In the phase III randomized ALSYMPCA trial of radium-223 in patients with castration-resistant prostate cancer and bone metastases, improved survival with radium-223 is accompanied by significant quality of life benefits, measured by EQ-5D and FACT-P, including a higher percentage of patients with meaningful improvement in quality of life and a slower decline in quality of life over time.
Keywords: castration-resistant prostate cancer, health-related quality of life, patient-reported outcomes, radium-223 dichloride, α-emitter
Abstract
Background
Radium-223 dichloride (radium-223), a first-in-class α-emitting radiopharmaceutical, is recommended in both pre- and post-docetaxel settings in patients with castration-resistant prostate cancer (CRPC) and symptomatic bone metastases based on overall survival benefit demonstrated in the phase III ALSYMPCA study. ALSYMPCA included prospective measurements of health-related quality of life (QOL) using two validated instruments: the general EuroQoL 5D (EQ-5D) and the disease-specific Functional Assessment of Cancer Therapy-Prostate (FACT-P).
Patients and methods
Analyses were conducted to determine treatment effects of radium-223 plus standard of care (SOC) versus placebo plus SOC on QOL using FACT-P and EQ-5D. Outcomes assessed were percentage of patients experiencing improvement, percentage of patients experiencing worsening, and mean QOL scores during the study.
Results
Analyses were carried out on the intent-to-treat population of patients randomized to receive radium-223 (n = 614) or placebo (n = 307). The mean baseline EQ-5D utility and FACT-P total scores were similar between treatment groups. A significantly higher percentage of patients receiving radium-223 experienced meaningful improvement in EQ-5D utility score on treatment versus placebo {29.2% versus 18.5%, respectively; P = 0.004; odds ratio (OR) = 1.82 [95% confidence interval (CI) 1.21–2.74]}. Findings were similar for FACT-P total score [24.6% versus 16.1%, respectively; P = 0.020; OR = 1.70 (95% CI 1.08–2.65)]. A lower percentage of patients receiving radium-223 experienced meaningful worsening versus placebo measured by EQ-5D utility score and FACT-P total score. Prior docetaxel use and current bisphosphonate use did not affect these findings. Treatment was a significant predictor of EQ-5D utility score, with radium-223 associated with higher scores versus placebo (0.56 versus 0.50, respectively; P = 0.002). Findings were similar for FACT-P total score (99.08 versus 95.22, respectively; P = 0.004).
Conclusions
QOL data from ALSYMPCA demonstrated that improved survival with radium-223 is accompanied by significant QOL benefits, including a higher percentage of patients with meaningful QOL improvement and a slower decline in QOL over time in patients with CRPC.
introduction
Patients with castration-resistant prostate cancer (CRPC) and bone metastases often present with symptoms such as pain, fatigue, anorexia, and, rarely, spinal cord compression, contributing to rapid and significant deterioration in health-related quality of life (QOL) and mortality [1–4]. Radium-223 dichloride (radium-223), a first-in-class α-emitting radiopharmaceutical, has demonstrated overall survival (OS) benefit in patients with CRPC and symptomatic bone metastases and no visceral metastases. Clinical practice guidelines recommend radium-223 in both pre- and post-docetaxel settings [5, 6]. Radium-223 approval was based on results from ALSYMPCA, a phase III, randomized, double-blind, placebo-controlled trial. In ALSYMPCA, radium-223 versus placebo prolonged OS by 3.6 months [hazard ratio (HR) 0.70; 95% confidence interval (CI) 0.58–0.83] and prolonged time to first symptomatic skeletal event (SSE) by 5.8 months (HR 0.66; 95% CI 0.52–0.83) [7, 8]. Radium-223 was associated with positive effects on QOL and a favorable safety profile with a low myelosuppression incidence [9–12].
Clinical practice guidelines recognize radium-223 as having an OS benefit and preserving QOL [5, 6, 13, 14]. The ALSYMPCA design included prospective QOL measurements using two validated instruments: EuroQoL EQ-5D, designed for the general population, and Functional Assessment of Cancer Therapy-Prostate (FACT-P), a disease-specific questionnaire for patients with prostate cancer [15, 16].
The goal of this ALSYMPCA data analysis was to determine effects of radium-223 plus standard of care (SOC) versus placebo plus SOC on the percentage of patients experiencing improvement or worsening in QOL while on treatment and observed over time.
methods
ALSYMPCA was a multicenter trial conducted at 136 sites (NCT00699751). Detailed methodology was reported elsewhere [7]. The primary end point was OS; QOL was a secondary clinical benefit end point. Descriptions of study conduct, patients, and treatment regimens are in Supplementary Materials, available at Annals of Oncology online.
health-related QOL assessments
QOL was assessed using EQ-5D [17] and FACT-P [15] questionnaires, completed by patients at randomization, on treatment at weeks 16 and 24, and at study discontinuation. During the first 28 weeks of follow-up, EQ-5D was completed every 2 months, and FACT-P was administered once at week 44 (22 weeks after last study drug administration). During the remaining follow-up period, EQ-5D was completed every 4 months, 6 times in total (although because of death and loss to follow-up, no EQ-5D data were collected at the 148-week visit).
