Abstract
Chimeric antigen receptors (CARs) are engineered fusion proteins constructed from antigen recognition, signaling, and costimulatory domains that can be expressed in cytotoxic T cells with the purpose of reprograming the T cells to specifically target tumor cells. CAR T-cell therapy uses gene transfer technology to reprogram a patient's own T cells to stably express CARs, thereby combining the specificity of an antibody with the potent cytotoxic and memory functions of a T cell. In early phase clinical trials, CAR T cells targeting CD19 have resulted in sustained complete responses within a population of otherwise refractory patients with B-cell malignancies and, more specifically, have shown complete response rates of ≈90% in patients with relapsed or refractory acute lymphoblastic leukemia. Given this clinical efficacy, preclinical development of CAR T-cell therapy for a number of cancer indications has been actively investigated, and the future of the CAR T-cell field is extensive and dynamic. Several approaches to increase the feasibility and safety of CAR T cells are currently being explored, including investigation into mechanisms regulating the persistence of CAR T cells. Additionally, numerous early-phase clinical trials are now investigating CAR T-cell therapy beyond targeting CD19, especially in solid tumors. Trials investigating combinations of CAR T cells with immune checkpoint blockade therapies are now beginning and results are eagerly awaited. This review evaluates several of the ongoing and future directions of CAR T-cell therapy.
Introduction
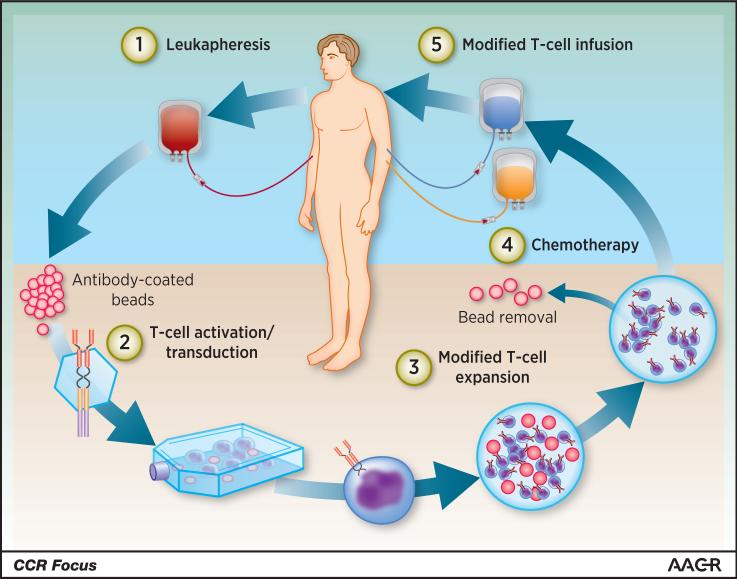
Over the past few decades, our understanding of the role of the immune system in cancer has grown considerably and so has the technology to purify and manipulate specific immune cell types with a goal of treating disease. The transfer of genetically engineered immune cells as a form of cellular therapy has been investigated as a treatment option for HIV and cancer (1, 2). Chimeric antigen receptor (CAR) T-cell therapy uses gene transfer technology to reprogram a patient's T cells to express CARs (Figure 1), thereby directing T cells’ cytotoxic potential against tumor cells that would otherwise be ignored (3). CARs are engineered fusion proteins that contain an extracellular antigen-binding domain composed of a single-chain variable fragment derived from an antibody and intracellular signaling domains, which are involved in the initiation of T-cell signaling and downstream T-cell effector functions (4). First-generation CARs consisted of only the T-cell receptor complex CD3ζ chain domain and antigen recognition domains, showed minimal clinical success, and were characterized by very low levels of engraftment in patients (5, 6). Second-generation CARs containing costimulatory domains, typically either CD28 or 4-1BB, were hypothesized and shown to augment CAR T-cell survival and proliferation (7-9). The inclusion of a costimulatory domain dramatically increased the antitumor efficacy and persistence of CAR T cells (3, 10, 11). Interest and investment in the development of CAR T-cell therapy is rapidly increasing in both academia and industry, with multiple ongoing clinical trials as well as many expectations for the future of the field. Although CAR T-cell therapies are on a fast track to approval by the US Food and Drug Administration for B-cell malignancies, there is active investigation into building better CAR T cells for treating hematologic malignancies and solid tumors.
Figure 1.
Overview of CAR T-cell therapy in the clinic. A patient's T cells are harvested through leukapheresis, followed by T-cell activation on antibody-coated beads serving as artificial dendritic cells. The activated T cells are then genetically reprogrammed ex vivo by transduction with a construct encoding the CAR, and the CAR T cells are further expanded ex vivo. When the CAR T-cell product had been prepared and passed all quality control testing, the patient receives lymphodepleting chemotherapy and CAR T cell infusion. © Novartis Pharmaceuticals.
Current CARs: Clinical Overview
The most clinical data using CAR T-cell therapy have been generated with CD19-specific CAR T cells in patients with relapsed or refractory B-cell malignancies, many of whom have no curative option other than hematopoietic stem cell transplant. CD19 is highly and uniformly expressed on B cells, starting early in development and continuing through all mature stages except plasma cells. CD19 is also expressed on B-cell malignancies developing in the bone marrow (B-cell acute lymphoblastic leukemia [ALL]) and secondary lymphoid organs (chronic lymphocytic lymphoma, diffuse large B-cell lymphoma, and follicular lymphoma) (12).
The CD19 CAR T-cell field has become highly competitive in recent years, with several pharmaceutical companies developing partnerships with academic institutions. Complete response rates of ≈90% have been observed in both pediatric and adult patients with relapsed or refractory ALL who were treated with CD19 CAR T-cells expressing either a CD28 or a 4-1BB costimulatory domain (13-17). Overall response rates of 50%-100% have been recently observed in patients with diffuse large B-cell lymphoma, follicular lymphoma, or chronic lymphocytic lymphoma who were treated with the CD19 CAR T-cell therapy CTL019 (18, 19). While CD19 is not highly expressed on terminally differentiated plasma cells, CD19 CAR T cells may have clinical benefit in patients with multiple myeloma; this is hypothesized to be due to continual repopulation of malignant plasma cells from a malignant B-cell precursor (20, 21). Although CD19-specific CAR T cells have demonstrated considerable efficacy in B-cell malignancies, treatment of other hematologic malignancies will require further CAR T-cell target identification and validation. Targets currently under investigation are summarized in Table 1.
Table 1.
