Significance
Early events during Proteus mirabilis-mediated urinary tract infection have not been well defined. Here we demonstrate that, in contrast to uropathogenic Escherichia coli, P. mirabilis rarely forms bladder intracellular communities. Rather, in the bladder lumen it establishes large urease- and mannose-resistant Proteus-like fimbriae-dependent clusters, which draw a massive neutrophil response and are precursors to urinary stones.
Keywords: Proteus mirabilis, urinary tract infection, urease, fimbriae, bladder stones
Abstract
The catheter-associated uropathogen Proteus mirabilis frequently causes urinary stones, but little has been known about the initial stages of bladder colonization and stone formation. We found that P. mirabilis rapidly invades the bladder urothelium, but generally fails to establish an intracellular niche. Instead, it forms extracellular clusters in the bladder lumen, which form foci of mineral deposition consistent with development of urinary stones. These clusters elicit a robust neutrophil response, and we present evidence of neutrophil extracellular trap generation during experimental urinary tract infection. We identified two virulence factors required for cluster development: urease, which is required for urolithiasis, and mannose-resistant Proteus-like fimbriae. The extracellular cluster formation by P. mirabilis stands in direct contrast to uropathogenic Escherichia coli, which readily formed intracellular bacterial communities but not luminal clusters or urinary stones. We propose that extracellular clusters are a key mechanism of P. mirabilis survival and virulence in the bladder.
The pathogen Proteus mirabilis is linked with catheter-associated urinary tract infections (UTIs), where it can cause complications including crystalline biofilms, urinary stones, pyelonephritis, and septicemia (1–3). Although numerous studies have identified aspects of P. mirabilis biology that are important for infection, the P. mirabilis pathogenic life cycle is poorly understood. Moreover, we lack a mechanistic understanding of the contributions of essential virulence factors. For example, although mannose-resistant Proteus-like (MR/P) fimbriae are required to maximally colonize the bladder and kidneys and are important for hemagglutination, biofilm formation, and auto-aggregation in vitro, their binding target and in vivo role remain elusive (4–6).
Visualization of P. mirabilis in experimentally infected mice at 48 h postinfection (hpi) and beyond found P. mirabilis in the urinary tract individually, in groups, and embedded in urinary stones (5, 7–9). Urolithiasis, the process of stone formation, requires urease, which releases ammonia and increases urinary pH via urea hydrolysis, resulting in the precipitation of calcium and magnesium compounds into urinary stones (10, 11). However, early stages in stone development remained unobserved in vivo, and it was unclear how this process was connected to early events in colonization and pathogenesis.
The early colonization events of uropathogenic Escherichia coli (UPEC), a major cause of uncomplicated UTI, have been delineated. Upon bladder entry, UPEC attaches to superficial urothelial cells (umbrella cells) that line the bladder lumen (12). The apical surface of umbrella cells is covered in 2D crystalline plaques formed by heterotetramers of four uroplakin proteins (UPIa, UPIb, UPII, and UPIIIa) (13). UPEC adheres to UPIa on the apical surface with type 1 fimbriae, which triggers the uptake of UPEC by umbrella cells (12). Once inside umbrella cells, UPEC can multiply and form organized groups of tightly packed bacteria known as intracellular bacterial communities (IBCs) (14). These IBCs may completely fill the umbrella cell before the cell either ruptures or is exfoliated, causing a rapid increase in umbrella cell turnover (14–16). In addition to intracellular replication, UPEC can multiply extracellularly in the bladder lumen, reaching up to 107 colony forming units (cfu)/mL (17, 18).
In this study, we compare the initial events of P. mirabilis bladder infection with the well-established life cycle of UPEC. Although initial attachment and invasion of P. mirabilis resembles that of UPEC, P. mirabilis forms IBCs that are significantly rarer and smaller than UPEC IBCs. Instead, the majority of P. mirabilis bacteria are in large, extracellular clusters in the bladder lumen. These clusters promote extensive neutrophil infiltration and deposition of calcium ions, a crucial initial step in urolithiasis. Importantly, cluster development requires both MR/P fimbriae and urease. This work elucidates a P. mirabilis life style that is distinct from UPEC’s and uncovers functions for two critical virulence factors in infection.
Results
P. mirabilis Rapidly Invades the Urothelium but Rarely Establishes IBCs.
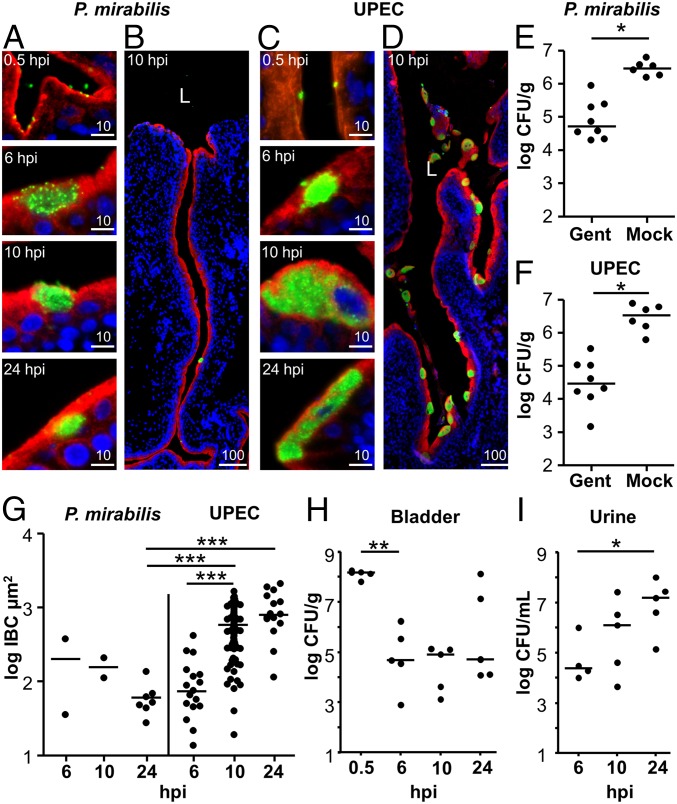
Previous studies of P. mirabilis pathogenesis did not document events within the first 24 h of infection. To investigate the in vivo localization of P. mirabilis during the early stages of pathogenesis, we visualized bacteria and the urothelial surface—defined by UPIIIa staining—in sections of infected murine bladders during the first 24 hpi. We found that P. mirabilis attached to the urothelial surface by 0.5 hpi. In some instances, P. mirabilis and UPIIIa staining overlapped, suggesting that P. mirabilis may invade the urothelium within this time frame (Fig. 1A). However, we infrequently observed intracellular bacteria at later time points; although P. mirabilis formed IBCs at 6, 10, and 24 hpi (Fig. 1A), IBCs were rare (Table 1 and Fig. 1B) and small (Fig. 1G).
Fig. 1.
P. mirabilis invades the urothelium, but infrequently forms IBCs. (A–D) Representative images of P. mirabilis (A and B) and UPEC (C and D) attachment and invasion. Bacteria (green), UPIIIa (red), and DNA (blue) show localization of the bacteria relative to the apical surface of the urothelium. (Scale bars, 10 µm.) (B and D) A regional view of the bladder section containing the 10 hpi IBC shown in A and C, respectively. (Scale bars, 100 µm.) L, bladder lumen. (E and F) Quantification of P. mirabilis (E) and UPEC (F) bladder invasion at 0.5 hpi following either ex vivo gentamicin treatment (Gent) or mock treatment (Mock) (n = 6–8). *P < 0.05. (G) Size of P. mirabilis and UPEC IBCs. Every observed IBC was measured from P. mirabilis-infected sections, as well as from UPEC-infected sections at 6 and 24 hpi. However, due to the high number of IBCs in UPEC-infected mice at 10 hpi, sizes were determined from two sections, each from a different mouse. See Table 1 for details on the IBC frequency relative to the number of sections and mice. (H and I) Quantification of the cfu detected during P. mirabilis infection of the bladder (H) and urine (I). Each data point represents an individual animal.
Table 1.
Quantification of IBCs in infected bladders
| Observations | P. mirabilis | UPEC | ||||
| 6 hpi | 10 hpi | 24 hpi | 6 hpi | 10 hpi | 24 hpi | |
| IBCs | 2 | 2 | 7 | 17 | 337 | 20 |
| Sections examined | 16 | 34 | 39 | 10 | 9 | 11 |
| Mice with IBCs | 1 | 1 | 2 | 2 | 2 | 2 |
| Mice with clusters | 0 | 2 | 5 | 0 | 0 | 0 |
| Total mice | 4 | 7 | 7 | 3 | 2 | 2 |
As a comparison, we examined UPEC in our model system. UPEC also attached to and appeared to invade the urothelial surface by 0.5 hpi (Fig. 1C). By 6 hpi, most detected bacteria were intracellular (Fig. 1C). At 10 hpi, IBCs were larger and more abundant, and the bladder lumen contained shed epithelial cells with IBCs (Fig. 1 C and D). Finally, at 24 hpi, we observed a decrease in the number of IBCs, consistent with the exfoliation of epithelial cells containing IBCs (Table 1). Notably, UPEC IBCs at 10 and 24 hpi were significantly larger than P. mirabilis IBCs at their most abundant time point (Fig. 1G, 24 hpi).
