Significance
IL-1 is a pleiotropic cytokine involved in a myriad of autoinflammatory disorders. In proline-serine-threonine phosphatase-interacting protein 2 (Pstpip2cmo) mice, IL-1β provokes autoinflammatory osteomyelitis. Here, we demonstrated the redundant roles of Nod like receptor family, pyrin domain containing 3 (NLRP3) inflammasome with caspase 8 in the processing of IL-1β in Pstpip2cmo mice. Identification of redundancy between NLRP3 inflammasome and caspase 8 is fundamental in our understanding of the inflammasomes and alternative modes of IL-1β regulation in osteomyelitic disease. Moreover, IL-1β signaling connects distinct compartments in promoting the disease, identifying previously unidentified checkpoints that could be targeted for therapeutic purposes in similar disease settings. Thus, our studies have unraveled the complex IL-1β regulatory network in vivo in a mouse model of osteomyelitis that will be useful for autoinflammatory diseases in general.
Keywords: NLRP3, inflammasome, caspase 8, caspase 1, PSTPIP2
Abstract
Missense mutation in the proline-serine-threonine phosphatase-interacting protein 2 (Pstpip2) gene results in the development of spontaneous chronic bone disease characterized by bone deformity and inflammation that is reminiscent of patients with chronic multifocal osteomyelitis (cmo). Interestingly, this disease is specifically mediated by IL-1β but not IL-1α. The precise molecular pathways that promote pathogenic IL-1β production in Pstpip2cmo mice remain unidentified. Furthermore, how IL-1β provokes inflammatory bone disease in Pstpip2cmo mice is not known. Here, we demonstrate that double deficiency of Nod like receptor family, pyrin domain containing 3 (NLRP3) and caspase 8 in Pstpip2cmo mice provides similar protection as observed in caspase-1 and caspase-8–deficient Pstpip2cmo mice, demonstrating redundant roles for the NLRP3 inflammasome and caspase 8 in provoking osteomyelitic disease in Pstpip2cmo mice. Consistently, immunofluorescence studies exhibited distinct caspase-1 and caspase-8 puncta in diseased Ptpn6spin neutrophils. Data from our chimera studies demonstrated that IL-1β produced by hematopoietic cells is sensed by the radioresistant compartment to promote bone disease. Furthermore, our results showed that the IL-1β signaling is unidirectional and feedback signaling of IL-1β onto the hematopoietic compartment is not important for disease induction. In conclusion, our studies have uncovered the combined actions of the NLRP3 inflammasome and caspase 8 leading to IL-1β maturation and the directionality of IL-1β in driving disease in Pstpip2cmo mice.
Autoinflammatory bone diseases that include osteoporosis, Paget’s disease, arthritis, periodontal disease, and chronic recurrent multifocal osteomyelitis (CRMO) are a major health burden to human health. The incidence of these diseases is projected to rise due to the increase in human life expectancy and changes in diet. IL-1 therapies have shown some promise in alleviating disease symptoms; however, IL-1 therapies target both IL-1β and IL-1α, which have known distinct functions (1–3). Furthermore, the signaling pathways that underlie IL-1–mediated autoinflammation in these bone diseases are mostly unknown.
Proline-serine-threonine phosphatase-interacting protein 2 (Pstpip2) gene is located on chromosome 18 in both mouse and human. PSTPIP2 is a member of the Fes/CIP4 homology-bin/amphiphysin/rvs (F-BAR) family of proteins also known as the pombe cdc15 homology family proteins (4). The F-bar domain of PSTPIP2 has been known to interact with phosphatidylinositol bisphosphate, whereas its C-terminal domain binds protein tyrosine phosphatases of the PEST (a domain rich in proline, glutamic acid, serine, and threonine) family (5–7). Recent studies have shown that PSTPIP2 can also interact with inhibitory enzymes CsK and SHIP1 (8). Whereas human patients with genetic mutations in the PSTPIP2 gene have not been identified, missense L98P mutation in the gene Pstpip2 in mice results in severe autoinflammatory disease of the bones that mimics CRMO in humans and thus these mice are referred to as Pstpip2cmo mice (9–11). Patients with CRMO are mostly composed of children and present with a wide range of symptoms ranging from bone inflammation, destruction, and deformity (12, 13). However, the current treatment options for CRMO are limited to the use of nonsteroidal antiinflammatory drugs (NSAIDs) and bisphosphonates (14). Pstpip2cmo mice have proved to be a valuable tool in understanding the molecular mechanisms involved in instigation of CRMO and bone diseases in general. Using Pstpip2cmo mice as a model to understand the etiology of bone diseases, we previously demonstrated that IL-1R signaling completely protected the progression of disease in these mice (1). Adding to these studies, we and others showed that IL-1β, but not IL-1α, was important for induction of bone disease in these mice (1, 15). More recent studies from our laboratory elucidated a rather surprising redundant role for caspase 1 and caspase 8 in the processing of IL-1β (2). The redundant roles of caspase 1 and caspase 8 suggest that these caspases are functioning independently in separate complexes to process the pathogenic cytokine IL-1β, unlike several other studies where caspase 1 and caspase 8 have been proposed to be in the same complex (16). In this study, we sought to understand the upstream molecules that assemble the caspase-1 and caspase-8 complexes. Furthermore, we undertook an innovative chimeric approach to elucidate the IL-1R signaling feedback loop in hematopoietic and radioresistant compartments and determine how IL-1β provoked inflammatory osteomyelitis in Pstpip2cmo mice.
We demonstrated that the caspase-1 complex is assembled by NLRP3, whereas caspase 8 does not engage the TNF signaling pathways, suggesting involvement of an alternate complex. Importantly, the NLRP3 inflammasome and caspase 8 play redundant roles in IL-1β processing and inducing disease in Pstpip2cmo mice. Activation of distinct caspase-1 and -8 complexes within the neutrophils provoked excessive IL-1β production. Finally, using bone marrow chimeras, we uncovered the complete IL-1β signaling loop, whereby IL-1β produced by neutrophils (hematopoieitic compartments) is sensed by IL-1R on radioresistant cells to provoke autoinflammation and bone disease.