Selected solid and hematological tumor targets other than CD19 that are currently being investigated as targets for CAR T cells.
| Target | Indication | Preclinical Antitumor Efficacy | Clinical Efficacy/Safety | Ongoing Trials |
|---|---|---|---|---|
| Solid tumor targets | ||||
| EGFRvIII (38, 70) | Glioma, glioblastoma, head and neck cancer | Human glioma cells in vitro and ex vivo, glioblastoma xenograft model | None reported |
NCT02209376 NCT01454596 |
| ERBB2 (42, 71, 72) | Glioblastoma, sarcoma | Tumor cell lines, breast cancer xenograft model | Stable disease for 12 weeks to 14 months in 24% of pts (n=17) |
NCT00902044 NCT01109095 NCT00889954 |
| Mesothelin (43, 62, 73, 74) | Mesothelioma, pancreatic cancer, ovarian cancer, lung cancer | Mesothelioma xenograft model | Stable disease in 67% of pts at day 28 (n = 6) |
NCT02159716 NCT02414269 NCT01583686 |
| Carbonic anhydrase IX (75, 76) | RCC | RCC cells in vitro | Liver toxicity affected several pts | None |
| PSMA (folate hydrolase 1) (40, 44) | Prostate cancer | Prostate adenocarcinoma murine model | Stable disease in 50% of pts treated (n = 4) | NCT01140373 |
| FAP (77-79) | Mesothelioma | Murine pancreatic cancer model, lung cancer xenograft model | None reported | NCT01722149 |
| Carcinoembryonic antigen (80) | Lung, colorectal, gastric, breast, and pancreatic cancers | Murine model of liver metastases | None reported | NCT02349724 |
| 5T4 (trophoblast glycoprotein) (81) | Solid tumors | Murine models of melanoma and colon carcinoma | None reported | None |
| Hematologic malignancy targets | ||||
| CD22 (82, 83) | B-ALL, B-NHL | B-ALL cell lines, xenograft model | MRD-negative CR in 29% of evaluable pediatric pts with ALL; CRS occurred in 57% of pts (n=7) |
NCT02315612 NCT02588456 |
| BCMA (TNFRSF17) (84, 85) | MM | MM primary cells and cell lines, xenograft model | ORR in 33% of pts (n=12) |
NCT02215967 NCT02546167 |
| CD123 (IL3RA) (86) | AML | Primary AML in immunodeficient mice | None reported |
NCT02159495 NCT02623582 |
| CS1 (SLAM7) (87) | AML, MM | Primary MM cells in vitro, MM xenograft model | None reported | NCT02203825 |
| CD138 (syndecan 1) (88) | MM | Primary MM cells in vitro, MM xenograft model | None reported | NCT01886976 |
| Kappa light chain of Ig (89) | MM, CLL, lymphoma | B-CLL cells in vivo and in vitro | None reported | NCT00881920 |
| ROR1 (90, 91) | Hematologic and solid tumors | Primary B-CLL and MCL cells in vitro, RCC cell lines, breast cancer cell lines | None reported | NCT02194374 |
AML, acute myeloid leukemia; BCMA, B-cell maturation antigen; CLL, chronic lymphocytic leukemia; CR, complete response; CRS, cytokine release syndrome; EGFRvIII, epidermal growth factor receptor vIII; FAP, fibroblast activation protein alpha; HPV, human papillomavirus; Ig, immunoglobulin; IL3RA, interleukin 3 receptor subunit alpha; MCL, mantle cell lymphoma; MM, multiple myeloma; MRD, minimal residual disease; NHL, non-Hodgkin lymphoma; ORR, overall response rate; PSMA, prostate-specific membrane antigen; RCC, renal cell carcinoma; ROR1, receptor tyrosine kinase-like orphan receptor 1; TCR, T-cell receptor.
Regulating CAR T-cell Persistence: Strategies Currently Under Investigation in the Clinic
A major differentiating factor among the current CARs that have been investigated in clinical trials has been the level of CAR T-cell persistence. Early studies using CAR T cells did not include a lymphodepletion step, which may have contributed to their very short persistence and poor antitumor activity. Lymphodepletion is now included in most CAR T-cell therapy protocols; however, variable levels of persistence are observed both between and within clinical trials. One strategy to improve lymphodepletion and CAR T-cell persistence is through increasing the intensity of lymphodepletion, which leads to depletion of regulatory T cells and greater engraftment of the infused T cells (22-24). The selective depletion of regulatory T cells along with administration of cytokines to support CAR T-cell function is also under investigation (25). Further research is needed to determine which lymphodepletion methods result in optimal CAR T-cell persistence and antitumor benefit.
An important question in the field is the degree to which the costimulatory domains included in the CAR affect CAR T-cell persistence. A recent study showed that certain CAR T cells containing a CD28 costimulatory domain increased the expression of T-cell exhaustion-related genes, while the 4-1BB (TNFSF9) costimulatory domain with the same antigen specificity ameliorated this exhausted phenotype (26). This may explain why in recent clinical trials of patients with relapsed or refractory ALL, CAR T cells expressing a CD28 domain have been reported to persist for up to 3 months, while CAR T cells with a 4-1BB domain persist for up to five years, and more than 6 months in most cases (13, 17, 19). Other costimulatory domains have also been investigated for inclusion in CARs, most notably OX40 (TNFRSF4) and ICOS, which both resulted in improved target cell lysis in vitro when compared with CARs lacking a costimulatory domain (8, 27). Indeed, third-generation CARs containing two costimulatory domains have recently entered into clinical trials. While it is possible that future CARs incorporating multiple costimulatory domains will result in increased CAR T-cell antitumor activity, more studies are required to better understand the kinetics of each of the costimulatory domains and their relative effects in the clinic. Incorporation of large numbers of signaling domains may also lower vector titers due to large transgenes, lower the expression of the CAR at the cell surface, or result in otherwise decreased functionality due to the requirement for accessory signaling apparatus proximal to the cell membrane.
The optimal duration of persistence of CAR T cells is unknown, and may in fact be different for CD19-directed CAR T cells than for CAR T cells directed to other malignancies. Long-term persistence of CD19-directed CAR T cells has both the advantage of ongoing disease surveillance and the disadvantage of long-term B-cell aplasia. In addition to the CAR-encoded signaling domains, other factors may affect the persistence of CAR T cells, such as the cell culture system used during manufacturing, the mode of gene transfer and associated promoters, and the functionality and phenotype of the input T cells, which in turn may be affected by age, disease, and prior therapies.
A method to potentially increase the proliferation and long-term activity of CAR T cells in vivo involves using T cells specific for an antigen associated with chronic viral infection as the starting cell population for CAR T-cell manufacturing. In a murine model using cytomegalovirus (CMV)-specific T cells that were engineered to express a CD19-specific CAR, vaccination of the mice with CMV peptide resulted in enhanced proliferation and antitumor activity of the CAR T cells due to stimulation through the endogenous CMV-specific T cell receptor (28). Similar approaches have been investigated using CAR T cells derived from Epstein-Barr virus- or Adenovirus-specific T cells (29, 30). Several trials are ongoing to investigate the safety and efficacy of virus-specific CAR T cells in patients, including NCT00709033, NCT01430390, and NCT01109095.