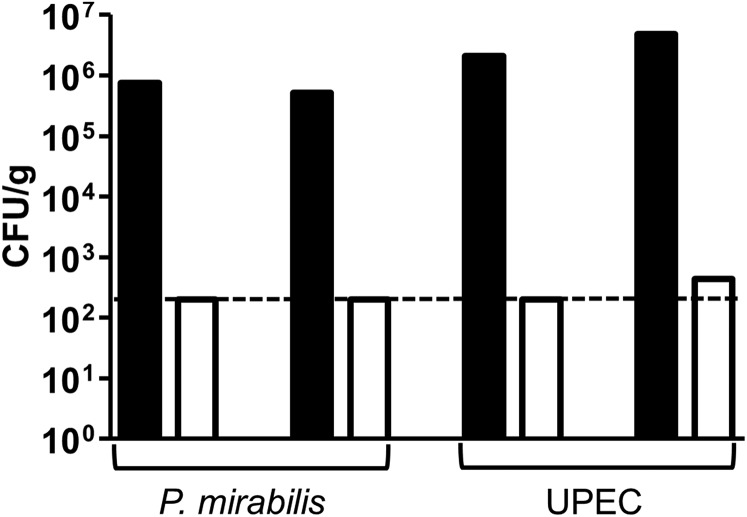
Because we observed evidence of invasion, we used an ex vivo gentamicin protection assay to enumerate intracellular bacteria. Both P. mirabilis and UPEC invaded the bladder urothelium by 0.5 hpi, as shown by a significant difference between gentamicin- and mock-treated bladders (P = 0.0107 and 0.0194, respectively), and both bacterial species had a similar amount of invasion (5.3 × 104 and 3.0 × 104 cfu/g tissue, respectively) (Fig. 1 E and F). When bladders were homogenized before gentamicin treatment, we rarely detected viable bacteria, indicating that gentamicin treatment effectively kills accessible bacteria (Fig. S1).
Fig. S1.
Gentamicin treatment kills accessible bacteria. To test the efficacy of the gentamicin treatment protocol, one-half of a murine bladder was homogenized in 1.2 mL DMEM. A total of 500 µL of the homogenized bladder was either mock-treated or treated with 100 µg/mL gentamicin at 37 °C for 1 h with gentle shaking. The homogenates were washed three times with PBS, serially diluted, and plated (n = 2 per bacterial species). Black bars, mock treatment. White bars, gentamicin treatment. The 200 cfu/g limit of detection is indicated with a dashed line.
To assess bacterial colonization over time, we enumerated the total P. mirabilis load in murine bladders at 0.5, 6, 10, and 24 hpi (Fig. 1H). At 0.5 hpi, infection was tightly clustered at a median of 1.5 × 108 cfu/g bladder. By 6 hpi, the bacterial burden decreased significantly to an average of 4.8 × 104 cfu/g bladder (P = 0.0079), whereas the variability in bacterial burden increased (Fig. 1H). Between 6 and 24 hpi the bacterial burden in the urine (P = 0.0317), but not in the bladder tissue, increased significantly (Fig. 1I). Individual mice with higher bladder bacterial burdens emerged at 24 hpi, suggesting the beginning of divergent disease courses (Fig. 1H). Notably, we observed differences in the bacterial load when bladders were homogenized immediately (1.5 × 108 cfu/g) (Fig. 1H) versus when they were homogenized after mock treatment and washes (2.9 × 106 cfu/g) (Fig. 1E), suggesting that at this early time point a large proportion of P. mirabilis weakly associate with the bladder surface.
P. mirabilis Forms Large Extracellular Clusters in the Bladder Lumen.
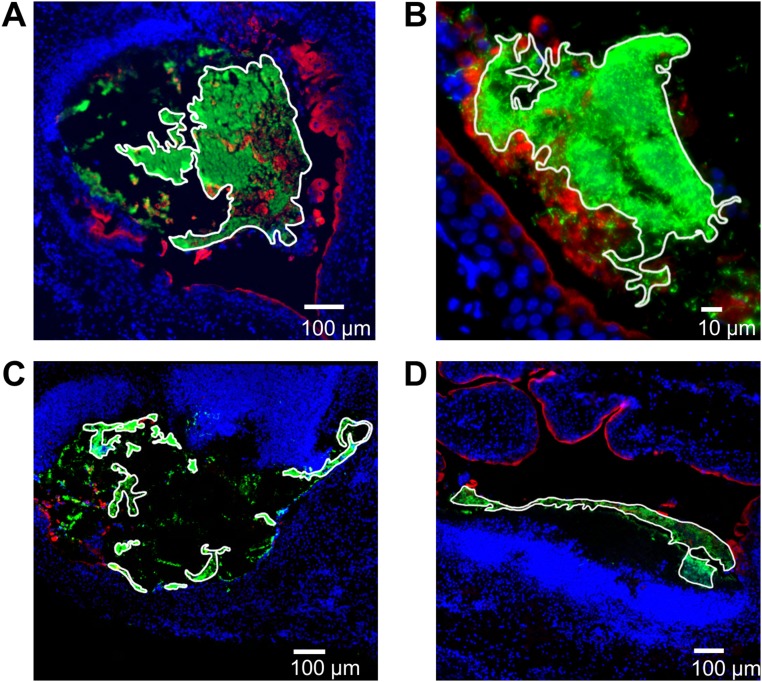
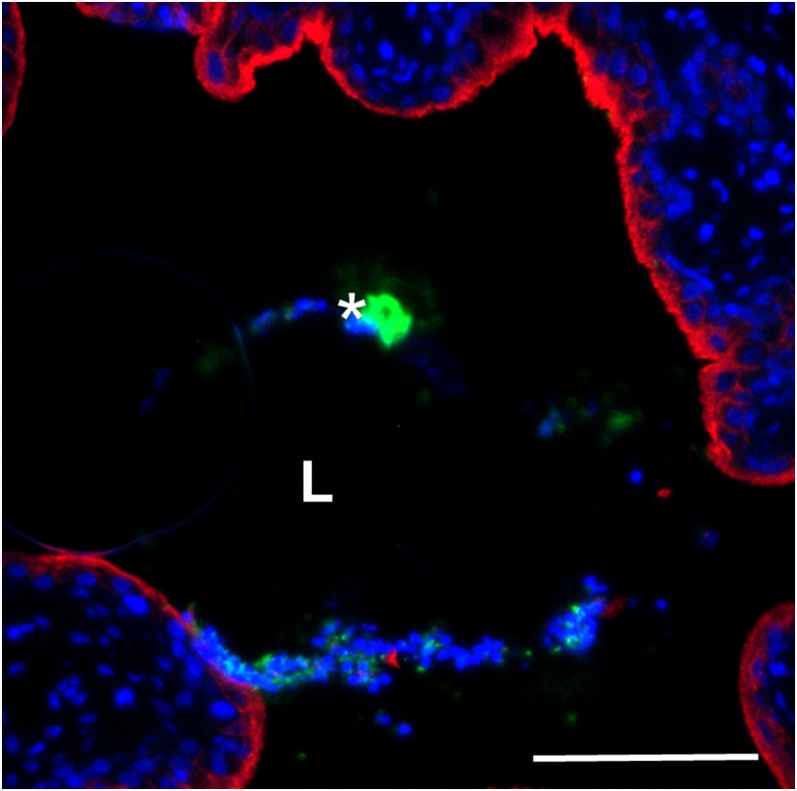
Remarkably, although P. mirabilis was difficult to detect using microscopy at 6 hpi, by 24 hpi P. mirabilis formed large extracellular clusters (median 5.9 × 104 µm2, n = 6) (Fig. S2) in the bladder lumen of the majority (five of seven) of infected mice (Fig. 2 A and C). Mice at 10 hpi displayed an intermediate phenotype with clusters detected in two of seven mice (Fig. 2 B and C). Generally, clusters were directly adjacent to the bladder surface, associated with urothelial destruction (as shown by minimal surface-associated uroplakin staining adjacent to the cluster), and had a large, unusual region of dense DAPI staining near the cluster. However, at both 10 hpi (Fig. 2B) and 24 hpi (Fig. S3), one of the detected clusters was unattached to the urothelium, showed limited urothelial damage, and had normal DAPI staining. We suspect that these two clusters represent either early stages of cluster formation or successful disruption of cluster development by the host. Clusters were rarely observed in voided urine (Fig. S4). Because the formation of these exceptionally large luminal bacterial clusters during UTI was an intriguing finding, we focused further studies on this phenotype.