The EQ-5D comprises five ordinal categorical responses (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) used to determine a country-specific utility score, a higher score indicating better QOL [17]. This analysis used UK tariffs to calculate utility scores.
FACT-P (version 4), a validated 39-item questionnaire, has four subscales for physical, emotional, functional, and social well-being and one for prostate cancer symptoms (PCS); these constitute a total score, a higher score indicating better QOL. Pain-related questions from the PCS were used to estimate a pain-related score (PRS) (see Supplementary Materials, available at Annals of Oncology online).
statistical analyses
Although ALSYMPCA included a pre-planned descriptive comparison of QOL, all hypothesis-testing analyses reported here were post hoc. Analyses were carried out on the intent-to-treat population, but limited to patients with a baseline assessment and ≥1 post-baseline assessment (i.e. weeks 16 and/or 24 or both). Three analyses were conducted using the EQ-5D utility score (utility score) and FACT-P total score: a responder analysis investigating treatment effects on percentage of patients experiencing meaningful QOL improvement on treatment; a similar on-treatment analysis of percentage of patients experiencing meaningful QOL worsening; and an overall analysis (using data collected during treatment and follow-up) investigating treatment effects on mean QOL and mean change from baseline over time [18]. For all analyses, when treatment differences were significant (P < 0.05) for FACT-P total score, subscales were evaluated to determine which were associated with the differences. When a significant QOL difference between treatment groups was found, regression models with docetaxel status (yes/no) and interaction terms were used to determine whether the treatment effect was different between pre-docetaxel and post-docetaxel subgroups; similar models were used to determine whether treatment effect was different between current bisphosphonate use (yes/no) subgroups [19].
When defining ‘meaningful improvement’ (or ‘meaningful worsening’), the upper limit of the minimally important difference (MID) range was used [15, 20, 21, 22], minimizing the likelihood of falsely identifying a patient as having improvement (or worsening). The utility score MID was 0.1; MIDs for FACT-P total score and subscales are shown in supplementary Table S1, available at Annals of Oncology online. χ2 tests were used to test for an association between treatment and likelihood of experiencing meaningful improvement (i.e. responder) or worsening in QOL [19]. Except for PRS, patients experiencing a QOL increase ≥MID from baseline at week 16 and/or week 24 were considered responders. Conversely, those experiencing a decrease in QOL score ≥MID from baseline at these time points were considered to have experienced worsening QOL. For PRS, a responder was defined as having a score increase of ≥2 without initiating opioid treatment. A patient initiating opioid use after baseline was assumed to have worsening pain and therefore to be a non-responder from that time forward.
Mixed-effect analysis of covariance (ANCOVA) regression models assessed the average treatment effect on utility score and FACT-P total score throughout the study. Covariates or main effects used were QOL score at baseline and on treatment at week 16 or 24, total alkaline phosphatase (ALP) concentration (≥220 or <220 U/l), current bisphosphonate use (yes/no), and prior docetaxel use (yes/no). Time was a random effect. A linear regression model, with the same covariates and main effects as the mixed-effect model, was used to conduct ANCOVA to provide descriptive assessments of treatment effect on utility score and FACT-P total score and subscales at each assessment time point. In both models, the dependent variable was mean change in score from baseline. To obtain estimates of the mean QOL on study, regression models were also run using mean score as a dependent variable [23].
Missing data were assumed to be missing completely at random for all analyses; no imputation was done to estimate missing observations. To investigate potential selection bias due to missing data, pattern-mixture models were used to re-evaluate the mixed-effect ANCOVA analyses and test for evidence of differential response based on extent of missing data [24]. The regression model included a variable for extent of missing data (classifying patients as having complete versus incomplete data), and interaction terms with this variable and each respective covariate. Statistical tests were conducted to determine whether missing data status was a significant predictor of treatment effect on utility score and FACT-P total score. If so, this would provide evidence of bias in the mixed-effect ANCOVA analysis.
results
All analyses were carried out on the intent-to-treat population of 921 patients randomized to receive either radium-223 (n = 614) or placebo (n = 307). At baseline, most patients (>93%) completed EQ-5D and FACT-P questionnaires. Completion rates decreased with each subsequent assessment because of study discontinuation, loss to follow-up, or death, with slightly higher completion rates in the radium-223 group than in the placebo group at post-baseline visits (supplementary Table S2, available at Annals of Oncology online).