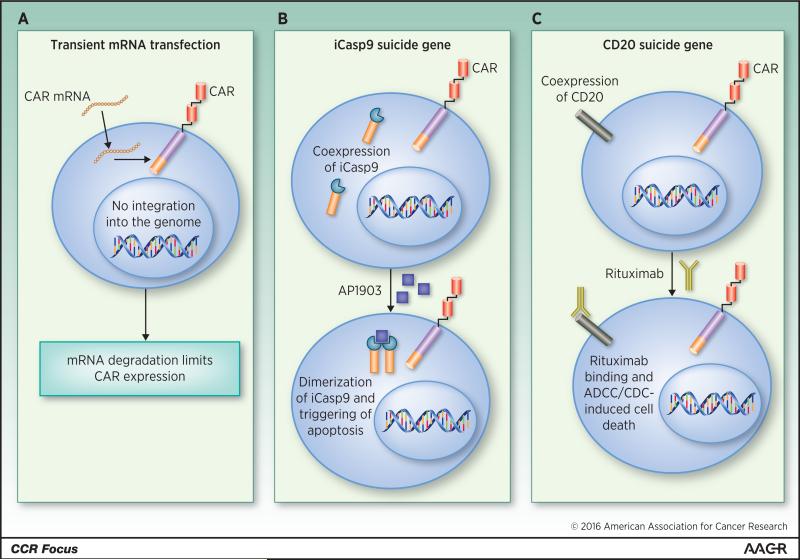
In some cases, particularly in the development of novel targets, transient CAR T-cell persistence may be desired. CARs can be expressed for a short duration through transfection of T cells with mRNA encoding the CAR, instead of using a viral vector, which permanently integrates the CAR into the genome (Figure 2A). RNA transfection is a fast and efficient procedure, requiring only 1 day of T-cell activation before transfection and resulting in very high (>40%) transfection efficiency (31). As mRNA is unable to integrate into the host genome, mRNA transfection results in a short-lived population of CAR T cells, which would require multiple doses to achieve an area under the curve that could be considered a therapeutic dose. Suboptimal scheduling of doses may result in generation of an immune or allergic response against the CAR, especially if a murine single-chain variable fragment is used (32). CARs transfected into T cells using mRNA are currently being investigated in early clinical trials at the University of Pennsylvania (NCT02624258, NCT01837602, NCT02277522, NCT02623582).
Figure 2.
Strategies to regulate CAR T-cell persistence. A. Activated T cells are transfected with mRNA encoding a CAR, resulting in expression of the CAR on the surface of the T cell until the mRNA is degraded. B. T cells are transduced with a construct containing and CAR as well as iCasp9. Administration of AP1903 causes dimerization of iCasp9 within the CAR T cells and subsequent apoptosis, resulting in specific depletion of the CAR T cells. C. T cells are transduced with a construct containing a CAR and the CD20 protein. Upon administration of the CD20-specific antibody rituximab, the CAR T cells (and all other CD20-expressing cells) are depleted. ADCC, antibody-dependent cellular cytotoxicity; CD20, cluster of differentiation 20; CDC, complement-dependent cytotoxicity; mRNA, messenger RNA.
Regulating CAR T-cell Persistence: Strategies on the Horizon
One of the beauties of CAR T cells is that they are “living drugs”: once infused, physiologic mechanisms maintain T-cell homeostasis, memory formation, and antigen-driven expansion. However, imperfect human intervention may lead to T cells that target an undesired tissue or proliferate to greater levels than necessary and therapeutic. As CAR T cells become incorporated into standard therapies, it may be useful to design them with patient- or physician-controlled persistence mechanisms, either “ON” switches or “suicide” switches. For technical reasons, suicide switches are easier to incorporate into T cells. One of the fastest-acting and clinically tested suicide gene strategies is the inducible caspase 9 (iCasp9) system (Figure 2B) (33). Cells transduced with iCasp9 can be depleted by administration of a synthetic small molecule that dimerizes iCasp9 promolecules, triggering activation of the apoptotic pathway (34). Induction of iCasp9 dimerization and T-cell depletion via the administration of the small molecule AP1903 is a strategy that has been used in patients with graft-versus-host disease, demonstrating the feasibility of this approach (35).
CAR T cells may also be depleted through the co-expression of a protein for which a depleting antibody is already in clinical use, i.e. CD20 or EGFR (Figure 2C). Administration of the depleting antibody is expected to deplete target-expressing CAR T cells if therapy-related toxicity arises or if “cure” has been achieved and maintained (36). To our knowledge, neither of these strategies have been clinically tested as a CAR T cell-depleting method in patients, but CARs containing an EGFR transgene are currently under clinical investigation at several centers (NCT02028455, NCT02159495, NCT01865617). Most investigators have preferred to manage toxicities with either cytokine blockade or corticosteroids, or both, rather than permanently ablate a potentially curative (and expensive) therapy.
A recent paper demonstrated proof-of-concept for the first “ON-switch”-based CAR T cells; here the signaling apparatus of the CAR was separated and each end was fused to a dimerizing domain similar to the basis of iCasp9, where the full CAR is reconstituted only in the presence of a tacrolimus-based drug (37). The clinical feasibility of such a system is likely to yield interesting results.
Despite the fast-track regulatory pathway for CD19-directed CAR T cells, there are still many areas of CAR design and genetic modifications of T cells that could broaden the therapeutic window and applicability of genetically-modified T cells for adoptive immunotherapy. For example, CARs have typically contained a single-chain variable fragment sequence derived from a mouse antibody, but humanized antibody fragments may be less immunogenic; this may be particularly important for CAR T cells directed to any antigen other than B cells, because serologic immune responses to the CAR could limit their functionality (38). A particularly exciting approach will be to target multiple antigens, so that Boolean gating such as “antigen 1 AND 2” or “antigen 1 NOT 2” can be employed to more specifically target tumor tissues. Although these approaches are in very early stages, one could imagine engineering T cells by using either several distinct CAR constructs in one T cell or a single bi-specific construct (39-41).
