Significance
There are no Food and Drug Administration approved drugs available for preventing or treating Argentine hemorrhagic fever (AHF), and the current treatment option is limited to administration of immune plasma. With the expanding clinical use of monoclonal antibodies (mAbs) for acute and chronic conditions, it has become clear that mAbs offer a highly specific, potent, and generally safe drug platform for antivirals, and may be a useful alternative to immune plasma. Here, we show that mAbs are effective in the Junin virus guinea pig model and likely to be an economical therapy for AHF.
Keywords: Junin, therapy, immunotherapy, hemorrhagic fever
Abstract
Countermeasures against potential biothreat agents remain important to US Homeland Security, and many of these pharmaceuticals could have dual use in the improvement of global public health. Junin virus, the causative agent of Argentine hemorrhagic fever (AHF), is an arenavirus identified as a category A high-priority agent. There are no Food and Drug Administration (FDA) approved drugs available for preventing or treating AHF, and the current treatment option is limited to administration of immune plasma. Whereas immune plasma demonstrates the feasibility of passive immunotherapy, it is limited in quantity, variable in quality, and poses safety risks such as transmission of transfusion-borne diseases. In an effort to develop a monoclonal antibody (mAb)-based alternative to plasma, three previously described neutralizing murine mAbs were expressed as mouse-human chimeric antibodies and evaluated in the guinea pig model of AHF. These mAbs provided 100% protection against lethal challenge when administered 2 d after infection (dpi), and one of them (J199) was capable of providing 100% protection when treatment was initiated 6 dpi and 92% protection when initiated 7 dpi. The efficacy of J199 is superior to that previously described for all other evaluated drugs, and its high potency suggests that mAbs like J199 offer an economical alternative to immune plasma and an effective dual use (bioterrorism/public health) therapeutic.
Junin virus (JUNV), a member of the genus Arenavirus, is the causative agent of Argentine hemorrhagic fever (AHF). Although still confined to Argentina, its geographical distribution has expanded since its discovery in 1958. As an endemic virus spread by native ineradicable rodent populations, JUNV could be acquired during natural outbreaks for bioterror purposes and could naturally spread outside of its current range. The relatively slow onset of AHF with its unspecific symptoms that may delay diagnosis, coupled with its debilitating hemorrhagic phase, make JUNV a serious threat to public health (1, 2). With the use of an attenuated vaccine manufactured in Argentina (3) in high-risk individuals, the incidence of AHF has declined, but cases continue to be reported. Untreated, AHF has a mortality rate of 20–30%; however, treatment with immune plasma within 8 d of symptoms reduces the mortality rate to 1% (4, 5).
Investigators have evaluated a variety of potential alternatives to immune plasma in guinea pigs, the most commonly used JUNV animal model. Testing the hypothesis that a low dose of cyclophosphamide could lead to an enhanced immune response, Ponzinibbio et al., found a small survival benefit (17%) and delay to death against a uniformly lethal JUNV challenge (6). The antiviral, ribavirin, has been tested in multiple studies, but no more than 50% survival was observed with high doses starting 1 h after infection (7). More recently, favipiravir (T-705), an antiviral approved for use against influenza in Japan and evaluated clinically against Ebola virus in Guinea, was found to provide up to 78% survival in the guinea pig model when treatment was initiated 2 d after infection (8). However, none of these experimental agents have proven as efficacious as immune plasma or convalescent serum, which have been shown to provide 100% protection to guinea pigs when delivered as late as 6 d after infection (9).
With the expanding clinical use of monoclonal antibodies (mAbs) for acute and chronic conditions, it has become clear that mAbs offer a highly specific, potent, and generally safe (especially for nonhuman antigen targets) drug platform for antivirals and may be a useful alternative to immune plasma (5). In this study, three previously described anti-JUNV glycoprotein (GP) neutralizing mAbs (10) were evaluated. These mAbs were chimerized, expressed transiently in transgenic Nicotiana benthamiana (11), and evaluated in vitro and in the guinea pig model.
Results
Production of the Chimeric mAbs in N. benthamiana.
The three mAbs were expressed by using the magnICON system (12) to vacuum infiltrate 1–2 kg (per manufacturing run) of transgenic N. benthamiana capable of generating mammalian-like N-glycans (13). Yields of the three mAbs postprotein A affinity chromatography were 226 ± 29 mg/kg for J199 (n = 5 production runs), 243 ± 77 mg/kg for J200 (n = 3), and 170 ± 56 mg/kg for J202 (n = 3). The N-glycosylation profiles of the mAbs (Table 1) were consistent with previous reports of mAbs purified from the transgenic N. benthamiana (14, 15), with greater than 75% of the GnGn glycoform.
Table 1.
N-linked glycans on the anti-JUNV mAbs
| mAb | % N-glycoform | |||||
| GnGn | GnM | GnGnF | Man8 | Man9 | Agly | |
| J199 | 78.16 | 2.71 | 1.38 | 0.78 | 0.57 | 16.40 |
| J200 | 78.54 | 2.70 | 1.01 | 0.53 | 0.57 | 16.64 |
| J202 | 82.56 | 3.49 | 1.22 | 0.40 | 0.34 | 11.98 |
N-glycosylation profile as determined by LS-ESI-MS. Numbers represent the presence of the different glycoforms in %. Agly, aglycosylated. The N-glycan nomenclature used is from www.proglycan.com.
Neutralization Potency of the Chimeric JUNV mAbs.
