Abstract
The first step in the development of a bacterial biofilm is contact with the surface on which the microbe will form this community. We review recent progress on ‘surface sensing’, and engage the question of ‘how does a microbe know it is on a surface?’
‘See me, feel me, touch me…’,
The Who
O chestnut tree, great rooted blossomer,
Are you the leaf, the blossom or the bole?
O body swayed to music, O brightening glance,
How can we know the dancer from the dance?
W. B. Yeats
The first step in the formation of a bacterial biofilm is contact with the surface on which the microbe will eventually form this community, raising the intriguing question: ‘How does a microbe know it is on a surface?’ This is a question that has greatly interested biofilm researchers for decades. For example, Zobell and Allen in their classic 1935 paper [1] postulated that bacteria might need a film of bacteria or nutrients to provide the necessary signal for the bacterium to recognize a suitable surface for attachment. More recent studies, by Prigent-Combaret, Lejeune and colleagues (1999), and review articles by Rasika Harshey (1994) and Tuson and Wiebel (2013) directly discussed the topic of‘surface sensing’ by bacteria [2,3,4•]. In this review, we will frame the concept of surface sensing using work on a variety of microbes, and focus on recent work using Pseudomonas aeruginosa on simple flat surfaces as a model system that strongly supports the hypothesis that bacteria can indeed sense contact with a surface (recent interesting work on complex surfaces with microscale patterns and topographies [5,6] are worthy of a separate review). We conclude by suggesting avenues of investigation that can elucidate the mechanism(s) of surface sensing in bacteria, and ultimately, provide a deep understanding of how bacteria make the transition from planktonic growth to initial steps in the formation of a biofilm.
‘Surface sensing’ can mean a great many behaviors: examples include the workings of the apparatus that allows perception of the proximal presence of a surface, to that of the apparatus that allows selection of different surfaces for attachment, to the biochemical cascade and physical consequences that follow the recognition of the surface. Not surprisingly, surface sensing has historically been conceptualized differently for different microbes. While there has not been a concerted effort to define mechanisms of surface sensing in microbes, this topic has received attention in the literature using a variety of model microbes. A pioneering 1988 paper by McCarter and Silverman described a ‘flagellar dynamometer’ in Vibrio parahaemolyticus — that is, a mechanism whereby decreased rotation of the polar flagellum upon surface contact or in high viscosity initiates a signal transduction pathway that triggers swarming motility with lateral flagella [7]. The concept underlying this model is that the restriction of flagellar rotation when this appendage binds to the substratum serves as a signal that the microbe has contacted a surface, a conclusion supported by the observation that incubation of planktonic cells in a highly viscous liquid also triggers the ‘surface’ program. In recent years, the McCarter lab has linked this process to cyclic diguanylate (cyclic di-GMP or c-di-GMP) [8–10] and, analogous to work in P. aeruginosa [11–13], has shown that an inverse relationship between motility and biofilm formation is mediated by c-di-GMP in V. parahaemolyticus [14]. A role for the flagellum in surface sensing has also been invoked in other microbes. The Belas group, using Proteus as a model, has implicated FliL, a hook-basal body protein as an important component in the surface sensory transduction pathway that controls swarmer cell gene transcription, especially the 14 C-terminal amino acids of this protein [15]. Similarly, the Stanley-Wall and Kearns groups have shown that flagellum rotation is a mechanical trigger that activates DegS–DegU, a two-component system that regulates a range of behaviors, including bio-film formation [16••,17].
Stress pathways can play a role in surface sensing. The Rather (2012) and Silhavy (2002) labs have implicated the periplasmic stress pathways (Rcs and Cpx) as surface sensors [18,19], with perturbation of the membrane(s), cell wall and/or periplasmic space probably triggering these canonical stress systems, thereby perhaps alerting the microbe to cell-to-substratum contact. Prigent-Com-baret, Lejeune and colleagues identified loci that were specifically up-regulated when cells were grown on an abiotic surface and concluded that 38% of genes where differentially expressed in planktonic versus biofilm populations [2].
That surface sensing can incorporate multiple stimuli is exemplified by the observation that nutrients can signal the existence of a surface. A report by Monds, O'Toole and colleagues showed that biofilm formation by P. fluorescens is positively regulated by the presence of inorganic phosphate [20]. Interestingly, in the environments occupied by P. fluorescens, most inorganic phosphate is found complexed in iron minerals; thus there is essentially no free phosphate [21]. These investigators postulated that inorganic phosphate functionally serves as a signal for ‘surface’. As mentioned above, Zobell and Allen also postulated a ‘conditioning film’ of bacteria and/ or nutrients as catalyzing attachment [1], an idea that has been advocated in a number of systems, including in the context of oral cavity biofilms better known as plaque [22].
