Abstract
Animals need a well-functioning immune system to protect themselves against pathogens. The immune system, however, is costly and resource trade-offs with other demands exist. For migratory animals several (not mutually exclusive) hypotheses exist. First, migrants reduce immune function to be able to allocate resources to migration. Second, migrants boost immune function to cope with more and/or novel pathogens encountered during migration. Third, migrants reallocate resources within the immune system. We tested these hypotheses by comparing baseline immune function in resident and migratory common blackbirds (Turdus merula), both caught during the autumn migration season on the island of Helgoland, Germany. Indices of baseline innate immune function (microbial killing capacity and haptoglobin-like activity) were lower in migrants than in residents. There was no difference between the groups in total immunoglobulins, a measure of baseline acquired immune function. Our study on a short-distance avian migrant supports the hypothesis that innate immune function is compromised during migration.
Keywords: immunity, migrant, resident, trade-off, eco-immunology
1. Introduction
An animal's health is continuously challenged by potentially harmful viruses, bacteria and other pathogens. The immune system is a major physiological component of self-maintenance that promotes survival by reducing the probability of disease-related mortality [1]. However, the immune system is costly in terms of its production, maintenance and activation [2,3]. For migratory animals, it has therefore been hypothesized that they need to reduce immune function during the physiologically demanding migration seasons [4,5]. A contrasting hypothesis proposes that migrants need to boost immune function because they encounter more and/or different pathogens during migration [6,7]. It is also possible that trade-offs are made within the immune system itself in that available resources are reallocated within the immune system [8]. Only few studies have tested these hypotheses using indices of baseline immune function (i.e. not those in response to an artificial immunological challenge). Some studies suggest that baseline immune function can be compromised during (avian) migration because several indices of immune function were lower during migration than during breeding [9–11]. Also, flying in a wind tunnel reduced migrants' baseline immune function [12]. Others, however, support the hypothesis that migrants boost immune function during migration. In captive red knots (Calidris canutus) baseline immune function peaked during migratory periods [13], and in partial migratory skylarks (Alauda arvensis), migratory individuals showed higher indices of immune function than resident individuals when measured during the breeding season [14].
The above studies, however, measured immune function in captivity, compared different seasons, or sampled migrants and residents outside the migration seasons. What is missing is a study that compares migrants and residents during migration at a single location. Such a study system is needed to ultimately test if migrants and residents differ in immune function, because it eliminates spatio-temporal variation in environmental factors, such as food availability or parasite exposure, which are known to affect immune function [15–17]. Here, we performed such a study by comparing multiple indices of baseline immune function between resident and migrating common blackbirds (Turdus merula, blackbird hereafter) on the island of Helgoland during the autumn migration season. During migration, birds from Scandinavian breeding populations use the island as a stopover site and mix with the local sedentary birds [18]. Consequently, during these periods resident and migrant blackbirds encounter similar environmental conditions. We quantified three parameters of baseline innate and acquired immune function and compared residents with migrants.
2. Material and methods
(a). Field procedures
Blackbirds were caught on Helgoland (54°11′ N, 07°55′ E) throughout October 2014. Immediately after capture birds were blood sampled from the wing vein. The plasma was separated within 4 h of capture and frozen at −20°C until assaying. Birds were sexed and aged (1st year or adult) on plumage (after [19]), ringed and fitted with a unique combination of four colour rings for later identification in the field. Fat stores were scored according to [20] on a scale from 0 to 8.
Migrants were separated from residents combining two approaches. First, blackbirds ringed (only with a metal ring) on Helgoland in the breeding seasons preceding our sampling were assumed to be residents. This assumption appears valid as all 12 individuals falling into this category were re-sighted by us on Helgoland after we colour-ringed them in October 2014. Second, 14 newly caught individuals were considered resident because they were re-sighted more than 9 days after initial trapping. We chose 9 days as a cut-off point, because 95% of 1307 re-traps of supposedly migrant blackbirds on Helgoland occurred within 9 days from first trapping (O. Hüppop 2015, unpublished data). Newly caught birds that were not re-sighted (n = 45) or re-sighted only within 9 days of colour-ringing (n = 6) were considered migrants. Searches for colour-ringed birds were made almost daily from the start of catching until four weeks after the last bird was colour-ringed. Helgoland is tiny (1 km2) and the 14 newly ringed residents were usually re-sighted rapidly and multiple times after colour-ringing (median number of days until first re-sighting was 3 (range: 0–21), and the median number of re-sightings during the study period was 4.5 (range: 1–18). Therefore, we are confident that we re-sighted practically all resident colour-ringed blackbirds. Furthermore, immigration and emigration rates in the Helgoland blackbird population are very low [21], increasing the likelihood of accurate assignment of status.
