Abstract
Objective
The objective of this study was to identify clinicopathologic features that are associated with an increased risk of recurrence for borderline ovarian tumors (BOT).
Methods
We performed a retrospective review of all patients treated for BOT at our institution from 1979–2008. Progression-free survival (PFS) was defined as the time of diagnosis to time of recurrence/death or last follow-up. The Kaplan-Meier method was used to calculate the PFS rate and Wilcoxon Gehan test was performed to identify prognostic factors.
Results
A total of 266 patients were identified. The median age was 43 years (range 15–94 years). The majority of patients (68.4%) had FIGO stage I disease and serous histology (73.7%). Only 23 (8.6%) patients developed recurrent disease. The median PFS was 19 years and the median follow-up was 4 years. Abnormal baseline CA-125 (>35 U/ml), advanced stage, age at diagnosis, and invasive implants were associated with decreased PFS. Of the 196 patients with serous BOT, those with a micropapillary pattern had a 3-year PFS of 75.9% (95%CI 55.6–87.8) compared with 94.3% (95% CI 88.4–97.3) for patients without micropapillary pattern (P<0.001).
Conclusion
Age at diagnosis, an elevated preoperative CA125, invasive implants, and micropapillary histology were clinical factors associated with increased risk of recurrence in women with BOT. Including these clinicopathologic features will likely identify patients at higher risk for recurrence, for whom development of new treatment strategies would be appropriate.
Background
Borderline ovarian tumors (BOT) were first described in 1929 [1] and further characterized by the World Health Organization in 1973 [2]. They comprise approximately 10% of ovarian epithelial neoplasms and differ from ovarian epithelial neoplasms both in pathologic characteristics and clinical behavior [3–6]. Long-term follow-up is beneficial as 5-year survival rates are 99% for stage I, 98% for stage II, 96% for stage III, and 77% for stage IV disease. [7] Various risk factors for recurrence have been established [8–15]. These include stage, presence of invasive implants, residual disease, and micropapillary features.
The median age of patients with BOT is lower than the median age of patients with invasive ovarian epithelial carcinoma. As a result, conservative surgery is an important consideration in premenopausal patients as well as patients who desire fertility. Prior studies have suggested that conservative surgery is a reasonable option in the surgical management of stage I BOT but is associated with increased risk of recurrence. [15–24]
The objective of this study was to report the experience of BOT at our institution. Clinical characteristics and outcomes in these patients will be described. In addition, we will examine the role of fertility-sparing surgery and lymphadenectomy.
Methods
After Institutional Review Board (IRB) approval was obtained, we identified all patients with BOT treated at our institution from 1979–2008. Not all patients were diagnosed at our institution; some presented for further management after surgery at an outside institution. Medical records including operative reports, pathology and laboratory reports, and chemotherapy records were reviewed and data were extracted. The pathology specimens from patients who were diagnosed at an outside institution were all reviewed at our institution. It is our hospital policy to confirm all outside pathology reports by institution review of submitted specimens.
Stage at initial diagnosis was designated based on the International Federation of Gynecology and Obstetrics (FIGO) staging system for ovarian carcinoma. [25] Pathology reports were used to determine histology, presence of micropapillary features, presence of invasive or non-invasive implants, and involvement of lymph nodes. Operative reports were reviewed to determine procedures performed as well as intraoperative findings including presence of ascites and residual disease. Fertility-sparing surgery was defined as surgery that resulted in retention of the uterus and at least one ovary.
Progression-free survival (PFS) was defined as the time of diagnosis to the time of recurrence/death or last follow-up. Overall survival (OS) was defined as the time of diagnosis to the time of death or last follow-up. Patients were observed for recurrence, which was defined with clinical or CA-125 criteria according to Rustin criteria. [26] Kaplan Meier method was used to estimate PFS rate. Univariate analysis with P values was generated applying the Cox proportional hazards model for continuous variables and the Wilcoxon-Gehan test for categorical variables. The association between stage and other categorical variables was tested using Fisher’s exact test.