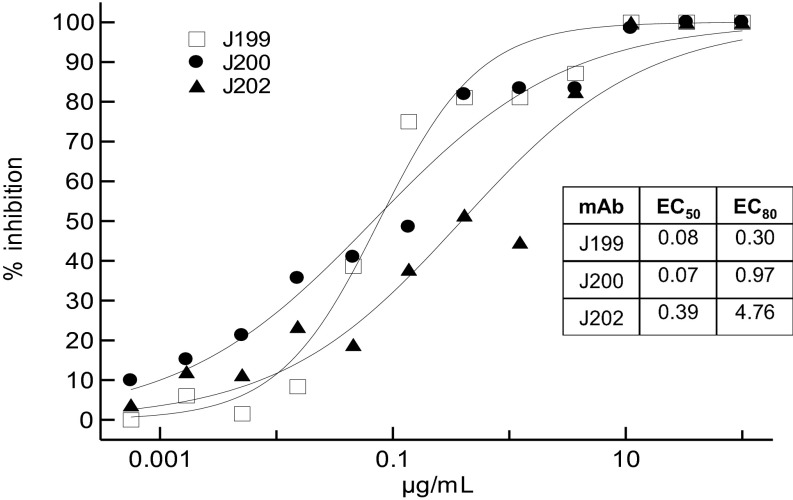
The three chimeric anti-JUNV mAbs were tested in a plaque neutralization assay by using the Candid #1 strain. Neutralization titers had been reported for the fully murine mAbs in ascites fluid (10), but because the concentration of the mAbs was not reported, the mAbs relative potency was unknown. As Fig. 1 illustrates, J199 and J200 had similar high neutralization potency (PRN50: 76 and 68 ng/mL, respectively), whereas J202 was the least potent of the three (PRN50: 388 ng/mL).
Fig. 1.
Activity of the JUNV mAbs in a plaque reduction neutralization assay with strain Candid #1. The percent inhibition of viral infection is displayed on the y axis. EC values are in μg/mL. Plotted points are the average of two replicates.
Efficacy of the Chimeric JUNV mAbs in the Guinea Pig Model.
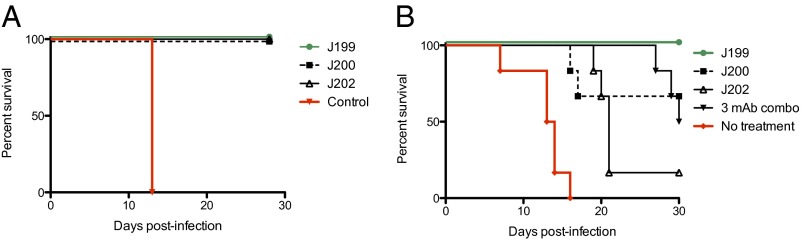
In a pilot experiment, outbred guinea pigs were administered an i.p. challenge with JUNV (Romero strain). Two days later, animals received an i.p. dose of 10 mg of mAb or were not treated. The 10-mg (∼20 mg/kg) dose used throughout these pilot studies was selected as a convenient dose consistent with dosing of the two mAbs approved by the Food and Drug Administration (FDA) for infectious disease indications (Synagis for respiratory syncytial virus is dosed at 15 mg/kg and Raxibacumab for anthrax is dosed at 40 mg/kg). As Fig. 2A illustrates, all animals treated with one of the three anti-JUNV GP mAbs survived lethal challenge, whereas untreated control animals succumbed to infection (P < 0.05 by Mantel–Cox).
Fig. 2.
Survival of guinea pigs infected with JUNV. (A) Animals were treated 2 d after infection with 10 mg of mAb (∼20 mg/kg; n = 5 per group) or untreated (n = 3). (B) Animals (n = 6 per group) were treated 7 and 11 d after infection with 10 mg of mAb (3.33 mg of each in the case of the “3 mAb combo” group) or untreated (n = 6).
To better distinguish the protective efficacy conferred by the three mAbs, a second experiment was performed in which guinea pigs were treated 7 and 11 d after infection. Treated animals received either 10 mg of one of the mAbs, or 10 mg of an equimolar mixture of all three of the mAbs (Fig. 2B). J199 provided 100% protection (P < 0.001 compared with control), J200 provided partial protection (67%; P < 0.01), J202 provided minimal protection (17%), and the three mAb mixture provided 50% protection. Although all controls succumbed to disease by day 16 after infection, mAb-treated animals that did not survive experienced a delay to death. Many of the treated animals experiencing a delay to death exhibited symptoms of neurological disease between days 18 and 30 (e.g., impaired hind leg use).
