Abstract
Background
Medication non-adherence is extremely common for osteoporosis, however no clear methods exist for identifying patients at-risk of this behavior. We developed a clinical prediction rule to predict medication non-adherence for women prescribed osteoporosis treatment.
Methods
Women undergoing bone mineral density testing and fulfilling WHO criteria for osteoporosis were invited to complete a questionnaire and then followed for one year. Adjusted logistic regression models were examined to identify variables associated with very low adherence (medication possession ratio < 20%). The weighted variables, based on the logistic regression, were summed and the score compared with the proportion of subjects with very low adherence.
Results
142 women participated in the questionnaire and were prescribed an osteoporosis medication. After one year, 36% (n = 50) had very low adherence. Variables associated with very low adherence included: prior non-adherence with chronic medications, agreement that side effects are concerning, agreement that she is taking too many medications, lack of agreement that osteoporosis is a worry, lack of agreement that a fracture will cause disability, lack of agreement that medications help her stay active, and frequent use of alcohol. When combined into a summative score, 36 of the 58 subjects (62%) with 7 or more points on the score demonstrated very low adherence. This compares with 14 of the 84 (17%) subjects with fewer than 7 points (c-statistic = 0.74).
Conclusions
We developed a brief clinical prediction rule that was able to discriminate between women likely (and unlikely) to experience very low adherence with osteoporosis medications.
Keywords: Osteoporosis, medication adherence, epidemiology, clinical prediction rule, health beliefs
INTRODUCTION
Osteoporosis is a major public health issue – 50% of women and 25% of men aged 50 years and over will sustain fractures during their lifetime. Fractures reduce quality of life, cause pain and loss of function, and appear to increase mortality.1–3 In addition, they are associated with substantial costs. Recent estimates for the US are that there were more than 2 million incident fractures at a direct medical cost of $17 billion in 2005; these are projected to rise by almost 50% over the next 15 years.4
The last fifteen years has seen the development of many effective pharmacologic treatments for osteoporosis. All such treatments have demonstrated the ability to improve bone mineral density and reduce fractures compared with placebo in randomized controlled trials.5 The use of these treatments remains sub-optimal, with under-treatment of at-risk patients by clinicians and very low adherence by patients.6–8 Across many populations from numerous settings, fewer than 50% of patients remain adherent with treatment one year after initiation.9–11
Adherence can be improved, but it is not simple. A Cochrane Collaborative review found that less than half of all randomized controlled trials targeting medication adherence produced improvement in clinical outcomes.12 In the osteoporosis arena, several adherence interventions have shown some promise.13 Reduction in the frequency of medication dosing has been found to improve adherence, but only in non-randomized studies.10, 11 In addition, the introduction of educational counseling appears to improve adherence.14, 15
Because some patients remain moderately or highly adherent to their medications, it would be most cost-effective to target adherence-enhancing strategies toward persons at high risk of non-adherence. Our goal was to determine potential predictors of very low adherence with osteoporosis medications. We combined these predictors in a clinical prediction rule that might be useful for identifying patients likely to experience very low adherence with osteoporosis medications.
METHODS
Study Cohort
Women enrollees of one health plan undergoing bone mineral density (BMD) who fulfilled the WHO criteria for OP and had not been treated with an OP drug in the prior 6 months were invited to complete a questionnaire about osteoporosis and grant permission for review of medical and pharmacy records. The study invitation did not discuss medication adherence. Consenting women filled the survey out in their home and were then followed for the subsequent year to examine their BMD results and use of osteoporosis medications. Women were not systematically shown their BMD results, although most providers shared this information with their patients. Our study focuses on the participating women who were prescribed a medication for osteoporosis during the subsequent twelve months and were still in the health plan at month twelve. Such medications included bisphosphonates (alendronate, ibandronate, risedronate, and zoledronic acid), calcitonin, hormone replacement therapy, raloxifene, and teriparatide. Five women using intravenous preparations were subsequently excluded from analyses. Since we have access to the electronic medical record, we were able to include subjects that were prescribed a medication for osteoporosis but never filled it at the pharmacy. The study protocol was approved by the Saint Vincent Hospital, Fallon Clinic, Fallon Community Health Plan Institutional Review Board/Research Review Committee.
