Significance
Inhibition of the VEGF/VEGF receptor (VEGFR) pathway has failed to increase overall survival in phase III trials in patients with glioblastoma (GBM). Previously we identified the angiopoietin-2 (Ang-2)/TEK receptor tyrosine kinase (Tie-2) pathway as a potential driver of resistance to VEGF inhibition in GBM. Here we show that dual inhibition of VEGFRs and Ang-2 inhibits tumor growth and prolongs vessel normalization compared with VEGFR inhibition alone, resulting in improved survival in murine GBM models. Furthermore, by blocking macrophage recruitment, we demonstrate that macrophages contribute to the beneficial effects of dual therapy.
Keywords: anti-angiogenic therapy, tumor microenvironment, tumor immunity, colony-stimulating factor 1, macrophage
Abstract
Glioblastomas (GBMs) rapidly become refractory to anti-VEGF therapies. We previously demonstrated that ectopic overexpression of angiopoietin-2 (Ang-2) compromises the benefits of anti-VEGF receptor (VEGFR) treatment in murine GBM models and that circulating Ang-2 levels in GBM patients rebound after an initial decrease following cediranib (a pan-VEGFR tyrosine kinase inhibitor) administration. Here we tested whether dual inhibition of VEGFR/Ang-2 could improve survival in two orthotopic models of GBM, Gl261 and U87. Dual therapy using cediranib and MEDI3617 (an anti–Ang-2–neutralizing antibody) improved survival over each therapy alone by delaying Gl261 growth and increasing U87 necrosis, effectively reducing viable tumor burden. Consistent with their vascular-modulating function, the dual therapies enhanced morphological normalization of vessels. Dual therapy also led to changes in tumor-associated macrophages (TAMs). Inhibition of TAM recruitment using an anti–colony-stimulating factor-1 antibody compromised the survival benefit of dual therapy. Thus, dual inhibition of VEGFR/Ang-2 prolongs survival in preclinical GBM models by reducing tumor burden, improving normalization, and altering TAMs. This approach may represent a potential therapeutic strategy to overcome the limitations of anti-VEGFR monotherapy in GBM patients by integrating the complementary effects of anti-Ang2 treatment on vessels and immune cells.
Glioblastoma (GBM) is the most common and aggressive primary malignant brain tumor in adults (1). Based on promising phase II trials, bevacizumab (Avastin), a VEGF monoclonal antibody, was approved by the Food and Drug Administration in 2009 as salvage monotherapy for recurrent GBM (rGBM) (1–5). However, three randomized phase III trials of bevacizumab in combination with chemoradiation in newly diagnosed GBM patients and in combination with chemotherapy in rGBM patients failed to prolong overall survival despite the extension of progression-free survival (6–8). A randomized trial of cediranib, a pan-VEGF receptor (VEGFR) tyrosine kinase inhibitor, with or without lomustine also failed to show improved survival in patients with rGBM (9). Inhibition of VEGFR2 transiently “normalized” GBM vasculature and improved the outcome of radiotherapy in mice bearing GBM (10). Vascular normalization also reduced intracranial edema, contributing to the survival benefit in animal models (11). Similarly, treatment with cediranib induced transient vessel normalization in patients with rGBM, effectively reducing edema (12, 13). Unfortunately, resistance to anti-VEGF therapy develops rapidly in patients with GBM. Modulation of other angiogenic, growth, and survival programs in response to VEGF-signaling blockade may mediate this resistance (1, 14–16). Thus, there is an urgent need to develop novel combinations targeting such resistance pathways to increase survival in GBM.
Angiopoietin-2 (Ang-2) is often up-regulated in GBMs (17–19) and is thought to play a role in GBM angiogenesis (17, 20, 21). Ang-2 is a member of the angiopoietin family that signals primarily through the tyrosine kinase receptor TEK receptor tyrosine kinase (Tie-2). Ang-1 and Ang-2 play important, often complementary, roles in maintaining normal vasculature through the modulation of vessel stability (22). Ang-2–mediated vessel destabilization can be either pro- or antiangiogenic in a context-dependent manner (17, 23–26). Increased Ang-2 expression may be an escape mechanism to anti-VEGF therapy. In both clinical and preclinical GBM studies, Ang-2 levels decline temporarily following inhibition of the VEGF pathway but later rebound as tumors become resistant to therapy (10, 12). We have shown that ectopic expression of Ang-2 in a GBM animal model compromised the survival benefit of VEGF-signaling inhibition by impairing vessel normalization and edema control (27). Moreover, tumor autopsy tissues from rGBM patients treated with anti-VEGF therapy showed abnormally high levels of Ang-2 (28), and the Ang-1/Ang-2 ratio correlated positively with survival (29) and vascular normalization (12). Therefore, we hypothesized that dual inhibition of VEGF and Ang-2 signaling could prolong the window of normalization or normalize vessels to a greater extent and thereby enhance the survival benefit of anti-VEGF therapy.