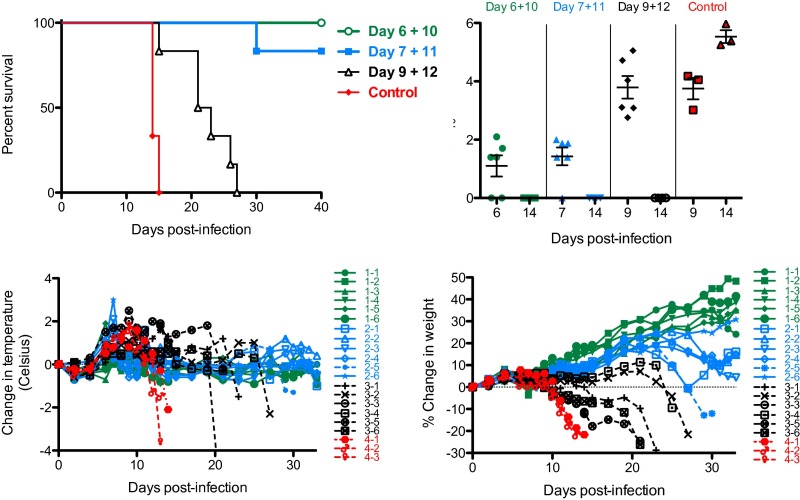
Because of its superior efficacy (Fig. 2B), J199 was selected for further evaluation. To determine the breadth of the therapeutic window, groups of guinea pigs were treated starting 6, 7, or 9 d after infection and received a second dose 3–4 d later. When treatment began on day 6 or 7 after infection, 100% (P < 0.001) or 83% (P < 0.005) of animals survived (Fig. 3, Top Left). In contrast, when treatment was initiated 9 d after infection, all animals succumbed, but a delay to death was observed (P < 0.01). A detectable viral load was observed in 67%, 83%, and 100% of animals on day 6, 7, and 9 after infection (plasma was sampled before mAb dosing), respectively (Fig. 3, Top Right). At day 14 after infection, untreated control animals had a mean plasma viral load of 3.4 ± 0.4 × 105 pfu/mL, whereas none of the animals treated with J199 had detectable virus. The absence of detectable virus on day 14 in treated animals could indicate that either no virus was present or that any virus that was present was neutralized by J199 and, therefore, undetectable in the plaque assay. Illness in all animals was associated with weight loss and a rapid temperature drop before death (Fig. 3, Bottom). Fever, presumably from the viral infection, peaked on day 6 for the day 6/10 treatment group (average of +0.3 °C from baseline), on day 7 for the day 7/11 group (+1.4 °C), and on day 9 for the day 9/12 (+1.5 °C) and untreated control groups (+1.4 °C). We speculate that the earlier fever in treated animals may have been due to physiologic responses to the killing of JUNV-infected host cells upon administration of the mAb.
Fig. 3.
Therapeutic window study with J199. (Top Left) Survival of guinea pigs infected with JUNV. Animals (n = 6 per mAb-treated group; n = 3 for untreated controls) were treated with 10 mg of J199 at different points after infection. (Top Right) Plasma viral load as determined by plaque assay. Day 6, 7, and 9 samples were obtained before dosing with mAb. (Bottom Left) Change in temperature over time. (Bottom Right) Percent change in weight over time. In the bottom graphs, dashed lines indicate data curves from animals that succumbed to infection. 1–1 through 1–6 indicate the six individual animals in the Day 6+10 treatment group; 2–1 through 2–6 for the Day 7+11 group; 3–1 through 3–6 for the Day 9+12 group; and 4–1 through 4–3 for the three animals in the control group.
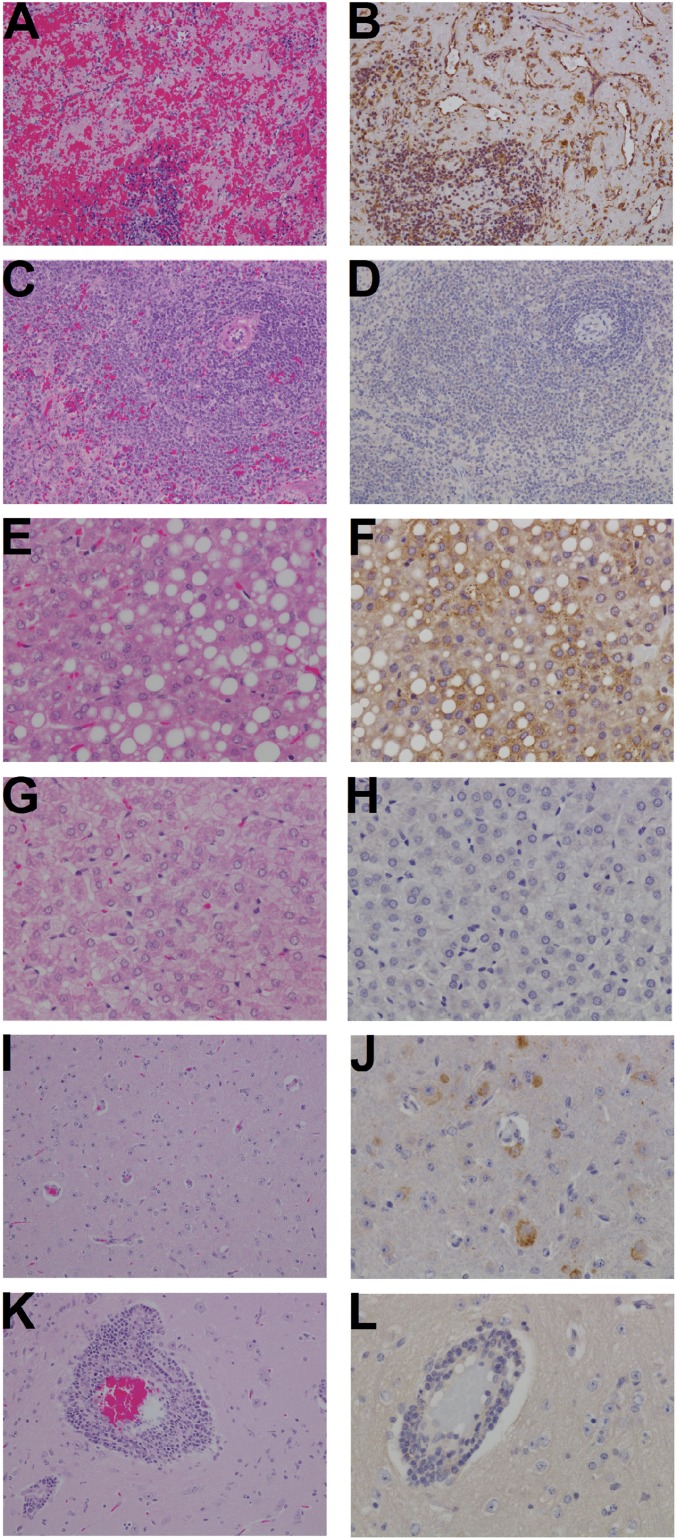
The three nontreated control guinea pigs all had high viral loads in liver (> 6.78 log10 pfu/g), spleen (> 6.78 log10 pfu/g) and brain tissue (5.64 ± 0.56 log10 pfu/g) on day 13 or 14 after infection. None of the treated animals that survived to the study endpoint (day 40) had virus detected in any of these tissues. Virus was detectable in brain tissue (3 of 3) of treated animals that succumbed to infection (days 21 and 30) at levels ranging from 2.70 to 5.32 log10 pfu/g.