responder analysis
A significantly higher percentage of patients receiving radium-223 had improved utility scores on treatment versus placebo (both with SOC) (29.2% versus 18.5%, respectively; P = 0.004), corresponding to an odds ratio (OR) of 1.82 (95% CI 1.21–2.74). Similarly, a significantly higher percentage of patients receiving radium-223 had a meaningful improvement in FACT-P total score on treatment [24.6% versus 16.1%, respectively; P = 0.020; OR = 1.70 (95% CI 1.08–2.65)] (Figure 1). Subgroup analyses (OR and 95% CI) for all patients and by ALSYMPCA trial stratification factors (baseline ALP, current bisphosphonate use, and prior docetaxel use) for FACT-P total score, PRS, and utility score are shown in supplementary Figure S1, available at Annals of Oncology online.
Figure 1.
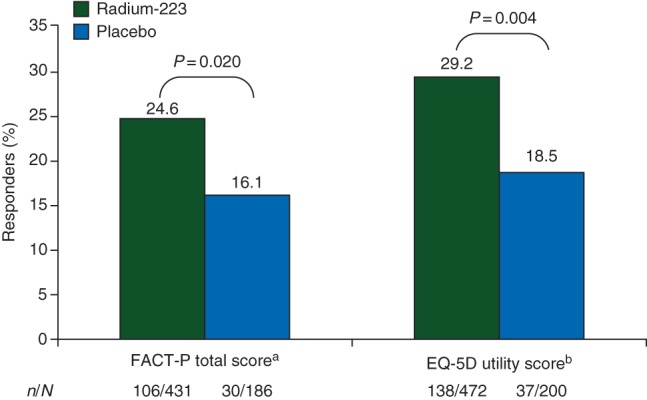
Percentage of patients experiencing a meaningful improvement in EQ-5D utility score or FACT-P total score while on treatment (week 16 and/or week 24). EQ-5D, EuroQoL 5D; FACT-P, Functional Assessment of Cancer Therapy-Prostate. aA responder was defined as a patient having an increase in FACT-P total score of ≥10 from baseline at either week 16 and/or week 24. bA responder was defined as a patient having an increase in EQ-5D utility score of ≥0.1 from baseline at either week 16 and/or week 24.
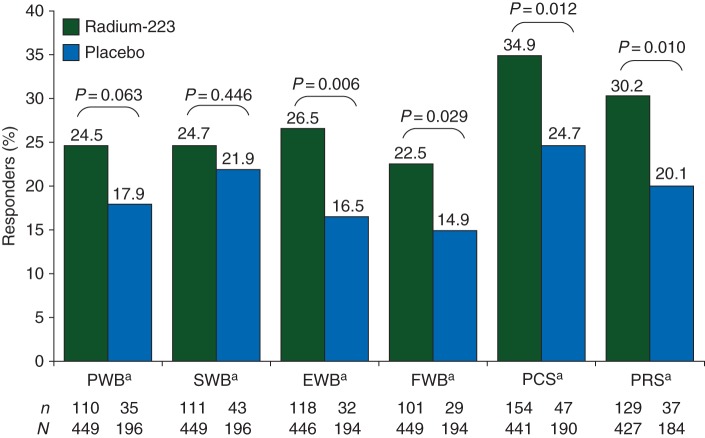
Analysis of FACT-P subscales indicated that responder rates for FACT-P total score were driven by improvements in three of five subscales (emotional and functional well-being, and PCS), and one approached significance (physical well-being) (Figure 2). In a more detailed analysis of pain (using PRS), both groups had a relatively low meaningful improvement in pain (radium-223: 30.2%; placebo: 20.1%; P = 0.010) (Figure 2). Table 1 shows per-visit details of improvement rates underlying this analysis.
Figure 2.
Percentage of patients experiencing a meaningful improvement in Functional Assessment of Cancer Therapy-Prostate (FACT-P) subscale scores while on treatment (week 16 and/or week 24). EWB, emotional well-being; FWB, functional well-being; PCS, prostate cancer subscale; PRS, pain-related score; PWB, physical well-being; SWB, social/family well-being. aA responder was defined as a patient having an increase in score of ≥3 points from baseline at either week 16 and/or week 24. bFor the pain-related score of the PCS, a responder was defined as a patient having an increase in score of ≥2 from baseline at either week 16 and/or week 24. A patient who initiated opioid use after baseline was assumed to be a non-responder from that time point forward.
Table 1.