From the discussion above, flagella can play complex and diverse roles in surface sensing. However, recent work has shown that type IV pili (TFP) can also play important roles (we refer the reader to recent reviews on the molecular machinery of TFP [23] and surface behavior of TFP [24]). Vibrio cholerae interact with a broad range of substrata with their TFP, including surfaces of phytoplankton, zooplankton, and crustaceans [25,26], in addition to non-nutritive, abiotic surfaces. Although V. cholerae has diverse types of TFP at its disposal, including man-nose-sensitive hemagglutinin (MSHA) pili, virulence-associated toxin co-regulated pili (TCP), and chitin-regulated pili (ChiRP), this organism is not known to exhibit ‘twitching’ surface motility typical of TFP-driven bacteria. Our work suggests that TFP can be used for surface sensing and surface selection: V. cholerae use their polar flagellum and MSHA pili synergistically to scan a surface mechanically before surface attachment. V. cholerae skims over a surface using flagellum-driven swimming. Recent work has shown that different interactions between MSHA pili on these skimming cells and the surface can create two types of motility: (1) ‘Orbiting motility’ consists of near-circular trajectories that allow cells to naturally loiter over surface regions that interact more strongly with MSHA pili; these pili brush against a surface and generate frictional forces that cause centripetal acceleration. (2) ‘Roaming motility’ consists of low curvature trajectories that allow cells to ‘fly over’ surface regions that weakly interact with MSHA pili. In this context, TFP interactions effectively facilitate mechanical scanning of a surface before V. cholerae attachment and subsequent biofilm development [27].
Surface sensing: P. aeruginosa is a sensational model system
The opportunistic pathogen P. aeruginosa has emerged as an important model for studying surface sensing, particularly in the context of the earliest stages of biofilm formation. It is important to note from the outset that it is still not clear how easily we can extrapolate what we have learned from this Gram-negative, motile gamma-proteobacterium to other microbes. For example, Staphylococcus aureus is another well-studied biofilm forming microbe — it is not clear if this Gram-positive, non-motile organism is capable of surface sensing or if it does so via a mechanism analogous to that employed by P. aeruginosa.
We believe that current data do indeed support the hypothesis that P. aeruginosa is capable of sensing and responding to a surface via specific programs, although many important questions remain. Our goals are to present the current data to inspire rigorous testing of the Pseudomonas surface sensing paradigm, and to share some thoughts on how to test this hypothesis going forward. For this review, rather than presenting the literature in chronological order, we lay out the pathway in the order in which we believe the events occur based on our current understanding.
First contact
What is the initial surface engagement event between P. aeruginosa and the surface to which it will attach? Current data suggest that TFP probably participate in surface engagement, and do so for multiple surface types. TFP of P. aeruginosa have been long known to berequired for early biofilm formation [28] and TFP, while occurring at low levels in planktonically grown cells, are robustly up regulated when P. aeruginosa is growing on an agar plate [29–31]. A study by Luo, Wong, O'Toole and colleagues took advantage of this observation and the fact that PilY1, a component of the TFP machinery, is up regulated when bacteria are grown on an agar surface. PilY1 is also required for the surface-specific up regulation of c-di-GMP upon surface growth; thus, PilY1 served as an excellent marker for the adaptation to a surface-associated lifestyle. A screen for mutants that altered pilY1 gene expression revealed mutations in the Pil-Chp system, previously reported as important for TFP biogenesis via production of cAMP; other loci required for TFP biosynthesis and regulation were also identified. Luo et al. found that starting within ∼2 h of the bacteria being transferred to an agar plate, cAMP level increased ∼6-fold, and this increase required PilJ, a protein with high sequence similarity to methyl accepting chemotaxis (MCP) proteins, as well as the TFP pilin, PilA [32••]. cAMP signaling in turn, is required to up-regulate TFP biogenesis. Thus, we suspect that low-level TFP production in planktonic cells primes these free-swimming organisms to use its pilus ‘feel’ for the surface. Once contact is initiated, TFP production is stimulated via the Pil-Chp system as is production of cAMP and the Vfr-cAMP-mediated up regulation of genes required for TFP biogenesis. A simultaneous and important publication by Persat, Gitai et al. also posited a role for TFP in surface sensing, and in particular, as a ‘mechanosensor’ that drives cAMP signaling via the Pil-Chp system when this microbe encounters a solid surface [33••], thus confirming the findings described above. Furthermore, Persat, Gitai et al. argued that extension/retraction of TFP amplify cAMP signaling based on monitoring cAMP-dependent PaQa expression in a pilUT double mutant. By contrast, Luo et al. showed that loss of PilU function stimulated cAMP. While additional studies are required, both studies point to the role of TFP extension and/or retraction as required for modulating cAMP signaling.
Concomitant with signaling via the TFP and the Pil-Chp pathway, Luo et al. showed that the FimS/AlgR two-component system is also required for stimulation of surface-dependent, up regulation of TFP production. Thus, there may be multiple inputs required for these events. In this same report, bacterial two-hybrid analysis showed interaction between PilJ and the sensor histidine kinase FimS (also called AlgZ) and Gitai et al. showed that PilJ and PilA also interact [33••], indicating the possibility of a signaling protein complex participating in early surface sensing.