Results
A total of 266 patients were identified. Clinical and pathologic characteristics for the cohort are listed in table 1. The median age was 43 years (range 15–94 years). The majority of patients had early stage disease; 181 (68%) patients had stage I disease, 18 (6.8%) patients had stage II disease, and 67 (25.2%) patients had stage III or IV disease. The most common histology was serous with 196 (73.7%) patients, followed by mucinous in 64 (24.1%) patients, clear cell in 3 (1.1%) patients, and endometrioid in 3 (1.1%) patients. The median follow-up time was 3.7 years (range 0–22.8 years). At the time of last follow-up, 224 (84.2%) patients had no evidence of disease, 15 (5.6%) patients were alive with disease, 7 (2.6%) patients were dead of disease, 17 (6%) patients were dead of other causes, and 3 (1.1%) patients were lost to followup.
Table 1.
Clinicopathologic characteristics.
| N (%) | |
|---|---|
|
| |
| Total number of patients | 266 |
|
| |
| Median age (range) | 43 years (15–94) |
|
| |
| Stage | |
| I | 181 (68) |
| II | 18 (6.8) |
| III/IV | 67 (25.2) |
|
| |
| Histology | |
| Serous | 196 (73.7) |
| Mucinous | 64 (24.1) |
| Clear cell | 3 (1.1) |
| Endometrioid | 3 (1.1) |
|
| |
| Lymphadenectomy | |
| Yes | 128 (48.1) |
| No | 138 (51.9) |
| Median lymph nodes sampled (range) | 14.5 (1–45) |
|
| |
| Adjuvant chemotherapy | |
| Yes | 20 (7.5) |
| No | 249 (92.5) |
|
| |
| Median follow-up (range) | 3.7 years (0–22.8) |
|
| |
| Status at last follow-up* | |
| NED | 224 (84.2) |
| AWD | 15 (5.6) |
| DOD | 7 (2.6) |
| DOO | 17 (6) |
| Lost to follow-up | 3 (1.1) |
|
| |
| Recurrence | |
| Yes | 23 (8.6) |
| No | 243 (91.4) |
NED = no evidence of disease, AWD = alive with disease, DOD = dead of disease, DOO = dead of other causes
Fertility-sparing surgery was performed in 95 (35.7%) patients. There was a variety of procedures performed, including unilateral or bilateral cystectomy in 35 (29.4%) patients, unilateral salpingo-oophorectomy in 60 (50.4%) patients, and bilateral salpingo-oophorectomy in 24 (20.2%) patients. Of the 95 patients who underwent fertility-sparing surgery, 37 (39%) patients underwent completion surgery.
Lymphadenectomy was performed in 138 (51.9%) patients. The median number of lymph nodes removed was 14.5 (range, 1–45). Of the patients who underwent lymphadenectomy, 19 (14.8%) patients had positive nodes. A total of 20 patients received adjuvant chemotherapy for BOT after initial surgery. All of these patients had stage III disease. In addition, nine patients received chemotherapy for recurrent disease. All of these patients had recurrent disease with invasive adenocarcinoma. Eleven patients received chemotherapy after developing another malignancy of another disease site.
