Abstract
Objective
The objective of this study was to evaluate patterns of recurrence and prognostic factors as well as the role of adjuvant chemotherapy in stage II–IV ovarian SBT.
Methods
We performed a retrospective review of all patients with advanced-stage SBT treated at our institution from 1979–2008. Advanced stage was defined as FIGO stage II–IV. Progression-free survival (PFS) was defined as the time of diagnosis to time of recurrence/death or last follow-up. Kaplan-Meier method was used to report the PFS rate.
Results
A total of 80 stage II–IV patients were identified, of which 15 (19%) were stage II, 63 (79%) were stage III, and 2 (2.5%) were stage IV. The site of metastasis was pelvis in 15 patients (19%), omentum in 29 patients (36%), isolated lymph nodes in 2 patients (2.5%), lung in 1 patient (1%), axilla in 1 patient (1%), and multiple sites in 32 patients (40%). With a median follow-up of 4.8 years, 17 patients (21%) developed recurrent disease. Only patients with metastasis to the omentum or multiple sites developed recurrent disease. Of the 65 stage III/IV patients, 17 patients (26%) received adjuvant chemotherapy following diagnosis. The 3-year progression-free survival (PFS) was 89.9% (95% CI, 77.3–95.7) for patients who did not receive adjuvant chemotherapy compared with 70.6% (95% CI, 43.1–86.6) for patients who received adjuvant chemotherapy.
Conclusion
While advanced-stage ovarian SBT generally has a good prognosis, nearly 21% of patients develop recurrent disease with intermediate follow-up. It is unclear from these data if adjuvant chemotherapy influenced PFS.
Keywords: ovarian borderline tumor, chemotherapy, recurrence, advanced stage
Introduction
Ovarian serous borderline tumors (SBTs) are a separate subset of ovarian epithelial neoplasms. They differ from invasive ovarian epithelial neoplasms both in pathologic characteristics and clinical behavior [1–4], and they have an excellent prognosis overall. Various risk factors for recurrence include the presence of invasive implants, micropapillary pattern histology, DNA ploidy, and age [5–13].
Most ovarian SBTs present with stage I disease; however, SBTs can be associated with advanced-stage disease [14]. The optimal management of advanced-stage ovarian SBTs relies mainly on surgery. The role of adjuvant chemotherapy is debatable, particularly in stage III–IV cases. Surgery is an integral component to management of advanced-stage ovarian SBT. Some early studies have shown that chemotherapy in the adjuvant setting provides some treatment benefit [15–16], but other studies have refuted this [17–18].
The objective of this study was to evaluate clinical characteristics, patterns of recurrence, and outcomes of patients with advanced stage SBTs, and to describe the role of adjuvant chemotherapy in this select group of patients.
Methods
After Institutional Review Board (IRB) approval, we identified all patients with ovarian SBTs treated at our institution from 1979–2008. Not all patients were diagnosed at our institution as some patients presented for further management after initial surgery and diagnosis at an outside institution. We reviewed medical records, including operative reports, pathology and laboratory reports, and chemotherapy records, and extracted the relevant data. The pathology specimens from patients who were diagnosed at an outside institution were all reviewed at our institution.
Stage at initial diagnosis was designated based on the International Federation of Gynecology and Obstetrics (FIGO) staging system for ovarian carcinoma [19]. We defined advanced-stage disease as stage II–IV. Histology information was obtained from institutional pathology reports, and only patients with tumors of serous histology were included in this cohort. It is our hospital policy to confirm all outside pathology reports by institutional review of submitted specimens. From the pathology reports, sites of metastasis, presence of micropapillary features, presence of invasive or non-invasive implants, and spread to lymph nodes were noted. We reviewed operative reports to determine which procedures had been performed and to note any intraoperative findings, including presence of ascites and residual disease.