First cAMP then c-di-GMP
What is the impact of stimulating TFP production within ∼120 min of contacting a surface? The data suggests at least two consequences, which are probably related. First, TFP play a key role in modulating surface movement and the commitment to attach by impacting two different forms of motility [34–36]. TFP are used for highly persistent movement of the microbe (‘crawling’) that allows long distance displacement while the cell is oriented parallel to the surface. TFP can also mediate cell ‘walking’, wherein the microbe stands upright and appears to explore the local surface [35,36]. Bacteria can readily transition between these two modes of movement. Importantly, upright ‘walking’ allows the bacterium to attain a ‘launch mode’ that promotes detachment from the surface [35,36]; thus, TFP play a key role in the transition between reversible and irreversible attachment (Figure 1). Second, increased expression of the TFP biogenesis machinery also results in the up-regulation of the TFP-associate protein, PilY1. PilY1 appears to play two independent roles in P. aeruginosa biology. It has been implicated as a TFP-associated adhesin [37,38], and independent of this role, PilY1 is an outer membrane-associated protein that impacts surface-dependent stimulation of c-di-GMP production [32••]. In this context, mutations in pilY1 were first isolated in a screen for mutants that restored swarming motility and reduce biofilm formation by a strain with elevated c-di-GMP levels, implicating PilY1 in the inverse control of these two surface behaviors. The ability of PilY1 to modulate c-di-GMP levels once bacteria engage a surface appears to be dependent on its ability to be secreted and localized to the cell surface [39]. Interestingly, PilY1 also appears to be able to down-regulate cAMP signaling, probably via PilJ and/or FimS by a yet-to-be determined mechanism. A recent report also showed a role for high levels of c-di-GMP down-regulating cAMP [40,41], consistent with the concept that the production of these dinucleotides is coordinated. Together, these data may suggest that once downstream events in cAMP signaling are highly induced, downstream pathway components subsequently participate in feedback inhibition of this ATP-derived, cyclic nucleotide.
Figure 1.
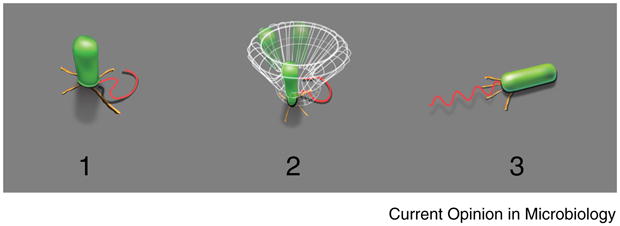
Examples of behavior for ‘reversibly’ and ‘irreversibly’ attached cells: the former is characterized by an out-of-plane cell orientation (1,2); the latter by cell orientations parallel to the surface (3). The TFP (yellow) and flagella (red) play important roles in the transition between these states.
How does PilY1, which is localized to the outer membrane, impact c-di-GMP levels in the cytoplasm? Genetic studies implicate signaling through the TFP ‘alignment complex’ (the PilMNOP proteins) to the inner membrane (IM)-localized SadC diguanylate cyclase; this model still needs further testing [32••]. Importantly, PilY1-mediat-ed, up regulation of c-di-GMP results in repression of flagellar-dependent swarming motility; thus, the cell shuts off flagellar function when up-regulating TFP biogenesis, allowing a hierarchical control of these motility appendages as cells transition into a biofilm. Additionally, the Gitai lab has investigated the role of two proteins in P. aeruginosa, PocA/B, in the proper localization and function of flagellar machinery and TFP-dependent twitching machinery; they suggest that these proteins may also help with surface-contact sensing [42]. Finally, in addition to its role in regulating early biofilm formation, a study lead by Siryaporn and Gitai, in collaboration with the O'Toole lab, showed that PilY1 plays a role in stimulating virulence upon contact with host cells, although this PilY1-mediated impact on pathogenesis does not appear to require the same factors (i.e., SadC, c-di-GMP) that control the surface-sensing pathway we describe above [39,43•]. Furthermore, PilY1 has a so-called von willibrand A domain that has been implicated in mechanosensing in eukaryotes; deletion of the VWA domain appears to lock PilY1 in a constitutively signaling conformation [33••], confirming a role for this domain of PilY1 in signal transduction. Our current understanding of P. aeruginosa surface sensing is summarized in Figure 2.
Figure 2.