The Future of CAR T-Cell Therapy: Moving Beyond Hematologic Malignancies and Into Solid Tumors
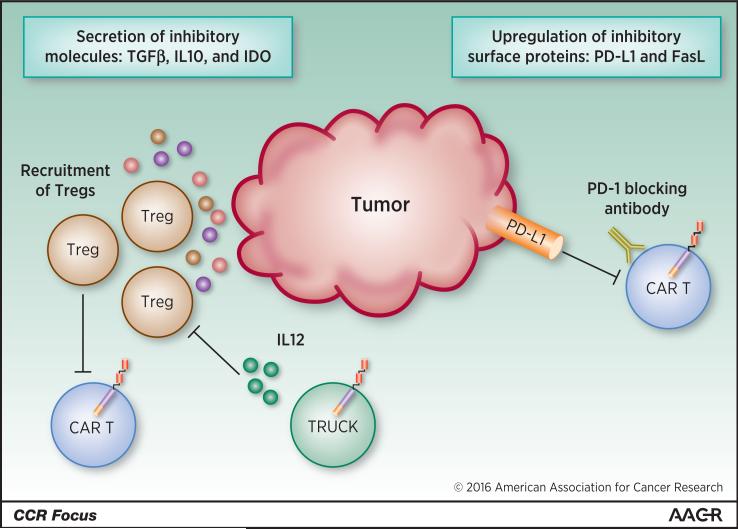
While CAR T-cell therapy has demonstrated high response rates in patients with leukemia or lymphoma, solid tumors present unique challenges for the use of cellular therapies. Several trials investigating CAR T-cell therapy in solid tumors have been initiated, but efficacy has been low. Best responses recently reported in trials using CAR T cells specific for the solid tumor antigens mesothelin, PSMA, or ERBB2 were stable disease in 24-67% of patients (42-44). The efficacy of CAR T-cell therapy in solid tumors may be reduced due to several factors. In contrast to hematologic malignancies, the solid tumor microenvironment is composed of immune cells, endothelial cells, fibroblasts, extracellular matrix molecules, and cytokines. This microenvironment not only reduces access of modified T cells to the entire mass of a solid tumor, but also plays a role in negative regulatory signaling that may limit CAR T-cell efficacy (Figure 3). For example, tumor stroma cells often produce molecules such as TGF-β, IL-10, and indoleamine-2,3-dioxygenase, which promote suppression of an effector T cell response by regulatory T cells (see Zarour in this CCR Focus (45)). Expressing TGF-β dominant-negative receptor II (DNRII) in T cells may be a method to reduce the influence of TFG-β on CAR T-cell therapy for solid tumors. Preclinical studies showed that cells expressing TGF-β DNRII had increased function, survival, and antitumor activity than cells that were not modified to express TGF-β DNRII (46, 47).
Figure 3.
Factors influencing CAR T-cell activity in the immunosuppressive solid tumor microenvironment. FasL, Fas ligand; IL, interleukin; PD-1, programmed death-1; PD-L1, programmed death-1 ligand-1; Treg, regulatory T cell; TRUCK, T cell redirected for universal cytokine-mediated killing.
Solid tumor cells also often upregulate immune checkpoint ligands such as PD-L1, which dampens an effector T-cell response when engaged with its receptor PD-1 (48) and could lead to inhibition of CAR T-cell therapies in the tumor microenvironment. It is possible that the combination of CAR T cells and PD-1 blockade may yield the greatest benefit for CAR T-cell therapy of solid tumors, as CAR T cells generally target only one (or at most two) surface molecules, whereas checkpoint blockade has the potential to unleash the endogenous T-cell response, which is better equipped to sense the entire range of neo-antigens resulting from tumor-specific mutations (see Türeci and colleagues in this CCR Focus (49)). Antibodies blocking the PD-1/PD-L1 pathway have recently been approved by the FDA for use in certain solid tumors, and combination of one of these antibodies with CAR T-cell therapy may enhance efficacy of CAR T cells in solid tumors.
An additional strategy for reducing the immunosuppressive effects of the solid tumor microenvironment involves using T cells redirected for universal cytokine-mediated killing (TRUCKs), which are CAR T cells engineered to secrete the proinflammatory cytokine IL-12, which can activate an innate immune response against the tumor (50). Additionally, IL-12 inhibits the action of regulatory T cells and myeloid-derived suppressor cells, which block antitumor T-cell responses (51, 52). However, high levels of IL-12 can be very toxic, as demonstrated in clinical trials studying the use of recombinant human IL-12 as a therapy (53). Therefore, if large amounts of IL-12 are produced endogenously, as in the case of an infection that triggers T cells through their endogenous T-cell receptors, IL-12–secreting CAR T cells may contribute to pathologic levels of IL-12 in the patient.
Solid tumors can also be difficult for immune cells to penetrate if the right combination of chemokines and their receptors is not present to facilitate T-cell migration into tissues, and this may be a primary reason for difficulty in using CAR T-cell therapy for solid tumors. In an alternative CAR engineering approach, CAR T cells may be directed to the tumor through co-expression of chemokine receptors such as CXCR2 or CCR4 (54, 55). In addition, a recent preclinical study showed that macrophages residing outside of the tumor microenvironment regulate infiltration of T cells into pancreatic tumors in mice (56); strategies to reduce the immune privilege provided by intratumoral and extratumoral macrophages may enhance the success of CAR T-cell therapy in some solid tumor types.
Targets and Solid Tumor Types Currently Investigated for Therapy With CAR T Cells
The single greatest challenge in targeting solid tumors is the identification of suitable target antigens. Many antigens identified in solid tumors are also expressed at low levels on healthy tissues, and the negative effects of long-term CAR T-cell–mediated attack of these tissues may outweigh the antitumor benefits provided by the therapy. While these on-target, off-tumor effects are also a problem when treating B-cell malignancies, the risks of B-cell aplasia are less severe than T-cell–mediated attack of vital organs. Long-term strategies for accurately and effectively targeting solid tumors may require further engineering of the CAR or combinatorial antigen recognition approaches (40). Alternatively, the identification of antigens that arise from mutations present in the tumor but not in healthy tissues may enable the development of CARs with greater specificity for solid tumors. Table 1 shows a list of many investigational targets for CAR T cells, and details of selected targets are described below.
EGFRvIII
Signaling through EGFR promotes cell proliferation, motility, and adhesion (57). A common deletion in EGFR creates EGFRvIII, a constitutively active variant (57). EGFRvIII is not expressed by normal tissues, but is expressed in some cases of glioblastoma (58). CAR T cells targeting EGFRvIII are currently investigated in clinical trials at the University of Pennsylvania (Penn) and the National Cancer Institute (NCI). The trial at Penn is examining the use of EGFRvIII-specific CAR T cells in patients with residual or recurrent glioma (NCT02209376). This study estimates an enrollment of 12 patients, with a primary completion date in 2016. Preliminary results indicate that CAR T-cell manufacturing is feasible in this patient population, and that infusions are well-tolerated in most patients. The phase 1/2 trial underway at the NCI is studying EGFRvIII-targeted CAR T cells for patients with refractory malignant glioma or glioblastoma (NCT01454596). The NCI study estimates an enrollment of up to 107 patients over a 7-year period, with a primary completion date in 2018. It is too early to determine clinical benefit, an endpoint that is particularly challenging to assess in patients with diseases like glioblastoma, for whom imaging-based responses are difficult to interpret and routine tumor biopsies could entail significant risk.
