18 months or 15-19 years of age, United States and Brazil.
In figure 4c, black lines indicate impact of vaccination at 12-18 months of age only and gray lines indicate impact of combined schedule with vaccination at 12-18 months of age and 15-19 years of age.
Sensitivity analyses
In our sensitivity analyses, the predicted reductions in cCMV infections would not change substantially with the assumption of 5-year latency duration instead of 20 years (Supplementary material – Table 3, columns a vs. b, and columns c vs. d). Assuming no vaccine protection against non-primary infections, the predicted reductions in cCMV infections would be smaller with vaccination strategies targeting adolescents or adults (Supplementary material – Table 3, columns a vs. c, for example). Assuming different contact mixing matrices, vaccination at age 12-18 months only or combined with vaccination at age 15-19 years would result in smaller reductions in cCMV infections with pattern I from Azevedo’s study, (Supplementary material – Table 3, columns a vs. e), and greater reductions with the Polymod matrix (Supplementary material – Table 3, columns a vs. g).
DISCUSSION
Using a mathematical model of CMV epidemiology parameterized with data from the United States and Brazil, we assessed the potential impact of vaccination on CMV seroprevalence and cCMV infections. Concurrent vaccination at ages 12-18 months and 15-19 years would have the greatest impact on reducing the number of cCMV infections overall, both in populations with moderate and high baseline maternal seroprevalence. Our model suggests that such a vaccination strategy, assuming a vaccine with 70% efficacy, 90% coverage and 5-year duration of protection, could prevent nearly 30%-50% of cCMV infections during the 10-50 years after vaccine introduction. Better understanding the relative contribution of type of maternal infection to overall burden of cCMV infection and cCMV disease, the main focus of vaccine prevention [20, 23], is critical.
Our analyses represent significant progress beyond the work of Griffiths [24] and Azevedo [11]. We incorporated type of maternal infection into the model and explored how the impact of CMV vaccination might vary by population-specific reproduction numbers and baseline seroprevalence. Azevedo found that vaccination at age 2-6 months may increase overall cCMV infections if vaccine-induced immunity wanes before 20 years [11]. With vaccination at age 12-18 months and vaccine duration of protection <20 years, our model predicted a decrease in the overall number of cCMV infections and a potential increase in the number of cCMV infections due to primary maternal infection because of a shift in the age of primary infection into childbearing age in Brazil. Because primary maternal infections are more likely to result in maternal-to-fetus transmission and appear to be more likely to result in cCMV disease than for non-primary infections [10], further investigation into the impact that increases in numbers of cCMV infections due to primary maternal infection might have on total cCMV disease burden is needed. Our model suggests that this potential perverse effect could be ameliorated by a combined vaccination schedule at age 12-18 months and 15-19 years. We did not predict a perverse effect from CMV vaccination in the US-based simulations. However, further investigation into the potential for a perverse effect in sub-groups in the US population with a disproportionate burden of CMV infection [15, 19] should to be considered in future planning of vaccination strategies.
The proportion of cCMV infections due to maternal reinfection vs. reactivation is not well understood, nor is the relative contribution of either of them to cCMV disease [20]. A study from Brazil found that nearly half of the mothers who delivered an infant with cCMV infection had evidence of infection with more than one CMV strain before or during the affected pregnancy and 18% seroconverted to a new strain during the affected pregnancy [25]. As such, a vaccine that provides protection to both CMV seronegative and seropositive individuals would have the greatest potential for reducing the number of cCMV infections in populations with high baseline maternal CMV seroprevalence. Encouraging results from studies of the glycoprotein B/MF59 vaccine indicate that not only did it have a 50% efficacy against primary CMV infection [26], it was also capable of boosting immunity in CMV-seropositive women [27, 28].