Outcome
The study outcome was very low medication adherence, defined as a medication possession ratio (MPR) < 20%, including women who never filled a prescribed medication. The MPR is a commonly used measure of adherence and was defined as the percentage of days during follow-up with medication available.16 We did not assess persistence. Follow-up began with the first medication dispensing or the date of the prescription for the women who did not pick up any medication. Available medication was judged based on the number of days supply dispensed, as determined by the pharmacy claims collected by Fallon Community Health Plan. The MPR for women prescribed an osteoporosis medication but who never received it was zero. If dispensed medications overlapped in their duration, we assumed that use was not concurrent. The maximum MPR was 100%. Subjects were not excluded if they switched between osteoporosis medications.
We chose < 20% as the definition of very low adherence for two reasons. First, while the precise relation is not clear, several studies suggest that reduced medication adherence correlates with a reduced fracture reduction effectiveness. However, it would be hard to imagine that at an MPR < 20% the medications produce any clinically important benefit. Second, by defining the outcome at such a low MPR, we hope that clinicians and/or the health delivery systems will be motivated to use the clinical prediction rule we develop to intervene. Sensitivity analyses were also performed for a threshold of MPR < 40% and < 80% to determine if the significant predictors varied by MPR level.
Potential Predictors
Potential predictors came from either responses to the questionnaire or previously collected health care claims.
Questionnaire
The questionnaire, described in earlier work,17 contained a variety of items, including sociodemographics, health behaviors, osteoporosis knowledge, and perceived osteoporosis severity (see Appendix for complete questionnaire). Most items used a 5-category Likert scale, ranging from “strongly agree” to “strongly disagree”.
Health care claims
We examined the health care utilization or claims data for the 12 months prior to the survey to determine each subject’s use of preventive services, such as colonoscopy, mammography, Pap smear, pneumococcal vaccine, and influenza vaccine. A Charlson comorbidity index was calculated based on published algorithms for health care claims data.18 As well, the pharmacy claims data were assessed for the filling of at least two prescriptions for chronic medical conditions, such as diabetes, hypertension, or hyperlipidemia. Subjects with these diagnoses but less than two prescription fillings were considered to have had prior non-adherence.
Statistical Analyses
We first examined the baseline characteristics of study subjects. The MPR was calculated during the follow-up year and the distribution of MPRs for the entire study cohort was assessed. Baseline characteristics and questionnaire responses were compared among subjects who experienced very low adherence versus the remaining cohort.
Four domains of potential predictors were identified: sociodemographic, health care factors, osteoporosis factors, and osteoporosis beliefs. Univariate and multivariable logistic regression models were examined for each domain. Variables with p-values ≤ 0.10 or odds ratios (ORs) > 2.0 or < 0.5 in the domain-specific models were considered for inclusion in the full multivariable logistic regression. Only variables with p-values < 0.10 were included in the final model for very low adherence. In secondary analyses, these variables were also tested with adherence < 40% and < 80% as the outcome.
The variables remaining in the fully adjusted model were used to develop a preliminary clinical prediction rule for very low adherence. We weighted each variable based on its OR and then summed the weights to create a score. In this manner, each subject in the study cohort was assigned a score, the distribution of scores was assessed, and the proportion with very low adherence was examined for each score. The area under the receiver operating characteristic curve (the c-statistic) was used to assess the predictive ability of the model. All analyses were carried out in SAS (Cary NC).
RESULTS
465 women were invited to participate. 142 women were prescribed medications and consented to participate. Ninety-seven (68%) were aged 65 and over, all were Caucasian, and 77% reported at least a high school education. Sixty-three percent of women made at least four visits to a health care provider in the prior year and the average number of medications used was 4.5. Only six women were diabetic, but 38% were using a lipid lowering agent and 39% were using an anti-hypertensive medication. During the year prior to enrollment, the cohort engaged in many preventive health services, such as mammography (74%), colonoscopy (32%), and influenza vaccines (49%).