Results
NLRP3 Inflammasome Plays a Redundant Role with Caspase 8 to Drive Disease in Pstpip2cmo Mice.
Whereas initial studies suggested that NLRP3, Apoptosis-associated speck-like protein containing a card (ASC), and caspase 1 (inflammasome) were dispensable for IL-1β–mediated disease in Pstpip2cmo mice (1, 15), our recent studies suggest that caspase 1 plays a redundant role with caspase 8 in promoting IL-1β secretion and disease in Pstpip2cmo mice (2). In line with these findings, we found no role for several proteases (2) or cathepsin B and cathepsin G (Fig. S1). Interestingly, deficiency of cathepsin C significantly delayed progression of disease in Pstpip2cmo mice, although most of the mice developed disease (Fig. S1). Whether cathepsin C plays a redundant role with other cathepsins or caspase 1 and caspase 8 is not known and will be investigated in the future. Our detailed analysis of these mice showed that complete deficiency of both caspase 1 and caspase 8 in Pstpip2cmo mice provides protection in more than 70% of the mice up to 120 d, although some mice demonstrate delayed clinical signs of disease (Fig. 1 A and B). Interestingly, haploinsufficiency of caspase 8 or caspase 1 in respective caspase-1– and caspase-8–deficient Pstpip2cmo mice significantly delayed progression of the disease (Fig. 1 A and B). To determine whether NLRP3 played a redundant role with caspase 8, we generated Pstpip2cmo mice that lacked both NLRP3 and caspase 8. Whereas NLRP3- or caspase-8 single-deficient mice developed severe disease, the mice lacking both molecules show significant protection from progression of arthritis and bone disease, suggesting a redundant role of NLRP3 with caspase 8 (Fig. 1 C and D). These data demonstrate that NLRP3, akin to caspase 1, plays a redundant role with caspase 8 to provoke bone disease in Pstpip2cmo mice, suggesting NLRP3 inflammasome as the caspase-1 complex that plays a redundant role with caspase 8.
Fig. S1.
Cathepsins are dispensable for progression of disease in Pstpip2cmo mice. Pstpip2cmo mice were bred with Ctsb−/−, Ctsc−/−, and Ctsg−/− mice to generate doubly deficient Pstpip2cmo × Ctsb−/−, Pstpip2cmo × Ctsc−/−, and Pstpip2cmo × Ctsg−/− mice. Pstpip2cmo mice deficient in these cathepsins were followed and scored for disease incidence. Disease curves were analyzed using the log-rank (Mantel–Cox) test. ****P < 0.0001.
Fig. 1.
NLRP3 inflammasome plays redundant roles with caspase 8 to promote osteomyelitis. (A and B) Pstpip2cmo, Pstpip2cmo × Rip3−/− × Casp1−/− × Casp8+/−, Pstpip2cmo × Rip3−/− × Casp1+/− × Casp8−/−, and Pstpip2cmo × Rip3−/− × Casp1−/− × Casp8−/− mice were generated by breeding Pstpip2cmo mice with Rip3−/− × Casp1−/− × Casp8−/− mice. Disease-free curves (A) and footpad images depicting bone deformity and inflammation (B) from the indicated Pstpip2cmo strains of mice on day 150. (C and D) Pstpip2cmo × Nlrp3−/− mice were bred with Ripk3−/− × Casp8−/− mice to generate Pstpip2cmo × Ripk3−/− × Casp8−/− × Nlrp3−/− mice. Disease scores (A) and footpad images of Pstpip2cmo and Pstpip2cmo × Ripk3−/− × Casp8−/− × Nlrp3−/− mice on day 150 are shown. Disease curves were analyzed using the log-rank (Mantel–Cox) test. ***P < 0.001 and ****P < 0.0001.
Recent studies published from our laboratory and others have shown that caspase 8 and caspase 1 can localize in the same inflammasome puncta containing ASC to process IL-1β (16–18). However, inflammasome-independent functions for caspase 8 in processing IL-1β have also been described (19–22). Our observation that deficiency of either caspase 1 or caspase 8 did not prevent disease progression in Pstpip2cmo mice indicates that the two caspases are part of distinct and independently activated complexes (Fig. 1 and ref. 2). Neutrophils are critical for disease induction in Pstpip2cmo mice, because the deletion of neutrophils can completely prevent disease in Pstpip2cmo mice (2). Thus, we asked whether caspase-1 and caspase-8 puncta could be visualized in neutrophils. Interestingly, we observed a significantly higher number of both caspase-1 and caspase-8 puncta in Pstpip2cmo neutrophils compared with WT controls (Fig. 2). Whereas most of the neutrophils contained either caspase-1 or caspase-8 puncta alone, we also observed some cells that contained both caspase-1 and caspase-8 puncta. Altogether, these data suggest that NLRP3 inflammasome plays a redundant role with a caspase-8 complex in promoting disease in Pstpip2cmo mice.
Fig. 2.
Caspase 1 and caspase 8 form independent complexes in Pstpip2cmo neutrophils. (A) Neutrophils were isolated from bone marrow cells of Pstpip2cmo and their littermate controls by centrifugation in Percoll. Isolated neutrophils were plated on chambered glass slides and stained with caspase-1 and caspase-8 antibody. The caspase-1 and caspase-8 staining in the neutrophils was visualized by immunofluorescence microscopy. DAPI was used to stain the nucleus of the cells. (B) Percent of cells with caspase-1 and caspase-8 puncta. Data represent means ± SEM and are representative of at least three independent experiments. Student’s t test was used to test significance. *P < 0.05.
TNF-Signaling Pathway Does Not Engage Inflammatory Caspase 8 in Pstpip2cmo Mice.