Of the 266 patients in the cohort, only 23 (8.6%) developed recurrent disease. Of these patients who developed recurrent disease, 14 (60.9%) had recurrent disease with invasive adenocarcinoma, 8 (34.8%) had recurrent disease with borderline histology, and 1 (4.3%) had recurrent disease of unknown histology. The median progression-free survival was 19 years and the 3-year PFS for the cohort was 92.7% (95% CI 88–95.6). Univariate analysis was performed to identify prognostic factors for progression-free survival. These are outlined in table 2. Age at diagnosis, stage, baseline CA125, presence of invasive implants, and residual disease were significant prognostic factors. For patients with borderline tumor of serous histology, micropapillary pattern was found to be a significant prognostic factor. Three-year PFS was 97.1% (95% CI 92.4–98.9) for patients with stage I disease, 93.3% (95% CI 61.3–99) for stage II disease, 83.8% (95% CI 71.2–91.3) for stage III or IV disease (P=0.029). Patients with normal baseline CA-125 (≤ 35) had a 3-year PFS of 96.7% (95% CI 91.3–98.7) compared with 86.9% (95% CI 71.3–94.3) for patients with elevated baseline CA125 (P=0.003). Survival curves for stage and baseline CA-125 are demonstrated in figures 1 and 2, respectively. Three-year PFS was 77.2% (95% CI 56–89) for patients with invasive implants and 95.8% (95% CI 91.4–98) for patients without invasive implants (P<0.001). Patients with any residual disease had a 3-year PFS of 70% (95% CI 32.9–82.9) compared with 95% (95% CI 90.6–97.4) for patients with no residual disease (P<0.001). Figures 3 and 4 demonstrate survival curves for invasive implants and residual disease, respectively. Of the 196 patients with borderline tumor of serous histology, those with a micropapillary pattern had a 3-year PFS of 75.9% (95% CI 55.6–87.8) compared with 94.3% (95% CI 88.4–97.3) for patients without micropapillary pattern (P<0.001). Fertility-sparing surgery was performed in 95 patients and the 3-year PFS rate was 98.7% (95% CI 91.2–99.8) compared with 89.9% (95% CI 83.3–94) for patients who did not have fertility-sparing surgery (P=0.034) Of the 95 patients who underwent fertility-sparing surgery, the 3-year PFS was 100% for the 37 patients who underwent completion surgery compared with 97.7% (95% CI 84.6–99.7) for 58 patients who did not have completion surgery (P=0.018). Other factors that were not found to be associated with PFS were histology (serous versus other), presence of ascites, and lymphadenectomy performed.
Table 2.
Univariate analysis for PFS.
| N (%) | 3-year RFS (95% CI) | P | |
|---|---|---|---|
|
| |||
| Median PFS for entire cohort | 266 | 92.7 (88–95.6) | |
|
| |||
| Age | 0.008* | ||
|
| |||
| Stage | 0.029* | ||
| I | 182 (68.4) | 97.1 (92.4–98.9) | |
| II | 18 (6.8) | 93.3 (61.3–99) | |
| III/IV | 66 (24.8) | 83.8 (71.2–91.3) | |
|
| |||
| CA125 | 0.003 | ||
| ≤ 35 | 143 (53.8) | 96.7 (91.3–98.7) | |
| >35 | 50 (18.8) | 86.9 (71.3–94.3) | |
| NA | 73 (27.4) | ||
|
| |||
| Histology | 0.079 | ||
| Serous | 196 (73.7) | 90.8 (84.9–94.5) | |
| Other | 70 (26.3) | 100 | |
|
| |||
| Invasive implants | <0.001 | ||
| Yes | 36 (13.5) | 77.2 (56–89) | |
| No | 229 (86.1) | 95.8 (91.4–98) | |
| NA | 1 (0.4) | ||
|
| |||
| Ascites | 0.227 | ||
| Yes | 51 (19.2) | 92.9 (87.5–96) | |
| No | 215 (80.8) | 92.5 (78.4–97.5) | |
|
| |||
| Lymphadenectomy | 0.394 | ||
| Yes | 128 (48.1) | 92.8 (85.5–96.5) | |
| No | 138 (51.9) | 92.7 (85.2–96.5) | |
|
| |||
| Micropapillary features (serous histology) | <0.001 | ||
| Yes | 36 (18.4) | 75.9 (55.6–87.8) | |
| No | 160 (81.6) | 94.3 (88.4–97.3) | |
|
| |||
| Residual disease | <0.001 | ||
| Yes | 11 (4.1) | 70 (32.9–82.9) | |
| No | 250 (94) | 95 (90.6–97.4) | |
| Unknown | 5 (1.9) | ||
|
| |||
| Fertility-sparing surgery | 0.034 | ||
| Yes | 95 (35.7) | 98.7 (91.2–99.8) | |
| No | 171 (64.3) | 89.9 (83.3–94) | |
|
| |||
| Completion surgery after fertility-sparing surgery | 0.018 | ||
| Yes | 37 (38.9) | 100 | |
| No | 58 (61.1) | 97.7 (84.6–99.7) | |
P-values were obtained using Cox proportional hazards model; other p values obtained using Wilcoxon Gehan test
Figure 1.