Percentage of patients with a meaningful improvement or meaningful worsening in health-related quality of life as measured by EQ-5D utility score and FACT-P total score
| Meaningful change | EQ-5D utility score |
FACT-P total score |
||
|---|---|---|---|---|
| Radium-223, n/N (%) | Placebo, n/N (%) | Radium-223, n/N (%) | Placebo, n/N (%) | |
| Improvementa | ||||
| Week 16 | 114/460 (24.8) | 28/194 (14.4) | 85/407 (20.9) | 24/177 (13.6) |
| Week 24 | 75/343 (21.9) | 20/131 (15.3) | 57/314 (18.2) | 10/120 (8.3) |
| Worseningb | ||||
| Week 16 | 109/460 (23.7) | 76/194 (39.2) | 129/407 (31.7) | 68/177 (38.4) |
| Week 24 | 104/343 (30.3) | 59/131 (45.0) | 120/314 (38.2) | 58/120 (48.3) |
| Stable | ||||
| Week 16 | 237/460 (51.5) | 90/194 (46.4) | 193/407 (47.4) | 85/177 (48.0) |
| Week 24 | 164/343 (47.8) | 52/131 (39.7) | 137/314 (43.6) | 52/120 (43.3) |
aMeaningful improvement was defined as a ≥0.1 increase in EQ-5D utility score or ≥10 increase in FACT-P total score at either week 16 and/or week 24. Patients could have improvement or worsening at different visits.
bMeaningful worsening was defined as a ≥0.1 decrease in EQ-5D utility score or ≥10 decrease in FACT-P total score at either week 16 and/or week 24. Patients could have improvement or worsening at different visits.
EQ-5D, EuroQoL 5D; FACT-P, Functional Assessment of Cancer Therapy-Prostate.
Logistic regression analysis investigating consistency of treatment effect across docetaxel subgroups (pre-docetaxel versus post-docetaxel) indicated no difference in treatment effect on improvement in utility score (P = 0.178) or FACT-P total score (P = 0.637). Similar analysis across current bisphosphonate use (yes/no) subgroups found no difference in treatment effect on improvement in utility score (P = 0.977) or FACT-P total score (P = 0.800).
percentage worsening analysis
A lower percentage of patients receiving radium-223 experienced a meaningful worsening in utility score versus placebo (both with SOC) [36.0% versus 54.0%, respectively; P < 0.001; OR = 0.48 (95% CI 0.34–0.67)]. For FACT-P total score, the percentage of patients experiencing meaningful worsening was nominally different [44.3% versus 51.6%, respectively; P = 0.095; OR = 0.75 (95% CI 0.53–1.05)]. Because no significant difference was seen in FACT-P total score, comparisons of treatment effect for subscales and PRS were not investigated. Table 1 shows per-visit details of worsening rates underlying this analysis.
Logistic regression analysis investigating treatment effect across docetaxel subgroups (pre-docetaxel versus post-docetaxel) indicated no difference in treatment effect on worsening based on utility score (P = 0.248) or FACT-P total score (P = 0.698). Similar analysis across bisphosphonate use (yes/no) subgroups found no difference in treatment effect on worsening of utility score (P = 0.069) or FACT-P total score (P = 0.746).
treatment effects on mean QOL score and change from baseline
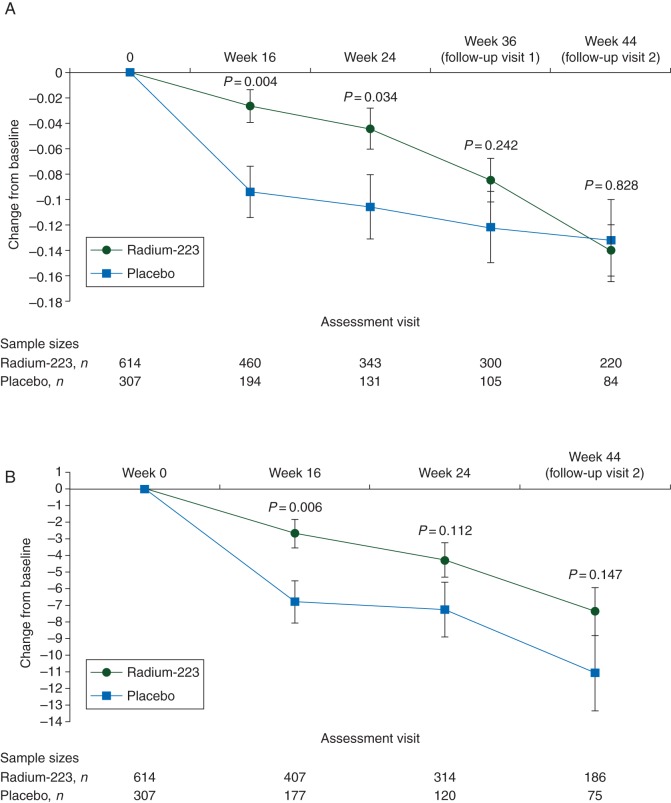
The mean baseline utility and FACT-P total scores were similar between treatment groups. Using all EQ-5D data collected throughout the study period (on-treatment and follow-up), treatment was a significant predictor of utility score, with radium-223 associated with higher scores versus placebo (0.56 versus 0.50; P = 0.002). The mean change from baseline in utility score was −0.10 with radium-223 and −0.16 with placebo (P = 0.002). For FACT-P total score, radium-223 was associated with a higher score (99.08 versus 95.22; P = 0.004) and a significantly smaller decrease from baseline versus placebo (−4.83 versus −8.69; P = 0.004). The mean changes in utility and FACT-P total scores from baseline over time (Figure 3; supplementary Figure S2, available at Annals of Oncology online shows changes in FACT-P subscales) indicate that higher QOL observed with radium-223 patients was a direct consequence of a slower rate of decline in QOL with radium-223 versus placebo. Differences in the mean score for radium-223 - placebo for all patients and by ALSYMPCA trial stratification factors (baseline ALP, current bisphosphonate use, and prior docetaxel use) for FACT-P total score, PRS, and utility score are shown in supplementary Figure S3, available at Annals of Oncology online.