Fig. S2.
Determination of cluster size. For each cluster observed, the paraffin section with the largest bacterial cross-section was selected for sizing. FIJI software was used to outline the contiguous edge of the cluster and calculate the enclosed area. Here, the outlines were emphasized using Photoshop. (A and B) Clusters shown in Fig. 2. (C and D) Clusters shown in Fig. 4.
Fig. 2.
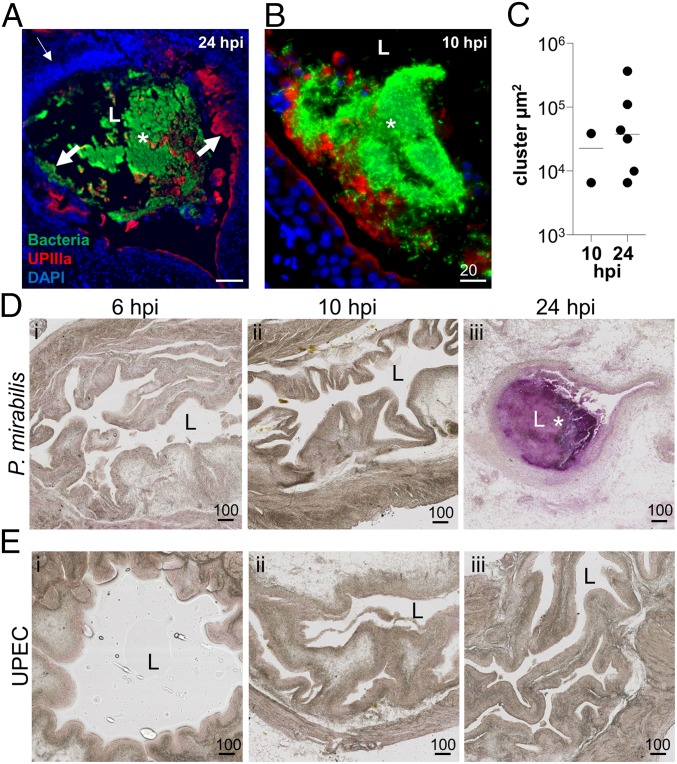
P. mirabilis extracellular clusters are precursors to stone formation. (A and B) A representative image of a P. mirabilis cluster at 24 hpi (A). (Scale bar, 100 µm.) (B) A P. mirabilis cluster at 10 hpi with limited urothelial destruction and normal DAPI staining. (Scale bar, 20 µm.) In both A and B, staining of bacteria (green), UPIIIa (red), and DNA (blue) show accumulation of bacterial clusters at the bacteria–bladder interface. An asterisk indicates an extracellular cluster; L, bladder lumen. The thin arrow indicates a region with increased DAPI signal, whereas thick arrows indicate areas of extensive urothelial damage. (C) Area of every extracellular cluster detected in P. mirabilis-infected murine bladders (n = 7 mice each at 10 and 24 hpi). (D and E) Detection of mineral deposition using Alizarin Red staining of (D) P. mirabilis- and (E) UPEC-infected bladder sections at (i) 6, (ii) 10, and (iii) 24 hpi. (Scale bars, 100 µm.) L, bladder lumen; an asterisk indicates extracellular cluster (purple staining).
Fig. S3.
P. mirabilis minor cluster at 24 hpi. The staining of bacteria (green), UPIIIa (red), and DNA (blue) show a small cluster, unattached to the urothelium, with limited nonurothelial DAPI signal. The asterisk indicates an extracellular cluster; L, bladder lumen. (Scale bar, 100 µm.)
Fig. S4.
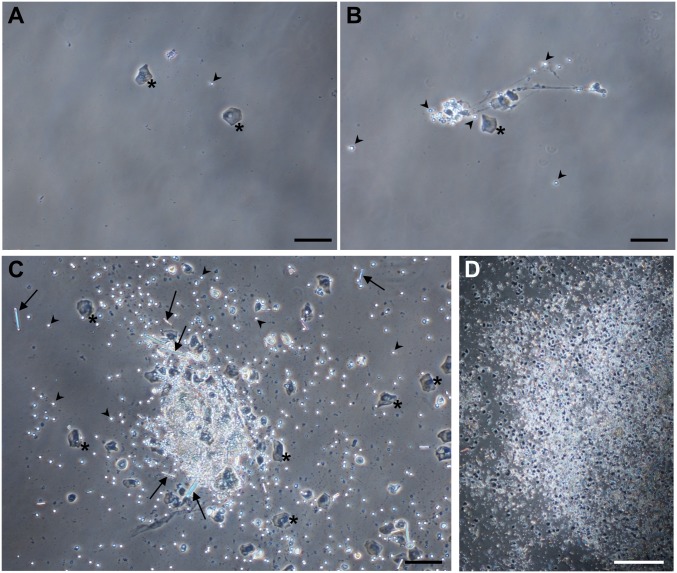
Visualization of clusters in voided urine. Mice (n = 7) were infected with wild-type P. mirabilis. At 24 hpi, urine was collected and Giemsa-stained for microscopic viewing. (A) Typical urine image with single epithelial cells, leukocytes, and planktonic bacteria but no clusters. (B) A mass of leukocytes and epithelial cells connected by stringy material is suggestive of NET formation; this image is from the same mouse as in A. (C and D) Urine from one mouse was visibly thicker and contained multiple clusters consisting of epithelial cells, leukocytes, bacteria, and rod-shaped crystalline material. Asterisks indicate an epithelial cell; arrowhead, leukocyte; arrow, crystalline material; bacteria are among the pinpoint background dots. (Scale bars in A–C, 100 μm; in D, 500 μm.)
P. mirabilis Extracellular Clusters Are an Early Stage of Urolithiasis.
A classic characteristic of P. mirabilis UTI is the development of urinary stones. Although mature stones have been imaged, early stones were undocumented in vivo (11). We hypothesized that concentrated urease activity within clusters would cause localized deposition of struvite and carbonate apatite. To test this hypothesis, we used Alizarin Red staining, which primarily detects calcium salts, but can also detect magnesium and other minerals (8). In support of our hypothesis, Alizarin Red strongly stained sections of P. mirabilis-infected bladders that contained extracellular clusters at 24 hpi (Fig. 2D). Conversely, P. mirabilis-infected bladders without clusters, regardless of the timing, were unstained by Alizarin Red (Fig. 2D). As expected, because UPEC does not produce urease, sections of highly infected bladders with many IBCs remained Alizarin Red-negative (Fig. 2E). These data support our hypothesis that P. mirabilis clusters result in mineral deposition that serves as an early step in stone formation.