Progression-free survival (PFS) was defined as the time of diagnosis to time of recurrence/death or last follow-up. Recurrence was defined with clinical or CA-125 criteria according to the Rustin criteria [20]. The Kaplan-Meier method was used to estimate PFS rates, and univariate analysis with P values were generated using the log-rank test. Statistical analyses were performed using SAS ® analytical software.
Results
A total of 80 stage II–IV patients were identified. The clinicopathologic characteristics for this cohort are described in Table 1. The median age at diagnosis was 41 years (range, 16–80 years). Fifteen patients (19%) had stage II disease, 63 (79%) had stage III disease, and 2 (2.5%) had stage IV disease at diagnosis. At the time of initial diagnosis, the site of metastasis was the pelvis in 15 patients (19%), omentum in 29 patients (36%), isolated lymph nodes in 2 patients (2.5%), lung in 1 patient (1%), axilla in 1 patient (1%), and multiple sites in 32 patients (40%). Of the 80 patients in the cohort, 25 (31%) had tumor histology with micropapillary features and 19 (24%) had invasive implants. Forty-four patients (55%) had lymph node sampling at the time of surgery. Of these 44 patients, 28 (64%) had positive lymph nodes. Adjuvant chemotherapy was given in 17 patients (21%). Because our cohort of patients were treated over a 30-year time period, a variety of intravenous and intraperitoneal chemotherapy regimens were given. Intravenous chemotherapy agents included cytoxan, cisplatin, adriamycin, paclitaxel, and carboplatin. Intraperitoneal chemotherapy agents included mitoxantrone, etoposide, carboplatin, cisplatin, and paclitaxel.
Table 1.
Clinicopathologic characteristics
| N (%) | |
|---|---|
|
| |
| Total number of patients | 80 |
|
| |
| Median age at diagnosis, years (range) | 41.1 (16.8–79.6) |
|
| |
| Stage | |
| II | 15 (19) |
| III | 63 (79) |
| IV | 2 (2.5) |
|
| |
| Sites of metastasis | |
| Pelvis | 15 (19) |
| Omentum | 29 (36) |
| Isolated lymph nodes | 2 (2.5) |
| Lung | 1 (1) |
| Axilla | 1 (1) |
| Multiple | 32 (40) |
|
| |
| Micropapillary features | |
| Yes | 25 (31) |
| No | 55 (69) |
|
| |
| Implants | |
| Invasive | 19 (24) |
| Non-invasive | 60 (75) |
| Unknown | 1 (1) |
|
| |
| Lymph nodes | |
| Positive | 28 (35) |
| Negative | 16 (20) |
| Not done | 36 (45) |
|
| |
| Ascites | |
| Yes | 32 (40) |
| No | 48 (60) |
|
| |
| Residual disease | |
| Yes | 8 (10) |
| No | 69 (86) |
| Unknown | 3 (4) |
|
| |
| Adjuvant chemotherapy | |
| Yes | 17 (21) |
| No | 63 (79) |
Table 2 outlines the follow-up and recurrence data. The median follow-up time was 4.8 years (range, 0.05–22.84 years). At the time of last follow-up, 50 patients (62.5%) had no evidence of disease, 10 (12.5%) were alive with disease, 4 (5%) were dead of disease, 4 (5%) were dead of other causes, and 12 (15%) were lost to follow-up. Of the 80 patients in the cohort, 17 (21%) developed recurrent disease—11 (65%) developed recurrent disease with invasive or low-grade serous carcinoma, 5 (29%) developed recurrent disease with borderline histology, and 1 (6%) developed recurrent disease with unknown histology.
Table 2.