Additionally, we recently demonstrated that vascular normalization leads to efficient immune cell recruitment and promotes an immuno-stimulatory microenvironment in murine breast tumors (30). Given that tumor-associated macrophage (TAM) infiltration is an important determinant of tumor progression and response to therapy in GBM and other tumors (31–33), we further hypothesized that TAMs may play a role in the response to dual therapy.
In this study we combined an Ang-2–neutralizing monoclonal antibody that targets both human and murine Ang-2, MEDI3617 (MedImmune) (34), with cediranib (AstraZeneca Pharmaceuticals) and assessed the effects of dual therapy on survival, tumor growth, vascular normalization, and TAM phenotype in two orthotopic models of GBM in mice: Gl261 (a syngeneic, hypovascular murine GBM model) and U87 (a highly angiogenic human GBM model).
Results
Dual Anti-VEGFR/Ang-2 Therapy Extends Survival Compared with Cediranib Monotherapy.
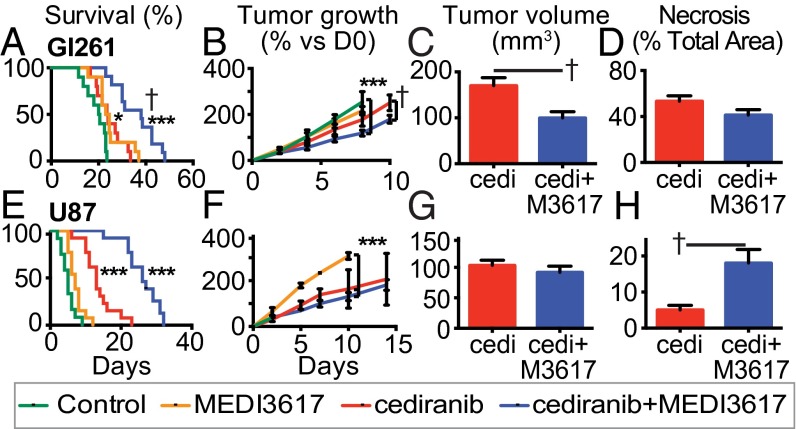
We treated mice bearing orthotopic Gl261 or U87 tumors with control IgG, MEDI3617, cediranib, or cediranib+MEDI3617 dual therapy. Consistent with our previous experience in GBM (10, 11, 27), treatment with cediranib alone significantly increased survival of Gl261-bearing mice compared with control (median 24 d vs. 20 d). MEDI3617 treatment also increased median survival (24 d). Strikingly, dual therapy significantly prolonged survival compared with the monotherapy and control arms (38 d) (Fig. 1A and Table S1). Similarly, dual therapy significantly increased mouse survival in U87-bearing mice (median 26 d) compared with mice treated with cediranib alone (13 d), MEDI3617 alone (7 d), or control treated mice (5 d) (Fig. 1E and Table S1).
Fig. 1.