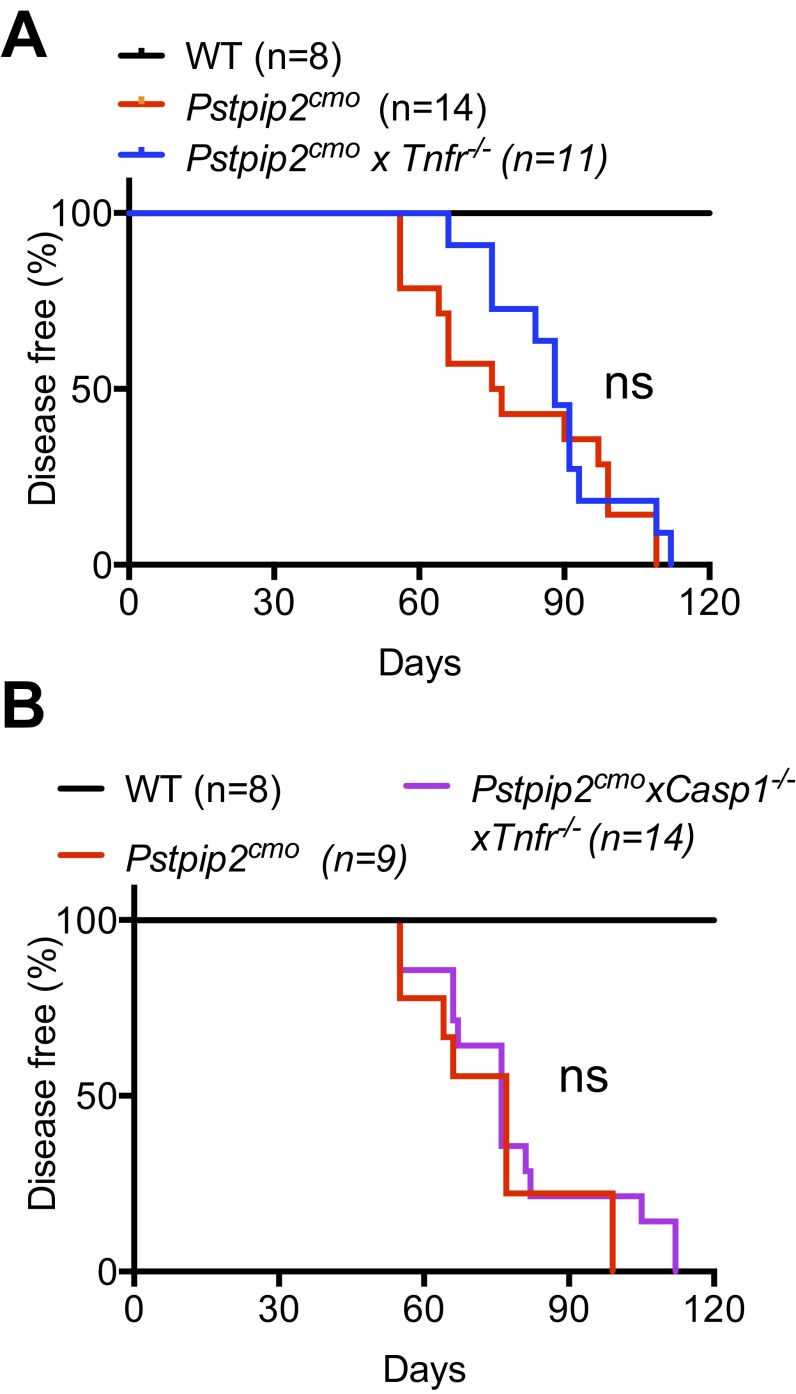
One of the most well-established pathways that lead to caspase-8 activation is the TNF-signaling pathway. Engagement of TNF receptor induces formation of the death complex that consists of FADD–caspase 8, whereby caspase 8 is activated, ultimately resulting in apoptotic cell death. Whereas TNF–TNF-R engagement is known to induce apoptosis, under certain conditions caspase-8–mediated processing of IL-1β and IL-18 is more prominent (20). Moreover, anti-TNF therapy has also been implicated as a potential therapeutic against inflammatory bone diseases in clinics (23, 24). Thus, we proposed that the TNF-signaling pathway engages inflammatory caspase 8 in Pstpip2cmo mice. Pstpip2cmo × Tnfr−/− mice developed disease with kinetics similar to Pstpip2cmo mice, demonstrating the dispensable role of the TNF-signaling pathway in disease progression (Fig. S2A). To further assess whether TNF-R engages caspase 8 to play a redundant role with caspase 1 in Pstpip2cmo mice, we generated Pstpip2cmo × Tnfr−/− × Casp1−/− mice. However, double deficiency of TNF-R and caspase 1 failed to provide significant protection in Pstpip2cmo mice (Fig. S2B). Thus, we concluded from these data that TNF-R signaling is dispensable and does not engage inflammatory caspase-8 complex in diseased Pstpip2cmo mice.
Fig. S2.
The TNF-signaling pathway is not involved in Pstpip2cmo-mediated bone disease. (A) Incidence of disease in Pstpip2cmo mice and Pstpip2cmo mice deficient in TNF-R (Pstpip2cmo × Tnfr−/− mice). (B) Incidence of disease in Pstpip2cmo and Pstpip2cmo × Casp1−/− × Tnfr−/− mice. Disease curves were analyzed using the log-rank (Mantel–Cox) test. ns, not significant.
IL-1β Secretion by Hematopoietic Cells Is Necessary and Required for Disease Progression in Pstpip2cmo Mice.