Follow-up data
| Median 3-year RFS rate | 84.9 (73.8–91.6) |
|
| |
| Median follow-up, years (range) | 4.8 (0.05–22.84) |
|
| |
| Status at time of last follow-up* | |
| NED | 50 (62.5) |
| AWD | 10 (12.5) |
| DOD | 4 (5) |
| DOO | 4 (5) |
| Lost to follow-up | 12 (15) |
|
| |
| Recurrence | |
| Yes | 17 (21) |
| No | 63 (79) |
RFS, recurrence-free survival; NED, no evidence of disease; AWD, alive with disease; DOD, dead of disease; DOO, dead of other causes
The 3-year PFS rate for the entire cohort was 84.9% (95% CI, 73.8–91.6). Univariate analysis of various factors was assessed with RFS. These factors are outlined in Table 3. The 3-year PFS rate was 91.7% (95% CI, 53.9–98.8) for stage II patients and 83.6% (95% CI, 70.8–91.1) for stage III/IV patients (P=0.093). The 3-year PFS rate was 72.4 (95% CI, 48.3–86.6) for patients with tumors of micropapillary features and 91.1 (95% CI, 78–96.6) for patients without micropapillary features (P= 0.023). The 3-year PFS rate was 66.7 (95% CI, 40.4–83.4) for patients with invasive implants and 93.6 (95% CI, 81.5–97.9) for patients with non-invasive implants (P=0.005). We further characterized patients according to residual disease. Eight patients (10%) had residual disease at initial surgery, 69 (86%) had no residual disease, and for 3 (4%) patients, it was unclear if there was residual disease at initial surgery. The 3-year PFS rate was 71.4 (95% CI, 25.8–92) for patients with residual disease and 89.4 (95% CI, 77.9–95.1) for patients with no residual disease at initial surgery. Univariate analysis for residual disease was not performed as the number of patients with residual disease was small.
Table 3.
Analysis of recurrence-free survival
| N (%) | 3-year RFS (95% CI) | P | |
|---|---|---|---|
|
| |||
| Stage | 0.093 | ||
| II | 15 (19) | 91.7 (53.9–98.8) | |
| III/IV | 65 (81) | 83.6 (70.8–91.1) | |
|
| |||
| Micropapillary type | 0.023 | ||
| Yes | 25 (31) | 72.4 (48.3–86.6) | |
| No | 55 (69) | 91.1 (78–96.6) | |
|
| |||
| Invasive implants | 0.005 | ||
| Yes | 19 (24) | 66.7 (40.4–83.4) | |
| No | 61 (76) | 93.6 (81.5–97.9) | |
|
| |||
| Residual disease | N/A | ||
| Yes | 8 (10) | 71.4 (25.8–92) | |
| No | 69 (86) | 89.4 (77.9–95.1) | |
| Unknown | 3 (4) | ||
|
| |||
| Adjuvant chemotherapy (all patients) | N/A | ||
| Yes | 17 (21) | 70.6 (43.1–86.6) | |
| No | 63 (79) | 89.9 (77.3–95.7) | |
|
| |||
| Adjuvant chemotherapy (patients without residual disease) | N/A | ||
| Yes | 15 (22) | 80 (50–93.1) | |
| No | 54 (78) | 92.7 (79–97.6) | |
None of the patients with stage II disease received adjuvant chemotherapy. The 3-year PFS rate was 89.9% (95% CI, 77.3–95.7) for patients who did not receive adjuvant chemotherapy compared with 70.6% (95% CI, 43.1–86.6) for patients who received adjuvant chemotherapy. As demonstrated in Figure 1, there is no benefit of adjuvant chemotherapy for RFS. Interestingly, none of the patients with residual disease at initial surgery received chemotherapy. Of the 69 patients with no residual disease, the 3-year PFS rate was 80% (95% CI, 50–93.1) for patients who received chemotherapy and 92.7% (95% CI, 79–97.6) for patients who did not receive adjuvant chemotherapy. Individual chemotherapy agents were not examined as there was a wide variety used among this cohort.
Fig. 1.