The three nontreated control animals had detectable pathology in the liver, spleen, and brain tissue on day 13 or 14 after infection (Table 2 and Fig. 4). Significant immunolabeling for JUNV antigen was identified in all three control animals, specifically in hepatocytes, in endothelium and mononuclear cells within the red and white pulp of the spleen, and in individual to clustered neurons, often associated with glial nodules. Surviving J199-treated animals necropsied on day 40 after infection (Table 2 and Fig. 4) displayed pathology that was minor in the liver, absent in the spleen, and with more central nervous system involvement than observed in the untreated controls that succumbed to infection on day 13 or 14. Significant immunolabeling for JUNV antigen was identified in 3/5 and 2/2 guinea pigs treated on days 7+11 and 9+12, respectively, only in individual to clustered neurons, often associated with glial nodules.
Table 2.
Histopathology summary
| Histopathology findings | Controls (untreated) | J199-treated groups | ||
| Day 6+10 | Day 7+11 | Day 9+12 | ||
| Succumbed/euthanized on: | Day 13 or 14 | Day 40 | Day 40 | Day 21 |
| Pathology findings | ||||
| Hepatocellular vacuolar degeneration | 3/3 | 3/6 | 1/5 | NT |
| Hepatocellular necrosis | 3/3 | 1/6 | 1/5 | NT |
| Kupffer cell hyperplasia | 3/3 | 6/6 (mild) | 5/5 (mild) | NT |
| Splenic lymphoid depletion | 3/3 | 0/6 | 0/5 | NT |
| Lymphoplasmacytic meningoencephalitis and diffuse gliosis* | 0/3 | 5/6 | 5/5 | 2/2 (mild) |
| Diffuse gliosis only | 3/3 | 1/6 | 0/5 | 0/2 |
| Immunohistochemistry findings | ||||
| JUNV antigen detected in liver | 3/3 | 0/6 | 0/5 | NT |
| JUNV antigen detected in spleen | 3/3 | 0/6 | 0/5 | NT |
| JUNV antigen detected in brain (neurons, often associated with glial nodules) | 3/3 | 0/6 | 3/5 | 2/2 |
Control animals evaluated histologically at earlier time points (day 13 or 14) uniformly had diffuse gliosis only, indicating an initial response of the neural tissues to the JUNV virus but not enough time to recruit a peripheral inflammatory response to the neural tissues. Most animals evaluated histologically at a later time point (day 21 and day 40) show the ability to recruit a peripheral inflammatory response manifesting as inflammation in the meninges and cuffing of vessels within the brain parenchyma (lymphoplasmacytic meningoencephalitis). In treated animals, however, those treated on day 6+10 lack JUNV antigen, whereas those treated at day 7+11 have approximately half of the animals, and all of the animals treated at day 9+12 showing JUNV antigen associated with the inflammation. NT, not tested.
Fig. 4.
Spleen, liver, and brain histopathology of JUNV-infected guinea pigs. A, B, E, F, I, and J are tissues from infected control animal 4–1 (Fig. 3) euthanized on day 14. C, D, G, H, K, and L are from animal 1–2 (treated with J199 on day 6+10) euthanized at study termination on day 40. A, C, E, G, I, and K are tissues stained with hematoxylin and eosin stain (H&E) and B, D, F, H, J, and L are immunohistochemistry (IHC) detecting JUNV antigen. In total, the images demonstrate that the control animal has extensive lesions as visualized with H&E and JUNV-specific antigen is associated with these lesions as determined by IHC. (A) Spleen: diffuse lymphoid depletion, degeneration, and hemorrhage. (B) Spleen: diffuse cytoplasmic immunolabeling of mononuclear cells and endothelium for JUNV antigen (brown). (C) Spleen: no significant lesions. (D) Spleen: no significant immunolabeling. (E) Liver: diffuse hepatocellular vacuolar degeneration and kupffer cell hyperplasia. (F) Liver: punctate cytoplasmic immunolabeling of hepatocytes and kupffer cells. (G) Liver: reactive kupffer cell hyperplasia. (H) Liver: no JUNV antigen detected. (I) Brain: diffuse gliosis. (J) Brain: diffuse cytoplasmic immunolabeling of neurons. (K) Brain: reactive lymphocytic perivascular cuffing and diffuse gliosis. (L) Brain: no JUNV antigen detected. (Magnification: A–D, I, and K, 20x; E–H, J, and L, 40x.)