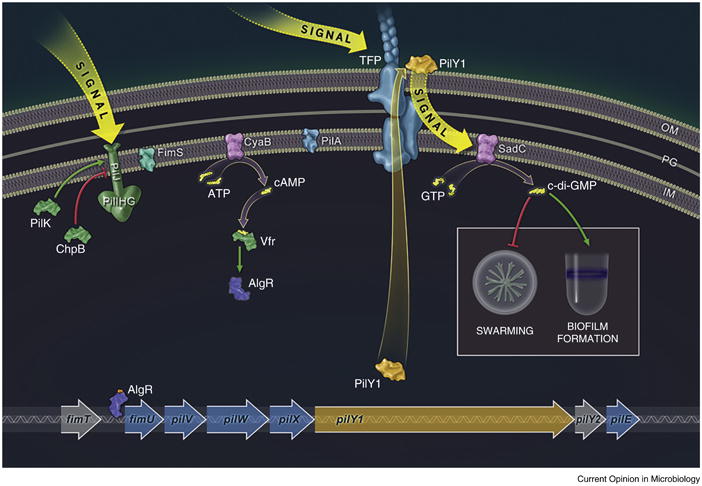
Surface sensing in Pseudomonas aeruginosa. A schematic model for cAMP and c-di-GMP signaling during early biofilm formation is shown. Subsequent to initial interaction with a surface, uncharacterized signals resulting from surface contact are detected by the chemotaxis-like protein PilJ and the type IV pilus (TFP), and transduced via the sensor histidine kinase FimS (also called AlgZ) and the adenylate cyclase CyaB, resulting in the surface-dependent increase in cAMP levels. PilK is the methyltransferase for PilJ, while ChpB serves as a demethylase; the methylation state of PilJ modulates its activity. cAMP, together with the transcription factor Vfr, are required for the transcription of the genes coding for FimS and its cognate response regulator AlgR. Phosphorylated AlgR is required for the increased transcription of several operons required for TFP biogenesis, including the fimUpilVpilXpilY1pilY2pilE operon. Thus, surface contact can establish a positive feedback loop whereby PilJ/TFP-mediated signals result in the increased production of TFP; TFP are in turn required for surface-associated twitching motility. Furthermore, as outlined in the text, bacterial two-hybrid studies indicate physical interactions between PilJ and FimS as well as between PilJ and PilA, suggesting that there may be an IM signaling complex required to sense and transduce surface signals. Subsequent to the stimulation of cAMP levels and transcriptional up-regulation of TFP-associated genes, the levels of PilY1 are increased. PilY1 is found on the bacterial cell surface and requires the TFP for its efficient secretion. Cell surface PilY1 can signal to SadC (by an unknown mechanism) to boost c-di-GMP levels, thereby repressing surface swarming motility and favoring the production of sessile cells and the formation of a biofilm. Additional details of this pathway can be found in Luo et al. [32••]. The WspR chemotaxis-like system controls later exopolysaccharide (EPS) production, also through the surface-dependent stimulation of c-di-GMP (not shown) [44–46].
Figure illustration and design copyright 2015 William Scavone and used with permission.
The many functions of exopolysaccharide (EPS)
For those cells that commit to irreversible attachment, production of an EPS probably plays a crucial role; P. aeruginosa PA14 produces Pel polysaccharide while P. aeruginosa PAO1 produces Psl. Most current work on EPS has employed P. aeruginosa PAO1 thus we focus on findings from that strain here. Studies from the Har-wood group using P. aeruginosa PAO1 have implicated Wsp, a chemotaxis-like system for regulating c-di-GMP levels, in regulating Psl production in response to surface sensing [44–46]. Thus, at least two different chemotaxis-like systems (Pil-Chp and Wsp) play crucial roles in early biofilm formation. The exciting studies from the Harwood group demonstrate a need for clustering of components of the Wsp system, which occurs preferentially in surface-grown cells, to stimulate c-di-GMP synthesis; but how and why this clustering occurs remains an open question. The Wsp system impacts biofilm macrocolony development, rather than initial attachment [47], a finding consistent with our unpublished analysis of wsp mutants which do not show an early-attachment defect in P. aeruginosa PA14. Thus, the Wsp system seems to function downstream of cAMP and PilY1-mediated c-di-GMP signaling. What role(s) does EPS play in establishing the early biofilm? It appears that the Psl EPS of P. aeruginosa PAO1 has up to four discreet functions. First, Psl appears to be required to establish and maintain irreversible attachment [48], presumably because Psl can act as an adhesin in at least some circumstances. Second, using single cell tracking combine with a fluorescent lectin to label Psl, Zhao, Parsek, Wong and colleagues showed that P. aeruginosa TFP-driven surface motility is guided by a web of collectively secreted Psl: the more Psl is deposited on a given patch of surface, the more likely a bacterium will visit that patch, and the more likely Psl will be secreted there again. Through this cycle of positive reinforcement and Psl accumulation, microcolonies are formed [49••]. Third, and perhaps related to its second role, Irie, Parsek and colleagues [50] proposed that Psl could serve as a signal to stimulate biofilm formation. While the precise mechanism of Psl-stimulated biofilm formation is still to be determined, the authors proposed that the activity of the diguanylate cyclases SiaD and SadC is stimulated by Psl, resulting in increased c-di-GMP levels, which in turn promotes production of biofilm-promoting factors. How extracellular Psl might stimulate the IM-localized SadC and SiaD is an intriguing question, although SiaD is part of a transmembrane signaling complex that includes the outer membrane protein SiaA [51]. Finally, Psl also stabilizes cell–cell interactions in P. aeruginosa PAO1 as the biofilm continues to mature; the Pel EPS plays this role in P. aeruginosa PA14 [52] in part by cross-linking extracellular DNA in the extracellular matrix [53•]. Overall, cell tracking studies showed that Psl is crucial to develop microcolonies, the stage in biofilm maturation downstream from irreversible attachment [49••].