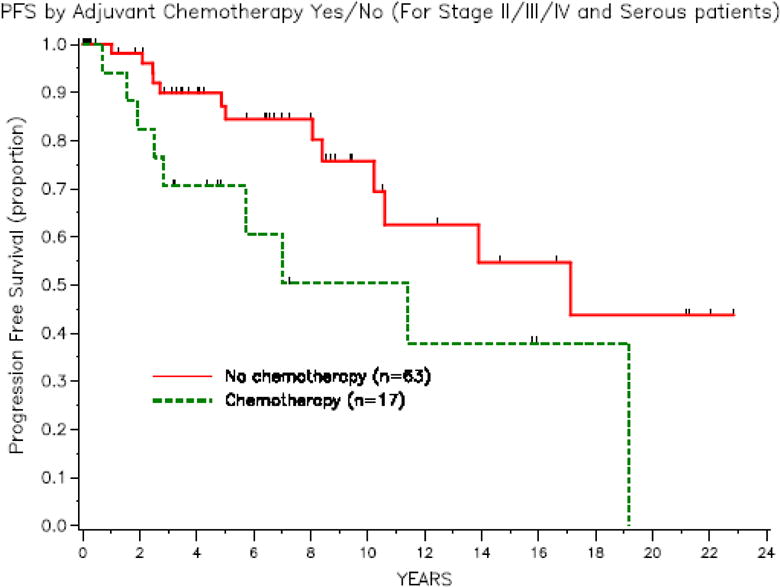
Of the 63 stage III patients, we evaluated sites of metastasis as this is a heterogeneous group. The only patients who developed recurrence had omental involvement or multiple sites of disease at the time of initial diagnosis. Of the 29 patients with the omentum as the only site of metastasis, 8 (28%) developed recurrence. Thirty-two patients had multiple sites of disease, and of these, 9 (28%) developed recurrence. None of the patients with isolated nodal disease developed recurrence. The 2 patients with stage IV disease had isolated metastasis to the lung or axilla, and neither patient developed recurrent disease. Both patients underwent surgical resection of metastatic disease. Evaluation of sites of metastases with significant prognostic factors (micropapillary type histology, invasive implants, and residual disease) found no significant associations.
Discussion
The management of advanced-stage ovarian borderline tumors is controversial. Surgery is a mainstay in the management of all ovarian borderline tumors and includes hysterectomy, bilateral salpingo-oophorectomy, peritoneal washings and biopsies, and resection of any gross disease. There are several purposes of surgery—staging of disease is necessary to determine prognosis and risk of recurrence[1, 14]; pathologic evaluation is necessary to identify prognostic factors such as invasive vs non-invasive implants and micropapillary pattern [5, 8, 21–23]; and in patients who desire childbearing, including patients with advanced disease [14, 24, 27], fertility-sparing surgery can be considered [1, 11, 24–26].
The role of adjuvant chemotherapy in advanced-stage ovarian SBTs is less defined, as reported response rates vary widely. We previously reported on a cohort of patients with SBTs who received platinum-based chemotherapy [16]. In that report, 21 patients with stage III or IV ovarian SBTs received platinum-based chemotherapy after initial cytoreductive surgery. Of the patients who underwent second-look laparotomy, 2 (29%) patients with macroscopic residual disease after initial cytoreductive surgery had complete response to chemotherapy. A compilation of literature in that report found a 26% complete response rate in patients with macroscopic residual disease at initial cytoreductive surgery. A Gynecologic Oncology Group (GOG) study evaluated adjuvant chemotherapy in 32 patients with stage III ovarian BT [17]. Fourteen patients underwent second-look laparotomy, and 2 patients (25%) with residual disease at initial surgery had a complete response. Furthermore, a study of 73 patients with ovarian SBTs and non-invasive implants reported a 5% complete response rate to chemotherapy in patients with macroscopic residual disease [22]. In addition, a study of 39 patients with ovarian SBTs with invasive implants reported a 14% complete response rate to chemotherapy in patients with macroscopic residual disease [21]. In that study, platinum-based chemotherapy was associated with a significantly shorter PFS.