ERBB2
ERBB2 is part of the EGFR family and is overexpressed in several types of cancer. Three phase 1 dose-escalation trials using ERBB2-specifc CAR T cells are ongoing at Baylor College of Medicine (NCT00902044, NCT01109095, NCT00889954). These trials are investigating ERBB2-specific CAR T cells in patients with ERBB2-positive malignancies including glioblastoma multiforme and sarcoma (59). In patients with sarcoma, ERBB2-specific CAR T cells persisted for at least 6 weeks in 7 of the 9 patients treated with the highest doses of CAR T cells (42). Additionally, no dose-limiting toxicity was observed in any of the patients (42), suggesting that ERBB2-specific CAR T cells may be a good therapeutic approach in patients with metastatic or recurrent sarcoma. However, the levels of engraftment and penetration into tumor were low, and clinical benefit is not obvious.
Mesothelin
Mesothelin is a surface glycoprotein of unknown function that is overexpressed by mesotheliomas, pancreatic cancers, ovarian cancers, and lung cancers (60), and CAR T cells targeting mesothelin have also been studied in several clinical trials. Because mesothelin is expressed in several tissues, targeted therapies such as CAR T-cell therapy have the potential to cause off-target effects and should be used with caution. In a phase 1 clinical trial conducted at Penn, patients with mesothelin-expressing tumors who had progressed after first-line therapy were treated with T cells transiently transfected with mRNA for a mesothelin-targeted CAR (61). The mesothelin-specific CAR used in this study was a second-generation CAR containing a CD3ζ domain and a 4-1BB costimulatory domain. These short-lived mesothelin-specific CAR T cells displayed modest antitumor activity in two patients, demonstrating feasibility of transient mRNA transfection and the utility of mesothelin as an antigen for CAR T-cell recognition (61).
An additional phase 1 trial ongoing at Penn involves using a lentiviral-transduced mesothelin-specific CAR (NCT02159716). This clinical trial, which began in June 2014, is currently enrolling patients with chemotherapy-refractory malignant pancreatic adenocarcinoma, epithelial ovarian cancer, and malignant epithelial pleural mesothelioma. Early results from 6 patients in this trial have shown 4 patients with stable disease at day 28 following CAR T-cell infusion (43). There were no acute adverse events associated with CAR T-cell infusion, and the lentiviral-transduced mesothelin-targeted CAR T cells showed improved persistence compared with the mesothelin-targeted CARs expressed through mRNA transfection (43).
A recent preclinical study at Memorial Sloan-Kettering Cancer Center compared intrapleurally administered and systemically administered mesothelin-specific CAR T cells in a model of pleural malignancy (62). The intrapleurally administered CAR T cells were found to have superior antitumor activity, persistence, and intratumoral accumulation compared with the systemically administered CAR T cells (14, 62). A phase 1 clinical trial at Memorial Sloan-Kettering Cancer Center will soon begin testing the safety of intrapleural administration of mesothelin-specific CAR T cells in patients with pleural malignancies (NCT02414269) (14, 62). In addition, the NCI is conducting a clinical trial using retrovirally transduced mesothelin-specific CAR T cells in patients with metastatic pancreatic cancer, mesothelioma, and ovarian cancer (NCT01583686).
PSCA and PSMA
Prostate stem cell antigen (PSCA) and prostate-specific membrane antigen (PSMA) have been investigated as CAR T-cell targets in several preclinical studies. PSCA is a cell surface protein that is overexpressed on several solid tumor types, including prostate, pancreatic, and kidney cancers (63). PSCA-specific CAR T cells showed efficacy in a pancreatic cancer xenograft model and were reactive against prostate tumor cells in vitro and in vivo (64, 65). Therefore, PSCA represents an antigen that may be used to target multiple tumor types; however, targeting PSCA may result in off-tumor, on-target toxicity in organs unaffiliated with the tumor that express low levels of PSCA, such as the placenta and kidney (66, 67). CAR T cells recognizing PSMA have also demonstrated efficacy in vitro and in vivo (68, 69). Additionally, both PSCA and PSMA were targeted in a preclinical study that showed that CAR T cells can be engineered to recognize only tumors expressing both antigens, thereby increasing CAR T-cell specificity and reducing off-tumor effects (40). A phase 1 clinical trial (NCT01140373) using PSMA-specific CAR T cells in patients with metastatic prostate cancer resulted in stable disease in 2 of 4 patients treated (44).
Concluding Remarks
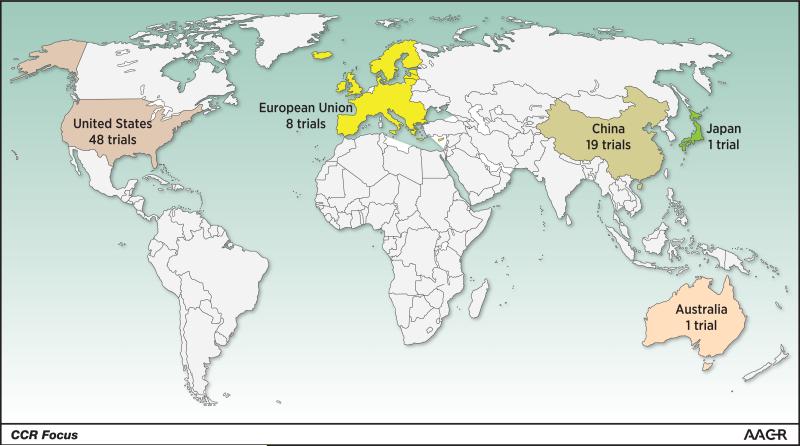
Immune-based therapy for cancer is undergoing rapid growth both in academic research laboratories and in industry-sponsored clinical trials. CAR T-cell therapy is now global, although most open trials are located in the US and China (Figure 4). This promises to be an exciting advance in the fields of cancer immunotherapy and cellular therapy. The impressive response rates observed in clinical trials of CD19 CAR T cells have led to the rapid proliferation of preclinical studies testing new targets and methods to make CAR T-cell therapy safer and more broadly applicable to various tumor types. Strategies to genetically modify T cells to improve their targeting, enhance their tissue penetration, and control their expansion and persistence are all ways to make better CAR T cells.
Figure 4.
Heat map indicating geographic location of ongoing or completed trials testing CAR T cells. From search term “chimeric antigen receptor;” source: http://clinicaltrials.gov, accessed January 8, 2016. Of these 92 trials, 80 have been sponsored by academic institutions, while 12 trials are either sponsored by industry or have an industry partner listed as a collaborator on http://clinicaltrials.gov.