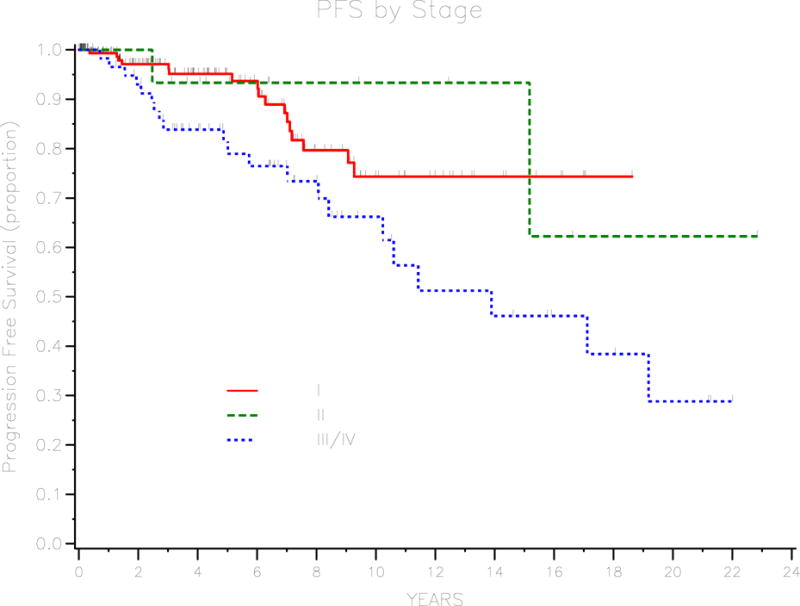
Survival curve for PFS by stage.
Figure 2.
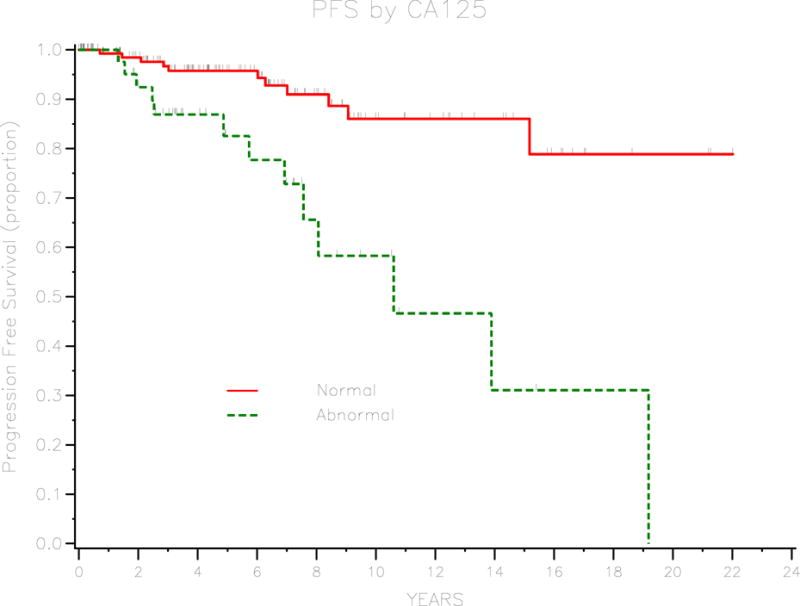
Survival curve for PFS by CA125 level.
Figure 3.
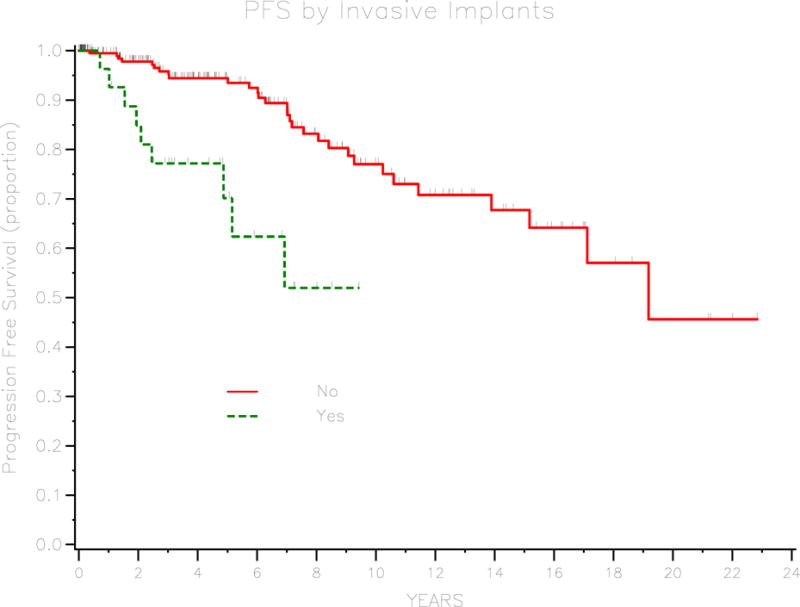
Survival curve for PFS by invasive implants.