The 80 patients in our cohort had an overall good prognosis; only 4 patients (5%) died from disease. As a result, we evaluated recurrence as a marker of prognosis, and even with advanced-stage disease, the 3-years RFS rate was 84.9% and 17 patients (21%) developed recurrence. In ovarian BT, long follow-up periods are required to evaluate recurrence risk. Recurrence rates of approximately 30% have been reported with 10-year follow-up [21–22] and 44% with 15-year follow-up [28]. Patients in this cohort with invasive implants or micropapillary type tumors appeared to have a higher recurrence risk. Interestingly, none of the patients who had residual disease after initial surgery received chemotherapy. As a result, it is not possible to evaluate for response rate to adjuvant chemotherapy.
There is increasing evidence that adjuvant chemotherapy may not be beneficial in ovarian SBTs, even in advanced stage. Reported response rates are low relative to response rates seen with invasive epithelial ovarian carcinomas. In our cohort, there did not appear to be a survival benefit in patients who received adjuvant chemotherapy. Interestingly adjuvant chemotherapy appears to increase the risk of recurrence. However, it is important to note that the chemotherapy agents used varied widely and with all retrospective studies, there is inherent selection bias that may influence this finding. Even in the patients with no residual disease at initial surgery, there did not appear to be a survival benefit with adjuvant chemotherapy.
Micropapillary type and invasive implants are both significant prognostic factors for recurrence. Residual disease may be associated with increased recurrence risk; however, due to the small number of patients, statistical assessment of significance was not possible. Only patients who had multiple sites of metastases or omental metastasis developed recurrence. While our data is limited by small numbers, none of the patients with nodal-only metastasis or isolated distant metastasis (axilla or lung) developed recurrence.
Our study is limited by weaknesses inherent to all retrospective studies. Referral bias is another weakness, as approximately half of the patients in the cohort were initially diagnosed at an outside hospital and then presented to our institution; however, most of these patients presented at our institution shortly afterward their initial diagnosis (up to several months). With a long study period included, the cohort of patients is heterogeneous with a variety of chemotherapy drugs and administrations (intravenous and intraperitoneal). Finally, we had a significant number of patients (15%) with short follow-up, and our overall median follow-up time was only 4.8 years. A longer follow-up period would have better assessed recurrence and survival.
Advanced ovarian SBTs have a good prognosis, with excellent survival. Recurrence risk is not negligible; nearly 21% of patients recurred, with a median follow-up time of 5 years. Adjuvant chemotherapy does not appear to impact risk of recurrence.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Karin K. Shih: no conflicts
Qin C. Zhou: no conflicts
Carol Aghajanian: no conflicts
Jae Huh: no conflicts
Robert A. Soslow: no conflicts
Jessica C. Morgan: no conflicts
Alexia Iasonos: no conflicts
Dennis S. Chi: no conflicts
Richard R. Barakat: no conflicts
Nadeem R. Abu-Rustums: no conflicts
References