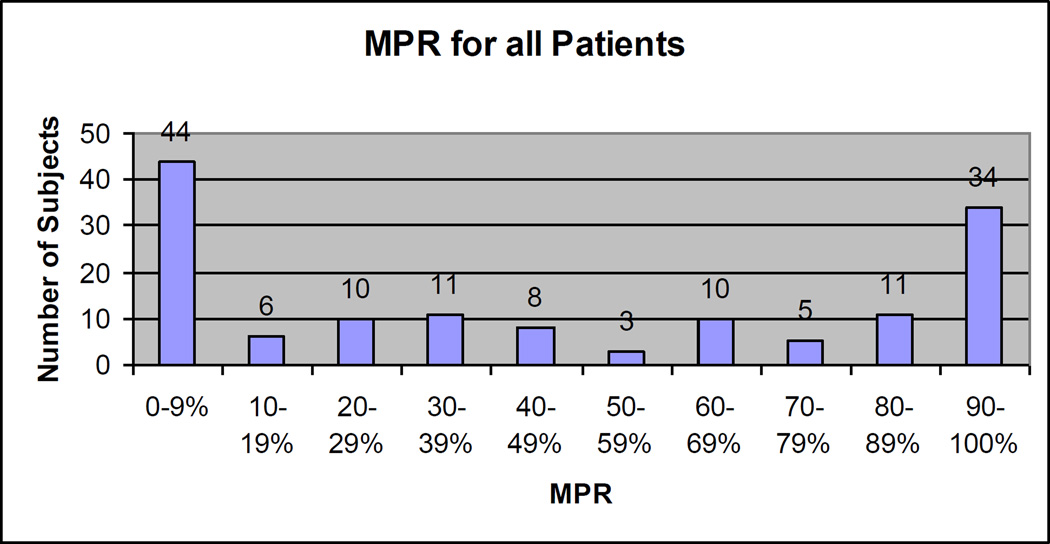
The distribution of MPRs is shown in Figure 1. The mean MPR was 55% and the median 54% (interquartile range 16 – 92%). Approximately one-third of women (n = 45) had MPRs of 80% and greater. Fifty women (35%) had MPRs < 20% and constitute the group with very low adherence. Within this group were 16 women who never filled their osteoporosis prescription, the remaining 34 women stopped filling their prescriptions regularly during the year of follow-up
Figure 1.
The figure illustrates the frequency of different medication possession ratios (MPR), in 10% bands for all 142 subjects.
MPR = days with available medication / days in study during one year of follow-up.
A number of sodiodemographic and health care factors differed slightly between women with very low adherence and the other women (see Table 1). Women with very low adherence were slightly younger, used fewer medications, and were less likely to have high blood pressure. Those with very low adherence were slightly more likely to have had an influenza vaccine and more likely to have a score of at least one on the Charlson comorbidity index. As well, alcohol consumption of at least 2 drinks per day was more common in the very low adherence group.
Table 1.
Sociodemographics and Health Care Factors, by Adherence Level
| Very low adherence (MPR<20%) (n = 50) |
Others (n = 92) |
|
|---|---|---|
| Sociodemographic | ||
| Age, < 65 | 15 (30%) | 30 (33%) |
| 65–74 | 19 (38%) | 22 (24%) |
| 75–84 | 14 (28%) | 27 (19%) |
| 85+ | 2 (4%) | 13 (14%) |
| Education, Less than high school | 12 (24%) | 20 (22%) |
| High school | 16 (32%) | 33 (36%) |
| Beyond high school | 22 (44%) | 38 (42%) |
| Health Care Factors | ||
| Office visits, < 4 | 18 (36%) | 35 (38%) |
| 4+ | 32 (64%) | 57 (62%) |
| Hospitalization, Any | 6 (12%) | 10 (11%) |
| None | 44 (88%) | 82 (89%) |
| Number of different medications, < 5 | 30 (60%) | 41 (45%) |
| 5+ | 20 (40%) | 51 (55%) |
| Hypertension, None | 35 (70%) | 52 (57%) |
| Yes | 15 (30%) | 40 (43%) |
| Diabetes, None | 48 (96%) | 88 (96%) |
| Yes | 2 (4%) | 4 (4%) |
| Lipid lowering treatments, None | 33 (66%) | 55 (60%) |
| Yes | 17 (34%) | 37 (40%) |
| Colonoscopy, None | 33 (66%) | 64 (70%) |
| Yes | 17 (34%) | 28 (30%) |
| Mammogram, None | 11 (22%) | 26 (28%) |
| Yes | 39 (78%) | 66 (72%) |
| Influenza vaccine, None | 23 (46%) | 50 (54%) |
| Yes | 27 (54%) | 42 (46%) |
| Pneumococcal vaccine, None | 48 (96%) | 88 (96%) |
| Yes | 2 (4%) | 4 (4%) |
| Charlson comorbidity score, zero | 19 (40%) | 42 (48%) |
| 1+ | 29 (60%) | 45 (52%) |
| Alcohol consumption of 2 or more drinks per day | ||
| No days per week | 35 (70%) | 76 (83%) |
| 1 – 2 days per week | 8 (16%) | 13 (14%) |
| 3 – 4 days per week | 4 (8%) | 3 (3%) |
| 5+ days per week | 3 (6%) | 0 (0%) |
Health care factors measured in health care claims data from 12 months prior to survey. None of the differences between the two columns are statistically significant, i.e., p < 0.05.