Acknowledgments
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals. We thank Judith Murphy, PhD, for providing editorial assistance with this manuscript.
Grant Support: M.V. Maus is supported by the NCI of the NIH under award number K08166039.
Footnotes
Disclosure of Potential Conflicts of Interest: M.V. Maus and C.H. June report receiving a commercial research grant from Novartis Pharmaceuticals and have ownership interest in patents on CAR T cells, which are owned by the University of Pennsylvania and licensed to Novartis Pharmaceuticals. No other potential conflicts of interest were disclosed.
References
- 1.Qian X, Wang X, Jin H. Cell transfer therapy for cancer: past, present, and future. J Immunol Res. 2014;2014:525913. doi: 10.1155/2014/525913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014;370(10):901–10. doi: 10.1056/NEJMoa1300662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finney HM, Lawson AD, Bebbington CR, Weir AN. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol. 1998;161(6):2791–7. [PubMed] [Google Scholar]
- 5.Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, et al. Adoptive immunotherapy for indolent non-hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112(6):2261–71. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12(20 Pt 1):6106–15. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18(4):676–84. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 8.Finney HM, Akbar AN, Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol. 2004;172(1):104–13. doi: 10.4049/jimmunol.172.1.104. [DOI] [PubMed] [Google Scholar]
- 9.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121(5):1822–6. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17(8):1453–64. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Snyder KM, Suhoski MM, Maus MV, Kapoor V, June CH, et al. 4-1BB is superior to CD28 costimulation for generating CD8+ cytotoxic lymphocytes for adoptive immunotherapy. J Immunol. 2007;179(7):4910–8. doi: 10.4049/jimmunol.179.7.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheuermann RH, Racila E. CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy. Leuk Lymphoma. 1995;18(5-6):385–97. doi: 10.3109/10428199509059636. [DOI] [PubMed] [Google Scholar]
- 13.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadelain M. CARs: from assembly to distribution.. Symposia Presentation; Proceedings of the 106th Annual Meeting of the American Association for Cancer Research; Philadelphia, PA. 2015 Apr 18-22; Philadelphia (PA): AACR; 2015. [Google Scholar]
- 15.Jensen MC. Advancing CAR T cell immunotherapy in pediatric oncology.. Scientific Session Presentation; Proceedings of the 106th Annual Meeting of the American Association for Cancer Research; Philadelphia, PA. 2015 Apr 18-22; Philadelphia (PA): AACR; 2015. [Google Scholar]
- 16.Curran K, Riviere I, Kobos R, Kernan N, Boulad F, Prockop S, et al. Chimeric antigen receptor (CAR) T cells targeting the CD19 antigen for the treatment of pediatric relapsed B cell ALL [abstract].. Proceedings of the 56th ASH Annual Meeting and Exposition; San Francisco, CA. 2014 Dec 6-9; Washington (DC): American Society of Hematology; 2014. Abstract nr 3716. [Google Scholar]
- 17.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuster SJ, Svoboda J, Nasta S, Porter DL, Mato A, Shah GD, et al. Phase IIa trial of chimeric antigen receptor modified T cells directed against CD19 (CTL019) in patients with relapsed or refractory CD19+ lymphomas. J Clin Oncol. 2015;33(suppl) abstr 8516. [Google Scholar]
- 19.Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaidos A, Barnes CP, Cowan G, May PC, Melo V, Hatjiharissi E, et al. Clinical drug resistance linked to interconvertible phenotypic and functional states of tumor-propagating cells in multiple myeloma. Blood. 2013;121(2):318–28. doi: 10.1182/blood-2012-06-436220. [DOI] [PubMed] [Google Scholar]
- 21.Garfall AL, Maus MV, Hwang WT, Lacey SF, Mahnke YD, Melenhorst JJ, et al. Chimeric antigen receptor T cells against CD19 for multiple myeloma. N Engl J Med. 2015;373(11):1040–7. doi: 10.1056/NEJMoa1504542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao X, Ahmadzadeh M, Lu YC, Liewehr DJ, Dudley ME, Liu F, et al. Levels of peripheral CD4(+)FoxP3(+) regulatory T cells are negatively associated with clinical response to adoptive immunotherapy of human cancer. Blood. 2012;119(24):5688–96. doi: 10.1182/blood-2011-10-386482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rapoport AP, Stadtmauer EA, Aqui N, Badros A, Cotte J, Chrisley L, et al. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nat Med. 2005;11(11):1230–7. doi: 10.1038/nm1310. [DOI] [PubMed] [Google Scholar]
- 24.Rapoport AP, Stadtmauer EA, Aqui N, Vogl D, Chew A, Fang HB, et al. Rapid immune recovery and graft-versus-host disease-like engraftment syndrome following adoptive transfer of costimulated autologous T cells. Clin Cancer Res. 2009;15(13):4499–507. doi: 10.1158/1078-0432.CCR-09-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui Y, Zhang H, Meadors J, Poon R, Guimond M, Mackall CL. Harnessing the physiology of lymphopenia to support adoptive immunotherapy in lymphoreplete hosts. Blood. 2009;114(18):3831–40. doi: 10.1182/blood-2009-03-212134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21:581–90. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen CJ, Yang YX, Han EQ, Cao N, Wang YF, Wang Y, et al. Chimeric antigen receptor containing ICOS signaling domain mediates specific and efficient antitumor effect of T cells against EGFRvIII expressing glioma. J Hematol Oncol. 2013;6:33, 8722–6-33. doi: 10.1186/1756-8722-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Wong CW, Urak R, Mardiros A, Budde LE, Chang WC, et al. CMVpp65 vaccine enhances the antitumor efficacy of adoptively transferred CD19-redirected CMV-specific T cells. Clin Cancer Res. 2015;21(13):2993–3002. doi: 10.1158/1078-0432.CCR-14-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakazawa Y, Huye LE, Salsman VS, Leen AM, Ahmed N, Rollins L, et al. PiggyBac-mediated cancer immunotherapy using EBV-specific cytotoxic T-cells expressing HER2-specific chimeric antigen receptor. Mol Ther. 2011;19(12):2133–43. doi: 10.1038/mt.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun J, Huye LE, Lapteva N, Mamonkin M, Hiregange M, Ballard B, et al. Early transduction produces highly functional chimeric antigen receptor-modified virus-specific T-cells with central memory markers: a production assistant for cell therapy (PACT) translational application. J Immunother Cancer. 2015;3:5. doi: 10.1186/s40425-015-0049-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabinovich PM, Komarovskaya ME, Wrzesinski SH, Alderman JL, Budak-Alpdogan T, Karpikov A, et al. Chimeric receptor mRNA transfection as a tool to generate antineoplastic lymphocytes. Hum Gene Ther. 2009;20(1):51–61. doi: 10.1089/hum.2008.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maus MV, Haas AR, Beatty GL, Albelda SM, Levine BL, Liu X, et al. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res. 2013;1(1):26–31. doi: 10.1158/2326-6066.CIR-13-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24(6):1160–70. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. 2014;257(1):107–26. doi: 10.1111/imr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365(18):1673–83. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Introna M, Barbui AM, Bambacioni F, Casati C, Gaipa G, Borleri G, et al. Genetic modification of human T cells with CD20: a strategy to purify and lyse transduced cells with anti-CD20 antibodies. Hum Gene Ther. 2000;11(4):611–20. doi: 10.1089/10430340050015798. [DOI] [PubMed] [Google Scholar]