- 1.Barakat RR. Borderline tumors of the ovary. Obstet Gynecol Clin North Am. 1994;21:93–105. [PubMed] [Google Scholar]
- 2.Silva EG, Kurman RJ, Russell P, Scully RE. Symposium: ovarian tumors of borderline malignancy. Int J Gynecol Pathol. 1996;15:281–302. [PubMed] [Google Scholar]
- 3.Hart WR. Borderline epithelial tumors of the ovary. Mod Pathol. 2005;18(Suppl 2):S33–50. doi: 10.1038/modpathol.3800307. [DOI] [PubMed] [Google Scholar]
- 4.Kurman RJ, Seidman JD, Shih IM. Serous borderline tumours of the ovary. Histopathology. 2005;47:310–5. doi: 10.1111/j.1365-2559.2005.02186.x. [DOI] [PubMed] [Google Scholar]
- 5.Eichhorn JH, Bell DA, Young RH, Scully RE. Ovarian serous borderline tumors with micropapillary and cribriform patterns: a study of 40 cases and comparison with 44 cases without these patterns. Am J Surg Pathol. 1999;23:397–409. doi: 10.1097/00000478-199904000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Seidman JD, Kurman RJ. Ovarian serous borderline tumors: a critical review of the literature with emphasis on prognostic indicators. Hum Pathol. 2000;31:539–57. doi: 10.1053/hp.2000.8048. [DOI] [PubMed] [Google Scholar]
- 7.Bristow RE, Gossett DR, Shook DR, Zahurak ML, Tomacruz RS, Armstrong DK, Montz FJ. Micropapillary serous ovarian carcinoma: surgical management and clinical outcome. Gynecol Oncol. 2002;86:163–70. doi: 10.1006/gyno.2002.6736. [DOI] [PubMed] [Google Scholar]
- 8.Deavers MT, Gershenson DM, Tortolero-Luna G, Malpica A, Lu KH, Silva EG. Micropapillary and cribriform patterns in ovarian serous tumors of low malignant potential: a study of 99 advanced stage cases. Am J Surg Pathol. 2002;26:1129–41. doi: 10.1097/00000478-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Prat J, De Nictolis M. Serous borderline tumors of the ovary: a long-term follow-up study of 137 cases, including 18 with a micropapillary pattern and 20 with microinvasion. Am J Surg Pathol. 2002;26:1111–28. doi: 10.1097/00000478-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Morice P, Camatte S, Rey A, Atallah D, Lhommé C, Pautier P, et al. Prognostic factors for patients with advanced stage serous borderline tumours of the ovary. Ann Oncol. 2003;14:592–8. doi: 10.1093/annonc/mdg173. [DOI] [PubMed] [Google Scholar]
- 11.Zanetta G, Rota S, Chiari S, Bonazzi C, Bratina G, Mangioni C. Behavior of borderline tumors with particular interest to persistence, recurrence, and progression to invasive carcinoma: a prospective study. J Clin Oncol. 2001;19:2658–64. doi: 10.1200/JCO.2001.19.10.2658. [DOI] [PubMed] [Google Scholar]
- 12.Kaern J, Tropé CG, Kristensen GB, Abeler VM, Pettersen EO. DNA ploidy; the most important prognostic factor in patients with borderline tumors of the ovary. Int J Gynecol Cancer. 1993;3:349–58. doi: 10.1046/j.1525-1438.1993.03060349.x. [DOI] [PubMed] [Google Scholar]
- 13.Longacre TA, McKenney JK, Tazelaar HD, Kempson RL, Hendrickson MR. Ovarian serous tumors of low malignant potential (borderline tumors): outcome-based study of 276 patients with long-term (> or =5-year) follow-up. Am J Surg Pathol. 2005;29:707–23. doi: 10.1097/01.pas.0000164030.82810.db. [DOI] [PubMed] [Google Scholar]
- 14.Cadron I, Leunen K, Van Gorp T, Amant F, Neven P, Vergote I. Management of borderline ovarian neoplasms. J Clin Oncol. 2007;25:2928–37. doi: 10.1200/JCO.2007.10.8076. [DOI] [PubMed] [Google Scholar]
- 15.Fort MG, Pierce VK, Saigo PE, Hoskins WJ, Lewis JL., Jr Evidence for the efficacy of adjuvant therapy in epithelial ovarian tumors of low malignant potential. Gynecol Oncol. 1989;32:269–72. doi: 10.1016/0090-8258(89)90622-7. [DOI] [PubMed] [Google Scholar]