Dual cediranib+MEDI3617 therapy enhances survival and reduces tumor burden in Gl261 and U87 tumors compared with cediranib therapy alone. Mice bearing Gl261 (A–D) or U87 (E–H) tumors were treated with control (green traces), MEDI3617 (orange traces), cediranib (red traces/bars), or dual therapy (blue traces/bars). (A) In Gl261 tumors, both cediranib and MEDI3617 monotherapies led to significantly higher overall median survival (24 d) than control treatment (20 d) (cediranib *P = 0.017; MEDI3617 *P = 0.011; n = 10). Dual therapy (n = 11) led to a significantly higher median survival (38 d) than control (***P < 0.0001) or cediranib treatment (†P = 0.002). (B) There was a significant difference in the growth rate of tumors treated with dual therapy compared with both control-treated (***P < 0.0001) and cediranib-treated (†P = 0.0076) tumors as measured by OFDI. (C) Dual therapy-treated tumors were significantly smaller than cediranib-treated tumors at day 20 as measured by MRI (†P = 0.0089). (D) There was no change in the extent of necrosis at day 20 (P = 0.11). (E) In the U87 model, both cediranib and dual therapy-treated mice had a significantly higher overall median survival (26 d and 13 d, respectively; n = 13) than control-treated mice (5 d; n = 12; ***P < 0.0001). (F and G) There was no difference in tumor growth (F) or volume (G) between dual therapy-treated tumors and cediranib-treated tumors in the U87 model. (H) There was a significant increase in ischemic hypoxic changes (early necrosis) in dual therapy-treated tumors compared with cediranib-treated tumors at day 6 (†P = 0.030). cedi, cediranib; cedi+M3671, cediranib+MEDI3617. Error bars represent the SEM. *P < 0.05, **P < 0.01, ***P < 0.001 compared with control unless otherwise indicated.
Table S1.
Median survival of U87 and Gl261 mice
| Treatment | n | Median | |
| U87 | |||
| Control | 12 | 5 | |
| MEDI3617 | 13 | 7*,† | |
| Cediranib | 13 | 13***,† | |
| MEDI3617 + cediranib | 13 | 26*** | |
| Gl261 | |||
| Control | 10 | 20 | |
| MEDI3617 | 10 | 24*,† | |
| Cediranib | 10 | 24*,† | |
| MEDI3617 + cediranib | 11 | 38*** | |
In both tumor models, dual therapy resulted in significantly higher survival than control treatment (Gl261: 38 d vs. 20 d; U87: 26 d vs. 5 d, ***P < 0.0001). In mice bearing Gl261 tumors, both cediranib and MEDI3617 monotherapies led to significantly higher overall median survival (24 d) than the control treatment (cediranib: *P = 0.017l; MEDI3617: *P = 0.011). In mice bearing U87 tumors, overall median survival with cediranib treatment (13 d) was significantly higher than with the control treatment (***P < 0.0001), and MEDI3617 alone also significantly increased survival compared with control treatment (7 d; *P = 0.014). In both tumor models, dual therapy resulted in significantly higher survival than seen with either monotherapy (Gl261: cediranib †P = 0.002, MEDI3617 †P = 0.0012; U87: cediranib †P < 0.0001, MEDI3617 †P < 0.0001).
Given the significant improvement in survival in mice treated with dual therapy over cediranib monotherapy, we next examined the impact of treatment on tumor growth. Gl261 tumors treated with either cediranib or dual therapy grew significantly more slowly than control tumors. Moreover, tumors treated with dual therapy grew more slowly than cediranib-treated tumors (Fig. 1B). This sustained delay in growth resulted in significantly smaller tumors near the median survival time of cediranib-treated mice (day 20) in the dual-therapy group compared with the cediranib group as measured by MRI (Fig. 1C). We also examined the extent of necrosis and observed no significant difference between Gl261 tumors treated with dual therapy or with cediranib monotherapy (Fig. 1D and Fig. S1). Thus, dual therapy delayed GBM progression by slowing the tumor growth rate, resulting in a lower viable tumor burden in Gl261-bearing mice treated with dual therapy than in mice treated with cediranib monotherapy.
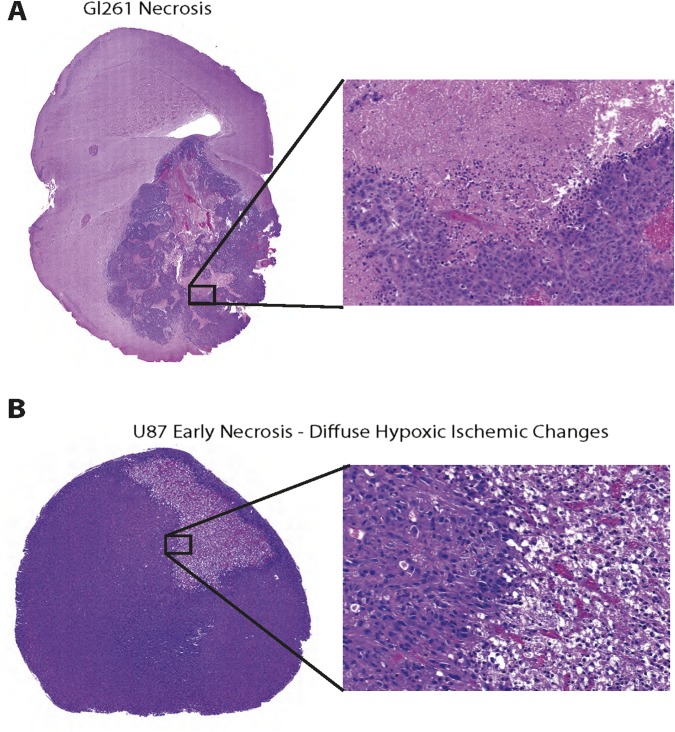
Fig. S1.