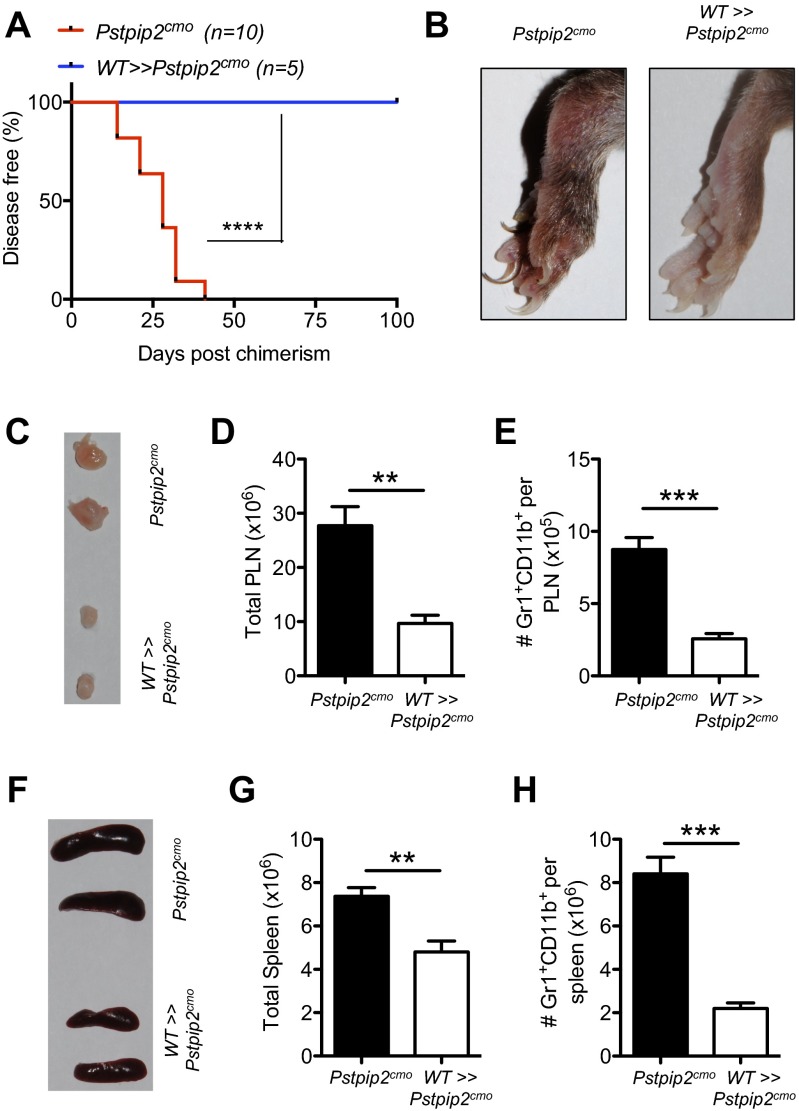
Our early studies clearly demonstrated that IL-1β, but not IL-1α, specifically provoked autoinflammatory disease in Pstpip2cmo mice (1). Herein, we have extended upon these studies to demonstrate how IL-1β provokes autoinflammatory disease in Pstpip2cmo mice. We generated bone marrow chimeras to test whether IL-1β in the hematopoietic or radioresistant compartment instigates disease in Pstpip2cmo mice. Pstpip2cmo>>WT mice developed disease, demonstrating the importance of the hematopoietic compartment in provoking disease. Concurrently, WT>>Pstpip2cmo chimeras fail to develop bone disease as evident by lack of arthritis, lymph node swelling, and accumulation of neutrophils (Fig. S3). We and others have previously shown that Pstpip2cmo × Il1b−/− DKO mice are completely protected from inflammation and disease (1, 14, 15). Interestingly, Pstpip2cmo>>Pstpip2cmo × Il1b−/− chimeras developed disease, suggesting IL-1β produced by radioresistant cells is dispensable for disease progression (Fig. 3 A and B). Conversely, Pstpip2cmo × Il1b−/−>>WT mice did not develop any significant disease, further strengthening the notion that hematopoietic IL-1β is sufficient and critical for progression of disease in Pstpip2cmo mice (Fig. 3 A and B). In line with the phenotype observed from the Pstpip2cmo chimeras, increased lymph node cellularity and granulocyte numbers were observed in the diseased mice (Pstpip2cmo>>WT and Pstpip2cmo>>Pstpip2cmo × Il1b−/− chimeras), compared with the protected mice (Pstpip2cmo × Il1b−/−>>WT chimeras) (Fig. 3 C and D).
Fig. S3.
Pstpip2 mutation in the hematopoietic cells is necessary for disease induction in Pstpip2cmo mice. (A) Disease incidence in Pstpip2cmo and WT>>Pstpip2cmo chimeras (donor>>recipient). (B) Footpad images of the indicated chimeras harvested on day 100 postchimerism. (C–E) Photographs of popliteal lymph nodes (C), total number of popliteal lymph node cells (D), and Gr1+CD11b+ neutrophils within the popliteal lymph nodes (E) are shown. (F–H) Photographs of spleen (F), total number of spleen cells (G), and Gr1+CD11b+ neutrophils within the spleen (H) are shown. Data in D, E, G, and H represent means ± SEM. Disease curves were analyzed using the log-rank (Mantel–Cox) test. The two-tailed Student’s t test was used to determine significance between groups. **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Fig. 3.
IL-1β secretion by hematopoietic cells provokes disease in Pstpip2cmo mice. (A) Disease incidence in Pstpip2cmo>>WT, Pstpip2cmo × Il1b−/−>>WT, and Pstpip2cmo>>Pstpip2cmo × Il1b−/− mice (donor>>recipient). (B) Footpad images of the indicated chimeras harvested on day 100 postchimerism showing the extent of footpad inflammation and bone disease. Total number of popliteal lymph node cells (C) and Gr1+CD11b+ neutrophils within the popliteal lymph nodes (D) from the indicated Pstpip2cmo/Il1b−/− chimeras. Data in C and D represent means ± SEM with n = 8 for Pstpip2cmo>>WT, n = 10 for Pstpip2cmo × Il1b−/−>>WT, and n = 8 for Pstpip2cmo>>Pstpip2cmo × Il1b−/− mice. Disease curves were analyzed using the log-rank (Mantel–Cox) test. The Mann–Whitney test was used to determine significance between groups. ****P < 0.0001.
IL-1R in the Radioresistant Compartment Is Critical for Disease Observed in Pstpip2cmo Mice.