Osteoporosis factors and osteoporosis beliefs also differed by adherence level (see Table 2). In general, women with very low adherence were less likely to agree with positive statements about osteoporosis medications and less likely to express concern with fractures.
Table 2.
Osteoporosis Factors and Health Beliefs by Adherence Level*
| Very low adherence (n = 50) |
Others (n = 92) |
|
|---|---|---|
| Osteoporosis factors | ||
| Do you have osteoporosis, No | 10 (20%) | 14 (15%) |
| Yes | 39 (80%) | 77 (85%) |
| Use a pillbox to remember OP medications, No | 17 (35%) | 30 (33%) |
| Yes | 31 (65%) | 60 (67%) |
| Doctor or nurse told you had osteoporosis, No | 10 (20%) | 21 (23%) |
| Yes | 40 (80%) | 70 (77%) |
| Has someone told you about your BMD results, No | 12 (24%) | 23 (25%) |
| Yes | 38 (76%) | 68 (75%) |
| How well do you think your OP medication is working, | ||
| Very well | 0 (17%) | 7 (12%) |
| Somewhat well | 4 (17%) | 5 (9%) |
| Not at all | 20 (83%) | 45 (79%) |
| Osteoporosis beliefs | ||
| I worry about having osteoporosis,* | ||
| Agree or strongly agree | 26 (54%) | 68 (65%) |
| Neutral, disagree, or strongly disagree | 22 (46%) | 22 (35%) |
| If I had a fracture, I could end up disabled* | ||
| Agree or strongly agree | 31 (62%) | 75 (71%) |
| Neutral, disagree, or strongly disagree | 19 (38%) | 16 (29%) |
| Medication can treat my osteoporosis | ||
| Agree or strongly agree | 30 (60%) | 69 (67%) |
| Neutral, disagree, or strongly disagree | 20 (40%) | 22 (33%) |
| Medications can make my bones stronger | ||
| Agree or strongly agree | 32 (67%) | 74 (74%) |
| Neutral, disagree, or strongly disagree | 16 (33%) | 18 (26%) |
| OP Medications can protect against a broken hip | ||
| Agree or strongly agree | 24 (48%) | 57 (56%) |
| Neutral, disagree, or strongly disagree | 26 (52%) | 35 (44%) |
| OP Medications can help me stay independent* | ||
| Agree or strongly agree | 25 (51%) | 63 (69%) |
| Neutral, disagree, or strongly disagree | 24 (49%) | 28 (31%) |
| OP medications are good for me* | ||
| Agree or strongly agree | 23 (47%) | 60 (66%) |
| Neutral, disagree, or strongly disagree | 26 (53%) | 31 (34%) |
| Worry about side effects of OP medications | ||
| Agree or strongly agree | 33 (67%) | 52 (57%) |
| Neutral, disagree, or strongly disagree | 16 (32%) | 39 (43%) |
| OP medications can help me stay active* | ||
| Agree or strongly agree | 24 (49%) | 67 (74%) |
| Neutral, disagree, or strongly disagree | 25 (51%) | 24 (26%) |
| There are better ways than medications to treat OP | ||
| Agree or strongly agree | 16 (33%) | 19 (21%) |
| Neutral, disagree, or strongly disagree | 33 (67%) | 73 (79%) |
| Doctors are too quick to give medications | ||
| Agree or strongly agree | 20 (40%) | 26 (28%) |
| Neutral, disagree, or strongly disagree | 30 (60%) | 66 (72%) |
| Doctors give medications when advice would be better | ||
| Agree or strongly agree | 17 (35%) | 21 (23%) |
| Neutral, disagree, or strongly disagree | 31 (65%) | 70 (77%) |
| I can take care of OP without medications | ||
| Agree or strongly agree | 6 (12%) | 3 (3%) |
| Neutral, disagree, or strongly disagree | 44 (88%) | 86 (97%) |
| Medications cause more problems than they solve | ||
| Agree or strongly agree | 18 (38%) | 27 (30%) |
| Neutral, disagree, or strongly disagree | 29 (62%) | 62 (70%) |
| I prefer not to take medications | ||
| Agree or strongly agree | 25 (50%) | 37 (41%) |
| Neutral, disagree, or strongly disagree | 25 (50%) | 53 (59%) |
| I am already taking too many medications | ||
| Agree or strongly agree | 15 (31%) | 28 (31%) |
| Neutral, disagree, or strongly disagree | 34 (69%) | 63 (69%) |
See Appendix for listing of exact working of survey items.