P. mirabilis Extracellular Clusters Induce an Extensive Neutrophil Response.
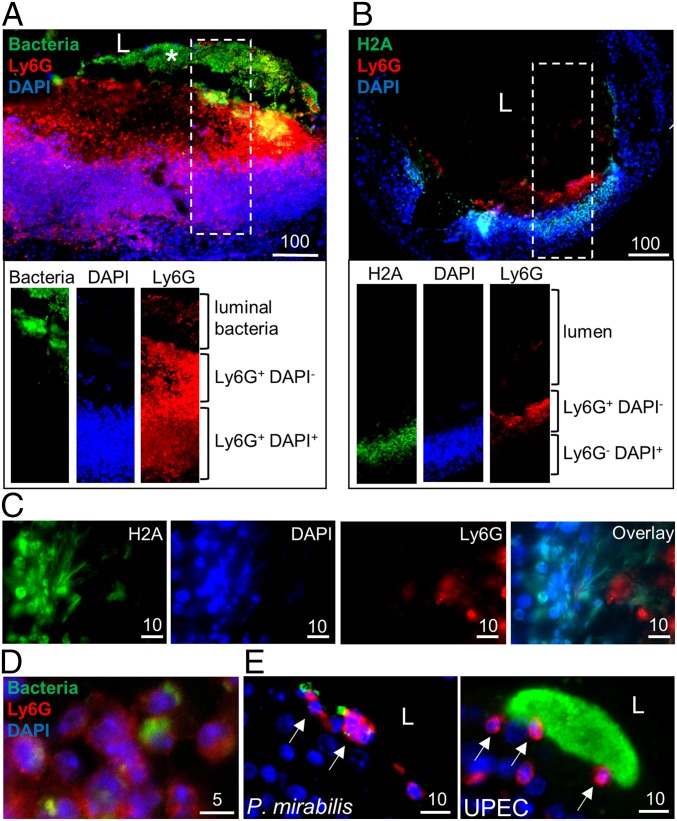
We reasoned that a large cluster of P. mirabilis cells associated with epithelial destruction in the bladder would induce a potent immune response; furthermore, neutrophil influx is critical for clearance of UPEC bladder infection (19). In support of this, we often observed (n = 5 of 6 clusters) a large mass of DAPI staining adjacent to P. mirabilis extracellular clusters at 24 hpi (Fig. 2A), which also stained positive for the surface-expressed, neutrophil-specific marker Ly6G (20, 21), in the densely stained DAPI region adjacent to bacterial clusters. We detected three unique patterns of Ly6G/DAPI staining in areas near clusters: Ly6G+DAPI+ (Fig. 3A), Ly6G+DAPI− (Fig. 3 A and B), and Ly6G−DAPI+ (Fig. 3B). We hypothesized that Ly6G+DAPI− and Ly6G−DAPI+ regions may represent neutrophil extracellular traps (NETs)—extracellular webs of DNA covered in antimicrobial peptides and proteins released by the neutrophil cell body—that trap and kill pathogens (22). To identify NETs, we stained for H2A, a histone marker that remains attached to eukaryotic DNA during NETosis, the process of NET formation (22, 23). Indeed, we observed colocalization of H2A and DAPI in areas adjacent to Ly6G+ staining (Fig. 3 B and C). Upon magnification, we observed a fiber-like pattern of overlapping H2A and DAPI staining independent of the membrane Ly6G staining, indicative of NET formation (Fig. 3C). The degree of separation between nuclear contents (H2A+DAPI+) and the membrane staining (Ly6G+) suggests that neutrophils may be undergoing vital NETosis, in which neutrophils continue to function as anuclear cytoplasts after NET expulsion (24, 25). We also observed a NET-like mass of neutrophils and epithelial cells in the urine of one mouse (Fig. S4B). Finally, we reasoned that Ly6G+DAPI+ regions could represent intact neutrophils: indeed, some of the Ly6G+DAPI+ cells appeared to have phagocytosed bacteria, a prototypical function of these innate immune cells (Fig. 3D).
Fig. 3.
P. mirabilis induces neutrophil recruitment and NET formation. (A and B) Visualization of neutrophil recruitment at extracellular clusters at 24 hpi. Individual channels for the region enclosed in the dashed rectangle are shown below at the same magnification. (Scale bars, 100 µm.) (C) Identification of NET formation in regions of neutrophil recruitment at 24 hpi. Individual channels representing nuclear stains (H2A and DAPI) and membrane stains (Ly6G) show the overlap of DAPI and H2A distant from Ly6G staining. (Scale bars, 10 µm.) (D) Neutrophil phagocytosis of P. mirabilis at 24 hpi. Arrows indicate neutrophils which have phagocytosed bacteria. (E) Individual neutrophil recruitment in sections of murine bladders infected with P. mirabilis without clusters at 24 hpi and UPEC-infected sections at 10 hpi. Bacteria (green), Ly6G (red), and DNA (blue) show neutrophils adjacent to bacteria. Arrows indicate intact neutrophils.
As a comparison, we assessed neutrophil influx in P. mirabilis-infected bladders in cluster-distal regions, as well as in UPEC-infected bladders. For UPEC, we found only individual, apparently intact neutrophils in the vicinity of bacteria, including IBCs, at 10 and 24 hpi (Fig. 3E). We saw a similar phenotype in regions of P. mirabilis-infected bladders distant from clusters at 10 and 24 hpi (Fig. 3E). We did not detect neutrophils in naive bladders, consistent with other reports (26, 27). Taken together, these data suggest that P. mirabilis clusters elicit a specific immune response that is robust and distinct from UPEC.
Formation of P. mirabilis Extracellular Clusters Requires Both MR/P Fimbriae and Urease.
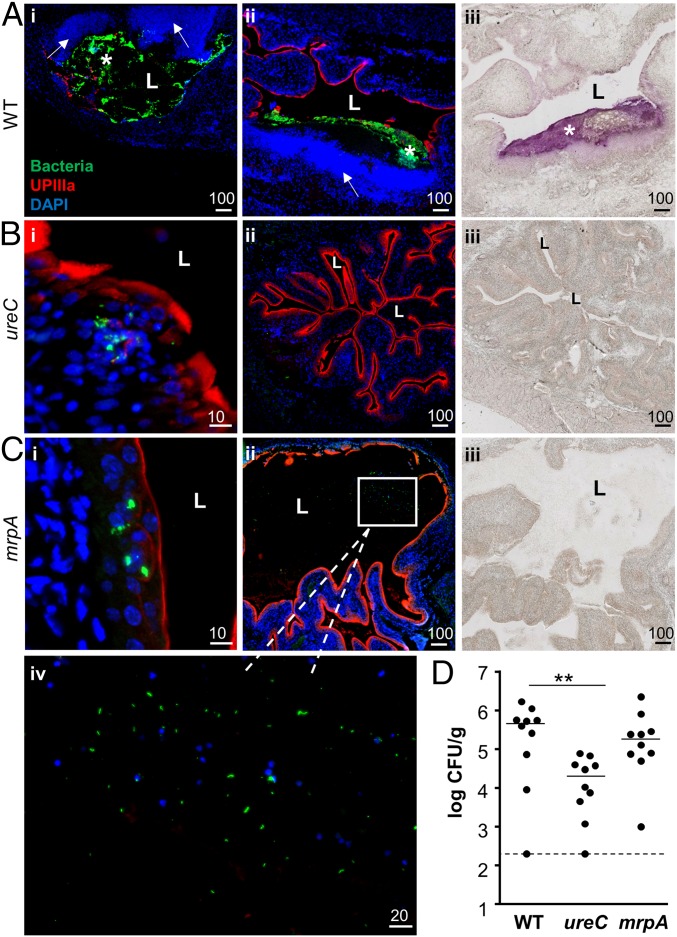
We next sought to identify bacterial factors that mediate cluster formation. Because our evidence suggested that these clusters are a precursor to stone formation, we compared the phenotype of a urease-negative P. mirabilis ureC mutant to the wild-type parent at 24 hpi. As expected, clusters in wild-type infected bladders detected by immunofluorescence closely corresponded with Alizarin Red-positive regions (Fig. 4A). In contrast, we did not observe Alizarin Red staining or extracellular clusters in sections from mice infected with the ureC mutant (n = 5) (Fig. 4B). Instead, bacteria were rarely observed and primarily in small intracellular groups (Fig. 4B). These results indicate that urease is important for the formation and/or maintenance of extracellular clusters as well as mineral deposition. We also compared the bacterial load of the ureC mutant to wild-type P. mirabilis at 24 hpi and found that the mutant had a 23-fold lower cfu/g than wild type (2.0 × 104 cfu/g tissue compared with 4.6 × 105 cfu/g tissue, respectively; P = 0.0091) (Fig. 4D). This is consistent with a previous study that reported a significant defect in bladder colonization at 48 hpi by the ureC mutant (11). In contrast, this mutant does not have an in vitro growth defect under a variety of culture conditions (Fig. S5). Overall, these data suggest that the ureC mutant is unable to thrive either intracellularly or extracellularly.
Fig. 4.
P. mirabilis mrpA and ureC mutants are defective in cluster formation. (A–C) Representative sections of (A) P. mirabilis wild-type, (B) ureC, and (C) mrpA-infected murine bladders at 24 hpi. The wild-type–infected bladders show two regional views of clusters, whereas the mutant-infected bladders show a close-up of the urothelial surface (B, i; C, i] and a regional view (B, ii; C, ii). A region with a loose aggregate of mrpA mutant bacteria is boxed in C, ii, and magnified in C, iv. Bacteria are in green, UPIIIa in red, and DAPI in blue. (Scale bars, in micrometers, are as marked.) (A–C, iii) Alizarin Red staining of a section proximate to the regional view shown by immunofluorescent staining. Only wild-type P. mirabilis-infected bladders contain significant mineral deposition. L, bladder lumen; an asterisk indicates an extracellular cluster. Arrows indicate regions with increased DAPI signal. (D) Quantification of P. mirabilis wild type and mutant bladder colonization at 24 hpi. Dashed line indicates the limit of detection (200 cfu/g). n = 10 mice per strain tested.
Fig. S5.