Figure 4.
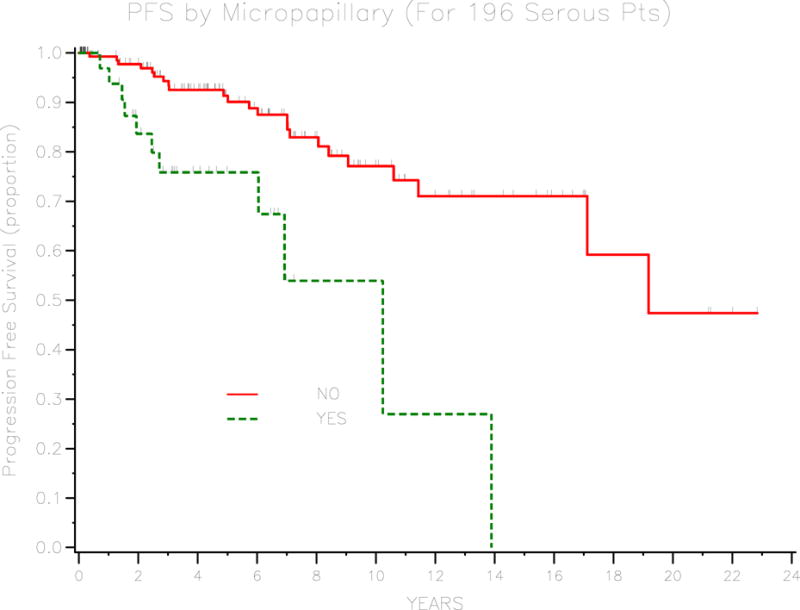
Survival curve for PFS by micropapillary type (serous only).
Multivariate analysis of these significant variables for PFS was not possible due to the relatively small number of recurrences. However, we assessed for degree of correlations between different significant factors such as stage and CA-125. As seen in supplemental table 1, stage at presentation was significantly associated with presence of invasive implants (P=0.001), conservative surgery (P<0.001), and preoperative CA-125 (P=0.006). Invasive implants and elevated CA125 were associated with higher stage disease, while conservative surgery was associated with early stage disease.
The median overall survival for the cohort was not reached. 3-year survival rate for the cohort was 98% (95% CI 94.7–99.2). Univariate analysis was performed to identify prognostic factors and these are outlined in Supplemental Table 2. Only baseline CA-125 and residual disease were significant prognostic factors. Patients with a normal baseline CA-125 had a 3-year-OS of 100% compared with 94.7% (95% CI 80.3–98.6) for patients with an abnormal baseline CA125 (P=0.002). Patients with no residual disease at initial surgery had a 3-year OS of 100% compared with 97.8 (95% CI 94.3–99.2) for patients with residual disease (P=0.001). Interestingly, stage of disease was not significant with 3-yr OS of 98.6% (95% CI 94.5–99.6) for stage I disease, 93.3% (95% CI 61.3–99) for stage II disease, and 98.2 (95% CI 88.0–99.7) for stage III or IV disease (P=NS). Histology, presence of invasive implants, ascites, lymphadenectomy performed, micropapillary pattern, and fertility-sparing surgery were not associated with overall survival.
Discussion
Borderline ovarian tumors are characterized by atypical epithelial proliferation without stromal invasion. The prognosis is excellent with 5-year survival of approximately 98% for stage I disease and 86–92% for advanced disease. [27] Surgery is an integral component of management. In addition, there is increasing evidence that fertility-sparing surgery is reasonable in patients who desire fertility. [17, 21–23] While overall prognosis of BOT is excellent, recurrent disease does occur. Clinical and pathologic prognostic factors include stage [14, 28], invasive implants [4–5, 12, 28–31], micropapillary type histology [5, 8–10, 13, 28], DNA ploidy [11], and residual disease [16].