Figure 3.
Mean change from baseline (least-squares mean ± standard error) over time in (A) EQ-5D utility score and (B) Functional Assessment of Cancer Therapy-Prostate total score. The analysis of covariance analysis was adjusted for baseline score, treatment, total alkaline phosphatase (≥ or <220 U/l), current use of bisphosphonates (yes/no), and prior use of docetaxel (yes/no).
The mean changes from baseline for each FACT-P subscale score were significantly different, favoring radium-223, for all except social well-being (Table 2). For PCS, containing items specific to prostate cancer, mean change from baseline in scores was significantly greater (indicating improved QOL) with radium-223 versus placebo (P = 0.009). Analysis of pain-related and non-pain-related PCS items indicated that treatment effects on pain were the primary reason for change in overall PCS score with radium-223 (Table 2).
Table 2.
Mean change from baseline in EQ-5D utility score, FACT-P total score, and pain-related and non-pain-related components of the FACT-P prostate cancer subscale (PCS) (ANCOVA analysis; entire trial period)
| Score | Mean score (radium-223; RA) | Change from baseline (radium-223; RA) | Mean score (placebo; PL) | Change from baseline (placebo; PL) | Difference (RA−PL) | P-value (RA–PL) |
|---|---|---|---|---|---|---|
| EQ-5Da | ||||||
| EQ-5D utility score | 0.56 | −0.10 | 0.50 | −0.16 | 0.06 | 0.002 |
| FACT-Pb | ||||||
| FACT-P total score | 99.08 | −4.83 | 95.22 | −8.69 | 3.86 | 0.004 |
| Physical well-being | 18.48 | −1.59 | 17.59 | −2.49 | 0.90 | 0.013 |
| Social/family well-being | 20.94 | −0.16 | 20.98 | −0.13 | −0.04 | 0.900 |
| Emotional well-being | 16.64 | −0.28 | 15.59 | −1.33 | 1.05 | <0.001 |
| Functional well-being | 15.39 | −1.81 | 14.53 | −2.67 | 0.86 | 0.017 |
| Prostate cancer score | 27.83 | −0.82 | 26.59 | −2.07 | 1.25 | 0.009 |
| Pain-related scorec | 8.38 | 0.29 | 7.63 | −0.46 | 0.75 | 0.006 |
| Non-pain-related component | 19.38 | −1.11 | 18.98 | −1.51 | 0.40 | 0.212 |
aEQ-5D utility scores were available for 220 radium-223 patients and 84 placebo patients at follow-up visit 2 (week 44).
bFACT-P total scores were available for 186 radium-223 patients and 75 placebo patients at follow-up visit 2 (week 44).
cHigher values for pain-related score indicated reduced pain.
Patient numbers at each visit are shown in supplementary Tables S1 and S2, available at Annals of Oncology online.
ANCOVA, analysis of covariance; EQ-5D, EuroQoL 5D; FACT-P, Functional Assessment of Cancer Therapy-Prostate.
Mixed-effect regression analysis investigating treatment effect within pre-docetaxel and post-docetaxel subgroups indicated no difference in treatment effect on mean change from baseline in utility (P = 0.714) or FACT-P total score (P = 0.785). Comparison of subgroups based on current bisphosphonate use (yes/no) found no difference in treatment effect on FACT-P total score (P = 0.309), but there was a difference in treatment effect on utility score (P = 0.034), with patients receiving bisphosphonates experiencing greater treatment benefit (0.61 versus 0.50, P < 0.001) than patients not currently receiving bisphosphonates (0.53 versus 0.50, respectively; P = 0.284). Per-visit descriptive analyses of least-squares mean change from baseline in utility score and FACT-P total score are in supplementary Tables S3 and S4, respectively, available at Annals of Oncology online.