Representative images of necrosis in Gl261 and U87 tumors. (A) Representative image of H&E staining to highlight necrosis found in Gl261 tumors at day 20. (B) Representative image of early necrosis exhibiting diffuse hypoxic ischemic changes in U87 tumors at day 6.
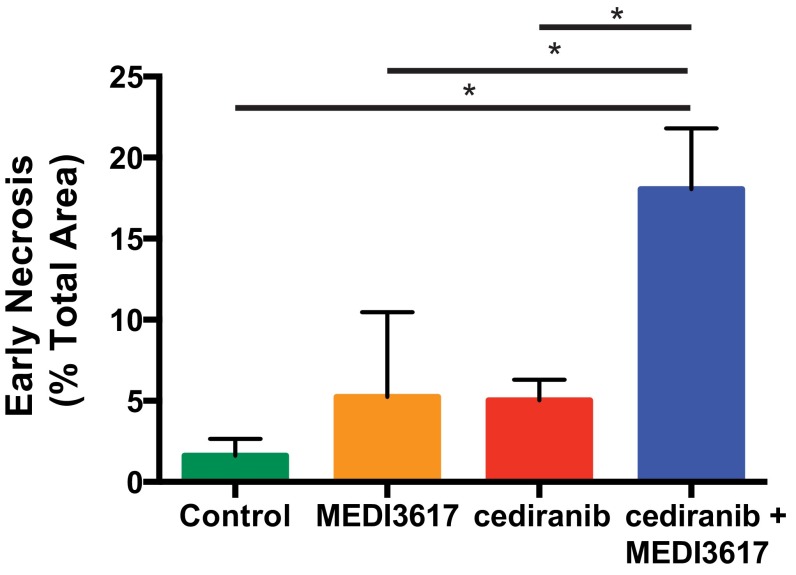
Interestingly, there was no detectable difference in the growth rate of U87 tumors treated with cediranib monotherapy or dual therapy (Fig. 1 F and G), suggesting that the inhibition of tumor growth was not a chief cause of the observed survival benefit. This finding is consistent with our previous study in the same tumor model (11). However, we detected a striking increase in areas of diffuse hypoxic ischemic change (i.e., early necrosis) (Fig. S1) by day 6 in the dual therapy-treated tumors as compared with both cediranib- and control-treated tumors (Fig. 1H and Fig. S2), resulting in a decreased viable tumor burden in mice treated with dual therapy.
Fig. S2.
Dual cediranib+MEDI3617 therapy increases the development of early necrosis in U87 tumors. Mice bearing U87 tumors were treated with control (green bar), MEDI3617 (orange bar), cediranib (red bar), or dual therapy (blue bar). H&E-stained tissues were analyzed for the area of diffuse hypoxic changes (early necrosis). *Dual therapy significantly increased early necrosis in U87 tumors compared with cediranib- (P = 0.0039), MEDI3617- (P = 0.043) and control-treated (P = 0.0026) tumors. Error bars represent the SEM.