In our cohort, we found similar results in patients presenting with stage I disease and serous histology. Overall prognosis was excellent; of the 266 patients in the cohort, only 7 died of disease, and 23 developed recurrent disease. With so few deaths from disease in our cohort, we assessed recurrence as the marker for prognosis. Significant univariate prognostic factors included age at diagnosis, stage, invasive implants, micropapillary histology, and residual disease. We also found that preoperative CA-125 was significantly associated with prognosis. CA-125 has not been previously identified as a prognostic factor for BOT. [32] Stage was found to be significantly associated with invasive implants, preoperative CA-125, and fertility-sparing surgery. In our cohort, stage was not associated with OS. This is likely due to the short median follow-up time and the expected disease course of BOT.
The role of surgery in the management of BOT includes staging of disease and resection of visible disease. Traditionally staging of disease involves hysterectomy, bilateral oophorectomy, omentectomy, peritoneal biopsies, pelvic and para-aortic lymphadenectomy, and peritoneal washings. In BOT, there is increasing evidence that fertility-sparing surgery is reasonable in patients who desire fertility. In our cohort, there was no difference in overall survival between patients who underwent fertility-sparing surgery and those who did not. In fact, patients who underwent conservative surgery had higher 3-year PFS rate than patients who did not undergo fertility-sparing surgery. We recognize that there is selection bias in this setting as the patients who underwent fertility-sparing surgery may have had more favorable prognosis such as early stage disease. Of the patients who underwent fertility-sparing surgery, risk of recurrence was higher in patients who did not undergo completion surgery, compared with those patients who had completion surgery. Previous reports have described an increased risk of recurrence with fertility-sparing surgery. However, there does not appear to be an impact on survival with fertility-sparing surgery. [15, 18–20, 22–24] As a result, fertility-sparing surgery was a reasonable option in the management of BOT in our cohort. After fertility has been completed, completion surgery can be offered to patients as it may be associated with decreased recurrence risk.
While lymphadenectomy is part of surgical staging in ovarian cancer, it has not been shown to be prognostically significant in BOT. [33] In our cohort, approximately half of the patients underwent lymphadenectomy. There was no difference in survival between the patients with respect to lymphadenectomy. This finding provides further evidence that routine lymphadenectomy may not be necessary with BOT.
Our study is limited by weaknesses inherent to all retrospective studies. Referral bias is another weakness as approximately half of the patients in the cohort were initially diagnosed at an outside hospital then presented to our institution. However, most of these patients presented at our institution shortly afterwards (up to several months). We had a relatively short median follow-up time of only 3.7 years. Longer follow-up period would have been desired to assess for recurrence and survival. With a median PFS of 19 years and a median follow-up time of 3.7 years, likely patients developed recurrence or death from disease after date of last follow-up. The short follow-up time likely contributes to the high percentage of patients who recurred with invasive cancer. In addition, with only a small number of recurrences (n=23) in our cohort of 266 patients, the sample size precluded the usefulness of multivariate analyses. Our cohort was selected from a study period of 20 years. During this study period, the treatment of BOT have remained relatively constant with surgical resection and staging as the cornerstone. However, chemotherapy regimens have changed throughout this period as well as specific pathologic diagnostic criteria for BOT. As a result, in the earlier time period of the study, likely the number of patients with micropapillary features was under-reported. Finally, the majority of patients presented with early stage disease; however, surgical staging and lymphadenectomy was performed in approximately half of the patients.
Borderline ovarian tumors have an excellent prognosis. Factors univariately associated with increased recurrence risk include age at diagnosis, stage, preoperative CA125, presence of invasive implants, and residual disease. In addition, ovarian serous borderline tumors with micropapillary features have an increased recurrence risk. Conservative surgery is not associated with increased recurrence risk; however, patients who undergo conservative surgery should be offered completion surgery after child-bearing is completed. Including these clinicopathologic features may identify patients at higher-risk for recurrence who require a more extended surveillance strategy.