It is interesting to contrast the behavior of P. aeruginosa with alphaproteobacteria. Caulobacter crescentus has a dimorphic life cycle with a motile swarmer cell that attaches to a surface, followed by differentiation into a nonmotile stalked cell ‘glued’ to a surface via a stalk tipped with a polysaccharide ‘holdfast’ [54]. Interestingly, cells arrive at the surface without the ‘holdfast’, and rapidly ‘deploy’ this primary adhesion (∼10 s) in response to surface contact [55–57]. Similar observations hold for other alphaproteobacteria such as Agrobacterium tumefaciens [58–60]. At present, it is not known precisely why P. aeruginosa goes through a long process of surface sensing, whereas Caulobacter and Agrobacterium make the decision so quickly. Perhaps part of the explanation is rooted in how surface sensing establishes what it is that cells need to do on the surface subsequently, but more work will have to be done to understand these differences. While we agree that it is useful to consider the possibility of cognate, conserved mechanisms for surface sensing among species, we argue that it is at least as interesting to think in terms of how diverse species have optimized surface sensing to suit their unique ecological circumstances, and how different model systems can mutually illuminate one another. There are many bacteria that engage surfaces, and we cannot understand potential underlying conserved mechanisms without well-understood model systems to inform our conceptual vocabularies.
Where do we go next?
We argue that surface sensing in P. aeruginosa is a complex, multi-step, cooperative process; microbes do not make the decision to commit to a biofilm lightly. At present, not much is known in general about how cells sense and select between different types of surfaces. V. cholerae for example, have at least three different types of pili, which allows them to interact with chemically distinct surfaces, so this can be one potential starting point. For Neisseria gonorrhoeae, mechanical forces are especially important, and also play a role in the organization of mixed colonies [61]. Surface sensing in P. aeruginosa probably involves both chemical cues (in the form of nutrient availability, polysaccharide sensing and probably other yet-to-be identified signals) and physical cues (in the form of TFP-mediated sensing), and seemingly piggybacking on at least two chemotaxis-like regulatory systems (Pil-Chp and Wsp).
We have seen from the discussion above that flagella and pili can act as sensors and start signaling programs via second messengers. However, recent work has shown that appendages are also controlled by second messengers; flagellum speed and direction in Eschericia coli can be influenced by c-di-GMP via YcgR [62–64]. In the broader context of bacterial developmental biology, oscillating levels of c-di-GMP impact the surface sensing induced developmental program in C. crescentus [65]. This leads to a kind of ‘chicken or egg’ conundrum if we imagine only chains of simple, causal relations consisting of stimulus and subsequent response. We hypothesize that this kind of logic is insufficient for understanding the emergence of qualitatively different, decisive behavior from complex systems. For example, the flagellum is both a sensor and an effector, so the demarcation between stimulus and response blurs. Seemingly simple ‘decisions’, such as the detection of a surface and the propensity to stay are necessarily cooperative and ‘self-consistent’ solutions to overlapping networks of development programs that integrate over many sensory inputs.
What are the crucial questions we need to explore going forward? First, TFP and the flagellum have been implicated as cell appendages that participate in sensing surface engagement; however, there is still a large knowledge gap in the mechanisms that translate these appendage-mediated signals to changes in cell behavior. Second, the participation of at least two chemosensory systems in these processes may indicate that surface sensing is a specialized form of chemotaxis; a question that should receive serious attention. Third, it appears that different signaling events can take different times to engage, especially when there are multiple pathways. Furthermore, these pathways appear to be ordered, and organized such that downstream pathways are not activated until upstream events run their course; is this hierarchical arrangement crucial for surface sensing? For example, it takes some finite time in order for PilY1-mediated flagellum inhibition to run its course and completely shut down the flagellum, so it is possible to have one or more transition states where we can have both flagellum and TFP working in some capacity. Therefore, in the simplest possible picture, we can have a sequence of four states. First, flagellum active, TFP probably inactive or present at low levels (e.g., free swimming cells); second, flagellum active but in the process of being inhibited, TFP active, defined as being able to retract (e.g., cells that first attach on the surface early in the biofilm life cycle), third, flagellum shut down, TFP active (e.g., twitching motility on the surface), and fourth, flagellum shut down, TFP shutdown (e.g., microcolonies, where EPS is now up regulated). Taken together, we believe that being able to connect phenotypic observations at single cell resolution to community level analyses will transform our understanding of the early events in biofilm formation and keep all of us busy for a long while!