- 37.Wu CY, Roybal KT, Puchner EM, Onuffer J, Lim WA. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science. 2015;350:aab4077. doi: 10.1126/science.aab4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson LA, Scholler J, Ohkuri T, Kosaka A, Patel PR, McGettigan SE, et al. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med. 2015;7(275):275ra22. doi: 10.1126/scitranslmed.aaa4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hegde M, Corder A, Chow KK, Mukherjee M, Ashoori A, Kew Y, et al. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol Ther. 2013;21(11):2087–101. doi: 10.1038/mt.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol. 2013;31(1):71–5. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5(215):215ra172. doi: 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed N, Brawley VS, Hegde M, Robertson C, Ghazi A, Gerken C, et al. Human epidermal growth factor receptor 2 (HER2) -specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol. 2015;33(15):1688–96. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanyi JL, Haas AR, Beatty GL, Morgan MA, Stashwick CJ, O'Hara MH, et al. Safety and feasibility of chimeric antigen receptor modified T cells directed against mesothelin (CART-meso) in patients with mesothelin expressing cancers [abstract].. Cancer Res; Proceedings of the 106th Annual Meeting of the American Association for Cancer Research; Philadelphia, PA. 2015 Apr 18-22; Philadelphia (PA): AACR; 2015. Abstract nr CT105. [Google Scholar]
- 44.Slovin SF, Wang X, Hullings M, Arauz G, Bartido S, Lewis JS, et al. Chimeric antigen receptor (CAR+) modified T cells targeting prostate-specific membrane antigen (PSMA) in patients (pts) with castrate metastatic prostate cancer (CMPC). J Clin Oncol. 31(suppl 6):2013. abstr 72. [Google Scholar]
- 45.Zarour HM. Reversing T-cell dysfunction and exhaustion in cancer. Clin Cancer Res. 2016;22:xxxx–xxxx. doi: 10.1158/1078-0432.CCR-15-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bollard CM, Rossig C, Calonge MJ, Huls MH, Wagner HJ, Massague J, et al. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood. 2002;99(9):3179–87. doi: 10.1182/blood.v99.9.3179. [DOI] [PubMed] [Google Scholar]
- 47.Zhang L, Yu Z, Muranski P, Palmer DC, Restifo NP, Rosenberg SA, et al. Inhibition of TGF-beta signaling in genetically engineered tumor antigen-reactive T cells significantly enhances tumor treatment efficacy. Gene Ther. 2013;20(5):575–80. doi: 10.1038/gt.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamid O, Carvajal RD. Anti-programmed death-1 and anti-programmed death-ligand 1 antibodies in cancer therapy. Expert Opin Biol Ther. 2013;13(6):847–61. doi: 10.1517/14712598.2013.770836. [DOI] [PubMed] [Google Scholar]
- 49.Tureci Ö , Vormehr M, Diken M, Kreiter S, Huber C, Sahin U. Targeting the heterogeneity of cancer with individualized neoepitope vaccines. Clin Cancer Res. 2016;22:xxxx–xxxx. doi: 10.1158/1078-0432.CCR-15-1509. [DOI] [PubMed] [Google Scholar]
- 50.Chmielewski M, Hombach AA, Abken H. Of CARs and TRUCKs: chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma. Immunol Rev. 2014;257(1):83–90. doi: 10.1111/imr.12125. [DOI] [PubMed] [Google Scholar]
- 51.King IL, Segal BM. Cutting edge: IL-12 induces CD4+CD25- T cell activation in the presence of T regulatory cells. J Immunol. 2005;175(2):641–5. doi: 10.4049/jimmunol.175.2.641. [DOI] [PubMed] [Google Scholar]
- 52.Steding CE, Wu ST, Zhang Y, Jeng MH, Elzey BD, Kao C. The role of interleukin-12 on modulating myeloid-derived suppressor cells, increasing overall survival and reducing metastasis. Immunology. 2011;133(2):221–38. doi: 10.1111/j.1365-2567.2011.03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood. 1997;90(7):2541–8. [PubMed] [Google Scholar]
- 54.Di Stasi A, De Angelis B, Rooney CM, Zhang L, Mahendravada A, Foster AE, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a hodgkin tumor model. Blood. 2009;113(25):6392–402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kershaw MH, Wang G, Westwood JA, Pachynski RK, Tiffany HL, Marincola FM, et al. Redirecting migration of T cells to chemokine secreted from tumors by genetic modification with CXCR2. Hum Gene Ther. 2002;13(16):1971–80. doi: 10.1089/10430340260355374. [DOI] [PubMed] [Google Scholar]
- 56.Beatty GL, Winograd R, Evans RA, Long KB, Luque SL, Lee JW, et al. Exclusion of T cells from pancreatic carcinomas in mice is regulated by Ly6C F4/80 extra-tumor macrophages. Gastroenterology. 2015;149:201–10. doi: 10.1053/j.gastro.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chu CT, Everiss KD, Wikstrand CJ, Batra SK, Kung HJ, Bigner DD. Receptor dimerization is not a factor in the signalling activity of a transforming variant epidermal growth factor receptor (EGFRvIII). Biochem J. 1997;324(Pt 3):855–61. doi: 10.1042/bj3240855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hinrichs CS, Rosenberg SA. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol Rev. 2014;257(1):56–71. doi: 10.1111/imr.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bielamowicz K, Khawja S, Ahmed N. Adoptive cell therapies for glioblastoma. Front Oncol. 2013;3:275. doi: 10.3389/fonc.2013.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kelly RJ, Sharon E, Pastan I, Hassan R. Mesothelin-targeted agents in clinical trials and in preclinical development. Mol Cancer Ther. 2012;11(3):517–25. doi: 10.1158/1535-7163.MCT-11-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res. 2014;2(2):112–20. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adusumilli PS, Cherkassky L, Villena-Vargas J, Colovos C, Servais E, Plotkin J, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med. 2014;6(261):261ra151. doi: 10.1126/scitranslmed.3010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zuo L, Zhang LF, Wu XP, Zhou ZX, Zou JG, He J, et al. Association of a common genetic variant in prostate stem cell antigen with cancer risk. Arch Med Sci. 2014;10(3):425–33. doi: 10.5114/aoms.2014.43736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abate-Daga D, Lagisetty KH, Tran E, Zheng Z, Gattinoni L, Yu Z, et al. A novel chimeric antigen receptor against prostate stem cell antigen mediates tumor destruction in a humanized mouse model of pancreatic cancer. Hum Gene Ther. 2014;25(12):1003–12. doi: 10.1089/hum.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hillerdal V, Ramachandran M, Leja J, Essand M. Systemic treatment with CAR-engineered T cells against PSCA delays subcutaneous tumor growth and prolongs survival of mice. BMC Cancer. 2014;14:30. doi: 10.1186/1471-2407-14-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gu Z, Thomas G, Yamashiro J, Shintaku IP, Dorey F, Raitano A, et al. Prostate stem cell antigen (PSCA) expression increases with high gleason score, advanced stage and bone metastasis in prostate cancer. Oncogene. 2000;19(10):1288–96. doi: 10.1038/sj.onc.1203426. [DOI] [PubMed] [Google Scholar]