Discussion
In this study, we produced three anti-JUNV GP neutralizing mouse-human chimeric mAbs in N. benthamiana (16). The mAbs exhibited potent neutralizing activity, and the neutralization potency appeared to correlate with protection in guinea pigs, with the most potent neutralizer providing the greatest efficacy. Although clinical dosing is based on the neutralization titer of immune plasma (4), there is some evidence that neutralization of free JUNV may not be the primary mechanism of action of IgG antibodies. Kenyon et al. found that F(ab′)2 had identical neutralizing activity to the IgG from which it was prepared. However, the F(ab′)2 provided no survival benefit to guinea pigs even when administered at doses greater than required for 100% protection with intact IgG. Further, no decrease in organ viral load was observed in F(ab′)2-treated animals compared with untreated infected controls (17). These results suggested that immune effector functions (i.e., complement or Fcγ receptor-mediated) are critical for therapeutic benefit by polyclonal antibodies in JUNV-infected animals. Importantly, human IgG can fix guinea pig complement and human IgG binds to guinea pig Fcγ receptors with similar affinity compared with human receptors (18). These observations support the use of the guinea pig model for studying the efficacy of antibodies with human constant regions (e.g., chimeric or fully human mAbs). The difference in efficacy between IgG and F(ab′)2 reported by Kenyon et al. is consistent with a growing body of evidence with a variety of viruses suggesting classic neutralization of free virus (i.e., antibody bound to virus sterically hinders binding to, fusion with, or internalization by a host target cell) may not be the primary mechanism by which anti-viral mAbs function in vivo, and that Fcγ receptor-mediated immune functions play a pivotal role (14, 15, 19–21). Although all three chimeric mAbs contained the same human Fc isotype (IgG1), the protective efficacy directly correlated with virus neutralization. This observation would suggest that either affinity directly correlates with neutralization for these three mAbs or that optimal protection in vivo requires both Fc-mediated immune functions as well as effective neutralization of free virus.
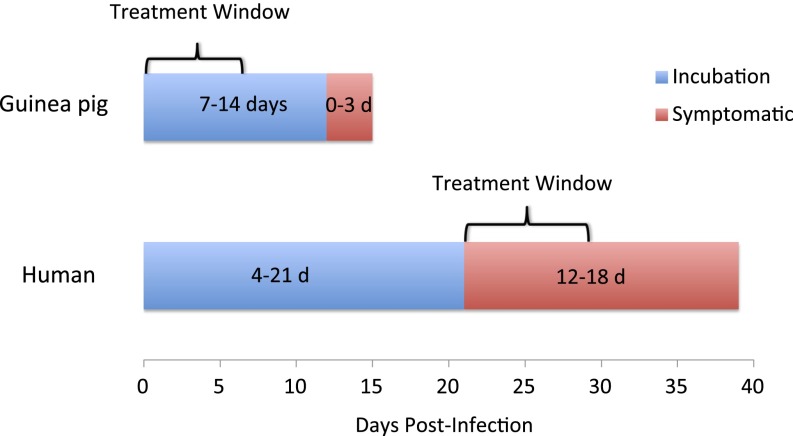
Consistent with previous reports (9), neurological involvement was observed in treated animals that experienced a delay to death, with hind leg paralysis the most commonly observed sign. None of the animals that received J199 on day 6 after infection had JUNV antigen detectable in the brain by immunohistochemistry (Table 1), suggesting that the mAb treatment was able to prevent infection of the central nervous system (CNS). However, when mAb treatment was delayed to day 7 or 9, 60% (3 of 5) and 100% (2 of 2) of animals, respectively, had JUNV antigen detected in brain tissue. These data indicate that viral access to the CNS occurs between days 6 and 9 after infection in the guinea pig model and is associated with a reduction in the benefit of mAb therapy (Fig. 3). These findings are consistent with clinical experience treating patients with immune plasma, although the treatment window appears to be greater in humans; treatment within 8 d after initial symptoms (as opposed to 7 d after exposure in the guinea pig) is generally successful (5) (Fig. 5). In humans, neurological involvement (late neurologic syndrome or LNS) is observed in a small percentage (∼10%) of patients treated with immune plasma, but has not been reported in patients who recovered without antibody treatment (22). Unlike the lethal nature of CNS involvement in the guinea pig model, LNS is not fatal in humans, although it is associated with significant morbidity. Serum IgG crosses the blood–brain barrier (BBB) but is found in cerebrospinal fluid (CSF) at 0.1–0.01% the concentration found in serum (23, 24); clinical experience and testing in the guinea pig model indicate that the reduced concentration is insufficient for therapeutic benefit. However, in one report in which guinea pigs were infected intracerebrally with a less virulent JUNV strain (XJ-44), a survival benefit was demonstrated by systemic treatment with immune serum on days 0 and 6 (9), suggesting the potential for antibodies to have a positive effect after the virus has breached the BBB. Increasing the permeability of the BBB to mAbs and other protein therapeutics is a highly active field of research in the pharmaceutical industry (25).
Fig. 5.