Acknowledgments
We thank Cynthia Whitchurch, Ramin Golestanian, and Lori Burrows for invaluable input. This study is supported by National Institutes of Health grant R37 AI83256-06 to GAO and the Human Frontiers in Science Program RGP0061/2013 and NIH R01AI102584 to GCLW.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Zobell CE, Allen EC. The significance of marine bacteria in the fouling of submerged surfaces. J Bacteriol. 1935;29:239–251. doi: 10.1128/jb.29.3.239-251.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prigent-Combaret C, et al. Abiotic surface sensing and biofilm-dependent regulation of gene expression in Eschericia coli. J Bacteriol. 1999;181:5993–6002. doi: 10.1128/jb.181.19.5993-6002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harshey RM. Bees aren't the only ones: swarming in gram-negative bacteria. Mol Microbiol. 1994;13:389–394. doi: 10.1111/j.1365-2958.1994.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 4•.Tuson HH, Weibel DB. Bacteria–surface interactions. Soft Matter. 2013;9:4368–4380. doi: 10.1039/C3SM27705D. An exellent review of how bacteria interact with surfaces from a biophysical perspective. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedlander RS, et al. Bacterial flagella explore microscale hummocks and hollows to increase adhesion. Proc Natl Acad Sci USA. 2013;110:5624–5629. doi: 10.1073/pnas.1219662110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holz C, et al. Bacterial motility and clustering guided by microcontact printing. Nano Lett. 2009;9:4553–4557. doi: 10.1021/nl903153c. [DOI] [PubMed] [Google Scholar]
- 7.McCarter L, et al. Flagellar dynamometer controls swarmer cell differentiation of V. parahaemolyticus. Cell. 1988;54:345–351. doi: 10.1016/0092-8674(88)90197-3. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira RB, et al. Vibrio parahaemolyticus ScrC modulates cyclic dimeric GMP regulation of gene expression relevant to growth on surfaces. J Bacteriol. 2008;190:851–860. doi: 10.1128/JB.01462-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira RB, et al. Output targets and transcriptional regulation by a cyclic dimeric GMP-responsive circuit in the Vibrio parahaemolyticus Scr network. J Bacteriol. 2012;194:914–924. doi: 10.1128/JB.05807-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gode-Potratz CJ, et al. Surface sensing in Vibrio parahaemolyticus triggers a programme of gene expression that promotes colonization and virulence. Mol Microbiol. 2011;79:240–263. doi: 10.1111/j.1365-2958.2010.07445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caiazza NC, et al. Inverse regulation of biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. J Bacteriol. 2007;189:3603–3612. doi: 10.1128/JB.01685-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Toole GA. How Pseudomonas aeruginosa regulates surface behaviors. Microbe. 2008;3:65–71. [Google Scholar]
- 13.Merritt JH, et al. SadC reciprocally influences biofilm formation and swarming motility via modulation of exopolysaccharide production and flagellarf unction. J Bacteriol. 2007;189:8154–8164. doi: 10.1128/JB.00585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YK, McCarter LL. ScrG, a GGDEF-EAL protein, participates in regulating swarming and sticking in Vibrio parahaemolyticus. J Bacteriol. 2007;189:4094–4107. doi: 10.1128/JB.01510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cusick K, et al. Perturbation of FliL interferes with Proteus mirabilis swarmer cell gene expression and differentiation. J Bacteriol. 2012;194:437–447. doi: 10.1128/JB.05998-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Cairns LS, et al. A mechanical signal transmitted by the flagellum controls signalling in Bacillus subtilis. Mol Microbiol. 2013;90:6–21. doi: 10.1111/mmi.12342. This manuscript examines the role of the flagellum in signaling a mechanical signal, and representes some of the best evidence that microbes can sense surface engagement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guttenplan SB, et al. The cell biology of peritrichous flagella in Bacillus subtilis. Mol Microbiol. 2013;87:211–229. doi: 10.1111/mmi.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgenstein RM, Rather PN. Role of the Umo proteins and the Rcs phosphorelay in the swarming motility of the wild type and an O-antigen (waaL) mutant of Proteus mirabilis. J Bacteriol. 2012;194:669–676. doi: 10.1128/JB.06047-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otto K, Silhavy TJ. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc Natl Acad Sci USA. 2002;99:2287–2292. doi: 10.1073/pnas.042521699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monds RD, et al. Phosphate-dependent modulation of c-di-GMP levels regulates Pseudomonas fluorescens Pf0-1 biofilmformation by controlling secretion of the adhesin LapA. Mol Microbiol. 2007;63:656–679. doi: 10.1111/j.1365-2958.2006.05539.x. [DOI] [PubMed] [Google Scholar]
- 21.Paul EA, Clark FE. Soil Microbiology and Biochemistry. San Diego, CA: Academic Press; 1989. [Google Scholar]