- 16.Barakat RR, Benjamin I, Lewis JL, Jr, Saigo PE, Curtin JP, Hoskins WJ. Platinum-based chemotherapy for advanced-stage serous ovarian carcinoma of low malignant potential. Gynecol Oncol. 1995;59:390–3. doi: 10.1006/gyno.1995.9956. [DOI] [PubMed] [Google Scholar]
- 17.Sutton GP, Bundy BN, Omura GA, Yordan EL, Beecham JB, Bonfiglio T. Stage III ovarian tumors of low malignant potential treated with cisplatin combination therapy (a Gynecologic Oncology Group study) Gynecol Oncol. 1991;41:230–3. doi: 10.1016/0090-8258(91)90314-u. [DOI] [PubMed] [Google Scholar]
- 18.Tropé C, Kaern J, Vergote IB, Kristensen G, Abeler V. Are borderline tumors of the ovary overtreated both surgically and systemically? A review of four prospective randomized trials including 253 patients with borderline tumors. Gynecol Oncol. 1993;51:236–43. doi: 10.1006/gyno.1993.1279. [DOI] [PubMed] [Google Scholar]
- 19.Benedet JL, Bender H, Jones H, 3rd, Ngan HY, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2000;70:209–62. [PubMed] [Google Scholar]
- 20.Rustin GJ, Nelstrop AE, Bentzen SM, Piccart MJ, Bertelsen K. Use of tumour markers in monitoring the course of ovarian cancer. Ann Oncol. 1999;10(Suppl 1):21–7. doi: 10.1023/a:1008351216605. [DOI] [PubMed] [Google Scholar]
- 21.Gershenson DM, Silva EG, Levy L, Burke TW, Wolf JK, Tornos C. Ovarian serous borderline tumors with invasive peritoneal implants. Cancer. 1998;82:1096–103. [PubMed] [Google Scholar]
- 22.Gershenson DM, Silva EG, Tortolero-Luna G, Levenback C, Morris M, Tornos C. Serous borderline tumors of the ovary with noninvasive peritoneal implants. Cancer. 1998;83:2157–63. doi: 10.1002/(sici)1097-0142(19981115)83:10<2157::aid-cncr14>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 23.Seidman JD, Kurman RJ. Treatment of micropapillary serous ovarian carcinoma (the aggressive variant of serous borderline tumors) Cancer. 2002;95:675–6. doi: 10.1002/cncr.10777. [DOI] [PubMed] [Google Scholar]
- 24.Camatte S, Morice P, Pautier P, Atallah D, Duvillard P, Castaigne D. Fertility results after conservative treatment of advanced stage serous borderline tumour of the ovary. BJOG. 2002;109:376–80. doi: 10.1111/j.1471-0528.2002.01359.x. [DOI] [PubMed] [Google Scholar]
- 25.Fortin A, Morice P, Thoury A, Camatte S, Dhainaut C, Madelenat P. Impact of infertility drugs after treatment of borderline ovarian tumors: results of a retrospective multicenter study. Fertil Steril. 2007;87:591–6. doi: 10.1016/j.fertnstert.2006.07.1503. [DOI] [PubMed] [Google Scholar]
- 26.Morice P, Camatte S, El Hassan J, Pautier P, Duvillard P, Castaigne D. Clinical outcomes and fertility after conservative treatment of ovarian borderline tumors. Fertil Steril. 2001;75:92–6. doi: 10.1016/s0015-0282(00)01633-2. [DOI] [PubMed] [Google Scholar]
- 27.Deffieux X, Morice P, Camatte S, Fourchotte V, Duvillard P, Castaigne D. Results after laparoscopic management of serous borderline tumor of the ovary with peritoneal implants. Gynecol Oncol. 2005;97:84–9. doi: 10.1016/j.ygyno.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Silva EG, Gershenson DM, Malpica A, Deavers M. The recurrence and the overall survival rates of ovarian serous borderline neoplasms with noninvasive implants is time dependent. Am J Surg Pathol. 2006;30:1367–71. doi: 10.1097/01.pas.0000213294.81154.95. [DOI] [PubMed] [Google Scholar]