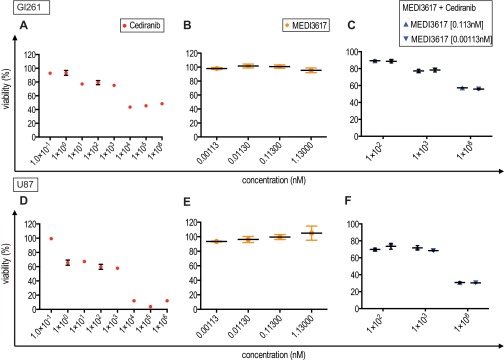
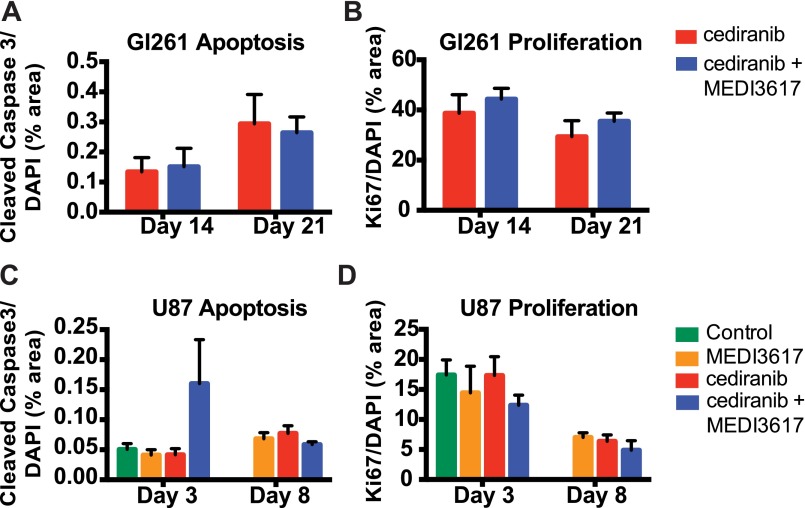
Direct antiproliferative effects were observed in vitro with high concentrations of cediranib (>103 nM), but MEDI3617 had no effect on cell viability in either Gl261 or U87 tumors (Fig. S3). However, histological analyses of tumor apoptosis and proliferation did not reveal significant changes (Fig. S4) in either U87 or Gl261 tumors, suggesting that alternative mechanisms are responsible for reduced viable tumor burden.
Fig. S3.
In vitro cell viability in Gl261 and U87 tumors treated with cediranib, MEDI3617, or dual cediranib+MEDI3617 therapy. Gl261 or U87 cells were treated with increasing concentrations of cediranib or MEDI3617. Cell viability was measured using an MTT assay after 72 h of incubation with therapies. (A and D) Cediranib concentrations ranged from 1–106 nM. Cediranib monotherapy was cytotoxic to cells at concentrations above 103 nM. (B and E) MEDI3617 concentrations ranged from 0.00113–1.13 nM. MEDI3617 had no effect on cell viability. (C and F) Cells were treated with dual-therapy regimens of cediranib (102 nM, 103 nM, or 106 nM) + MEDI3617 (0.113 nM or 0.00113 nM). As in cediranib monotherapy, dual therapy reduced cell viability at higher concentrations of cediranib. These effects were the same with different concentrations of MEDI3617, suggesting that the main cytotoxic effects are caused solely by cediranib.
Fig. S4.
Dual cediranib+MEDI3617 therapy does not affect tumor apoptosis or proliferation. Gl261 (A and B) and U87 (C and D) tissues were collected from mice treated with control (green bars), MEDI3617 (orange bars), cediranib (red bars), or dual therapy (blue bars) at various time points after beginning treatment. Sections were stained for cleaved caspase 3 (apoptosis) or Ki67 (proliferation) and DAPI (nuclei). (A and B) In Gl261 tissue, neither apoptosis (A) nor proliferation (B) was altered by dual therapy at days 14 or 21. (C and D) Similarly, in U87 tissue dual therapy had no effect on apoptosis (C) or proliferation (D). Error bars represent the SEM.
Dual Therapy Enhances Normalization in Gl261 Tumors Compared with Cediranib Monotherapy.