- 22.Kolenbrander PE, London J. Adhere today, here tomorrow: oral bacterial adherence. J Bacteriol. 1993;175:3247–3252. doi: 10.1128/jb.175.11.3247-3252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burrows LL. Pseudomonas aeruginosa twitching motility: type IV pili in action. Annu Rev Microbiol. 2012;66:493–520. doi: 10.1146/annurev-micro-092611-150055. [DOI] [PubMed] [Google Scholar]
- 24.Maier B, Wong GC. How bacteria use Type IV pili machinery on surfaces. Trends Microbiol. 2015;23:775–788. doi: 10.1016/j.tim.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Lipp EK, et al. Effects of global climate on infectious disease: the cholera model. Clin Microbiol Rev. 2002;15:757–770. doi: 10.1128/CMR.15.4.757-770.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meibom KL, et al. The Vibrio cholerae chitin utilization program. Proc Natl Acad Sci USA. 2004;101:2524–2529. doi: 10.1073/pnas.0308707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Utada AS, et al. Vibrio cholerae use pili and flagella synergistically to effect motility switching and conditional surface attachment. Nat Commun. 2014;5:4913. doi: 10.1038/ncomms5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 29.Kelly NM, et al. Pseudomonas aeruginosa pili as ligands for nonopsonic phagocytosis by fibronectin-stimulated macrophages. Infect Immun. 1989;57:3841–3845. doi: 10.1128/iai.57.12.3841-3845.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Speert DP, et al. Nonopsonic phagocytosis of nonmucoid Pseudomonas aeruginosa by human neutrophils and monocyte-derived macrophages is correlated with bacterial piliation and hydrophobicity. Infect Immun. 1986;53:207–212. doi: 10.1128/iai.53.1.207-212.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bradley DE. Evidence for the retraction of Pseudomonas aeruginosa RNA phage pili. Biochem Biophys Res Commun. 1972;47:142–149. doi: 10.1016/s0006-291x(72)80021-4. [DOI] [PubMed] [Google Scholar]
- 32••.Luo Y, et al. A hierarchical cascade of second messengers regulates Pseudomonas aeruginosa surface behaviors. mBio. 2015;6:e02456–14. doi: 10.1128/mBio.02456-14. This publication, along with Persat et al., describes for the first time the role of TFP-mediated cAMP signaling in a ‘surface sensing’ pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33••.Persat A, et al. Type IV pili mechanochemically regulate virulence factors in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2015;112:7563–7568. doi: 10.1073/pnas.1502025112. This publication, along with Luo et al., describes for the first time the role of TFP-mediated cAMP signaling in a ‘surface sensing’ pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin F, et al. Bacteria use type-IV pili to slingshot on surfaces. Proc Natl Acad Sci USA. 2011;108:12617–12622. doi: 10.1073/pnas.1105073108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conrad JC, et al. Flagella and pili-mediated near-surface single-cell motility mechanisms in P. aeruginosa. Biophys J. 2011;100:1608–1616. doi: 10.1016/j.bpj.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibiansky ML, et al. Bacteria use type IV pili to walk upright and detach from surfaces. Science. 2010;330:197. doi: 10.1126/science.1194238. [DOI] [PubMed] [Google Scholar]
- 37.Alm RA, et al. Fimbrial biogenesis genes of Pseudomonas aeruginosa: pilW and pilX increase the similarity of type 4 fimbriae to the GSP protein-secretion systems and pilY1 encodes a gonococcal PilC homologue. Mol Microbiol. 1996;22:161–173. doi: 10.1111/j.1365-2958.1996.tb02665.x. [DOI] [PubMed] [Google Scholar]
- 38.Heiniger RW, et al. Infection of human mucosal tissue by Pseudomonas aeruginosa requires sequential and mutually dependent virulence factors and a novel pilus-associated adhesin. Cell Microbiol. 2010;12:1158–1173. doi: 10.1111/j.1462-5822.2010.01461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuchma SL, et al. Cyclic-di-GMP-mediated repression of swarming motility by Pseudomonas aeruginosa: the pilY1 gene and its impact on surface-associated behaviors. J Bacteriol. 2010;192:2950–2964. doi: 10.1128/JB.01642-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almblad H, et al. The cyclic AMP-Vfr signaling pathway in Pseudomonas aeruginosa is inhibited by cyclic di-GMP. J Bacteriol. 2015;197:2190–2200. doi: 10.1128/JB.00193-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Almblad H, et al. Erratum for Almblad et al., The cyclic AMP-Vfr signaling pathway in Pseudomonas aeruginosa is inhibited by cyclic di-GMP. J Bacteriol. 2015;197:2731. doi: 10.1128/JB.00493-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cowles KN, et al. The putative Poc complex controls two distinct Pseudomonas aeruginosa polar motility mechanisms. Mol Microbiol. 2013;90:923–938. doi: 10.1111/mmi.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Siryaporn A, et al. Surface attachment induces Pseudomonas aeruginosa virulence. Proc Natl Acad Sci USA. 2014;111:16860–16865. doi: 10.1073/pnas.1415712111. This manuscript describes the link between surface contact and viruelnce in the model organisms Pseudomonas aeruginosa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guvener ZT, Harwood CS. Subcellular location characteristics of the Pseudomonas aeruginosa GGDEF protein, WspR, indicate that it produces cyclic-di-GMP in response to growth on surfaces. Mol Microbiol. 