Presentation of AHF in guinea pigs and humans. Systemically infected guinea pigs typically are asymptomatic for 7–14 d and then show a rapid decline to death within 0–3 d. In humans, infections, likely transmitted mucosally, are asymptomatic for 4–21 d, followed by a week of flu-like symptoms, and another week of either improvement or further decline.
A comparison of the efficacy of the different agents evaluated historically in the guinea pig model (Table 3) illustrates that antibody-based interventions are superior to the broad-spectrum agents that have been tested, providing a greater survival benefit and a greater therapeutic window. Indeed, recent studies have highlighted the potential of mAbs for intervening late in viral disease (26–29), even under conditions of high viral load. mAbs are generally more potent than broad-spectrum antivirals, but because of their high specificity, mAbs sacrifice broad-spectrum activity. For example, the JUNV mAbs described here do not cross-react with Machupo (10), a related arenavirus. In instances where broadly neutralizing mAbs cannot be identified, combining mAbs of different specificities into a mixture offers a promising approach to develop a drug with high specificity and broad-spectrum activity.
Table 3.
Comparison of survival benefit of candidate JUNV therapeutics in the guinea pig model
| Treatment | Timing of first dose with respect to challenge | Dose (per kg) | Dose frequency | Survival in treated | Survival in controls | Ref. |
| Ribavirin | +4 h | 60 mg | Daily for 24 d | 3/6 | 0/5 | 7 |
| Favipiravir | +1 d | 300 mg (oral) | 2x/day for 14 d | 2/10 | 0/10 | 8 |
| +2 d | 300 mg | 2x/day for 14 d | 7/9 | 1/9 | ||
| Cyclophosphamide | +3 d | 50/25 mg | +3 d/+10 d | 3/18 | 0/18 | 6 |
| Convalescent serum | +6 d | 30,000 TU* | Once | 6/6 | 0/20 | 9 |
| J199 | +6 d | 25 mg | +6/+10 d | 6/6 | 0/3 | |
| +7 d | 25 mg | +7/+11 d | 11/12 | 0/9 |
No less then 3,000 TU/kg is the recommended clinical dose (4). Dosing and challenge are all i.p. unless otherwise noted. Data are not comprehensive but rather highlight the best efficacy demonstrated by the treatment. Antibody-based treatments are highlighted in bold.
The data presented here suggest mAbs could be an efficacious replacement for immune plasma in AHF therapy. Additional virology studies must be performed including characterizing J199’s activity against a selection of clinical isolates (sequences of JUNV GPs in public databases have ≥97% identity) and examining the propensity of the mAb to select for functional escape mutants. Although J199 is at an early development stage for identifying clinical dosing, a preliminary estimate may be gleaned from clinical dosing with immune plasma, which is based on the neutralizing titer of the donor plasma (4). The currently recommended clinical dose is 3000 therapeutic units (TUs) (TU = mL × 1/PRN80 titer). With the high neutralization potency of J199 representing ∼3,333 TUs per mg, dosage for a 70-kg adult equates to 63 mg of J199. Commercial prices of most FDA-licensed mAb products range from $2–10/mg (30, 31), suggesting the commercial cost of treatment using a mAb like J199 could be under $200 with current manufacturing processes, and even less costly in the future given continuing improvements with the pharmacoeconomics of traditional and novel manufacturing systems. Thus, mAb therapy of AHF appears to be technically and economically feasible.
Materials and Methods
Production of the JUNV mAbs.
The mAbs described in Sanchez et al. (10) were given a simpler designation: GB-03-BE08 = J199; GD01-AG02 = J200; OD01-AA09 = J202. Genes containing the variable region sequences of J199, J200, and J202 were synthesized (Life Technologies) and subsequently cloned into plant expression vectors (TMV and PVX; Icon Genetics) containing codon-optimized human kappa and human IgG1 constant regions followed by transformation into Agrobacterium tumefaciens strain ICF320 (Icon Genetics). N. benthamiana plants genetically modified to produce highly homogenous mammalian N-glycans of the GnGn glycoform (13) were grown for 4 wk in an enclosed growth room (20–23 °C) and used for vacuum infiltration as described (16). Seven days after infiltration, the mAb was extracted from the leaf tissue and purified via protein A chromatography (16), then passed through an Acrodisc Unit with Mustang Q Membrane (Pall Life Sciences) by using a syringe for endotoxin reduction. Endotoxin levels were measured with Endosafe PTS (Charles River) and were less than 150 EU/mg. N-glycan analysis was performed by liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS) of tryptic glycopeptides (32). In short, bands that correspond to the heavy chain in Coomassie-stained SDS/PAGEs were excised, S-alkylated, digested with trypsin, and subsequently analyzed by LC-ESI-MS.
Plaque Reduction Neutralization Assay.
The mAbs were serially diluted threefold in infection medium (MEM supplemented with 1% FBS, L-gln; Pen/Strep) starting at 200 μg/mL. Junín virus strain Candid #1 (JUNV) was diluted in infection medium to 2,000 pfu/mL. Each dilution of the antibodies was mixed with an equal volume of JUNV to achieve a final concentration of 100 µg/mL and 100 pfu of JUNV. A virus-only control was incubated with medium alone. The dilutions were then incubated for 1 h at 37 °C in a humidified CO2 atmosphere. Vero cells seeded in 24-well plates to near confluency were infected with the dilutions for 1 h before 0.8% methylcellulose was added as overlay. After 8 d, plaques were visualized by staining the cell monolayer with crystal violet in 5% (vol/vol) glutaraldehyde. Effective concentrations 50 and 80 (EC50 and EC80) were calculated (μg/mL) relative to the virus-only control by using 4PL curve fit (XLfit model 205).
