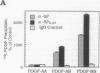
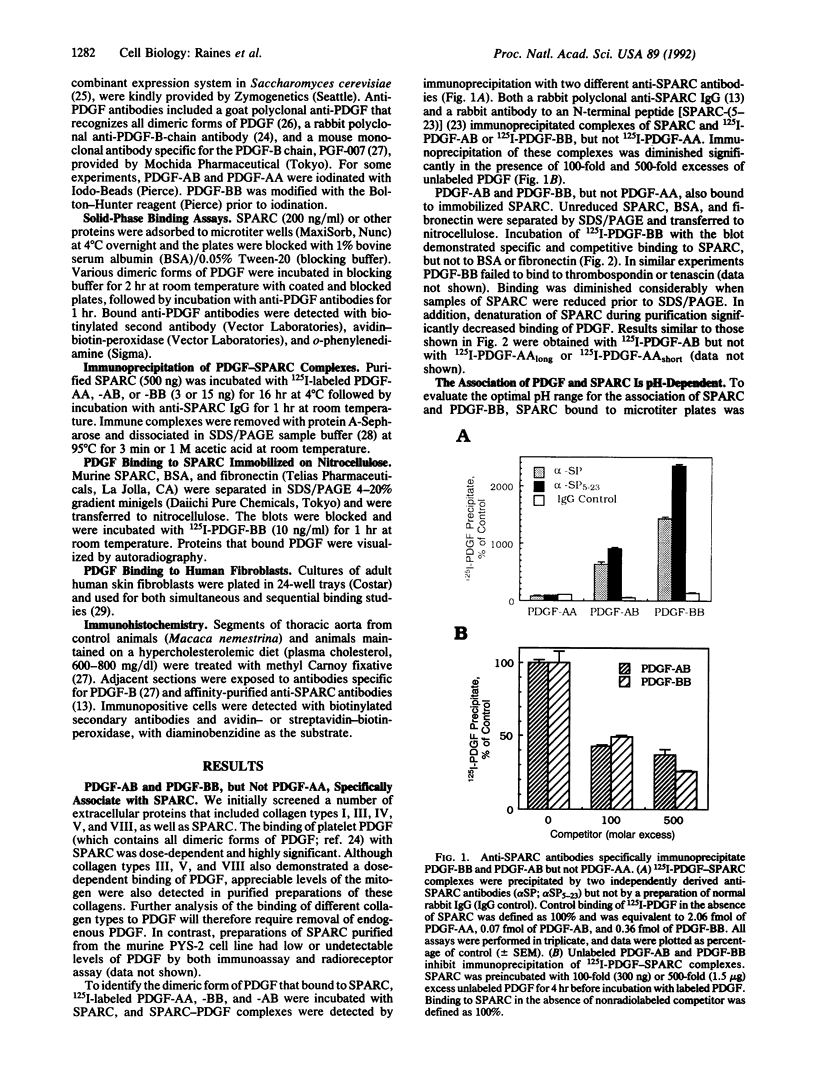
Abstract
Interactions among growth factors, cells, and extracellular matrix are critical to the regulation of directed cell migration and proliferation associated with development, wound healing, and pathologic processes. Here we report the association of PDGF-AB and -BB, but not PDGF-AA, with the extracellular glycoprotein SPARC. Complexes of SPARC and 125I-labeled PDGF-BB or -AB were specifically immunoprecipitated by anti-SPARC immunoglobulins. 125I-PDGF-BB and -AB also bound specifically to SPARC that was immobilized on microtiter wells or bound to nitrocellulose after transfer from SDS/polyacrylamide gels. The binding of PDGF-BB to SPARC was pH-dependent; significant binding was detectable only above pH 6.6. The interaction of SPARC with specific dimeric forms of PDGF affected the activity of this mitogen. SPARC inhibited the binding of PDGF-BB and PDGF-AB, but not PDGF-AA, to human dermal fibroblasts in a dose-dependent manner. The expression of SPARC and PDGF was minimal in most normal adult tissues but was increased after injury. Enhanced expression of both PDGF-B chain and SPARC was seen in advanced lesions of atherosclerosis. We suggest that the coordinate expression of SPARC and PDGF-B-containing dimers following vascular injury may regulate the activity of specific dimeric forms of PDGF in vivo.