2007;66:1459–1473. doi: 10.1111/j.1365-2958.2007.06008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hickman JW, Harwood CS. Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol Microbiol. 2008;69:376–389. doi: 10.1111/j.1365-2958.2008.06281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Connor JR, et al. Surface sensing and lateral subcellular localization of WspA, the receptor in a chemosensory-like system leading to c-di-GMP production. Mol Microbiol. 2012;86:720–729. doi: 10.1111/mmi.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hickman JW, et al. A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. Proc Natl Acad Sci USA. 2005;102:14422–14427. doi: 10.1073/pnas.0507170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jackson KD, et al. Identification of psl, a locus encoding a potential exopolysaccharide that Is essential for Pseudomonas aeruginosa PAO1 biofilm formation. J Bacteriol. 2004;186:4466–4475. doi: 10.1128/JB.186.14.4466-4475.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49••.Zhao K, et al. Psl trails guide exploration and microcolony formation in Pseudomonas aeruginosa biofilms. Nature. 2013;497:388–391. doi: 10.1038/nature12155. This publication demonstrates the ability of polysaccharides to drive microcoilony formation by a ‘rich get richer’ mechanism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Irie Y, et al. Self-produced exopolysaccharide is a signal that stimulates biofilm formation in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2012;109:20632–20636. doi: 10.1073/pnas.1217993109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klebensberger J, et al. SiaA and SiaD are essential for inducing autoaggregation as a specific response to detergent stress in Pseudomonas aeruginosa. Environ Microbiol. 2009;11:3073–3086. doi: 10.1111/j.1462-2920.2009.02012.x. [DOI] [PubMed] [Google Scholar]
- 52.Colvin KM, et al. The Pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathog. 2011;7:e1001264. doi: 10.1371/journal.ppat.1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jennings LK, et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosabiofilm matrix. Proc Natl Acad Sci U S A. 2015;112:11353–11358. doi: 10.1073/pnas.1503058112. Parsek and colleagues report that two key components of the biofilm matrix interact, probably helping to stabilize these communities. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsang PH, et al. Adhesion of single bacterial cells in the micronewton range. Proc Natl Acad Sci USA. 2006;103:5764–5768. doi: 10.1073/pnas.0601705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li G, et al. Surface contact stimulates the just-in-time deployment of bacterial adhesins. Mol Microbiol. 2012;83:41–51. doi: 10.1111/j.1365-2958.2011.07909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoffman MD, et al. Timescales and frequencies of reversible and irreversible adhesion events of single bacterial cells. Anal Chem. 2015;87:12032–12039. doi: 10.1021/acs.analchem.5b02087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bodenmiller D, et al. Development of surface adhesion in Caulobacter crescentus. J Bacteriol. 2004;186:1438–1447. doi: 10.1128/JB.186.5.1438-1447.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu J, et al. Genetic analysis of Agrobacterium tumefaciens unipolar polysaccharide production reveals complex integrated control of the motile-to-sessile switch. Mol Microbiol. 2013;89:929–948. doi: 10.1111/mmi.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y, et al. The Ctp type IVb pilus locus of Agrobacterium tumefaciens directs formation of the common pili and contributes to reversible surface attachment. J Bacteriol. 2014;196:2979–2988. doi: 10.1128/JB.01670-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feirer N, et al. A pterin-dependent signaling pathway regulates a dual-function diguanylate cyclase-phosphodiesterase controlling surface attachment in Agrobacterium tumefaciens. mBio. 2015;6:e00156. doi: 10.1128/mBio.00156-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oldewurtel ER, et al. Differential interaction forces govern bacterial sorting in early biofilms. eLife. 2015;4:e10811. doi: 10.7554/eLife.10811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boehm A, et al. Second messenger-mediated adjustment of bacterial swimming velocity. Cell. 2010;141:107–116. doi: 10.1016/j.cell.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 63.Paul K, et al. The c-di-GMP binding protein YcgR controls flagellar motor direction and speed to affect chemotaxis by a “backstop brake” mechanism. Mol Cell. 2010;38:128–139. doi: 10.1016/j.molcel.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fang X, Gomelsky M. A post-translational, c-di-GMP-dependent mechanism regulating flagellar motility. Mol Microbiol. 2010;76:1295–1305. doi: 10.1111/j.1365-2958.2010.07179.x. [DOI] [PubMed] [Google Scholar]
- 65.Abel S, et al. Bi-modal distribution of the second messenger c-di-GMP controls cell fate and asymmetry during the caulobacter cell cycle. PLoS Genet. 2013;9:e1003744. doi: 10.1371/journal.pgen.1003744. [DOI] [PMC free article] [PubMed] [Google Scholar]