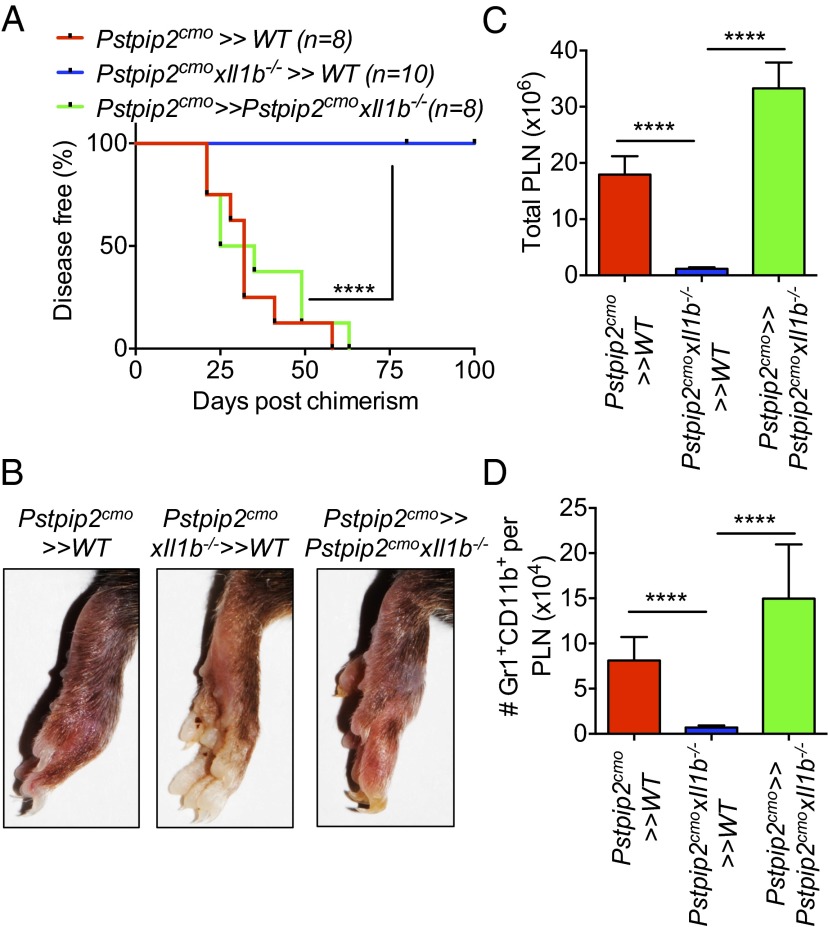
Our data suggest that dysregulated IL-1β produced by the hematopoietic compartment (most probably neutrophils) (2) is important for disease progression (Fig. 3). We next asked whether autocrine IL-1β signaling of the hematopoietic cells or whether IL-1β signaling in the radioresistant compartment was important for the progression of disease. Previous studies have shown that IL-1R deficiency provides complete protection from disease progression in Pstpip2cmo mice (1, 15). Whereas Pstpip2cmo>>WT chimeras developed full footpad inflammation, bone disease associated with increased lymph node cellularity and granulocyte numbers, Pstpip2cmo>>Pstpip2cmo × Il1r−/− chimeras were completely protected suggesting the importance of IL-1R in the radioresistant compartment (Fig. 4). To test whether hematopoieitic IL-1R was important for progression of disease, we generated Pstpip2cmo × Il1r−/−>>WT chimeras. These chimeras developed disease similar to Pstpip2cmo>>WT chimeras, which is interesting, given that the Pstpip2cmo × Il1r−/− mice are completely protected (Fig. 4). These data suggest that the immune cells from protected Pstpip2cmo × Il1r−/− mice are capable of inducing disease in WT mice and that IL-1R signaling in the hematopoietic compartment is dispensable for disease progression.
Fig. 4.
IL-1β signaling through IL-1R in the radioresistant cells is necessary for induction of disease in Pstpip2cmo mice. (A) Disease incidence in Pstpip2cmo>>WT, Pstpip2cmo × Il1r−/−>>WT, and Pstpip2cmo>>Pstpip2cmo × Il1r−/− mice (donor>>recipient). (B) Footpad images of the indicated chimeras harvested on day 100 postchimerism showing the extent of footpad inflammation and bone disease. Total numbers of popliteal lymph node cells (C) and Gr1+CD11b+ neutrophils within the popliteal lymph nodes (D) from the indicated Pstpip2cmo/Il1r−/− chimeras are shown. Data in C and D represent means ± SEM with n = 6 for Pstpip2cmo>>WT, n = 12 for Pstpip2cmo × Il1r−/−>>WT, and n = 7 for Pstpip2cmo>>Pstpip2cmo × Il1r−/− mice. Disease curves were analyzed using the log-rank (Mantel–Cox) test. The Mann–Whitney test was used to determine significance between groups. **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Discussion
Our current studies demonstrate that Pstpip2cmo mice deficient in both caspase 1 and caspase 8 are significantly protected from bone disease observed in Pstpip2cmo mice. Whereas single deficiency of either caspase 1 or caspase 8 does not provide protection (1, 2, 15), deficiency of both caspase 1 and caspase 8 in Pstpip2cmo mice provided significant protection from induction of bone disease (2). Furthermore, the effect of either caspase 1 or caspase 8 is dose-dependent, because haplodeficiency of either of these caspases delays the progression of disease (Fig. 1A). Recent studies have shown that caspase 1 and caspase 8 function together in the same inflammasome complex (16–18, 25) and independently (19–21), to process IL-1β and IL-18. Deficiency of ASC does not provide any protection from bone disease in Pstpip2cmo mice, suggesting that caspase 1 and caspase 8 function independently in this model of CRMO (1, 15). In support of this hypothesis, our microscopy data revealed distinct caspase-1 and caspase-8 puncta in neutrophils from diseased Pstpip2cmo mice that are rarely detected in the neutrophils from WT mice.
Caspase 1 is a prototypical caspase that is activated in the inflammasome complex. Our findings demonstrate that the caspase-1 complex contains NLRP3, and this complex plays a redundant role with caspase 8 in processing IL-1β, which is critical for disease induction. Generation of Pstpip2cmo mice deficient in both Casp8/Casp1 and Casp8/Nlrp3 has yielded several unexpected results. Whereas we and others had previously shown that NLRP3/caspase 1 and caspase 8 are dispensable for induction of disease in Pstpip2cmo mice (1, 15), our findings here established that both NLRP3/caspase 1 and caspase 8 are critical for progression of disease. Our studies also exclude the TNF-signaling pathway in engaging caspase 8; however, other TNF-family members such as CD95 and TRAIL might be involved and will be investigated in future studies (20, 26). Caspase 8 has also been shown to be activated in the Dectin–Syk–CARD9 complex and function as an IL-1β–processing protease (21), and it is possible that the Dectin–Syk–CARD9 pathway might engage caspase 8 in Pstpip2cmo mice. Our study highlights the complexity of these autoinflammatory bone diseases, where multiple pathways could be working in unison or independently.