Full text
PDF
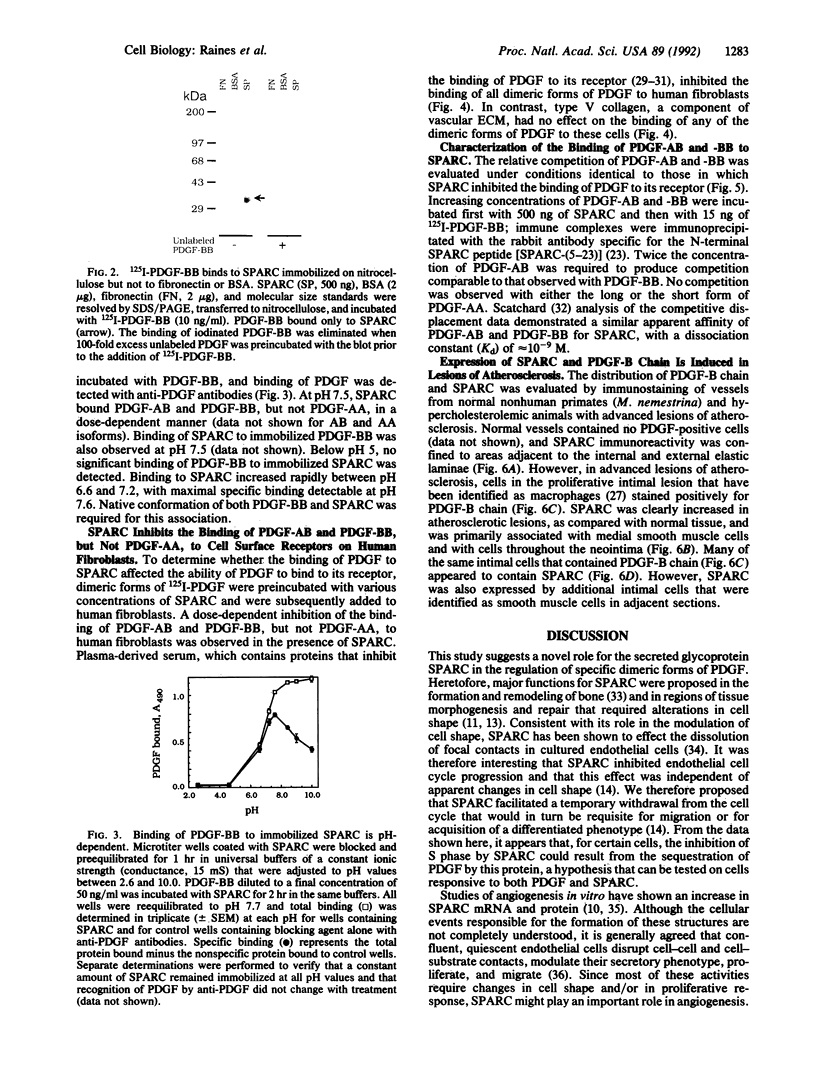

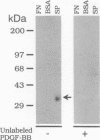
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Galanopoulos T., Neville-Golden J., Kiritsy C. P., Lynch S. E. Injury induces in vivo expression of platelet-derived growth factor (PDGF) and PDGF receptor mRNAs in skin epithelial cells and PDGF mRNA in connective tissue fibroblasts. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):565–569. doi: 10.1073/pnas.88.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar R. S., Boes M., Booth B. A., Dake B. L., Henley S., Hart M. N. The effects of platelet-derived growth factor in cultured microvessel endothelial cells. Endocrinology. 1989 Apr;124(4):1841–1848. doi: 10.1210/endo-124-4-1841. [DOI] [PubMed] [Google Scholar]
- Beitz J. G., Kim I. S., Calabresi P., Frackelton A. R., Jr Human microvascular endothelial cells express receptors for platelet-derived growth factor. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):2021–2025. doi: 10.1073/pnas.88.5.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolander M. E., Young M. F., Fisher L. W., Yamada Y., Termine J. D. Osteonectin cDNA sequence reveals potential binding regions for calcium and hydroxyapatite and shows homologies with both a basement membrane protein (SPARC) and a serine proteinase inhibitor (ovomucoid). Proc Natl Acad Sci U S A. 1988 May;85(9):2919–2923. doi: 10.1073/pnas.85.9.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheifetz S., Andres J. L., Massagué J. The transforming growth factor-beta receptor type III is a membrane proteoglycan. Domain structure of the receptor. J Biol Chem. 1988 Nov 15;263(32):16984–16991. [PubMed] [Google Scholar]
- Engel J., Taylor W., Paulsson M., Sage H., Hogan B. Calcium binding domains and calcium-induced conformational transition of SPARC/BM-40/osteonectin, an extracellular glycoprotein expressed in mineralized and nonmineralized tissues. Biochemistry. 1987 Nov 3;26(22):6958–6965. doi: 10.1021/bi00396a015. [DOI] [PubMed] [Google Scholar]
- Fellström B., Dimeny E., Larsson E., Klareskog L., Tufveson G., Rubin K. Importance of PDGF receptor expression in accelerated atherosclerosis-chronic rejection. Transplant Proc. 1989 Aug;21(4):3689–3691. [PubMed] [Google Scholar]
- Ferns G. A., Sprugel K. H., Seifert R. A., Bowen-Pope D. F., Kelly J. D., Murray M., Raines E. W., Ross R. Relative platelet-derived growth factor receptor subunit expression determines cell migration to different dimeric forms of PDGF. Growth Factors. 1990;3(4):315–324. doi: 10.3109/08977199009003674. [DOI] [PubMed] [Google Scholar]
- Funk S. E., Sage E. H. The Ca2(+)-binding glycoprotein SPARC modulates cell cycle progression in bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2648–2652. doi: 10.1073/pnas.88.7.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden M. A., Au Y. P., Kirkman T. R., Wilcox J. N., Raines E. W., Ross R., Clowes A. W. Platelet-derived growth factor activity and mRNA expression in healing vascular grafts in baboons. Association in vivo of platelet-derived growth factor mRNA and protein with cellular proliferation. J Clin Invest. 1991 Feb;87(2):406–414. doi: 10.1172/JCI115011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon M. Y., Riley G. P., Watt S. M., Greaves M. F. Compartmentalization of a haematopoietic growth factor (GM-CSF) by glycosaminoglycans in the bone marrow microenvironment. 1987 Mar 26-Apr 1Nature. 326(6111):403–405. doi: 10.1038/326403a0. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J. Heparin protects basic and acidic FGF from inactivation. J Cell Physiol. 1986 Sep;128(3):475–484. doi: 10.1002/jcp.1041280317. [DOI] [PubMed] [Google Scholar]