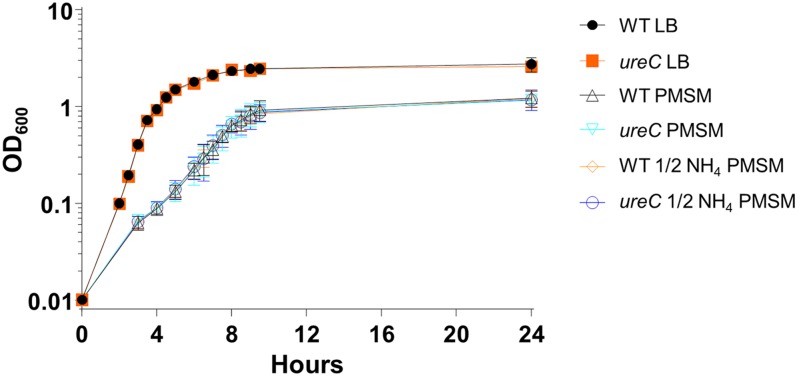
The ureC mutant does not have an in vitro growth defect. The ureC mutant has identical growth kinetics in LB medium compared with the wild-type parent strain HI4320, consistent with its initial report (53). The same was true when these strains were cultured in minimal medium, even if the sole nitrogen source, ammonium sulfate, was halved. Results are from three independent experiments. Although the ureC mutant is readily cultured in pooled human urine (28), the parent strain rapidly generates alkaline conditions (pH > 9.0) with consequent growth inhibition and mineral precipitation.
We also hypothesized that MR/P fimbriae might be important for cluster formation because they are essential for full bladder infection (4, 5), they are the most highly induced genes in bacteria isolated from the urine of infected mice at 24 hpi (28), and their expression in P. mirabilis or exogenously in E. coli results in autoaggregation (5, 6). Indeed, a P. mirabilis mrpA mutant failed to form typical extracellular clusters in murine bladders by 24 hpi. Instead, the bacteria were mostly in small intracellular groups (Fig. 4C, i). In one instance, we identified a loose Alizarin Red-negative luminal aggregation of the mrpA mutant embedded in a matrix of unknown material (Fig. 4C, ii and iv). Despite the distinct localization of mrpA mutant bacteria, the bladder cfu were not significantly different from wild type at 24 hpi (Fig. 4D; P = 0.4813).
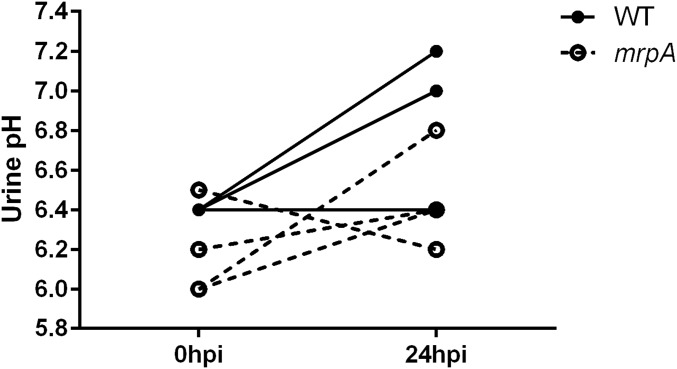
Because urease activity of intracellular bacteria might be excluded from the bladder lumen, we tested the pH of urine from infected mice 24 hpi. We found modest increases in pH for both wild-type and mrpA mutant infected mice, although the increase was less for mrpA (Fig. S6). We did not see the massive infiltration that we found around wild-type clusters in bladders infected with either the ureC or mrpA mutant (Fig. 4 A–C, ii). Together, these data support a role for both urease and MR/P fimbriae in cluster formation and subsequent urolithiasis.
Fig. S6.
Urinary pH during P. mirabilis UTI. Urinary pH was measured in mice before infection and 24 hpi. Connected lines show the change in pH in an individual mouse over time. The median increase in pH was 0.6 and 0.3 for wild type and the mrpA mutant, respectively.
Discussion
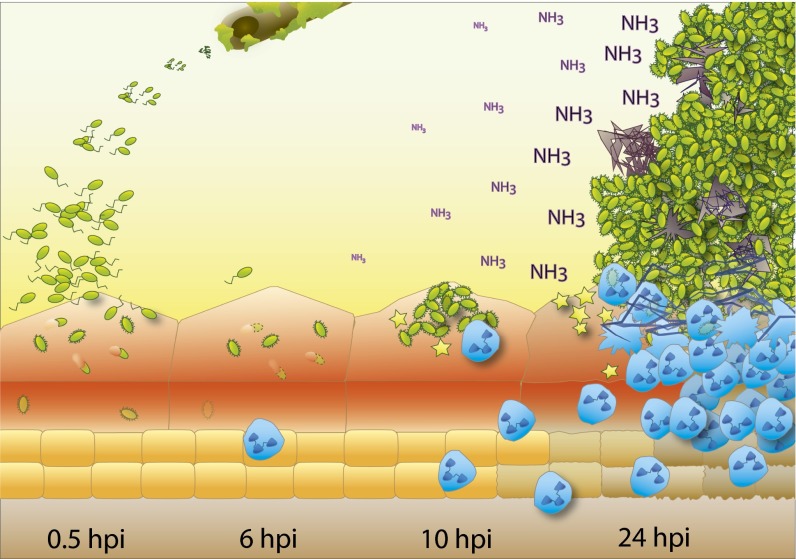
To our knowledge, our study is the first to examine the localization of P. mirabilis in the bladder during the first 24 h after infection. Despite initially invading the urothelium (Fig. 5, 0.5 hpi), P. mirabilis primarily occupied an extracellular niche as infection proceeded (Fig. 5, 6 hpi). P. mirabilis formed large luminal clusters, dependent on both MR/P fimbriae and urease, which are likely precursors to urinary stones and resulted in extensive neutrophil recruitment (Fig. 5, 24 hpi). Clusters were nonuniform, and in addition to bacteria contained urothelial cell debris, mineral deposits, and neutrophils. This study, to our knowledge, is the first systematic characterization of the cellular localization of a uropathogen that engages in a life style distinct from that of UPEC.
Fig. 5.
Model of initial bladder colonization and cluster development by P. mirabilis. P. mirabilis infections are frequently associated with urinary catheters (Top). At early time points after gaining access to the bladder (0.5 hpi), bacteria (green) adhere to and invade urothelial umbrella cells; however, by 6 hpi, intracellular bacteria have largely disappeared (dashed outlining). Instead, fimbriated bacteria begin to form clusters on the apical surface of umbrella cells (10 hpi), which continue to grow (24 hpi), leading to increased local concentrations of toxins (yellow stars) and urease-generated ammonia (NH3) as well as massive neutrophil infiltration (blue cells). The combined effect leads to crystalline mineral deposition (gray) and destruction of the urothelial surface.
What Is the Fate of Intracellular P. mirabilis?
Previous studies showed that P. mirabilis is highly invasive in cultured cells (29, 30), and we found that P. mirabilis invaded the bladder epithelium as effectively as UPEC at 0.5 hpi (Fig. 1E; Fig. 5, 0.5 hpi). However, at later time points, intracellular P. mirabilis was primarily found as small and infrequent IBCs, suggesting that IBC development is not a key strategy for P. mirabilis (Fig. 5, 6–24 hpi). It is unclear why P. mirabilis largely fails to develop into IBCs. One possibility is that umbrella cells expel invaded P. mirabilis into the lumen, a phenomenon shown to occur during UPEC infection (31). P. mirabilis could be poorly adapted for an intracellular life style, resulting in host-mediated bacterial death. Alternatively, the invasive capacity of P. mirabilis and survival of the intracellularly located mrpA mutant suggest that the intracellular compartment could be an important component of infection, perhaps by modulation of the host response to extracellular bacteria.
Luminal Clusters Are an Essential Precursor to Urolithiasis.
Our data suggest that extracellular clusters are an essential component of P. mirabilis infection: by 24 hpi, most bacteria were found in clusters that stained positive for mineral deposition (Fig. 2D; Fig. 4A; Fig. 5, 24 hpi). Based on this observation, we hypothesize that clusters correlate with urolithiasis and that bacteria themselves may be an essential component of urinary stones. Indeed, previous experiments showed that mature P. mirabilis-induced stones contain embedded bacteria and can serve as a reservoir for recurrent infections (8, 32, 33). We also note that clusters are not uniform in composition; although clusters may contain areas devoid of bacterial staining, these areas do not necessarily coincide with mineralization (note the serial sections in Fig. 4A, ii and iii). Important questions remain, including how frequently clusters develop into stones and which bacterial and host factors affect this process. Because extracellular clusters may mediate disease severity, it is imperative that future studies both identify the mechanism of their formation and determine whether this phenotype is prevalent in patients with P. mirabilis-dependent urolithiasis.