discussion
These analyses of ALSYMPCA QOL data consistently showed treatment benefit with radium-223 plus SOC versus placebo plus SOC, as measured by utility and FACT-P total scores. In patients receiving radium-223, a significantly higher percentage experienced meaningful improvement in QOL and a lower percentage experienced meaningful worsening in QOL versus placebo. Radium-223 treatment appeared to result in higher mean QOL scores on study versus placebo, a function of slower deterioration over time. This trend was consistent across four of five FACT-P subscales, indicating that treatment effects extend across multiple QOL domains. Analysis of pain-related and non-pain-related components of the PCS suggests that pain reduction primarily contributed to PCS treatment differences. This effect is consistent with other ALSYMPCA analyses showing that radium-223 significantly prolonged time to first opioid use [11], and that radium-223 was equally effective, regardless of opioid use at baseline [25]. These data are consistent with those of an earlier phase II study, showing pain response (reduced pain and stable analgesic consumption) in 71% of patients with CRPC receiving radium-223 [26].
Treatment effects in utility and FACT-P total scores appeared consistent across pre-docetaxel and post-docetaxel subgroups, and except for change from baseline in mean utility score, treatment benefit is consistent across current bisphosphonate use (yes/no) subgroups.
QOL deterioration over time for CRPC patients is well documented, and may be a prognostic factor for more rapid disease progression [1, 2]. Agents such as radium-223 that delay QOL deterioration or improve overall QOL and OS are valuable additions to treatment; there is evidence of a durable response to radium-223 over time, as measured by FACT-P total score and subscales. Recent analyses of QOL data from enzalutamide and abiraterone studies in CRPC patients documented the QOL decline in patients using FACT-P, and reported these agents' beneficial effects on QOL [27, 28]. However, several confounding factors make comparisons of QOL findings across these studies and ALSYMPCA difficult. Post hoc analysis of FACT-P data from the abiraterone trial, where 48% of patients were classified as responders, used the same meaningful improvement in FACT-P total score as the current radium-223 analysis, but the abiraterone analysis used only data from patients with a low FACT-P total score at baseline (i.e. those with the greatest chance for improvement). Furthermore, there were more FACT-P assessments (at cycles 1, 4, 7, 10, and every 6 cycles thereafter) than in ALSYMPCA because of longer treatment duration, giving patients more opportunities to be responders [28]. In addition, statistical analyses used across studies, and differing assumptions in each model, make direct comparisons of QOL findings challenging.
Limitations of the ALSYMPCA analyses reported here include the results being from post hoc analyses. However, collection of QOL data in ALSYMPCA was done prospectively, and measured using validated QOL instruments, one (FACT-P) specific for prostate cancer. If the assumption of data missing completely at random was not correct, some analyses may be biased. However, the pattern-mixture model analysis was done to detect evidence of informative missing, and no evidence was found. Decreases in EQ-5D and FACT-P completion rates over time were a potentially confounding factor, although a pattern-mixture model showed no evidence that missing data biased the analyses. Moreover, responder analysis used the upper limit of the clinically meaningful range for FACT-P subscales to minimize the likelihood of falsely identifying a patient as a responder. A limitation of the analysis of pain improvement is that once a patient initiated opioid therapy, significant increases or decreases in pain management could not be accurately assessed. Regarding multiple testing, a conservative approach using the Bonferroni correction (α = 0.008) when evaluating the three main analyses applied to utility and FACT-P total scores would change only the conclusion regarding FACT-P responder analysis, to non-significant (P = 0.020) after applying the correction. Even with these conservative approaches, significantly more patients receiving radium-223 experienced a meaningful increase in QOL.
The findings reported here and in the primary report of ALSYMPCA [7] suggest that in the clinical practice setting, radium-223 treatment was associated with both increased survival outcomes and clinically meaningful improvements in QOL. Furthermore, pain is often associated with SSEs (e.g. spinal cord compression) in CRPC patients, and radium-223 demonstrated an additional clinical benefit of delaying SSE occurrence (by 5.8 months). Analysis of utility scores in ALSYMPCA before/after SSE and before/after disease progression found that the radium-223 QOL benefit was greatest in stable disease and pre-SSE settings [29], suggesting that delay to first SSE is associated with better QOL. Overall, interest in monitoring QOL during treatment in patients with CRPC is increasing, and recommendations have been made to include patient-reported outcomes and QOL, using instruments such as FACT-P [30]. A recent report of the European Society for Medical Oncology (ESMO) validated tool to assess magnitude of clinical benefit scale (ESMO-MCBS), which assesses QOL and survival, rated radium-223 as having the highest level of clinical benefit [14]. These QOL instruments continue to be evaluated and refined in this population, especially in configuring questions addressing clinical relevance from the oncologist's perspective and symptoms of greatest concern to patients [31–34]. Examples of QOL instruments that may provide valuable insights in this CRPC setting are the EORTC QLQ-BM22 instrument for patients with bone metastases [35], and the FACT-Bone Pain [36].