Guinea Pig Model.
JUNV strain Romero (P3235) was obtained from Thomas Ksiazek (Galveston National Laboratory; GNL). Animal studies were completed under BSL-4 biocontainment at the GNL and were approved by the University of Texas Medical Branch (UTMB) Institutional Laboratory Animal Care and Use Committee in accordance with state and federal statutes and regulations relating to experiments involving animals and the Institutional Biosafety Committee. Female outbred Hartley guinea pigs (351–400 g) were purchased from Charles River Laboratories and subsequently quarantined and acclimatized before JUNV challenge. Transponders for temperature recording were implanted before the acclimatization period. Individual animals were infected with ∼1,000 pfu of JUNV by i.p. injection. mAbs were administered i.p. in volumes of 0.8–0.9 mL. Studies were concluded 28, 30, or 40 d after infection. JUNV titration was performed by conventional plaque assay on Vero E6 cells from plasma collected from guinea pigs at various times after infection (33). In brief, increasing 10-fold dilutions of the samples were adsorbed to Vero E6 monolayers in duplicate wells (200 μL). The limit of detection was 25 pfu/mL. Histopathology was performed on liver, spleen, and brain tissue by using H&E staining and immunohistochemistry. Specific anti-JUNV immunoreactivity was detected by using an anti-JUNV primary antibody (kindly provided by Thomas Ksiazek) at a 1:1600 dilution or 60 min. The tissues were processed for immunohistochemistry by using the Dako Autostainer. Secondary used was biotinylated goat anti-mouse IgG at 1:1600 for 30 min followed by Dako LSAB2 streptavidin-HRP (K1016) for 30 min. Slides were developed with Dako DAB chromagen (K3468) for 5 min and counterstained with Harris Hematoxylin for 1 min. Survival curves were analyzed with the log-rank (Mantel–Cox) test by using Prism (Version 5.0d).
Data and Materials Availability.
Chimeric anti-JUNV mAbs can be obtained through a Material Transfer Agreement with Mapp.
Acknowledgments
We thank Drs. Yuri Gleba and Victor Klimyuk (Icon Genetics) for access to the magnICON expression technology and Natalie Dobias and the Research Histopathology Core at UTMB for optimization of the immunohistochemistry protocol. This publication was made possible by National Institute of Allergy and Infectious Disease Grant AI111391.
Footnotes
Conflict of interest statement: K.J.W. and L.Z. are co-owners of Mapp Biopharmaceutical, Inc.
This article is a PNAS Direct Submission.
References
- 1.Grant A, et al. Junín virus pathogenesis and virus replication. Viruses. 2012;4(10):2317–2339. doi: 10.3390/v4102317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weissenbacher MC, de Guerrero LB, Boxaca MC. Experimental biology and pathogenesis of Junin virus infection in animals and man. Bull World Health Organ. 1975;52(4-6):507–515. [PMC free article] [PubMed] [Google Scholar]
- 3.Enria DA, Ambrosio AM, Briggiler AM, Feuillade MR, Crivelli E. Study Group on Argentine Hemorrhagic Fever Vaccine [Candid#1 vaccine against Argentine hemorrhagic fever produced in Argentina. Immunogenicity and safety] Medicina (B Aires) 2010;70(3):215–222. [PubMed] [Google Scholar]
- 4.Enria DA, Briggiler AM, Fernandez NJ, Levis SC, Maiztegui JI. Importance of dose of neutralising antibodies in treatment of Argentine haemorrhagic fever with immune plasma. Lancet. 1984;2(8397):255–256. doi: 10.1016/s0140-6736(84)90299-x. [DOI] [PubMed] [Google Scholar]
- 5.Enria DA, Briggiler AM, Sánchez Z. Treatment of Argentine hemorrhagic fever. Antiviral Res. 2008;78(1):132–139. doi: 10.1016/j.antiviral.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponzinibbio C, González P, Laguens RP. Protective effect of a low-dose of cyclophosphamide in experimental infection of guinea pigs with Junin virus. J Med Virol. 1989;29(2):146–151. doi: 10.1002/jmv.1890290213. [DOI] [PubMed] [Google Scholar]
- 7.Salazar M, et al. Effect of ribavirin on junin virus infection in guinea pigs. Zoonoses Public Health. 2012;59(4):278–285. doi: 10.1111/j.1863-2378.2011.01447.x. [DOI] [PubMed] [Google Scholar]
- 8.Gowen BB, et al. Favipiravir (T-705) inhibits Junín virus infection and reduces mortality in a guinea pig model of Argentine hemorrhagic fever. PLoS Negl Trop Dis. 2013;7(12):e2614. doi: 10.1371/journal.pntd.0002614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenyon RH, Green DE, Eddy GA, Peters CJ. Treatment of junin virus-infected guinea pigs with immune serum: Development of late neurological disease. J Med Virol. 1986;20(3):207–218. doi: 10.1002/jmv.1890200303. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez A, et al. Junin virus monoclonal antibodies: Characterization and cross-reactivity with other arenaviruses. J Gen Virol. 1989;70(Pt 5):1125–1132. doi: 10.1099/0022-1317-70-5-1125. [DOI] [PubMed] [Google Scholar]
- 11.Pogue GP, et al. Production of pharmaceutical-grade recombinant aprotinin and a monoclonal antibody product using plant-based transient expression systems. Plant Biotechnol J. 2010;8(5):638–654. doi: 10.1111/j.1467-7652.2009.00495.x. [DOI] [PubMed] [Google Scholar]