Our model relies on a number of key assumptions about CMV epidemiology for which data are lacking. Specifically, the susceptibility to reinfection and duration of latency and viral excretion following non-primary infections are unknown [8, 9, 25]. We did not incorporate the risk of maternal-to-fetal transmission of CMV by maternal infection type into our model because data are scarce for maternal reinfections or reactivations; as a result, the model may have overestimated the contribution of primary infection to cCMV infections. In sensitivity analyses, the estimated reductions in cCMV infections were similar when varying latency duration from 5 to 20 years. However, they were sensitive to whether the vaccine protects seropositive individuals against non-primary infections, when considering vaccination of adolescents or young adults, and to the contact mixing matrices used, which derived different the distributions of cCMV infections by type of maternal infection.
Although the focus of CMV vaccine trials for prevention of cCMV infection thus far has been mainly on prevention of primary infection in seronegative women of childbearing age [26], modeling suggests universal vaccination of infants could result in elimination of cCMV infection in populations with moderate and high baseline maternal seroprevalence. Such a strategy would require a vaccine that would be efficacious in the face of potential interference by maternal antibodies and early exposure to CMV as a result of breastfeeding. In the absence of vaccination during infancy, modeling suggests that vaccination strategies that include young children would result in greater declines in cCMV infection than those restricted only to adolescents or women of reproductive age. Designing and conducting vaccine trials that include infants or young children will require identification of clinically important and feasible-to-study endpoints that could lead to licensure of a vaccine with the purpose of preventing cCMV disease [20]. Future studies of CMV vaccines should evaluate effectiveness and duration of protection among young children and adolescents, in seronegative and seropositive individuals, and in settings of lower and higher force of infection.
Supplementary Material
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: All authors have no conflicts of interest
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- [1].Enders G, Bader U, Lindemann L, Schalasta G, Daiminger A. Prenatal diagnosis of congenital cytomegalovirus infection in 189 pregnancies with known outcome. Prenat Diagn. 2001;21:362–77. doi: 10.1002/pd.59. [DOI] [PubMed] [Google Scholar]
- [2].Boppana SB, Pass RF, Britt WJ, Stagno S, Alford CA. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. The Pediatric infectious disease journal. 1992;11:93–9. doi: 10.1097/00006454-199202000-00007. [DOI] [PubMed] [Google Scholar]
- [3].Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin Infect Dis. 2004;39:233–9. doi: 10.1086/421999. [DOI] [PubMed] [Google Scholar]
- [4].Grosse SD, Ortega-Sanchez IR, Bialek SR, Dollard SC. The Economic Impact of Congenital CMV Infection: Methods and Estimates. In: Reddehase MJ, editor. Cytomegaloviruses: From Molecular Pathogenesis to Intervention. Caister Academic Press; 2013. [Google Scholar]
- [5].Vaccines for the 21st Century: A Tool for Decisionmaking. The National Academies Press; 2000. [PubMed] [Google Scholar]
- [6].Schleiss MR. Cytomegalovirus vaccine development. Current topics in microbiology and immunology. 2008;325:361–82. doi: 10.1007/978-3-540-77349-8_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Garnett GP, Cousens S, Hallett TB, Steketee R, Walker N. Mathematical models in the evaluation of health programmes. Lancet. 2011;378:515–25. doi: 10.1016/S0140-6736(10)61505-X. [DOI] [PubMed] [Google Scholar]
- [8].Cannon MJ, Hyde TB, Schmid DS. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Reviews in medical virology. 2011;21:240–55. doi: 10.1002/rmv.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ross SA, Arora N, Novak Z, Fowler KB, Britt WJ, Boppana SB. Cytomegalovirus reinfections in healthy seroimmune women. The Journal of infectious diseases. 2010;201:386–9. doi: 10.1086/649903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fowler KB, Stagno S, Pass RF. Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA : the journal of the American Medical Association. 2003;289:1008–11. doi: 10.1001/jama.289.8.1008. [DOI] [PubMed] [Google Scholar]