Abbreviations: OP, osteoporosis. Several variables have a few missing responses and thus do not add up to the column total.
p < 0.05
Partial logistic regression models were examined for each of the four domains of interest – sociodemographic, health care factors, osteoporosis factors, and osteoporosis beliefs. The area under the receiver operating characteristic curves differed greatly for the four domains: c-statistics sociodemographic = 0.52, c-statistic health care factors = 0.69, c-statistic osteoporosis factors = 0.65, and osteoporosis beliefs = 0.81. Variables were selected from each of the domains based on p-values and ORs. The final multivariable model is shown in Table 3 (c-statistic = 0.79). The ORs for each of the variables provided a weight to create a score for the clinical prediction rule (see Table 3). When the model was re-run using an MPR < 80%, the c-statistic was 0.73 and the ORs were similar (see Supplemental Table).
Table 3.
Multivariable Logistic Regression For Very Low Adherence
| Predictor | Fully adjusted* odds ratios (95% CI) |
Weight for clinical prediction rule |
|---|---|---|
| Prior non-adherence | 9.42 (1.37 – 64.75) | 9 |
| Agree that side effects are concerning | 2.51 (0.99 – 6.37) | 3 |
| Agree that taking too many different medications | 1.72 (0.75 – 3.95) | 2 |
| Disagree that osteoporosis is a worry | 3.30 (1.28 – 8.50) | 3 |
| Disagree that a broken bone will cause disability | 2.51 (0.96 – 6.55) | 3 |
| Disagree that medications will help you stay active | 2.17 (0.92 – 5.11) | 2 |
| Drink ≥ 2 drinks per day on 3+ days per week | 8.85 (1.71 – 45.84) | 9 |
Very low adherence refers to a medication possession ratio < 20%. The variable selection is described in text. The fully adjusted odds ratios come from a model adjusted for all predictors noted above. The weights for the clinical prediction rule are proportional to the odds ratios and the total possible score for the clinical prediction rule = 31.
The covariate weights were used to give each subject a score on the clinical prediction rule. In Table 4, we list the number of subjects with each score and the n (%) with very low adherence at each score. At low scores, few subjects had very low adherence. However, there appeared to be a threshold at a score of 6 on the clinical prediction rule, above which 62% of subjects (36 of 58) experienced very low adherence. In a logistic regression model, the clinical prediction rule threshold of 6 demonstrated a c-statistic of 0.74.
Table 4.