- 12.Giritch A, et al. Rapid high-yield expression of full-size IgG antibodies in plants coinfected with noncompeting viral vectors. Proc Natl Acad Sci USA. 2006;103(40):14701–14706. doi: 10.1073/pnas.0606631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strasser R, et al. Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol J. 2008;6(4):392–402. doi: 10.1111/j.1467-7652.2008.00330.x. [DOI] [PubMed] [Google Scholar]
- 14.Zeitlin L, et al. Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc Natl Acad Sci USA. 2011;108(51):20690–20694. doi: 10.1073/pnas.1108360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiatt A, et al. Glycan variants of a respiratory syncytial virus antibody with enhanced effector function and in vivo efficacy. Proc Natl Acad Sci USA. 2014;111(16):5992–5997. doi: 10.1073/pnas.1402458111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeitlin L, et al. Prophylactic and therapeutic testing of Nicotiana-derived RSV-neutralizing human monoclonal antibodies in the cotton rat model. MAbs. 2013;5(2):263–269. doi: 10.4161/mabs.23281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenyon RH, Condie RM, Jahrling PB, Peters CJ. Protection of guinea pigs against experimental Argentine hemorrhagic fever by purified human IgG: Importance of elimination of infected cells. Microb Pathog. 1990;9(4):219–226. doi: 10.1016/0882-4010(90)90010-n. [DOI] [PubMed] [Google Scholar]
- 18.Bournazos S, DiLillo DJ, Ravetch JV. The role of Fc-FcγR interactions in IgG-mediated microbial neutralization. J Exp Med. 2015;212(9):1361–1369. doi: 10.1084/jem.20151267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boere WA, Benaissa-Trouw BJ, Harmsen M, Kraaijeveld CA, Snippe H. Neutralizing and non-neutralizing monoclonal antibodies to the E2 glycoprotein of Semliki Forest virus can protect mice from lethal encephalitis. J Gen Virol. 1983;64(Pt 6):1405–1408. doi: 10.1099/0022-1317-64-6-1405. [DOI] [PubMed] [Google Scholar]
- 20.Bournazos S, et al. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell. 2014;158(6):1243–1253. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmaljohn AL, Johnson ED, Dalrymple JM, Cole GA. Non-neutralizing monoclonal antibodies can prevent lethal alphavirus encephalitis. Nature. 1982;297(5861):70–72. doi: 10.1038/297070a0. [DOI] [PubMed] [Google Scholar]
- 22.Maiztegui JI, Fernandez NJ, de Damilano AJ. Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurological syndrome. Lancet. 1979;2(8154):1216–1217. doi: 10.1016/s0140-6736(79)92335-3. [DOI] [PubMed] [Google Scholar]
- 23.Reiber H. Dynamics of brain-derived proteins in cerebrospinal fluid. Clin Chim Acta. 2001;310(2):173–186. doi: 10.1016/s0009-8981(01)00573-3. [DOI] [PubMed] [Google Scholar]
- 24.Tabrizi M, Bornstein GG, Suria H. Biodistribution mechanisms of therapeutic monoclonal antibodies in health and disease. AAPS J. 2010;12(1):33–43. doi: 10.1208/s12248-009-9157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azad TD, et al. Therapeutic strategies to improve drug delivery across the blood-brain barrier. Neurosurg Focus. 2015;38(3):E9. doi: 10.3171/2014.12.FOCUS14758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geisbert TW, et al. Therapeutic treatment of Nipah virus infection in nonhuman primates with a neutralizing human monoclonal antibody. Sci Transl Med. 2014;6(242):242ra82. doi: 10.1126/scitranslmed.3008929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leyva-Grado VH, Tan GS, Leon PE, Yondola M, Palese P. Direct administration in the respiratory tract improves efficacy of broadly neutralizing anti-influenza virus monoclonal antibodies. Antimicrob Agents Chemother. 2015;59(7):4162–4172. doi: 10.1128/AAC.00290-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu X, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514(7520):47–53. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith SA, et al. Isolation and characterization of broad and ultrapotent human monoclonal antibodies with therapeutic activity against chikungunya virus. Cell Host Microbe. 2015;18(1):86–95. doi: 10.1016/j.chom.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelley B. Industrialization of mAb production technology: The bioprocessing industry at a crossroads. MAbs. 2009;1(5):443–452. doi: 10.4161/mabs.1.5.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hagel L, Jagschies G, Sofer G, (2008) Handbook of Process Chromatography: Development, Manufacturing, Validation and Economics (Elsevier, London), 2nd Ed.
- 32.Stadlmann J, Pabst M, Kolarich D, Kunert R, Altmann F. Analysis of immunoglobulin glycosylation by LC-ESI-MS of glycopeptides and oligosaccharides. Proteomics. 2008;8(14):2858–2871. doi: 10.1002/pmic.200700968. [DOI] [PubMed] [Google Scholar]
- 33.Jahrling PB. 1999. Filoviruses and arenaviruses. Manual of Clinical Microbiology, ed PR Murray EB, M Pfaller, FC Tenover, RH Yolken (Am Soc Microbiol, Washington), pp 1125–1136.