(b). Immunoassays
We measured two parameters of baseline innate immune function, an individual's first line of defence. We quantified the microbial killing capacity (against Escherichia coli) of plasma (hereafter BKA) following the method described by [22] with a few modifications. We used 3 µl of frozen plasma and mixed it in 4 µl of 105 E. coli solution. We measured bacteria growth at 600 nm using a microplate reader. We quantified haptoglobin-like activity (mg ml−1) in plasma samples using a commercially available colorimetric assay kit (TP801; Tri-Delta Diagnostics, Boonton, NJ, USA), which quantifies the haeme-binding capacity of plasma. We followed the ‘manual method’ instructions provided by the kit manufacturer with a few minor modifications following [23]. Furthermore, we measured one parameter of baseline acquired immune function. We quantified the total level of antibodies in plasma (total immunoglobulins) by means of an enzyme-linked immunosorbent assay (ELISA) following [24].
(c). Data analysis
For each immune parameter, we ran general linear models, using SPSS v. 23.0 (IBM, New York), containing the following variables: status (resident or migratory), date, sex, age and fat score. For haptoglobin-like activity, a ‘405 nm pre-scan value’ was added as a covariate to correct for plasma redness (after [23], who corrected at 450 nm). Model selection was done using stepwise backward elimination of non-significant terms (p > 0.05) in order of least significance. To normalize residuals, BKA was log transformed prior to analyses. Two small negative values of BKA were set to 1 to allow log transformation. Limited plasma volumes resulted in a reduced sample size for the immunoglobulin assay.
3. Results and discussion
Migrants had lower microbial killing capacity against E. coli (BKA) and haptoglobin-like activity than residents (table 1, figure 1). The two groups did not differ in total immunoglobulins (table 1, figure 1). Adult birds had higher total immunoglobulins than 1st year birds (table 1), which may reflect that it needs initial exposures (and hence time) to build up acquired immune function. No other age effects were observed. Date, sex and fat stores did not explain a significant amount of the variation in any of the immune indices (table 1). Within migrants, total immunoglobulins were not correlated with BKA (Pearson's r = 0.01, p = 0.97, n = 37), or residual (corrected for plasma redness) haptoglobin-like activity (Pearson's r = 0.13, p = 0.43, n = 37).
Table 1.
Model summaries examining three parameters of immune function of common blackbirds in relation to status (resident or migratory), date, sex, age and fat score. Variable statistics are given as in the step prior to removal from the model. The final model is in italic. All d.f. = 1. Reference categories were resident for status, male for sex and first year for age.
| model | variable | B ± s.e. | T | p-value |
|---|---|---|---|---|
| BKA (n = 77) | status | −0.274 ± 0.102 | −2.68 | 0.009 |
| date | 0.012 ± 0.008 | 1.61 | 0.11 | |
| sex | −0.146 ± 0.098 | −1.48 | 0.14 | |
| age | 0.086 ± 0.104 | 0.83 | 0.41 | |
| fat score | −0.034 ± 0.047 | −0.74 | 0.47 | |
| haptoglobin-like activity (n = 77) | status | −0.052 ± 0.010 | −5.03 | <0.001 |
| date | 0.001 ± 0.001 | 1.57 | 0.12 | |
| sex | −0.011 ± 0.010 | −1.07 | 0.29 | |
| age | 0.014 ± 0.010 | 1.35 | 0.18 | |
| fat score | −0.004 ± 0.005 | −0.85 | 0.40 | |
| 405 nm | 0.617 ± 0.023 | 26.36 | <0.001 | |
| immunoglobulins (n = 60) | status | −1.772 ± 1.311 | −1.39 | 0.17 |
| date | −0.124 ± 0.097 | −1.28 | 0.21 | |
| sex | −0.099 ± 1.30 | −0.08 | 0.94 | |
| age | 3.161 ± 1.296 | 2.44 | 0.018 | |
| fat score | 0.114 ± 0.629 | 0.18 | 0.86 |
Figure 1.