P. mirabilis Embedded in Extracellular Clusters Is Insulated from Neutrophil Attacks.
Clusters induce a rapid, extensive infiltration of neutrophils that, to our knowledge, is unlike any immune response reported in the literature (Fig. 3; Fig. 5, 24 hpi). Although the exact neutrophil staining pattern varied among clusters, we found recurring patterns of Ly6G+DAPI+, Ly6G+DAPI−, and Ly6G−DAPI+ staining. Upon closer examination, we identified instances of neutrophil attack on bacterial clusters via NETosis and phagocytosis. Indeed, a recent proteomic analysis suggested that NETs could occur specifically in response to P. mirabilis-mediated UTI (34). In addition to vital NETosis, regions with Ly6G staining but lacking nuclear DAPI signal may be the result of P. mirabilis toxins directly attacking infiltrating immune cells (35, 36). The potent immune response could be influenced by NLRP3-inflammasome activation; this pathway can be broadly activated by crystalline substances and specifically activated by P. mirabilis in the digestive tract (37, 38). It is also possible that neutrophils directly contribute to cluster formation, such as by providing materials for aggregation (i.e., DNA). Further studies of the immune response to P. mirabilis infection may examine the recruitment of additional innate immune effectors as well the mechanisms that P. mirabilis uses to evade immune cell attacks.
Both Urease and MR/P Fimbriae Are Essential for Extracellular Cluster Formation.
There are several explanations for why the ureC mutant does not form clusters. In addition to causing mineral deposition, the generation of ammonia by urease also results in changes to the pH and nitrogen availability in the bladder lumen. Each of these aspects of urease activity could contribute to P. mirabilis adaptation to the harsh environment of the urinary tract, culminating in a defect for the ureC mutant in cluster formation or maintenance. Although the requirement of urease for stone formation has been known for decades (39), its necessity for cluster formation is a novel finding.
Although the importance of MR/P fimbriae in vivo is well established, the mechanism of MR/P contribution to virulence remains enigmatic. Previous in vitro studies support a role for MR/P fimbriae in autoaggregation (5, 6), hemagglutination (6, 40), and biofilm formation (5), phenotypes that are reminiscent of the in vivo clusters; indeed, we found that the P. mirabilis mrpA mutant failed to form extracellular clusters. We hypothesize that MR/P initiates cluster formation through interactions with the bladder surface, mineral deposits, other P. mirabilis cells, or, most likely, a combination of all of the above. The exact mechanism by which MR/P functions and identification of the binding target(s) remains an active area of investigation.
mrpA Mutant Persists in an Intracellular Niche.
Surprisingly, although the mrpA mutant failed to form luminal clusters, it may be more successful at surviving intracellularly than wild-type cells. However, other studies clearly show that mrp mutants are defective in colonization by 7 d postinfection (4, 6, 41, 42), indicating that the intracellular life style is not an effective long-term strategy for this pathogen. The intracellular phenotype of the mrpA mutant could be due to several factors, including altered attachment resulting in increased invasion or a different invasion pathway. In support of this, a previous study suggested that MR/P fimbriae affect the localization of bacteria in the bladder (5). Alternatively, the mrpA mutant may elicit an altered immune response or modify other host–pathogen interactions. It is also possible that a polar effect on the transcriptional regulator MrpJ, which is encoded in the mrp operon, and known to regulate an array of virulence-associated genes, contributes to the phenotypes of the P. mirabilis mrpA mutant (41). Thus, mrpA mutant phenotypes may be dependent on the fimbrial structure, on MrpJ targets, or both. Additional in vivo and in vitro studies will be needed to define the exact roles of MR/P fimbriae, particularly in attachment, invasion, and intracellular survival.
P. mirabilis and UPEC Occupy Distinct Locations in the Bladder.
In this and other studies, UPEC establishes an intracellular niche (Fig. 1D; Table 1). Others have reported UPEC in an adherent layer on the luminal surface or as filamentous bacteria extruding from urothelial cells (16, 43, 44). We found that, unlike UPEC, P. mirabilis primarily resided in large luminal clusters (Figs. 2A and 5; Table 1). Interestingly, cocolonization of P. mirabilis and UPEC results in increased UPEC bacterial loads in the bladder (45), which could be the result of P. mirabilis clusters and/or stones providing an additional niche for UPEC to colonize. Similarly, P. mirabilis cocolonization with P. stuartii results in increased P. stuartii bacterial loads compared with monospecies infection (46). These data emphasize the need to further investigate the life style of P. mirabilis and other understudied uropathogens, particularly in the context of polymicrobial infections.
Concluding Remarks.
This study applied a technique commonly used to study UPEC pathogenesis to the understudied uropathogen P. mirabilis. We revealed that this pathogen uses a colonization pathway that is distinct from UPEC and results in a unique and robust immune response. By comparing colonization of wild-type and mutant P. mirabilis, we gained insight into the mechanisms of urease and MR/P contribution to virulence. These results provide a strong basis for future studies that will illuminate details of P. mirabilis invasion, life style, and interaction with the host that are essential for understanding and preventing P. mirabilis infection.
Materials and Methods
Bacterial Strains and Media.
All bacterial strains were grown at 37 °C with aeration in low-salt LB (per liter: 10 g tryptone, 5 g yeast extract, 0.5 g NaCl) or on LB solidified with 1.5% agar. Antibiotics were added as needed: ampicillin (100 µg/mL) and kanamycin (25 µg/mL). The strains used in this study are in Table S1 and details on mrpA mutant construction are in SI Materials and Methods.
Table S1.
Bacterial strains used in this study
Mouse Model of UTI.
Five- to seven-week-old female CBA/J mice (Jackson Laboratory) were infected with 1–2 × 108 cfu bacteria in PBS via transurethral catheter in accordance with New York University Langone Medical Center Institutional Animal Care and Use Committee-approved animal protocol 140204; details are in SI Materials and Methods.
Gentamicin Protection Invasion Assay.
The ex vivo gentamicin protection assay was performed as previously described with the following modifications (14). At 0.5 hpi, infected bladders were aseptically extracted, bisected, and washed with PBS. One bladder half was incubated with DMEM + 100 µg/mL gentamicin and the other half with DMEM alone. Both halves were incubated at 37 °C for 1 h, washed with PBS, homogenized, serially diluted, and plated. Details are in SI Materials and Methods.
Immunofluorescence and Alizarin Red Staining.
For immunofluorescence, 5-µm paraffin-embedded sections were processed and blocked, and antigens were detected with the appropriate primary and secondary antibodies and then incubated with DAPI solution (Invitrogen). Sections were visualized on a Nikon TE2000 inverted microscope, and images were processed and pseudocolored using FIJI (47). To detect early stages of mineral deposition, we modified the Alizarin Red S staining procedure previously used to stain urinary stones caused by P. mirabilis (8). Detailed methods of staining, imaging, and measurement of IBC and cluster size are in SI Materials and Methods.
Statistical Analyses.
To compare bacterial colonization, invasion, and IBC size, the nonparametric two-tailed Mann–Whitney test was used (GraphPad). Median values and statistical analyses with a P value ≤0.05 were reported. (*P ≤ 0.05, **P < 0.01, ***P < 0.001).
SI Materials and Methods
Mouse Model of UTI.
Female CBA/J mice (5–7 wk old) were transurethrally infected with bacteria in accordance with New York University Langone Medical Center (NYULMC) Institutional Animal Care and Use Committee-approved animal protocol 140204. The infection procedure was modified from that described in refs. 28 and 48 as follows: a bacterial suspension of 1–2 × 108 cfu in PBS, corresponding to an OD600 of ∼1.8 for P. mirabilis strains or OD600 of ∼4.5 for E. coli UTI89, was prepared from an overnight culture. Fifty microliters of the suspension was inoculated directly into the bladder of anesthetized mice via a sterile transurethral catheter attached to an infusion pump (Harvard Apparatus). Urine was collected by gentle abdominal massage within 2 h before sacrifice. To enumerate bacteria, urine and homogenized bladders were serially diluted in PBS and plated in LB agar. Urine pH was determined using Hydrion paper, pH 6.0–8.0 (Micro Essential).
Gentamicin Protection Invasion Assay.
The ex vivo gentamicin protection assay was modified from Mulvey et al. (14) as follows: at 0.5 hpi, infected bladders were aseptically extracted and bisected. Each bladder half was weighed and washed three times with PBS. One bladder half was incubated with DMEM with 4.5 g/L glucose, l-glutamine, and sodium pyruvate (Corning) + 100 µg/mL gentamicin; the other half was incubated in DMEM alone. Both halves were incubated at 37 °C for 1 h with gentle shaking. The bladders were then washed three times with PBS, homogenized in 0.5 mL PBS, serially diluted, and plated.