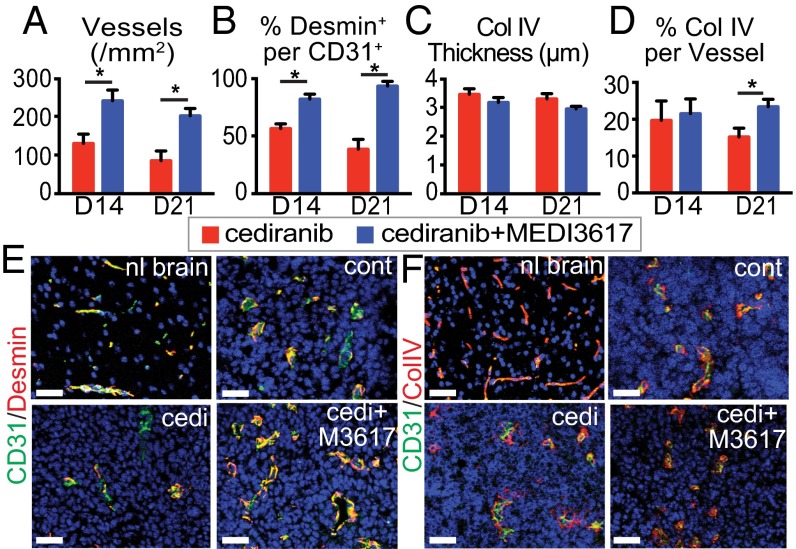
To determine the effects of cediranib+MEDI3617 dual therapy on the tumor vasculature, we assessed vessel morphology. In Gl261 tumors we focused our analysis on day 14 (the time point of the beginning of the end of cediranib-induced vascular normalization based on our preliminary studies) and day 21 (near the median survival of cediranib-treated mice) to identify the effects of prolonged dual therapy compared with cediranib monotherapy. Tumor microvessel density (MVD) and perivascular cell coverage were higher in the dual therapy group than in cediranib-treated tumors at day 14, but there were no differences in the extent of basement membrane (BM) coverage or thickness (Fig. 2). By day 21, tumor vessels were still structurally normalized, with significantly higher BM coverage, in the mice treated with dual therapy (Fig. 2). Overall the vessels in the dual therapy-treated tumors had a more normalized vessel structure than the vessels in the cediranib-treated tumors (Fig. 2 E and F).
Fig. 2.
Dual cediranib+MEDI3617 therapy extends vascular normalization in viable tissue compared with cediranib therapy alone. Gl261 tumors were collected from mice treated with cediranib (red bars) or dual therapy (blue bars) at days 14 or 21 after beginning treatment. Sections were stained for CD31 (for vessels), desmin (for perivascular cells), or collagen IV (for BM) and DAPI (for nuclei). (A, B, D) Both MVD (A) and perivascular cell coverage (the percentage of the desmin/CD31 double-positive area in the CD31+ area) (B) were higher in the dual therapy-treated tumors than in cediranib-treated tumors at days 14 and 21, but BM coverage (D) was higher only at day 21. (C) There was no significant difference in BM thickness among groups. (E) Representative images of CD31 (green)/desmin (red) staining in the normal brain (nl brain) and in control (cont)-, cediranib (cedi)-, and dual therapy (cedi+M3617)-treated tumors on day 21. (F) Representative images of CD31 (green)/collagen IV (red) staining. Error bars represent the SEM. *P < 0.05 compared with control unless otherwise indicated. (Scale bars, 50 μm.)
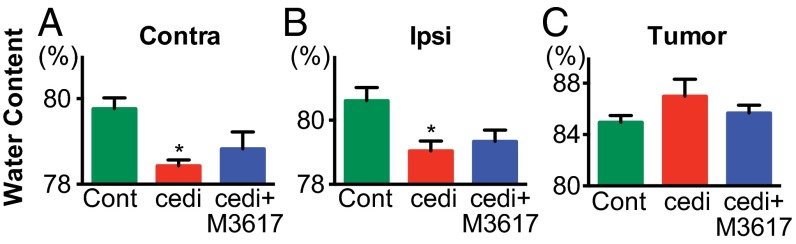
Because dual therapy induced sustained structural vessel normalization in Gl261, we next investigated whether these changes could translate to improved edema control. In addition to determining intratumoral edema, we assessed edema in both the ipsilateral hemisphere (to measure peritumoral edema) and the contralateral hemisphere (as a control for global changes in edema). In Gl261 tumors, both cediranib and dual therapy decreased edema in the ipsilateral and contralateral hemispheres compared with controls, although only cediranib had a statistically significant effect (Fig. 3 A and B; dual therapy P = 0.052). There was no difference among the treatment groups in edema within the tumor itself (Fig. 3C). These data suggest that edema control is not the major consequence of the improved normalization in dual therapy-treated tumors compared with cediranib-treated tumors.
Fig. 3.