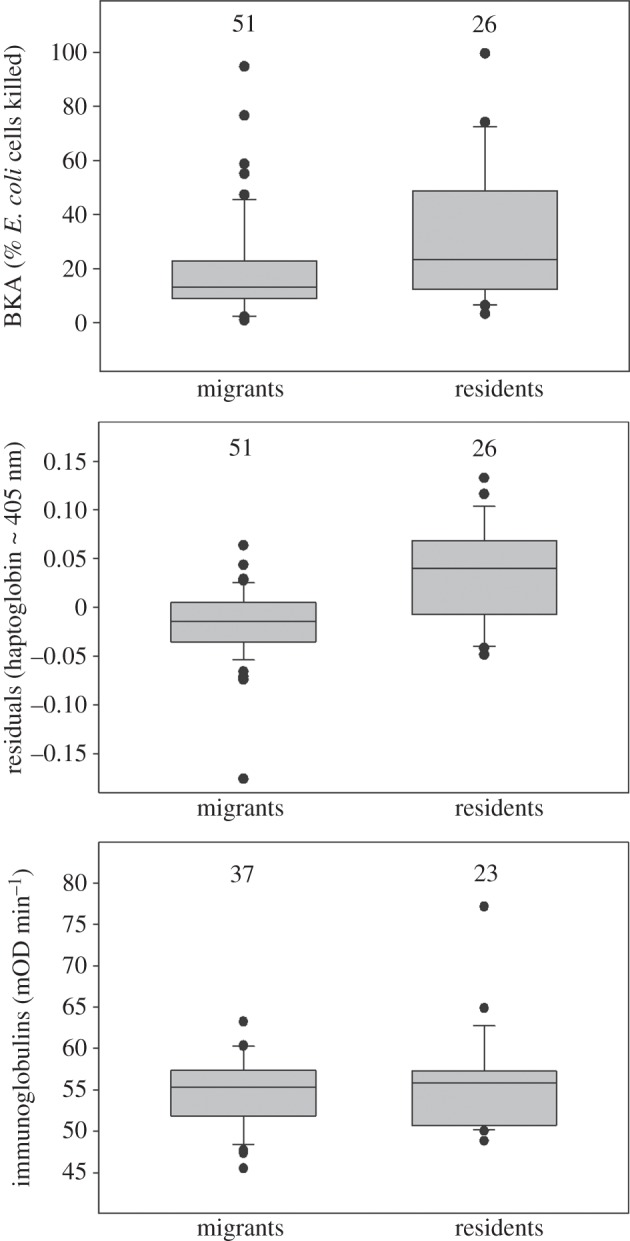
Boxplots of three parameters of immune function in resident and migratory common blackbirds sampled on Helgoland during autumn migration. For haptoglobin-like activity, the unstandardized residuals of the regression of a 405 nm pre-scan on haptoglobin are presented. Immunoglobulin level is presented as milli optical density (mOD). Plotted are the median (horizontal line in the box), 25th and 75th percentiles (horizontal box boundaries), and 10th and 90th percentiles (whiskers). Black dots indicate outliers. Numbers above boxes are sample sizes.
With our study we provide the first comparison of baseline immune function in wild migrant and resident birds sampled at the same location during the same time period. Our results thereby provide answers to previously posted, but untested hypotheses in eco-immunology. Our study clearly indicates that migrating blackbirds did not boost their innate immune defences. Instead, it lends support to the idea that immune function is compromised during migration as a consequence of physiological or energetic trade-offs [4]. However, only innate immune function (BKA and haptoglobin-like activity) was lower in migrants than in residents; baseline acquired (antibody-mediated) immunity, measured as total immunoglobulins, did not differ between migrants and residents. This may suggest that trade-offs are made within the immune system itself; during physiologically demanding periods animals could reallocate available resources from the ‘expensive’ (i.e. innate) to the ‘cheaper’ (i.e. acquired) components of the immune system as hypothesized by Lee [8]. However, the absence in migrants of a negative relationship between parameters of innate and acquired immune function does not support this hypothesis. That migration appears to compromise baseline innate immune function in blackbirds may seem surprising; migration by short-distance migrants is usually slow and considered less demanding as compared to long-distance migrants [25,26]. However, especially in partial migrants such as blackbirds, resource competition at stopover sites could be particularly high for migrating individuals if resident conspecifics dominate the best foraging sites. There are at least two alternative explanations for the difference in innate immune function between migrants and residents. First, previous exposure to parasites on the breeding grounds may have differed between the two groups because the migrants in our sample breed at higher latitudes. Even though differences in pathogen pressure are generally hypothesized for more extreme habitat differences (e.g. tropical versus temperate, temperate versus arctic), blackbirds breeding in Scandinavia might encounter fewer pathogens than Helgoland blackbirds, which may lead to a lower investment into immune function [17,27]. Second, residents may boost their immune function during migration seasons because incoming migrants might expose them to new pathogens [28]. Determining the annual patterns of immune function in Scandinavian and Helgoland blackbirds would allow the testing of these ideas.