- [11].Azevedo RS, Amaku M. Modelling immunization strategies with cytomegalovirus vaccine candidates. Epidemiology and infection. 2011;139:1818–26. doi: 10.1017/S0950268811000343. [DOI] [PubMed] [Google Scholar]
- [12].Sinclair J. Human cytomegalovirus: Latency and reactivation in the myeloid lineage. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2008;41:180–5. doi: 10.1016/j.jcv.2007.11.014. [DOI] [PubMed] [Google Scholar]
- [13].Vochem M, Hamprecht K, Jahn G, Speer CP. Transmission of cytomegalovirus to preterm infants through breast milk. The Pediatric infectious disease journal. 1998;17:53–8. doi: 10.1097/00006454-199801000-00012. [DOI] [PubMed] [Google Scholar]
- [14].Peckham CS, Johnson C, Ades A, Pearl K, Chin KS. Early acquisition of cytomegalovirus infection. Archives of disease in childhood. 1987;62:780–5. doi: 10.1136/adc.62.8.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis. 2010;50:1439–47. doi: 10.1086/652438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Almeida LN, Azevedo RS, Amaku M, Massad E. Cytomegalovirus seroepidemiology in an urban community of Sao Paulo, Brazil. Revista de saude publica. 2001;35:124–9. doi: 10.1590/s0034-89102001000200004. [DOI] [PubMed] [Google Scholar]
- [17].Heffernan JM, Smith RJ, Wahl LM. Perspectives on the basic reproductive ratio. J R Soc Interface. 2005;2:281–93. doi: 10.1098/rsif.2005.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Diekmann O, Heesterbeek JA, Roberts MG. The construction of next-generation matrices for compartmental epidemic models. J R Soc Interface. 2010;7:873–85. doi: 10.1098/rsif.2009.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Colugnati FA, Staras SA, Dollard SC, Cannon MJ. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC infectious diseases. 2007;7:71. doi: 10.1186/1471-2334-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Krause PR, Bialek SR, Boppana SB, Griffiths PD, Laughlin CA, Ljungman P, et al. Priorities for CMV vaccine development. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Haber M, Longini IM, Jr., Halloran ME. Measures of the effects of vaccination in a randomly mixing population. International journal of epidemiology. 1991;20:300–10. doi: 10.1093/ije/20.1.300. [DOI] [PubMed] [Google Scholar]
- [22].Mossong J, Hens N, Jit M, Beutels P, Auranen K, Mikolajczyk R, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS medicine. 2008;5:e74. doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Griffiths P, Plotkin S, Mocarski E, Pass R, Schleiss M, Krause P, et al. Desirability and feasibility of a vaccine against cytomegalovirus. Vaccine. 2013;31(Suppl 2):B197–203. doi: 10.1016/j.vaccine.2012.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Griffiths PD, McLean A, Emery VC. Encouraging prospects for immunisation against primary cytomegalovirus infection. Vaccine. 2001;19:1356–62. doi: 10.1016/s0264-410x(00)00377-7. [DOI] [PubMed] [Google Scholar]
- [25].Yamamoto AY, Mussi-Pinhata MM, Boppana SB, Novak Z, Wagatsuma VM, Oliveira Pde F, et al. Human cytomegalovirus reinfection is associated with intrauterine transmission in a highly cytomegalovirus-immune maternal population. American journal of obstetrics and gynecology. 2010;202:297 e1–8. doi: 10.1016/j.ajog.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pass RF, Zhang C, Evans A, Simpson T, Andrews W, Huang ML, et al. Vaccine prevention of maternal cytomegalovirus infection. The New England journal of medicine. 2009;360:1191–9. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sabbaj S, Pass RF, Goepfert PA, Pichon S. Glycoprotein B vaccine is capable of boosting both antibody and CD4 T-cell responses to cytomegalovirus in chronically infected women. The Journal of infectious diseases. 2011;203:1534–41. doi: 10.1093/infdis/jir138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schleiss MR. Could therapeutic vaccination of cytomegalovirus-seropositive persons prevent reinfection and congenital virus transmission? The Journal of infectious diseases. 2011;203:1513–6. doi: 10.1093/infdis/jir144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.