Immunofluorescence Staining and Imaging.
Isolated bladders were fixed for 1 h at room temperature in either 10% (wt/vol) formalin or 4% (wt/vol) paraformaldehyde, washed, dehydrated in ethanol and xylenes, paraffin-embedded, and sectioned. For immunofluorescence staining, 5-µm sections were deparaffinized, underwent antigen retrieval in 0.1 M sodium citrate, pH 6.0, and then blocked in PBS + 3% (wt/vol) BSA. Blocked sections were incubated in the following primary antibodies: α-E. coli polyclonal antibody PA125636 (which recognizes both E. coli and P. mirabilis; Pierce), 1:100 dilution; α-UPIIIa AU1 (49), 1:20 dilution; α-Ly6G IA8 (BioXCell) (21), diluted 1:10,000; and α-H2A antibody AB18255 (Abcam), 1:200 dilution. Primary antibodies were detected with the appropriate Alexa Fluor-conjugated antibodies (Invitrogen) diluted 1:200. After washing, sections were incubated in a 5-µg/mL DAPI solution (Invitrogen), washed, and mounted in Fluoro-Gel with Tris buffer (Electron Microscopy Sciences). Sections were visualized using 10×, 20×, and 40× objectives on a Nikon TE2000 inverted microscope coupled with a DS-Qi1 monochrome digital camera (Nikon). Images were processed and pseudocolored using FIJI (47).
Measurement of IBC and Cluster Size.
To determine the area occupied by IBCs and P. mirabilis extracellular clusters, images of infected bladder sections were analyzed in FIJI. IBCs were considered to be a group of 10 or more contiguous bacteria in an intact umbrella cell; if the bacterial population was too dense to allow visualization of bacterial rods, it was also considered an IBC. Clusters were similarly defined, except they were larger groups observed in the bladder lumen; because clusters spanned multiple bladder sections, sizing was conducted on the section with the largest cluster area. The bacterial signal from IBCs and clusters was outlined with a freehand selection (Fig. S2), and the resulting area reported.
Alizarin Red Detection of Mineralization.
We modified the Alizarin Red S staining procedure previously used to stain urinary stones caused by P. mirabilis (8) as follows. To prevent solubilization of calcium deposits during staining, Alizarin Red S staining and wash steps were performed in 1% KOH (50), and steps were performed quickly. Specifically, 2% Alizarin Red S (wt/vol) (Acros Organics) was dissolved in 1% KOH and filtered through a 0.22-µm PES filter. The 20-µm paraffin-embedded sections were briefly deparaffinized and hydrated [2 × 5 min in xylenes followed by a 3-min wash in 100%, 80%, and 50% (vol/vol) ethanol] and then gently flooded with 2% Alizarin Red S for 1 min. The slides were then washed three times in 1% KOH (1–2 min/wash), allowed to air-dry, and then mounted in Permount (Fisher). Slides were imaged at the NYULMC Histopathology Core with a Leica SCN400F slide scanner at 20× magnification.
Giemsa Stain of Murine Urine.
At 24 hpi, 5 μL of voided murine urine was mixed with 5 μL of Giemsa stain (Acros Organics) in PBS (0.8% wt/vol, diluted 1:40 before use), spotted on a glass slide, and coverslipped. Microscopic images were taken using a Nikon Diaphot 300 inverted microscope. Images were processed using FIJI software (47). Urine was examined from a total of seven mice.
Construction of a P. mirabilis mrpA Mutant.
MR/P fimbriae are encoded by the mrpABCDEFGHJ operon. We constructed an mrpA mutant by inserting a kanamycin-resistance gene using a modified TargeTron Gene Knockout System (Sigma) as described in ref. 51. Briefly, a group II intron was reprogrammed by mutagenic PCR to target mrpA using primers mrpA-IBS (AAAAAAGCTTATAATTATCCTTACATGGCACTGTTGTGCGCCCAGATAGGGTG), mrpA-EBS2 (TGAACGCAAGTTTCTAATTTCGGTTCCATGTCGATAGAGGAAAGTGTCT), and mrpA-EBS1d (CAGATTGTACAAATGTGGTGATAACAGATAAGTCACTGTTAATAACTTACCTTTCTT TGT) and then inserted into the plasmid pACD4K-C to create plasmid pMP231. This plasmid was electroporated into P. mirabilis HI4320 and then induced to jump from the plasmid into the mrpA gene in the P. mirabilis chromosome. An HI4320mrpA::kan mutant was identified by selection on kanamycin and confirmed by PCR of the mrpA locus using primers mrpA-TT-F (CTGCTTTAGCTGCAGATC) and mrpA-TT-R (TATCAGGAGTAATTGAGC).
In Vitro Fitness of the ureC Mutant.
To determine whether the ureC mutant has decreased fitness during nitrogen restriction, the mutant and wild-type parent strain HI4320 were cultured in LB, P. mirabilis minimal salts medium (PMSM) (52), or PMSM with the nitrogen source halved (0.5 g/L ammonium sulfate instead of 1.0 g/L). Cultures were incubated at 37 °C with aeration, and OD600 was measured at regular intervals. Three technical replicates were performed per experiment, and three independent experiments were conducted.
Acknowledgments
We thank Yi Liao, Xue-Ru Wu, Yan Liu, and Lisa Kuan for experimental advice and reagents and members of the New York University Urothelial Biology Group and the M.M.P. laboratory for stimulating discussion. This work was supported by National Institutes of Health (NIH) Grants AI083743 (to M.M.P.) and DK52206 and DK39753 (to T.-T.S.). The New York University Langone Medical Center Histopathology Core is partially supported by Perlmutter Cancer Center Grant P30CA016087 and NIH Grant UL1 TR00038.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1601720113/-/DCSupplemental.
References
- 1.Coker C, Poore CA, Li X, Mobley HLT. Pathogenesis of Proteus mirabilis urinary tract infection. Microbes Infect. 2000;2(12):1497–1505. doi: 10.1016/s1286-4579(00)01304-6. [DOI] [PubMed] [Google Scholar]
- 2.Stickler DJ. Bacterial biofilms in patients with indwelling urinary catheters. Nat Clin Pract Urol. 2008;5(11):598–608. doi: 10.1038/ncpuro1231. [DOI] [PubMed] [Google Scholar]
- 3.Schaffer JN, Pearson MM. Proteus mirabilis and urinary tract infections. Microbiol Spectr. 2015;3(5) doi: 10.1128/microbiolspec.UTI-0017-2013. :UTI-0017-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahrani FK, et al. Construction of an MR/P fimbrial mutant of Proteus mirabilis: Role in virulence in a mouse model of ascending urinary tract infection. Infect Immun. 1994;62(8):3363–3371. doi: 10.1128/iai.62.8.3363-3371.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jansen AM, Lockatell V, Johnson DE, Mobley HLT. Mannose-resistant Proteus-like fimbriae are produced by most Proteus mirabilis strains infecting the urinary tract, dictate the in vivo localization of bacteria, and contribute to biofilm formation. Infect Immun. 2004;72(12):7294–7305. doi: 10.1128/IAI.72.12.7294-7305.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Johnson DE, Mobley HLT. Requirement of MrpH for mannose-resistant Proteus-like fimbria-mediated hemagglutination by Proteus mirabilis. Infect Immun. 1999;67(6):2822–2833. doi: 10.1128/iai.67.6.2822-2833.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao H, Thompson RB, Lockatell V, Johnson DE, Mobley HLT. Use of green fluorescent protein to assess urease gene expression by uropathogenic Proteus mirabilis during experimental ascending urinary tract infection. Infect Immun. 1998;66(1):330–335. doi: 10.1128/iai.66.1.330-335.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, et al. Visualization of Proteus mirabilis within the matrix of urease-induced bladder stones during experimental urinary tract infection. Infect Immun. 2002;70(1):389–394. doi: 10.1128/IAI.70.1.389-394.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jansen AM, Lockatell CV, Johnson DE, Mobley HLT. Visualization of Proteus mirabilis morphotypes in the urinary tract: The elongated swarmer cell is rarely observed in ascending urinary tract infection. Infect Immun. 2003;71(6):3607–3613. doi: 10.1128/IAI.71.6.3607-3613.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mobley HLT, Island MD, Hausinger RP. Molecular biology of microbial ureases. Microbiol Rev. 1995;59(3):451–480. doi: 10.1128/mr.59.3.451-480.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson DE, et al. Contribution of Proteus mirabilis urease to persistence, urolithiasis, and acute pyelonephritis in a mouse model of ascending urinary tract infection. Infect Immun. 1993;61(7):2748–2754. doi: 10.1128/iai.61.7.2748-2754.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou G, et al. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: Evidence from in vitro FimH binding. J Cell Sci. 2001;114(Pt 22):4095–4103. doi: 10.1242/jcs.114.22.4095. [DOI] [PubMed] [Google Scholar]