Pstpip2cmo hematopoietic cells are critical and sufficient for induction of disease (1). Given that IL-1β is critical for induction of disease (1, 15), we asked whether hematopoietic cells were the major source of IL-1β. Our studies demonstrated that IL-1β secreted by the hematopoietic compartment is necessary and sufficient for disease induction and progression, and that IL-1β produced by the radioresistant compartment is dispensable. Whereas our chimeras only demonstrate whether hematopoietic or the radioresistant compartment are important for IL-1β secretion, based on previous studies (2), it could be posited that the major IL-1β–producing cells are the neutrophils. Indeed, anti-Ly6G Ab treatment (that depletes neutrophils) of Pstpip2cmo mice prevents induction of bone disease (2). Although it is possible that myeloid cells as well as other immune cells might partake in the induction and propagation of disease, neutrophils are essential instigators of the disease in Pstpip2cmo mice.
IL-1β is a pleiotropic cytokine that affects target cells to produce several important cytokines and chemokines that ultimately contribute to the induction of bone disease. Importantly, cytokines such as TNF-α, MCP-1, and M-CSF, which are highly up-regulated in Pstpip2cmo mice, are all at basal level in age-matched Pstpip2cmo × Il1b−/− mice (1). Thus, IL-1β is an apical cytokine that signals through target cells to amplify inflammatory milieu and provoke autoinflammatory bone disease in Pstpip2cmo mice. We generated chimeras from Pstpip2cmo and Il1r−/− mice to investigate the compartment that responds to IL-1β signal to instigate disease in Pstpip2cmo mice. Interestingly, IL-1β signaling in radioresistant cells, but not hematopoietic cells, is critical for disease induction in Pstpip2cmo mice. Adding to this observation, hematopoietic cells from protected Pstpip2cmo × Il1r−/− mice can induce disease in WT recipients (Pstpip2cmo × Il1r−/−>>WT chimera develop full disease), further confirming that autocrine IL-1β signaling in hematopoietic cells is not necessary for disease induction. Altogether, the chimera data generated in this study demonstrate the directionality of IL-1β signaling required for the induction of disease. Future studies will investigate the cell populations in the radioresistant compartments that respond to IL-1β signals to instigate the bone disease in Pstpip2cmo mice.
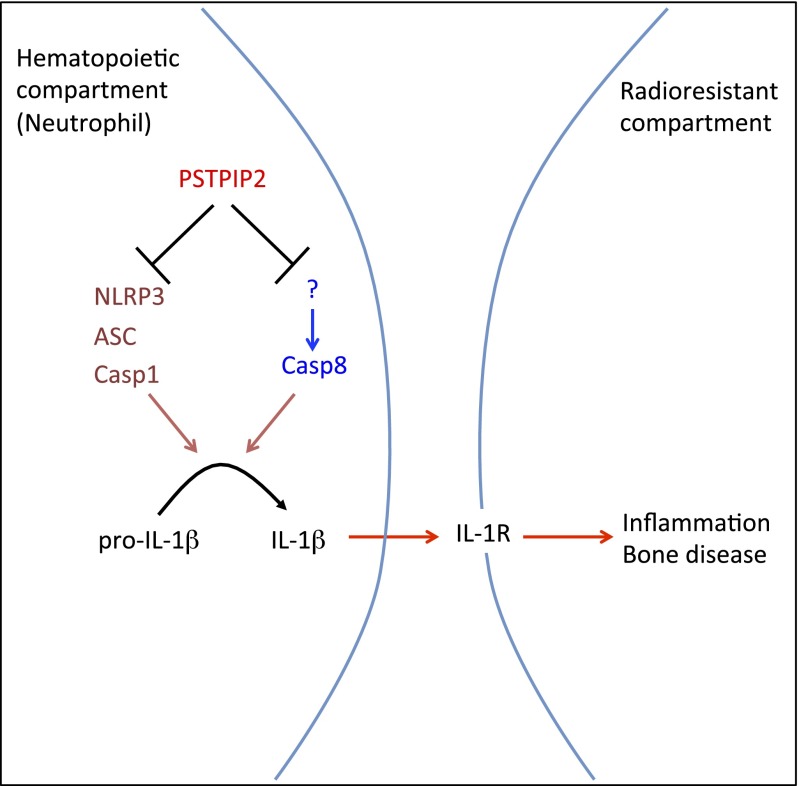
Chronic multifocal osteomyelitis is a severe autoinflammatory bone disease commonly observed in children and adolescents with severe consequences on the affected individuals resulting in permanent bone deformity (12). Our study highlights the complex redundancy between NLRP3 inflammasome and caspase 8 in processing IL-1β and instigating the debilitating bone disease in Pstpip2cmo mice (a mouse model for chronic multifocal osteomyelitis) (Fig. 5). We further demonstrate that IL-1β produced by hematopoietic cells signals through radioresistant cells to induce inflammation and bone disease in Pstpip2cmo mice. Our studies provide a previously unidentified description of upstream molecules as well as potential cellular targets that can be intercepted in the IL-1β pathway to treat this debilitating bone disease.
Fig. 5.
Model showing IL-1β–mediated inflammation and bone disease in Pstpip2cmo mice. Pstpip2cmo mutation in hematopoietic cells results in induction of distinct caspase-1 and caspase-8 complexes (most probably within the neutrophils), i.e., PSTPIP2 might actively suppress spontaneous formation of these complexes. Whereas caspase 1 is activated within the NLRP3 inflammasome complex, caspase 8 is activated in a yet unknown complex. Regardless, these caspases work in a redundant manner to cleave pro–IL-1β into their mature bioactive forms. Once secreted by the hematopoietic cells, bioactive IL-1β is sensed by IL-1R on radioresistant cells to promote inflammation and bone disease.