- 67.Reiter RE, Gu Z, Watabe T, Thomas G, Szigeti K, Davis E, et al. Prostate stem cell antigen: a cell surface marker overexpressed in prostate cancer. Proc Natl Acad Sci U S A. 1998;95(4):1735–40. doi: 10.1073/pnas.95.4.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Santoro SP, Kim S, Motz GT, Alatzoglou D, Li C, Irving M, et al. T cells bearing a chimeric antigen receptor against prostate-specific membrane antigen mediate vascular disruption and result in tumor regression. Cancer Immunol Res. 2015;3(1):68–84. doi: 10.1158/2326-6066.CIR-14-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther. 2010;18(2):413–20. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morgan RA, Johnson LA, Davis JL, Zheng Z, Woolard KD, Reap EA, et al. Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Hum Gene Ther. 2012;23(10):1043–53. doi: 10.1089/hum.2012.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao Y, Wang QJ, Yang S, Kochenderfer JN, Zheng Z, Zhong X, et al. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J Immunol. 2009;183(9):5563–74. doi: 10.4049/jimmunol.0900447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–51. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res. 2014;2(2):112–20. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70(22):9053–61. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lamers CH, Sleijfer S, van Steenbergen S, van Elzakker P, van Krimpen B, Groot C, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther. 2013;21(4):904–12. doi: 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weijtens ME, Willemsen RA, Valerio D, Stam K, Bolhuis RL. Single chain ig/gamma gene-redirected human T lymphocytes produce cytokines, specifically lyse tumor cells, and recycle lytic capacity. J Immunol. 1996;157(2):836–43. [PubMed] [Google Scholar]
- 77.Lo A, Wang LC, Scholler J, Monslow J, Avery D, Newick K, et al. Tumor-promoting desmoplasia is disrupted by depleting FAP-expressing stromal cells. Cancer Res. 2015;75:2800–10. doi: 10.1158/0008-5472.CAN-14-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lo A, Wang L-CS, Scholler J, Monslow J, Avery D, Evans RA, et al. Depleting cells expressing fibroblast activation protein disrupts tumor-promoting desmoplasia [abstract].. Cancer Res; Proceedings of the 106th Annual Meeting of the American Association for Cancer Research; Philadelphia, PA. 2015 Apr 18-22; Philadelphia (PA): AACR; 2015. Abstract nr 3187. [Google Scholar]
- 79.Wang LC, Lo A, Scholler J, Sun J, Majumdar RS, Kapoor V, et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol Res. 2014;2(2):154–66. doi: 10.1158/2326-6066.CIR-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Burga RA, Thorn M, Point GR, Guha P, Nguyen CT, Licata LA, et al. Liver myeloid-derived suppressor cells expand in response to liver metastases in mice and inhibit the anti-tumor efficacy of anti-CEA CAR-T. Cancer Immunol Immunother. 2015;64:817–29. doi: 10.1007/s00262-015-1692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiang HR, Gilham DE, Mulryan K, Kirillova N, Hawkins RE, Stern PL. Combination of vaccination and chimeric receptor expressing T cells provides improved active therapy of tumors. J Immunol. 2006;177(7):4288–98. doi: 10.4049/jimmunol.177.7.4288. [DOI] [PubMed] [Google Scholar]
- 82.Haso W, Lee DW, Shah NN, Stetler-Stevenson M, Yuan CM, Pastan IH, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013;121(7):1165–74. doi: 10.1182/blood-2012-06-438002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fry TJ, Stetler-Stevenson M, Shah NN, Yuan CM, Yates B, Delbrook C, et al. Clinical activity and persistence of anti-CD22 chimeric antigen receptor in children and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL) [abstract].. Proceedings of the 57th ASH Annual Meeting and Exposition; Orlando, FL. 2015 Dec 5-8; Washington (DC): American Society of Hematology; 2015. Abstract nr 1324] [Google Scholar]
- 84.Carpenter RO, Evbuomwan MO, Pittaluga S, Rose JJ, Raffeld M, Yang S, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19(8):2048–60. doi: 10.1158/1078-0432.CCR-12-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ali SA, Shi V, Wang M, Stroncek D, Maric I, Brudno JN, et al. Remissions of multiple myeloma during a first-in-humans clinical trial of T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor [abstract].. Proceedings of the 57th ASH Annual Meeting and Exposition; Orlando, FL. 2015 Dec 5-8; Washington (DC): American Society of Hematology; 2015. Abstract nr LBA-1. [Google Scholar]
- 86.Gill S, Tasian SK, Ruella M, Shestova O, Li Y, Porter DL, et al. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood. 2014;123(15):2343–54. doi: 10.1182/blood-2013-09-529537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chu J, He S, Deng Y, Zhang J, Peng Y, Hughes T, et al. Genetic modification of T cells redirected toward CS1 enhances eradication of myeloma cells. Clin Cancer Res. 2014;20(15):3989–4000. doi: 10.1158/1078-0432.CCR-13-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jiang H, Zhang W, Shang P, Zhang H, Fu W, Ye F, et al. Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol Oncol. 2014;8(2):297–310. doi: 10.1016/j.molonc.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108(12):3890–7. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hudecek M, Schmitt TM, Baskar S, Lupo-Stanghellini MT, Nishida T, Yamamoto TN, et al. The B-cell tumor-associated antigen ROR1 can be targeted with T cells modified to express a ROR1-specific chimeric antigen receptor. Blood. 2010;116(22):4532–41. doi: 10.1182/blood-2010-05-283309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hudecek M, Lupo-Stanghellini MT, Kosasih PL, Sommermeyer D, Jensen MC, Rader C, et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin Cancer Res. 2013;19(12):3153–64. doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]