In conclusion, analyses of EQ-5D and FACT-P data from the ALSYMPCA trial demonstrate that improved survival with radium-223 is accompanied by significant QOL benefits, including a higher percentage of patients with meaningful improvement in QOL and an overall slower decline in QOL over time.
funding
This work was supported by Bayer HealthCare, Whippany, NJ, USA. No grant numbers applied.
disclosure
The authors have the following conflicts of interest to disclose: SN: personal fees from Bayer Healthcare, outside the submitted work. PC: personal fees from Bayer HealthCare, outside the submitted work, as he is an employee of Bayer HealthCare. OS: grants and personal fees from Bayer AS (formerly Algeta ASA) and Bayer HealthCare, during the conduct of the study. NJV: personal fees from Bayer, Medivation, Janssen, Endocyte, Dava Oncology, Dendreon, Sanofi Aventis, and Pfizer, outside the submitted work. REC: grants from Bayer during the conduct of the study, and personal fees from Novartis, outside the submitted work. JMOS: grants and personal fees from Bayer, outside the submitted work. JR-S: personal fees from Bayer Pharma AG, during the conduct of the study. MS: personal fees from Bayer HealthCare, outside the submitted work, as he is an employee of Bayer HealthCare, and Bayer stock ownership. LZ: personal fees from Bayer HealthCare, outside the submitted work, as she is an employee of Bayer HealthCare. CP: grants and personal fees from Bayer and personal fees from BNIT, Janssen, Takeda, and Astellas, outside the submitted work.
Supplementary Material
acknowledgements
Medical writing and editorial assistance was provided by Yvonne E. Yarker, PhD, CMPP, and Joanna Sandilos-Rega, PhD, of SciStrategy Communications, funded by Bayer HealthCare.
references
- 1.Sullivan PW, Mulani PM, Fishman M, Sleep D. Quality of life findings from a multicenter, multinational, observational study of patients with metastatic hormone-refractory prostate cancer. Qual Life Res 2007; 16: 571–575. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan PW, Nelson JB, Mulani PM, Sleep D. Quality of life as a potential predictor for morbidity and mortality in patients with metastatic hormone-refractory prostate cancer. Qual Life Res 2006; 15: 1297–1306. [DOI] [PubMed] [Google Scholar]
- 3.Weinfurt KP, Li Y, Castel LD et al. . The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol 2005; 16: 579–584. [DOI] [PubMed] [Google Scholar]
- 4.Berruti A, Dogliotti L, Bitossi R et al. . Incidence of skeletal complications in patients with bone metastatic prostate cancer and hormone refractory disease: predictive role of bone resorption and formation markers evaluated at baseline. J Urol 2000; 164: 1248–1253. [PubMed] [Google Scholar]
- 5.NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer—v1.2015; http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 6.Fitzpatrick JM, Bellmunt J, Fizazi K et al. . Optimal management of metastatic castration-resistant prostate cancer: highlights from a European Expert Consensus Panel. Eur J Cancer 2014; 50: 1617–1627. [DOI] [PubMed] [Google Scholar]
- 7.Parker C, Nilsson S, Heinrich D et al. . Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369: 213–223. [DOI] [PubMed] [Google Scholar]
- 8.Sartor O, Coleman R, Nilsson S et al. . Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol 2014; 15: 738–746. [DOI] [PubMed] [Google Scholar]
- 9.Parker C, Heinrich D, Bottomley D et al. . Effects of radium-223 dichloride (radium-223) on health-related quality of life (QOL) outcomes in the phase 3 ALSYMPCA study in patients with castration-resistant prostate cancer (CRPC) and bone metastases. Presentation at: Eur J Cancer, 2013; 49 (suppl 2): Abstr 2878. [Google Scholar]
- 10.Hoskin P, Sartor O, O'Sullivan JM et al. . Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol 2014; 15: 1397–1406. [DOI] [PubMed] [Google Scholar]
- 11.Nilsson S, Tomblyn M, Cislo P et al. . Patient-reported quality of life analysis of radium-223 dichloride evaluating pain relief from the phase 3 ALSYMPCA study. J Clin Oncol 2014; 32: Abstr 5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nilsson S. Radium-223 dichloride for the treatment of bone metastatic castration-resistant prostate cancer: an evaluation of its safety. Expert Opin Drug Saf 2015; 14: 1127–1136. [DOI] [PubMed] [Google Scholar]
- 13.Basch E, Loblaw DA, Oliver TK et al. . Systemic therapy in men with metastatic castration-resistant prostate cancer: American Society of Clinical Oncology and Cancer Care Ontario clinical practice guideline. J Clin Oncol 2014; 32: 3436–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherny NI, Sullivan R, Dafni U et al. . A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann Oncol 2015; 26: 1547–1573. [DOI] [PubMed] [Google Scholar]