Methods
Mice and Breeding.
All mice were kept in a specific pathogen-free environment at the St. Jude Children’s Research Hospital Animal Facility. Pstpip2cmo mice were previously described and purchased from The Jackson Laboratory and were on the BALB/c background (9). All other mutant mice were on C57BL/6 background or backcrossed to C57BL/6 mice for at least 10 generations. Nlrp3−/− (27), Casp1−/− (27), Il1b−/− (28), Il1r−/− (29), Ripk3−/−Casp8−/− (30), Ctsb−/− (31), Ctsc−/− (32), Ctsg−/− (33), and Tnfr−/− (34) mice have been previously described. Pstpip2cmo mice (BALB/c background) were crossed with C57BL/6 mice to generate mixed background Pstpip2cmo mice for chimera experiments. Pstpip2cmo mice were crossed with the indicated genetic KO mice to test the role of a particular gene in bone disease. Littermate controls were used to determine whether genetic deletions influenced osteomyelitic bone disease development. Animal studies were conducted under protocols approved by St. Jude Children’s Research Hospital.
Neutrophil Isolation and Confocal Imaging.
Bone marrow was flushed from the tibias and femurs of WT and diseased Pstpip2cmo mice. Bone marrow cells were then passed through 18-gauge needles at least three times to make a single cell suspension. These cells were filtered through a 70-μm cell strainer to get rid of clumps, and neutrophils were purified from the interface of 62.5% Percoll (GE Healthcare) gradient after a 30-min centrifugation at 1,300 × g for 30 min at 4 °C.
Neutrophils were then plated onto four-chambered glass slides pretreated with poly-l-lysine. Neutrophils were then fixed in 4% (vol/vol) paraformaldehyde for 15 min at room temperature, followed by nonspecific blocking in goat serum (DAKO) containing 0.1% saponin (Sigma). Following blocking, neutrophils were gently washed three times and stained with either mouse Casp1 (AG-20B-0042-C100, Adipogen) or rat caspase-8 (1G12, Enzo) antibody for 1 h at 37 °C. Neutrophils were gently washed three times again and stained with secondary antibodies (goat anti-rat Alexa Fluor 488, goat anti-mouse Cy3 from Life Technologies) for 40 min at 37 °C. Neutrophils were then washed four times in warm PBS, air dried, and mounted with DAPI. The images were analyzed and imaged using a Nikon C2 confocal microscope. For each slide, ∼50 cells were counted three independent times (different regions) and cells positive for caspase-1 and caspase-8 puncta of these 50 cells were noted and represented as percentages. Three to four independent slides were used for each experiment. For statistical analysis, an average percentage of caspase-1 or caspase-8 puncta from each slide was used.
Generation of Chimeras.
Four- to six-week-old WT, Pstpip2cmo, Pstpip2cmo × Il1b−/−, and Pstpip2cmo × Il1r−/− mice were lethally irradiated with 900 rads. Six to eight hours after lethal irradiation, these mice received bone marrow cells from donor WT, Pstpip2cmo, Pstpip2cmo × Il1b−/−, and Pstpip2cmo × Il1r−/− mice. Briefly, bone marrow cells were harvested from tibias and femurs of mice and passed through 18-gauze needles to generate single cell suspension. After filtering through a 40-μm cell strainer, about 5 × 106 bone marrow cells were transferred via retroorbital sinus into the lethally irradiated mice. These mice were then followed twice weekly for signs of footpad inflammation and osteomyelitic bone disease.
Mice Harvest and Flow Cytometry Analysis.
Mice were killed using CO2 inhalation according to the approved guidelines from St. Jude Children’s Research Hospital. Following killing, popliteal lymph nodes and spleen were harvested. Single cell suspension of cells from lymph node and spleen were made as described previously (35). Cells were then stained for neutrophils [anti-Ly6G Percp5.5 Ab (Biolegend) and anti-CD11b eFlour450 Ab (eBioscience)] and analyzed using the BD FacsCalibur and FlowJo software.
Statistical Analysis.
All data are represented as mean ± SEM and all experiments were repeated at least two to three times before being reported. Survival curves were analyzed using the log-rank (Mantel–Cox) test. Two-tailed Student’s t test and Mann–Whitney u test were used to compare statistical significance between two groups. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Acknowledgments
We thank Drs. Shizuo Akira, Gabriel Nunez, Richard Flavell, John Bertin, David Chaplin, Douglas Green, Thomas Reinheckel, and Christine Pham for generous supply of mutant mice and Drs. Deepika Sharma, David Place, and Farrah Phillips for their help with editing the manuscript. P.G. is a postdoctoral fellow supported by a Paul Barrett Endowed Fellowship from St. Jude Children’s Research Hospital. T.-D.K. is supported by the National Institutes of Health (Grants AR056296, CA163507, and AI101935) and the American Lebanese Syrian Associated Charities.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1601636113/-/DCSupplemental.