Supplementary Material
Acknowledgements
We are much obliged to Thomas Klinner for fieldwork, Sinja Werner and Helgoland IfV staff for colour-band reading, and Cyndi Birberg for help with laboratory work. Ommo Hüppop kindly provided and analysed Helgoland blackbird trapping data. Dennis Hasselquist, Kevin Matson and two reviewers provided helpful suggestions on an earlier draft.
Ethics
All procedures were approved by the Ministry for Energy, Agriculture, the Environment and Rural Areas, Schleswig-Holstein, Germany (project number: V242-7224.123-11).
Data accessibility
The dataset supporting this article has been uploaded as the electronic supplementary material.
Authors' contributions
C.E. conceived of the study, collected data and performed statistical analyses. A.H. performed all laboratory analyses. C.E. and A.H. together wrote the article. Both authors approved of the current version, and agree to be accountable for its contents.
Competing interests
We have no competing interests.
Funding
A.H. was supported by a Rubicon PostDoc-Fellowship (825.13.022) from the Netherlands Organization for Scientific Research and is associated with the Centre for Animal Movement Research (CAnMove), which is financed by a Linnaeus grant no. (349-2007-8690) from the Swedish Research Council and Lund University.
References
- 1.Roitt IM, Brostoff J, Male DK. 1998. Immunology, 5th edn London, UK: Mosby. [Google Scholar]
- 2.Klasing KC. 2004. The costs of immunity. Acta Zool. Sin. 50, 961–969. [Google Scholar]
- 3.Hasselquist D, Nilsson JA. 2012. Physiological mechanisms mediating costs of immune responses: what can we learn from studies of birds? Anim. Behav. 83, 1303–1312. ( 10.1016/j.anbehav.2012.03.025) [DOI] [Google Scholar]
- 4.Buehler DM, Piersma T. 2008. Travelling on a budget: predictions and ecological evidence for bottlenecks in the annual cycle of long-distance migrants. Phil. Trans. R. Soc. B 363, 247–266. ( 10.1098/rstb.2007.2138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasselquist D. 2007. Comparative immunoecology in birds: hypotheses and tests. J. Ornithol. 148, S571–S582. ( 10.1007/s10336-007-0201-xER) [DOI] [Google Scholar]
- 6.Buehler DM, Tieleman BI, Piersma T. 2010. How do migratory species stay healthy over the annual cycle? A conceptual model for immune function and for resistance to disease. Integr. Comp. Biol. 50, 346–357. ( 10.1093/icb/icq055) [DOI] [PubMed] [Google Scholar]
- 7.Møller AP, Erritzoe J. 1998. Host immune defence and migration in birds. Evol. Ecol. 12, 945–953. ( 10.1023/A:1006516222343) [DOI] [Google Scholar]
- 8.Lee KA. 2006. Linking immune defenses and life history at the levels of the individual and the species. Integr. Comp. Biol. 46, 1000–1015. ( 10.1093/icb/icl049) [DOI] [PubMed] [Google Scholar]
- 9.Owen JC, Moore FR. 2006. Seasonal differences in immunological condition of three species of thrushes. Condor 108, 389–398. ( 10.1650/0010-5422(2006)108[389:SDIICO[2.0.CO;2) [DOI] [Google Scholar]
- 10.Hegemann A, Matson KD, Both C, Tieleman BI. 2012. Immune function in a free-living bird varies over the annual cycle, but seasonal patterns differ between years. Oecologia 170, 605–618. ( 10.1007/s00442-012-2339-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hegemann A, Matson KD, Versteegh MA, Villegas A, Tieleman BI. 2013. Immune response to an endotoxin challenge involves multiple immune parameters and is consistent among the annual-cycle stages of a free-living temperate zone bird. J. Exp. Biol. 216, 2573–2580. ( 10.1242/jeb.083147) [DOI] [PubMed] [Google Scholar]
- 12.Nebel S, Bauchinger U, Buehler DM, Langlois LA, Boyles M, Gerson AR, Price ER, McWilliams SR, Guglielmo CG. 2012. Constitutive immune function in European starlings, Sturnus vulgaris, is decreased immediately after an endurance flight in a wind tunnel. J. Exp. Biol. 215, 272–278. ( 10.1242/jeb.057885) [DOI] [PubMed] [Google Scholar]