- Hart C. E., Bailey M., Curtis D. A., Osborn S., Raines E., Ross R., Forstrom J. W. Purification of PDGF-AB and PDGF-BB from human platelet extracts and identification of all three PDGF dimers in human platelets. Biochemistry. 1990 Jan 9;29(1):166–172. doi: 10.1021/bi00453a022. [DOI] [PubMed] [Google Scholar]
- Hart C. E., Forstrom J. W., Kelly J. D., Seifert R. A., Smith R. A., Ross R., Murray M. J., Bowen-Pope D. F. Two classes of PDGF receptor recognize different isoforms of PDGF. Science. 1988 Jun 10;240(4858):1529–1531. doi: 10.1126/science.2836952. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Bäckström G., Ostman A., Hammacher A., Rönnstrand L., Rubin K., Nistér M., Westermark B. Binding of different dimeric forms of PDGF to human fibroblasts: evidence for two separate receptor types. EMBO J. 1988 May;7(5):1387–1393. doi: 10.1002/j.1460-2075.1988.tb02955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Platelet-derived growth factor: mechanism of action and possible in vivo function. Cell Regul. 1990 Jul;1(8):555–566. doi: 10.1091/mbc.1.8.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland P. W., Harper S. J., McVey J. H., Hogan B. L. In vivo expression of mRNA for the Ca++-binding protein SPARC (osteonectin) revealed by in situ hybridization. J Cell Biol. 1987 Jul;105(1):473–482. doi: 10.1083/jcb.105.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. S., Huang S. S., Deuel T. F. Specific covalent binding of platelet-derived growth factor to human plasma alpha 2-macroglobulin. Proc Natl Acad Sci U S A. 1984 Jan;81(2):342–346. doi: 10.1073/pnas.81.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D. E., Folkman J. How does extracellular matrix control capillary morphogenesis? Cell. 1989 Sep 8;58(5):803–805. doi: 10.1016/0092-8674(89)90928-8. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe M. L., Hasselaar P., Sage H. Differential expression of extracellular proteins is correlated with angiogenesis in vitro. Lab Invest. 1991 Feb;64(2):174–186. [PubMed] [Google Scholar]
- Ishai-Michaeli R., Eldor A., Vlodavsky I. Heparanase activity expressed by platelets, neutrophils, and lymphoma cells releases active fibroblast growth factor from extracellular matrix. Cell Regul. 1990 Oct;1(11):833–842. doi: 10.1091/mbc.1.11.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. D., Raines E. W., Ross R., Murray M. J. The B chain of PDGF alone is sufficient for mitogenesis. EMBO J. 1985 Dec 16;4(13A):3399–3405. doi: 10.1002/j.1460-2075.1985.tb04096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane T. F., Sage E. H. Functional mapping of SPARC: peptides from two distinct Ca+(+)-binding sites modulate cell shape. J Cell Biol. 1990 Dec;111(6 Pt 2):3065–3076. doi: 10.1083/jcb.111.6.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T., Heidaran M., Miki T., Popescu N., La Rochelle W., Kraus M., Pierce J., Aaronson S. Isolation of a novel receptor cDNA establishes the existence of two PDGF receptor genes. Science. 1989 Feb 10;243(4892):800–804. doi: 10.1126/science.2536956. [DOI] [PubMed] [Google Scholar]
- Raines E. W., Bowen-Pope D. F., Ross R. Plasma binding proteins for platelet-derived growth factor that inhibit its binding to cell-surface receptors. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3424–3428. doi: 10.1073/pnas.81.11.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines E. W., Dower S. K., Ross R. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science. 1989 Jan 20;243(4889):393–396. doi: 10.1126/science.2783498. [DOI] [PubMed] [Google Scholar]
- Raines E. W., Ross R. Identification and assay of platelet-derived growth factor-binding proteins. Methods Enzymol. 1987;147:48–64. doi: 10.1016/0076-6879(87)47098-5. [DOI] [PubMed] [Google Scholar]
- Roberts W. M., Look A. T., Roussel M. F., Sherr C. J. Tandem linkage of human CSF-1 receptor (c-fms) and PDGF receptor genes. Cell. 1988 Nov 18;55(4):655–661. doi: 10.1016/0092-8674(88)90224-3. [DOI] [PubMed] [Google Scholar]