References
- 1.Lukens JR, et al. Critical role for inflammasome-independent IL-1β production in osteomyelitis. Proc Natl Acad Sci USA. 2014;111(3):1066–1071. doi: 10.1073/pnas.1318688111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lukens JR, et al. Dietary modulation of the microbiome affects autoinflammatory disease. Nature. 2014;516(7530):246–249. doi: 10.1038/nature13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lukens JR, et al. RIP1-driven autoinflammation targets IL-1α independently of inflammasomes and RIP3. Nature. 2013;498(7453):224–227. doi: 10.1038/nature12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts-Galbraith RH, Gould KL. Setting the F-BAR: Functions and regulation of the F-BAR protein family. Cell Cycle. 2010;9(20):4091–4097. doi: 10.4161/cc.9.20.13587. [DOI] [PubMed] [Google Scholar]
- 5.Chitu V, et al. PSTPIP2 deficiency in mice causes osteopenia and increased differentiation of multipotent myeloid precursors into osteoclasts. Blood. 2012;120(15):3126–3135. doi: 10.1182/blood-2012-04-425595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsujita K, et al. Coordination between the actin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis. J Cell Biol. 2006;172(2):269–279. doi: 10.1083/jcb.200508091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y, Dowbenko D, Lasky LA. PSTPIP 2, a second tyrosine phosphorylated, cytoskeletal-associated protein that binds a PEST-type protein-tyrosine phosphatase. J Biol Chem. 1998;273(46):30487–30496. doi: 10.1074/jbc.273.46.30487. [DOI] [PubMed] [Google Scholar]
- 8.Drobek A, et al. PSTPIP2, a protein associated with autoinflammatory disease, interacts with inhibitory enzymes SHIP1 and Csk. J Immunol. 2015;195(7):3416–3426. doi: 10.4049/jimmunol.1401494. [DOI] [PubMed] [Google Scholar]
- 9.Chitu V, et al. Primed innate immunity leads to autoinflammatory disease in PSTPIP2-deficient cmo mice. Blood. 2009;114(12):2497–2505. doi: 10.1182/blood-2009-02-204925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferguson PJ, et al. A missense mutation in pstpip2 is associated with the murine autoinflammatory disorder chronic multifocal osteomyelitis. Bone. 2006;38(1):41–47. doi: 10.1016/j.bone.2005.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grosse J, et al. Mutation of mouse Mayp/Pstpip2 causes a macrophage autoinflammatory disease. Blood. 2006;107(8):3350–3358. doi: 10.1182/blood-2005-09-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acikgoz G, Averill LW. Chronic recurrent multifocal osteomyelitis: typical patterns of bone involvement in whole-body bone scintigraphy. Nucl Med Commun. 2014;35(8):797–807. doi: 10.1097/MNM.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 13.Hedrich CM, Hofmann SR, Pablik J, Morbach H, Girschick HJ. Autoinflammatory bone disorders with special focus on chronic recurrent multifocal osteomyelitis (CRMO) Pediatr Rheumatol Online J. 2013;11(1):47. doi: 10.1186/1546-0096-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson PJ, Laxer RM. New discoveries in CRMO: IL-1β, the neutrophil, and the microbiome implicated in disease pathogenesis in Pstpip2-deficient mice. Semin Immunopathol. 2015;37(4):407–412. doi: 10.1007/s00281-015-0488-2. [DOI] [PubMed] [Google Scholar]
- 15.Cassel SL, et al. Inflammasome-independent IL-1β mediates autoinflammatory disease in Pstpip2-deficient mice. Proc Natl Acad Sci USA. 2014;111(3):1072–1077. doi: 10.1073/pnas.1318685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurung P, Kanneganti TD. Novel roles for caspase-8 in IL-1β and inflammasome regulation. Am J Pathol. 2015;185(1):17–25. doi: 10.1016/j.ajpath.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurung P, et al. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol. 2014;192(4):1835–1846. doi: 10.4049/jimmunol.1302839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karki R, et al. Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection. Cell Host Microbe. 2015;17(3):357–368. doi: 10.1016/j.chom.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antonopoulos C, El Sanadi C, Kaiser WJ, Mocarski ES, Dubyak GR. Proapoptotic chemotherapeutic drugs induce noncanonical processing and release of IL-1β via caspase-8 in dendritic cells. J Immunol. 2013;191(9):4789–4803. doi: 10.4049/jimmunol.1300645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bossaller L, et al. Cutting edge: FAS (CD95) mediates noncanonical IL-1β and IL-18 maturation via caspase-8 in an RIP3-independent manner. J Immunol. 2012;189(12):5508–5512. doi: 10.4049/jimmunol.1202121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gringhuis SI, et al. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13(3):246–254. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- 22.Shenderov K, et al. Cutting edge: Endoplasmic reticulum stress licenses macrophages to produce mature IL-1β in response to TLR4 stimulation through a caspase-8- and TRIF-dependent pathway. J Immunol. 2014;192(5):2029–2033. doi: 10.4049/jimmunol.1302549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nat Rev Immunol. 2002;2(5):364–371. doi: 10.1038/nri802. [DOI] [PubMed] [Google Scholar]
- 24.Coates LC, Marzo-Ortega H, Bennett AN, Emery P. Anti-TNF therapy in ankylosing spondylitis: Insights for the clinician. Ther Adv Musculoskelet Dis. 2010;2(1):37–43. doi: 10.1177/1759720X09359728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Man SM, et al. Salmonella infection induces recruitment of Caspase-8 to the inflammasome to modulate IL-1β production. J Immunol. 2013;191(10):5239–5246. doi: 10.4049/jimmunol.1301581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mérino D, Lalaoui N, Morizot A, Solary E, Micheau O. TRAIL in cancer therapy: Present and future challenges. Expert Opin Ther Targets. 2007;11(10):1299–1314. doi: 10.1517/14728222.11.10.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanneganti TD, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440(7081):233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 28.Shornick LP, et al. Mice deficient in IL-1beta manifest impaired contact hypersensitivity to trinitrochlorobenzone. J Exp Med. 1996;183(4):1427–1436. doi: 10.1084/jem.183.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glaccum MB, et al. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol. 1997;159(7):3364–3371. [PubMed] [Google Scholar]
- 30.Oberst A, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471(7338):363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deussing J, et al. Cathepsins B and D are dispensable for major histocompatibility complex class II-mediated antigen presentation. Proc Natl Acad Sci USA. 1998;95(8):4516–4521. doi: 10.1073/pnas.95.8.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pham CT, Ley TJ. Dipeptidyl peptidase I is required for the processing and activation of granzymes A and B in vivo. Proc Natl Acad Sci USA. 1999;96(15):8627–8632. doi: 10.1073/pnas.96.15.8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacIvor DM, et al. Normal neutrophil function in cathepsin G-deficient mice. Blood. 1999;94(12):4282–4293. [PubMed] [Google Scholar]
- 34.Peschon JJ, et al. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160(2):943–952. [PubMed] [Google Scholar]
- 35.Gurung P, et al. An NLRP3 inflammasome-triggered Th2-biased adaptive immune response promotes leishmaniasis. J Clin Invest. 2015;125(3):1329–1338. doi: 10.1172/JCI79526. [DOI] [PMC free article] [PubMed] [Google Scholar]