- 13.Wu XR, Kong XP, Pellicer A, Kreibich G, Sun TT. Uroplakins in urothelial biology, function, and disease. Kidney Int. 2009;75(11):1153–1165. doi: 10.1038/ki.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mulvey MA, et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282(5393):1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 15.Anderson GG, et al. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301(5629):105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 16.Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun. 2001;69(7):4572–4579. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lane MC, et al. Role of motility in the colonization of uropathogenic Escherichia coli in the urinary tract. Infect Immun. 2005;73(11):7644–7656. doi: 10.1128/IAI.73.11.7644-7656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lloyd AL, Smith SN, Eaton KA, Mobley HLT. Uropathogenic Escherichia coli suppresses the host inflammatory response via pathogenicity island genes sisA and sisB. Infect Immun. 2009;77(12):5322–5333. doi: 10.1128/IAI.00779-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nielubowicz GR, Mobley HLT. Host-pathogen interactions in urinary tract infection. Nat Rev Urol. 2010;7(8):430–441. doi: 10.1038/nrurol.2010.101. [DOI] [PubMed] [Google Scholar]
- 20.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83(1):64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, El-Achkar TM, Wu XR. Tamm-Horsfall protein regulates circulating and renal cytokines by affecting glomerular filtration rate and acting as a urinary cytokine trap. J Biol Chem. 2012;287(20):16365–16378. doi: 10.1074/jbc.M112.348243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 23.Urban CF, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5(10):e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pilsczek FH, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185(12):7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 25.Yipp BG, et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med. 2012;18(9):1386–1393. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mora-Bau G, et al. Macrophages subvert adaptive immunity to urinary tract infection. PLoS Pathog. 2015;11(7):e1005044. doi: 10.1371/journal.ppat.1005044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haraoka M, et al. Neutrophil recruitment and resistance to urinary tract infection. J Infect Dis. 1999;180(4):1220–1229. doi: 10.1086/315006. [DOI] [PubMed] [Google Scholar]
- 28.Pearson MM, Yep A, Smith SN, Mobley HLT. Transcriptome of Proteus mirabilis in the murine urinary tract: Virulence and nitrogen assimilation gene expression. Infect Immun. 2011;79(7):2619–2631. doi: 10.1128/IAI.05152-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alamuri P, et al. Adhesion, invasion, and agglutination mediated by two trimeric autotransporters in the human uropathogen Proteus mirabilis. Infect Immun. 2010;78(11):4882–4894. doi: 10.1128/IAI.00718-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chippendale GR, Warren JW, Trifillis AL, Mobley HLT. Internalization of Proteus mirabilis by human renal epithelial cells. Infect Immun. 1994;62(8):3115–3121. doi: 10.1128/iai.62.8.3115-3121.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miao Y, Li G, Zhang X, Xu H, Abraham SN. A TRP channel senses lysosome neutralization by pathogens to trigger their expulsion. Cell. 2015;161(6):1306–1319. doi: 10.1016/j.cell.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabbuba NA, Mahenthiralingam E, Stickler DJ. Molecular epidemiology of Proteus mirabilis infections of the catheterized urinary tract. J Clin Microbiol. 2003;41(11):4961–4965. doi: 10.1128/JCM.41.11.4961-4965.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabbuba NA, et al. Genotyping demonstrates that the strains of Proteus mirabilis from bladder stones and catheter encrustations of patients undergoing long-term bladder catheterization are identical. J Urol. 2004;171(5):1925–1928. doi: 10.1097/01.ju.0000123062.26461.f9. [DOI] [PubMed] [Google Scholar]
- 34.Yu Y, et al. Diagnosing inflammation and infection in the urinary system via proteomics. J Transl Med. 2015;13:111. doi: 10.1186/s12967-015-0475-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swihart KG, Welch RA. Cytotoxic activity of the Proteus hemolysin HpmA. Infect Immun. 1990;58(6):1861–1869. doi: 10.1128/iai.58.6.1861-1869.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alamuri P, Mobley HLT. A novel autotransporter of uropathogenic Proteus mirabilis is both a cytotoxin and an agglutinin. Mol Microbiol. 2008;68(4):997–1017. doi: 10.1111/j.1365-2958.2008.06199.x. [DOI] [PubMed] [Google Scholar]
- 37.Seo SU, et al. Distinct commensals induce interleukin-1β via NLRP3 inflammasome in inflammatory monocytes to promote intestinal inflammation in response to injury. Immunity. 2015;42(4):744–755. doi: 10.1016/j.immuni.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ciraci C, Janczy JR, Sutterwala FS, Cassel SL. Control of innate and adaptive immunity by the inflammasome. Microbes Infect. 2012;14(14):1263–1270. doi: 10.1016/j.micinf.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griffith DP, Musher DM, Itin C. Urease. The primary cause of infection-induced urinary stones. Invest Urol. 1976;13(5):346–350. [PubMed] [Google Scholar]
- 40.Bahrani FK, Mobley HLT. Proteus mirabilis MR/P fimbriae: Molecular cloning, expression, and nucleotide sequence of the major fimbrial subunit gene. J Bacteriol. 1993;175(2):457–464. doi: 10.1128/jb.175.2.457-464.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bode NJ, et al. Transcriptional analysis of the MrpJ network: Modulation of diverse virulence-associated genes and direct regulation of mrp fimbrial and flhDC flagellar operons in Proteus mirabilis. Infect Immun. 2015;83(6):2542–2556. doi: 10.1128/IAI.02978-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X, Lockatell CV, Johnson DE, Mobley HLT. Identification of MrpI as the sole recombinase that regulates the phase variation of MR/P fimbria, a bladder colonization factor of uropathogenic Proteus mirabilis. Mol Microbiol. 2002;45(3):865–874. doi: 10.1046/j.1365-2958.2002.03067.x. [DOI] [PubMed] [Google Scholar]
- 43.Dhakal BK, Kulesus RR, Mulvey MA. Mechanisms and consequences of bladder cell invasion by uropathogenic Escherichia coli. Eur J Clin Invest. 2008;38(Suppl 2):2–11. doi: 10.1111/j.1365-2362.2008.01986.x. [DOI] [PubMed] [Google Scholar]
- 44.Justice SS, et al. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc Natl Acad Sci USA. 2004;101(5):1333–1338. doi: 10.1073/pnas.0308125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alteri CJ, Himpsl SD, Mobley HLT. Preferential use of central metabolism in vivo reveals a nutritional basis for polymicrobial infection. PLoS Pathog. 2015;11(1):e1004601. doi: 10.1371/journal.ppat.1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Armbruster CE, Smith SN, Yep A, Mobley HLT. Increased incidence of urolithiasis and bacteremia during Proteus mirabilis and Providencia stuartii coinfection due to synergistic induction of urease activity. J Infect Dis. 2014;209(10):1524–1532. doi: 10.1093/infdis/jit663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schindelin J, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hagberg L, et al. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun. 1983;40(1):273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang FX, et al. Organization of uroplakin subunits: Transmembrane topology, pair formation and plaque composition. Biochem J. 2001;355(Pt 1):13–18. doi: 10.1042/0264-6021:3550013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rigueur D, Lyons KM. Whole-mount skeletal staining. Methods Mol Biol. 2014;1130:113–121. doi: 10.1007/978-1-62703-989-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pearson MM, Mobley HLT. The type III secretion system of Proteus mirabilis HI4320 does not contribute to virulence in the mouse model of ascending urinary tract infection. J Med Microbiol. 2007;56(Pt 10):1277–1283. doi: 10.1099/jmm.0.47314-0. [DOI] [PubMed] [Google Scholar]
- 52.Belas R, Erskine D, Flaherty D. Transposon mutagenesis in Proteus mirabilis. J Bacteriol. 1991;173(19):6289–6293. doi: 10.1128/jb.173.19.6289-6293.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones BD, Lockatell CV, Johnson DE, Warren JW, Mobley HLT. Construction of a urease-negative mutant of Proteus mirabilis: Analysis of virulence in a mouse model of ascending urinary tract infection. Infect Immun. 1990;58(4):1120–1123. doi: 10.1128/iai.58.4.1120-1123.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mobley HLT, Warren JW. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J Clin Microbiol. 1987;25(11):2216–2217. doi: 10.1128/jcm.25.11.2216-2217.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]