- Romberg R. W., Werness P. G., Lollar P., Riggs B. L., Mann K. G. Isolation and characterization of native adult osteonectin. J Biol Chem. 1985 Mar 10;260(5):2728–2736. [PubMed] [Google Scholar]
- Ross R., Masuda J., Raines E. W., Gown A. M., Katsuda S., Sasahara M., Malden L. T., Masuko H., Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990 May 25;248(4958):1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- Rubin K., Tingström A., Hansson G. K., Larsson E., Rönnstrand L., Klareskog L., Claesson-Welsh L., Heldin C. H., Fellström B., Terracio L. Induction of B-type receptors for platelet-derived growth factor in vascular inflammation: possible implications for development of vascular proliferative lesions. Lancet. 1988 Jun 18;1(8599):1353–1356. doi: 10.1016/s0140-6736(88)92177-0. [DOI] [PubMed] [Google Scholar]
- Sage E. H., Bornstein P. Extracellular proteins that modulate cell-matrix interactions. SPARC, tenascin, and thrombospondin. J Biol Chem. 1991 Aug 15;266(23):14831–14834. [PubMed] [Google Scholar]
- Sage H., Vernon R. B., Decker J., Funk S., Iruela-Arispe M. L. Distribution of the calcium-binding protein SPARC in tissues of embryonic and adult mice. J Histochem Cytochem. 1989 Jun;37(6):819–829. doi: 10.1177/37.6.2723400. [DOI] [PubMed] [Google Scholar]
- Sage H., Vernon R. B., Funk S. E., Everitt E. A., Angello J. SPARC, a secreted protein associated with cellular proliferation, inhibits cell spreading in vitro and exhibits Ca+2-dependent binding to the extracellular matrix. J Cell Biol. 1989 Jul;109(1):341–356. doi: 10.1083/jcb.109.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela O., Moscatelli D., Sommer A., Rifkin D. B. Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J Cell Biol. 1988 Aug;107(2):743–751. doi: 10.1083/jcb.107.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela O., Rifkin D. B. Release of basic fibroblast growth factor-heparan sulfate complexes from endothelial cells by plasminogen activator-mediated proteolytic activity. J Cell Biol. 1990 Mar;110(3):767–775. doi: 10.1083/jcb.110.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarini P. R., Seyedin S. M. The high molecular weight receptor to transforming growth factor-beta contains glycosaminoglycan chains. J Biol Chem. 1988 Jun 15;263(17):8366–8370. [PubMed] [Google Scholar]
- Seifert R. A., Hart C. E., Phillips P. E., Forstrom J. W., Ross R., Murray M. J., Bowen-Pope D. F. Two different subunits associate to create isoform-specific platelet-derived growth factor receptors. J Biol Chem. 1989 May 25;264(15):8771–8778. [PubMed] [Google Scholar]
- Smits A., Hermansson M., Nistér M., Karnushina I., Heldin C. H., Westermark B., Funa K. Rat brain capillary endothelial cells express functional PDGF B-type receptors. Growth Factors. 1989;2(1):1–8. doi: 10.3109/08977198909069076. [DOI] [PubMed] [Google Scholar]
- Stenner D. D., Tracy R. P., Riggs B. L., Mann K. G. Human platelets contain and secrete osteonectin, a major protein of mineralized bone. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6892–6896. doi: 10.1073/pnas.83.18.6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termine J. D., Kleinman H. K., Whitson S. W., Conn K. M., McGarvey M. L., Martin G. R. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981 Oct;26(1 Pt 1):99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- Uchiyama A., Suzuki M., Lefteriou B., Glimcher M. Isolation and chemical characterization of the phosphoproteins of chicken bone matrix: heterogeneity in molecular weight and composition. Biochemistry. 1986 Nov 18;25(23):7572–7583. doi: 10.1021/bi00371a047. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Folkman J., Sullivan R., Fridman R., Ishai-Michaeli R., Sasse J., Klagsbrun M. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]