- 15.Cella D, Nichol MB, Eton D et al. . Estimating clinically meaningful changes for the Functional Assessment of Cancer Therapy-Prostate: results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health 2009; 12: 124–129. [DOI] [PubMed] [Google Scholar]
- 16.Esper P, Mo F, Chodak G et al. . Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology 1997; 50: 920–928. [DOI] [PubMed] [Google Scholar]
- 17.EuroQol—a new facility for the measurement of health-related quality of life. Health Policy 1990; 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 18.Sternberg CN, Molina A, North S et al. . Effect of abiraterone acetate on fatigue in patients with metastatic castration-resistant prostate cancer after docetaxel chemotherapy. Ann Oncol 2013; 24: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 19.Agresti A, Kateri M. Categorical Data Analysis. Berlin, Heidelberg, Germany: Springer; 2011. [Google Scholar]
- 20.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes 2007; 5: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cella D, Hahn EA, Dineen K. Meaningful change in cancer-specific quality of life scores: differences between improvement and worsening. Qual Life Res 2002; 11: 207–221. [DOI] [PubMed] [Google Scholar]
- 22.Yost KJ, Eton DT. Combining distribution- and anchor-based approaches to determine minimally important differences: the FACIT experience. Eval Health Prof 2005; 28: 172–191. [DOI] [PubMed] [Google Scholar]
- 23.Neter J, Kutner MH, Wasserman W, Nachtsheim CJ. Applied Linear Statistical Models. Chicago, IL: McGraw-Hill/Irwin; 1996: 958–1009; 1010–1044. [Google Scholar]
- 24.Hedeker D, Gibbons RD. Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychol Methods 1997; 2: 64–78. [Google Scholar]
- 25.Bottomley D, Vogelzang N, Coleman R et al. . Efficacy and safety of radium-223 dichloride by baseline opioid use in patients with castration-resistant prostate cancer and symptomatic bone metastases: results from ALSYMPCA. Presentation at: Global Congress on Prostate Cancer; 4–6 February; Rome, Italy 2015. [Google Scholar]
- 26.Nilsson S, Strang P, Aksnes AK et al. . A randomized, dose–response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur J Cancer 2012; 48: 678–686. [DOI] [PubMed] [Google Scholar]
- 27.Cella D, Ivanescu C, Holmstrom S et al. . Impact of enzalutamide on quality of life in men with metastatic castration-resistant prostate cancer after chemotherapy: additional analyses from the AFFIRM randomized clinical trial. Ann Oncol 2015; 26: 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harland S, Staffurth J, Molina A et al. . Effect of abiraterone acetate treatment on the quality of life of patients with metastatic castration-resistant prostate cancer after failure of docetaxel chemotherapy. Eur J Cancer 2013; 49: 3648–3657. [DOI] [PubMed] [Google Scholar]
- 29.Cislo P, Sartor O, Reuning-Scherer J et al. . Effects of radium-223 dichloride on health-related quality of life assessed by the EQ-5D utility scores in ALSYMPCA. Eur Urol 2015; 14: e673–e673a. Abstr 673. [Google Scholar]
- 30.Basch E, Abernethy AP, Mullins CD et al. . Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol 2012; 30: 4249–4255. [DOI] [PubMed] [Google Scholar]
- 31.Victorson DE, Beaumont JL, Rosenbloom SK et al. . Efficient assessment of the most important symptoms in advanced prostate cancer: the NCCN/FACT-P Symptom Index. Psychooncology 2011; 20: 977–983. [DOI] [PubMed] [Google Scholar]
- 32.Cella D, Rosenbloom SK, Beaumont JL et al. . Development and validation of 11 symptom indexes to evaluate response to chemotherapy for advanced cancer. J Natl Compr Canc Netw 2011; 9: 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones D, Zhao F, Fisch MJ et al. . The validity and utility of the MD Anderson Symptom Inventory in patients with prostate cancer: evidence from the Symptom Outcomes and Practice Patterns (SOAPP) data from the Eastern Cooperative Oncology Group. Clin Genitourin Cancer 2014; 12: 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quinten C, Martinelli F, Coens C et al. . A global analysis of multitrial data investigating quality of life and symptoms as prognostic factors for survival in different tumor sites. Cancer 2014; 120: 302–311. [DOI] [PubMed] [Google Scholar]
- 35.Chow E, Nguyen J, Zhang L et al. . International field testing of the reliability and validity of the EORTC QLQ-BM22 module to assess health-related quality of life in patients with bone metastases. Cancer 2012; 118: 1457–1465. [DOI] [PubMed] [Google Scholar]
- 36.FACT-BP quality of life measurement in patients with bone pain. www.facit.org/FACITOrg/Questionnaires 2004.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.