- 13.Buehler DM, Piersma T, Matson K, Tieleman BI. 2008. Seasonal redistribution of immune function in a migrant shorebird: annual-cycle effects override adjustments to thermal regime. Am. Nat. 172, 783–796. ( 10.1086/592865) [DOI] [PubMed] [Google Scholar]
- 14.Hegemann A, Marra PP, Tieleman BI. 2015. Causes and consequences of partial migration in a passerine bird. Am. Nat. 186, 531–546. ( 10.1086/682667) [DOI] [PubMed] [Google Scholar]
- 15.Mendes L, Piersma T, Hasselquist D, Matson KD, Ricklefs RE. 2006. Variation in the innate and acquired arms of the immune system among five shorebird species. J. Exp. Biol. 209, 284–291. ( 10.1242/jeb.02015) [DOI] [PubMed] [Google Scholar]
- 16.Buehler DM, Encinas-Viso F, Petit M, Vezina F, Tieleman BI, Piersma T. 2009. Limited access to food and physiological trade-offs in a long-distance migrant shorebird. II. Constitutive immune function and the acute-phase response. Physiol. Biochem. Zool. 82, 561–571. ( 10.1086/603635) [DOI] [PubMed] [Google Scholar]
- 17.Horrocks NPC, Hegemann A, Ostrowski S, Ndithia H, Shobrak M, Williams JB, Matson KD, Tieleman BI. 2015. Environmental proxies of antigen exposure explain variation in immune investment better than indices of pace of life. Oecologia 177, 281–290. ( 10.1007/s00442-014-3136-y) [DOI] [PubMed] [Google Scholar]
- 18.Dierschke J, Dierschke V, Huppop K, Huppop O, Jachmann KF. 2011. The birds of the Island of Helgoland. Helgoland, Germany: OAG Helgoland. [Google Scholar]
- 19.Svensson L. 1992. Identification guide to European passerines, 4th edn Stockholm, Sweden: L. Svensson. [Google Scholar]
- 20.Kaiser A. 1993. A new multi-category classification of subcutaneous fat deposits of songbirds. J. Field Ornithol. 64, 246–255. [Google Scholar]
- 21.Sacher T. 2009. Genetic differentiation and migration behaviour of an island population of the common blackbird (Turdus merula), PhD thesis, Institute of Avian Research, Wilhelmshaven, Germany.
- 22.French SS, Neuman-Lee LA. 2012. Improved ex vivo method for microbiocidal activity across vertebrate species. Biol. Open 1, 482–487. ( 10.1242/bio.2012919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matson KD, Horrocks NPC, Versteegh MA, Tieleman BI. 2012. Baseline haptoglobin concentrations are repeatable and predictive of certain aspects of a subsequent experimentally-induced inflammatory response. Comp. Biochem. Phys. A 162, 7–15. ( 10.1016/j.cbpa.2012.01.010) [DOI] [PubMed] [Google Scholar]
- 24.Sköld-Chiriac S, Nord A, Nilsson J, Hasselquist D. 2014. Physiological and behavioral responses to an acute-phase response in zebra finches: immediate and short-term effects. Physiol. Biochem. Zool. 87, 288–298. ( 10.1086/674789) [DOI] [PubMed] [Google Scholar]
- 25.Alerstam T, Lindström Å. 1990. Optimal bird migration: the relative importance of time, energy, and safety. In Bird migration: the physiology and ecophysiology (ed. Gwinner E.), pp. 331–351. Berlin, Germany: Springer. [Google Scholar]
- 26.La Sorte FA, Fink D, Hochachka WM, DeLong JP, Kelling S. 2013. Population-level scaling of avian migration speed with body size and migration distance for powered fliers. Ecology 94, 1839–1847. ( 10.1890/12-1768.1) [DOI] [PubMed] [Google Scholar]
- 27.Horrocks NPC, Hegemann A, Matson KD, Hine K, Jaquier S, Shobrak M, Williams SB, Tinbergen JM, Tieleman BI. 2012. Immune indexes of larks from desert and temperate regions show weak associations with life history but stronger links to environmental variation in microbial abundance. Physiol. Biochem. Zool. 85, 504–515. ( 10.1086/666988) [DOI] [PubMed] [Google Scholar]
- 28.van Dijk JGB, Hoye BJ, Verhagen JH, Nolet BA, Fouchier RAM, Klaassen M. 2014. Juveniles and migrants as drivers for seasonal epizootics of avian influenza virus. J. Anim. Ecol. 83, 266–275. ( 10.1111/1365-2656.12131) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting this article has been uploaded as the electronic supplementary material.