Very low adherence by score on non-adherence clinical prediction rule
| Total score | N with score |
N (%) with very low adherence |
N with score |
N (%) with very low adherence |
|---|---|---|---|---|
| 0 | 11 | 1 (9.1%) | ||
| 2 | 8 | 0 (0%) | ||
| 3 | 25 | 5 (20%) | 84 | 14 (17%) |
| 4 | 3 | 1 (33.3%) | ||
| 5 | 31 | 7 (22.6%) | ||
| 6 | 6 | 0 (0%) | ||
| 7 | 11 | 7 (63.6%) | ||
| 8 | 13 | 7 (53.9%) | ||
| 9 | 3 | 2 (66.7%) | ||
| 10 | 10 | 6 (60%) | ||
| 11 | 6 | 5 (83.3%) | ||
| 12 | 4 | 2 (50%) | ||
| 13 | 2 | 0 (0%) | 58 | 36 (62%) |
| 14 | 4 | 2 (50%) | ||
| 15 | 1 | 1 (100%) | ||
| 17 | 1 | 1 (100%) | ||
| 19 | 2 | 2 (100%) | ||
| 26 | 1 | 1 (100%) | ||
| Total | 142 | 50 (35.2%) | ||
DISCUSSION
We examined potential predictors of adherence with osteoporosis medications. Very low adherence (<20%) was chosen as an important category to predict since it would be hard to argue that patients receive any benefit from medications used in this way. Sociodemographic information, health care factors, osteoporosis factors and beliefs were assessed and seven predictors of very low adherence were identified. From the ORs calculated in an adjusted logistic regression model, a clinical prediction rule was developed. In this sample, the rule had a strong ability to discriminate between patients likely to experience very low adherence versus other patients.
This rule is preliminary and requires validation. In such a small sample, we were unable to do more than develop the rule. However, the variables have strong face validity and will likely relate to very low adherence in other populations. We chose < 20% as a cutoff for developing this rule because it is clear that such patients require an intervention to improve adherence. Some may consider it warranted to intervene at higher adherence levels. The appropriate threshold for adherence is not clear and so we chose a very low level. However, the findings were unchanged when we used 40% as a threshold and not 20%. Other factors that might be considered when determining the appropriate adherence threshold include the likelihood of patient response to a given intervention and cost-effectiveness of intervening in a given group.
Our methods are limited by several important factors. First, our sample was relatively small and consisted of white women who were all insured. It is unclear if the rule will apply to other groups of patients; testing in other groups is warranted to assess generalizability. Second, the group of potential predictors that we studied was broad but not all inclusive. Psychological factors, such as depression or anxiety, were not queried. In addition, complexity of medication regimens might play a role in very low adherence, but we did not inquire. Other factors to be tested in future work include the presence of osteoporosis versus osteopenia and a history of fracture. All women in our sample had osteoporosis and we had incomplete information about prior fractures. Third, we followed patients for only one year and non-adherence may occur beyond one year. Fourth, some of the non-adherence may have been recommended by physicians. We did not exclude subjects who switched between medicines to limit this issue.
While the goal of this paper was to develop a clinical prediction rule, some of the responses regarding osteoporosis beliefs in Table 2 were notable and require comment. Among the fifty women with very low adherence, 88% believed that they could take care of their osteoporosis without medications. Perhaps, this should not be a surprise among very low adherers, but this attitude suggests a lack of education about osteoporosis. A majority of this group also agreed that medications cause a lot of problems. One way to improve adherence may be to work on this presumed knowledge deficit.
If validated, the clinical prediction rule we developed could have value in practice. Patients starting osteoporosis medications could be queried by their physician, nurse, or pharmacist about the issues contained in the clinical prediction rule. Patients scoring above 6 points could be considered as good candidates for adherence-enhancing interventions. Such interventions might include medications with different administration schedules (i.e., less frequent dosing), increased education about the value of medications for osteoporosis, increased follow-up about medication adherence, or a medication reminder program. The ability to accurately target high-risk patients may increase the cost-effectiveness and lead to greater uptake of such adherence improvement programs.
Supplementary Material
Acknowledgments
Support: This project was supported by a grant to Fallon Clinic from Novartis Pharmaceuticals Corporation. Dr. Solomon receives salary support from Amgen for work on rheumatoid arthritis as well as support from the Arthritis Foundation, AHRQ, and the NIH (AR 055989, AR 047782) on osteoporosis and adherence. Dr. Brookhart is supported by a career development award from the National Institute on Aging (AG27400). He has received grant support from Amgen for unrelated projects and has served on scientific advisory boards for Amgen without receiving compensation.
Contributor Information
Daniel H. Solomon, Email: dsolomon@partners.org.
M. Alan Brookhart, Email: alan@brookhart.org.
Peter Tsao, Email: htsao1@partners.org.