Supplementary Material
Research Highlights (GYN-10-724R1).
Preoperative CA-125 level is associated with recurrence of borderline ovarian tumors
Recurrence risk is increased with micropapillary pattern tumors and invasive implants
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Taylor HJ. Malignant and semi-malignant tumors of the ovary. Surg Gynecol Obstet. 1929;48:204–230. [Google Scholar]
- 2.Serov SF, S R, Sobin LH. Histologic typing of ovarian tumors in international histologic classification of tumors (No 9) Geneva, Swizerland: World Health Organization; 1973. [Google Scholar]
- 3.Barakat RR. Borderline tumors of the ovary. Obstet Gynecol Clin North Am. 1994;21(1):93–105. [PubMed] [Google Scholar]
- 4.Hart WR. Borderline epithelial tumors of the ovary. Mod Pathol. 2005;18(Suppl 2):S33–50. doi: 10.1038/modpathol.3800307. [DOI] [PubMed] [Google Scholar]
- 5.Kurman RJ, Seidman JD, Shih IM. Serous borderline tumours of the ovary. Histopathology. 2005;47(3):310–5. doi: 10.1111/j.1365-2559.2005.02186.x. [DOI] [PubMed] [Google Scholar]
- 6.Silva EG, et al. Symposium: ovarian tumors of borderline malignancy. Int J Gynecol Pathol. 1996;15(4):281–302. [PubMed] [Google Scholar]
- 7.Trimble CL, Kosary C, Trimble EL. Long-term survival and patterns of care in women with ovarian tumors of low malignant potential. Gynecol Oncol. 2002;86(1):34–7. doi: 10.1006/gyno.2002.6711. [DOI] [PubMed] [Google Scholar]
- 8.Bristow RE, et al. Micropapillary serous ovarian carcinoma: surgical management and clinical outcome. Gynecol Oncol. 2002;86(2):163–70. doi: 10.1006/gyno.2002.6736. [DOI] [PubMed] [Google Scholar]
- 9.Deavers MT, et al. Micropapillary and cribriform patterns in ovarian serous tumors of low malignant potential: a study of 99 advanced stage cases. Am J Surg Pathol. 2002;26(9):1129–41. doi: 10.1097/00000478-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Eichhorn JH, et al. Ovarian serous borderline tumors with micropapillary and cribriform patterns: a study of 40 cases and comparison with 44 cases without these patterns. Am J Surg Pathol. 1999;23(4):397–409. doi: 10.1097/00000478-199904000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Kaern J, et al. DNA ploidy; the most important prognostic factor in patients with borderline tumors of the ovary. Int J Gynecol Cancer. 1993;3(6):349–358. doi: 10.1046/j.1525-1438.1993.03060349.x. [DOI] [PubMed] [Google Scholar]
- 12.Morice P, et al. Prognostic factors for patients with advanced stage serous borderline tumours of the ovary. Ann Oncol. 2003;14(4):592–8. doi: 10.1093/annonc/mdg173. [DOI] [PubMed] [Google Scholar]
- 13.Prat J, De Nictolis M. Serous borderline tumors of the ovary: a long-term follow-up study of 137 cases, including 18 with a micropapillary pattern and 20 with microinvasion. Am J Surg Pathol. 2002;26(9):1111–28. doi: 10.1097/00000478-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Seidman JD, Kurman RJ. Ovarian serous borderline tumors: a critical review of the literature with emphasis on prognostic indicators. Hum Pathol. 2000;31(5):539–57. doi: 10.1053/hp.2000.8048. [DOI] [PubMed] [Google Scholar]
- 15.Zanetta G, et al. Behavior of borderline tumors with particular interest to persistence, recurrence, and progression to invasive carcinoma: a prospective study. J Clin Oncol. 2001;19(10):2658–64. doi: 10.1200/JCO.2001.19.10.2658. [DOI] [PubMed] [Google Scholar]
- 16.Crispens MA, et al. Response and survival in patients with progressive or recurrent serous ovarian tumors of low malignant potential. Obstet Gynecol. 2002;99(1):3–10. doi: 10.1016/s0029-7844(01)01649-0. [DOI] [PubMed] [Google Scholar]