Devi Sundaresan, Email: devi.sundaresan@fallonclinic.org.
Susan E. Andrade, Email: SusanEAndrade@aol.com.
Kathleen Mazor, Email: Kathy.mazor@meyersprimary.org.
Robert Yood, Email: robert.yood@fallonclinic.org.
REFERENCES
- 1.Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality and costs. Journal of the American Geriatrics Society. 2003;51(3):364–370. doi: 10.1046/j.1532-5415.2003.51110.x. [DOI] [PubMed] [Google Scholar]
- 2.Melton LJ., 3rd Adverse outcomes of osteoporotic fractures in the general population. J Bone Miner Res. 2003 Jun;18(6):1139–1141. doi: 10.1359/jbmr.2003.18.6.1139. [DOI] [PubMed] [Google Scholar]
- 3.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. Jama. 2009 Feb 4;301(5):513–521. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 4.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007 Mar;22(3):465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 5.Cranney A, Guyatt G, Griffith L, et al. Meta-analyses of therapies for postmenopausal osteoporosis. IX: Summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocrine Reviews. 2002;23(4):570–578. doi: 10.1210/er.2001-9002. [DOI] [PubMed] [Google Scholar]
- 6.Solomon DH, Finkelstein JS, Katz JN, Mogun H, Avorn J. Underuse of osteoporosis medications in elderly patients with fractures. American Journal of Medicine. 2003;115(5):398–400. doi: 10.1016/s0002-9343(03)00357-7. [DOI] [PubMed] [Google Scholar]
- 7.Solomon DH, Morris C, Cheng H, et al. Medication use patterns for osteoporosis: an assessment of guidelines, treatment rates, and quality improvement interventions. Mayo Clinic Proceedings. 2005;80(2):194–202. doi: 10.4065/80.2.194. [DOI] [PubMed] [Google Scholar]
- 8.Solomon DH, Avorn J, Katz JN, et al. Compliance with osteoporosis medications. Archives of Internal Medicine. 2005;165(20):2414–2419. doi: 10.1001/archinte.165.20.2414. [DOI] [PubMed] [Google Scholar]
- 9.Kothawala P, Badamgarav E, Ryu S, Miller RM, Halbert RJ. Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc. 2007 Dec;82(12):1493–1501. doi: 10.1016/S0025-6196(11)61093-8. [DOI] [PubMed] [Google Scholar]
- 10.Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007 Aug;18(8):1023–1031. doi: 10.1007/s00198-006-0322-8. [DOI] [PubMed] [Google Scholar]
- 11.Rabenda V, Hiligsmann M, Reginster JY. Poor adherence to oral bisphosphonate treatment and its consequences: a review of the evidence. Expert Opin Pharmacother. 2009 Oct;10(14):2303–2315. doi: 10.1517/14656560903140533. [DOI] [PubMed] [Google Scholar]
- 12.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;(2):CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 13.Gleeson T, Iversen MD, Avorn J, et al. Interventions to improve adherence and persistence with osteoporosis medications: a systematic literature review. Osteoporos Int. 2009 Jun 5; doi: 10.1007/s00198-009-0976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook PF, Emiliozzi S, McCabe MM. Telephone counseling to improve osteoporosis treatment adherence: an effectiveness study in community practice settings. Am J Med Qual. 2007 Nov-Dec;22(6):445–456. doi: 10.1177/1062860607307990. [DOI] [PubMed] [Google Scholar]
- 15.Clowes JA, Peel NF, Eastell R. The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. Journal of Clinical Endocrinology & Metabolism. 2004;89(3):1117–1123. doi: 10.1210/jc.2003-030501. [DOI] [PubMed] [Google Scholar]
- 16.Choudhry NK, Shrank WH, Levin RL, et al. Measuring concurrent adherence to multiple related medications. Am J Manag Care. 2009 Jul;15(7):457–464. [PMC free article] [PubMed] [Google Scholar]
- 17.Yood RA, Mazor KM, Andrade SE, Emani S, Chan W, Kahler KH. Patient decision to initiate therapy for osteoporosis: the influence of knowledge and beliefs. J Gen Intern Med. 2008 Nov;23(11):1815–1821. doi: 10.1007/s11606-008-0772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of Clinical Epidemiology. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.