- 17.Fortin A, et al. Impact of infertility drugs after treatment of borderline ovarian tumors: results of a retrospective multicenter study. Fertil Steril. 2007;87(3):591–6. doi: 10.1016/j.fertnstert.2006.07.1503. [DOI] [PubMed] [Google Scholar]
- 18.Lim-Tan SK, Cajigas HE, Scully RE. Ovarian cystectomy for serous borderline tumors: a follow-up study of 35 cases. Obstet Gynecol. 1988;72(5):775–81. [PubMed] [Google Scholar]
- 19.Morice P, et al. Clinical outcomes and fertility after conservative treatment of ovarian borderline tumors. Fertil Steril. 2001;75(1):92–6. doi: 10.1016/s0015-0282(00)01633-2. [DOI] [PubMed] [Google Scholar]
- 20.Fauvet R, et al. Laparoscopic management of borderline ovarian tumors: results of a French multicenter study. Ann Oncol. 2005;16(3):403–10. doi: 10.1093/annonc/mdi083. [DOI] [PubMed] [Google Scholar]
- 21.Camatte S, et al. Fertility results after conservative treatment of advanced stage serous borderline tumour of the ovary. BJOG. 2002;109(4):376–80. doi: 10.1111/j.1471-0528.2002.01359.x. [DOI] [PubMed] [Google Scholar]
- 22.Tinelli R, et al. Feasibility, safety, and efficacy of conservative laparoscopic treatment of borderline ovarian tumors. Fertil Steril. 2009;92(2):736–41. doi: 10.1016/j.fertnstert.2008.07.1716. [DOI] [PubMed] [Google Scholar]
- 23.Tinelli R, et al. Conservative surgery for borderline ovarian tumors: a review. Gynecol Oncol. 2006;100(1):185–91. doi: 10.1016/j.ygyno.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 24.Suh-Burgmann E. Long-term outcomes following conservative surgery for borderline tumor of the ovary: a large population-based study. Gynecol Oncol. 2006;103(3):841–7. doi: 10.1016/j.ygyno.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Benedet JL, et al. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2000;70(2):209–62. [PubMed] [Google Scholar]
- 26.Rustin GJ, et al. Use of tumour markers in monitoring the course of ovarian cancer. Ann Oncol. 1999;10(Suppl 1):21–7. doi: 10.1023/a:1008351216605. [DOI] [PubMed] [Google Scholar]
- 27.Gershenson DM. Clinical management potential tumours of low malignancy. Best Pract Res Clin Obstet Gynaecol. 2002;16(4):513–27. doi: 10.1053/beog.2002.0308. [DOI] [PubMed] [Google Scholar]
- 28.Longacre TA, et al. Ovarian serous tumors of low malignant potential (borderline tumors): outcome-based study of 276 patients with long-term (> or =5-year) follow-up. Am J Surg Pathol. 2005;29(6):707–23. doi: 10.1097/01.pas.0000164030.82810.db. [DOI] [PubMed] [Google Scholar]
- 29.Gershenson DM, et al. Ovarian serous borderline tumors with invasive peritoneal implants. Cancer. 1998;82(6):1096–103. [PubMed] [Google Scholar]
- 30.Silva EG, et al. The recurrence and the overall survival rates of ovarian serous borderline neoplasms with noninvasive implants is time dependent. Am J Surg Pathol. 2006;30(11):1367–71. doi: 10.1097/01.pas.0000213294.81154.95. [DOI] [PubMed] [Google Scholar]
- 31.Silva EG, et al. Tumor recurrence in stage I ovarian serous neoplasms of low malignant potential. Int J Gynecol Pathol. 1998;17(1):1–6. doi: 10.1097/00004347-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Makar AP, et al. Evaluation of serum CA 125 level as a tumor marker in borderline tumors of the ovary. Int J Gynecol Cancer. 1993;3(5):299–303. doi: 10.1046/j.1525-1438.1993.03050299.x. [DOI] [PubMed] [Google Scholar]
- 33.Winter WE, 3rd, et al. Surgical staging in patients with ovarian tumors of low malignant potential. Obstet Gynecol. 2002;100(4):671–6. doi: 10.1016/s0029-7844(02)02171-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