Dual cediranib+MEDI3617 therapy promotes edema control in Gl261 tumors. Gl261-bearing mice (n = 5–7) were treated with control (green bars), cediranib (red bars), or dual therapy (blue bars). Water content (edema) was measured on day 10 after treatment start by wet/dry weight ratio in the contralateral hemisphere (Contra) (A), ipsilateral hemisphere (Ipsi) (B), and tumor (C). Cediranib monotherapy significantly decreased water content in both the contralateral (P = 0.029) and ipsilateral (P = 0.024) hemispheres. Dual therapy also decreased water content compared with control-treated tumors, although the decrease did not reach statistical significance (contralateral P = 0.095; ipsilateral P = 0.052). There was no significant difference in edema in any of the tissues treated with dual therapy as compared with cediranib treatment. cedi, cediranib; cedi+M3617, cediranib+MEDI3617; Cont, control. Error bars represent the SEM. *P < 0.05 compared with control unless otherwise indicated.
Dual Therapy Improves Structural Vessel Normalization in U87 Tumors Compared With Cediranib Monotherapy.
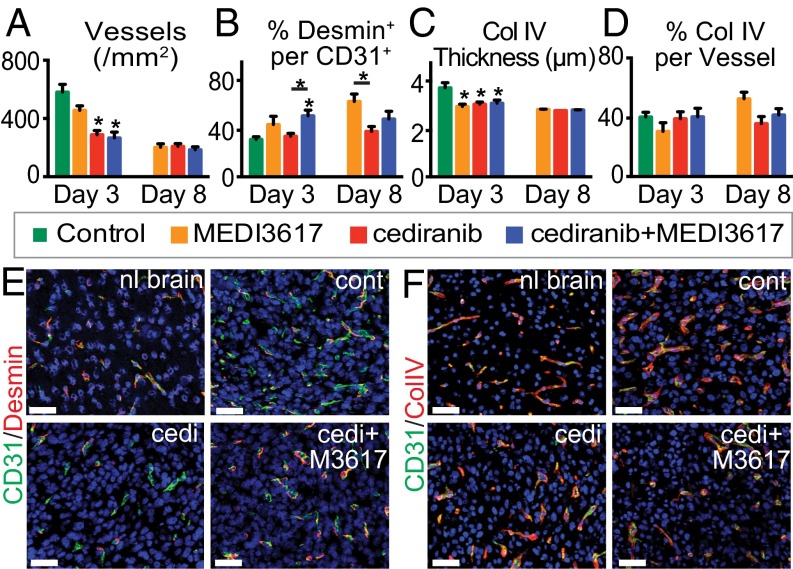
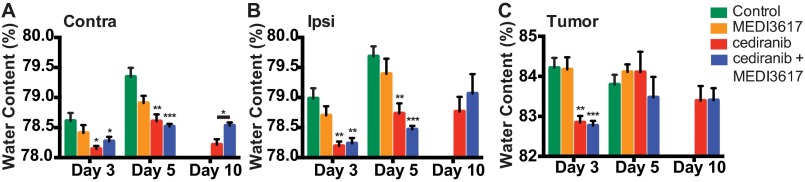
Vessels were analyzed at days 3 and 8 after treatment initiation to determine the effects of therapy during the previously defined time window of normalization (days 3–8) (10, 11, 27). Tumors treated with either cediranib alone or dual therapy displayed a significant decrease in MVD and BM thickness, but not BM coverage, at day 3 compared with control tumors (Fig. 4 A, C, D, and F). Perivascular cell coverage was significantly higher in dual therapy-treated tumors than in vessels from control- and cediranib-treated tumors (Fig. 4 B and E), suggesting a greater extent of vessel normalization after dual therapy. Indeed, after dual therapy GBM vessels exhibit better normalization (Fig. 4 E and F). These features were maintained in both the cediranib- and dual therapy-treated tumors at day 8. Notably, no control mice survived to day 8. MEDI3617 monotherapy induced some features of vessel normalization but did so in an inconsistent manner: Although there was no change in the MVD of MEDI3617-treated tumors, BM thickness was decreased compared with control tumors at day 3, and perivascular cell coverage was increased at day 8 (Fig. 4). Thus, as in Gl261 tumors, improved vascular normalization was observed in dual therapy-treated U87 tumors compared with cediranib-treated tumors. We also found that, as in Gl261 tumors, this vessel normalization with dual therapy did not translate into better edema control than seen with cediranib monotherapy (Fig. S5).
Fig. 4.
Dual cediranib+MEDI3617 therapy improves vessel normalization in U87 tumors as compared with cediranib monotherapy. U87 tissues were collected from mice treated with control (green bars), MEDI3617 (orange bars), cediranib (red bars), or dual cediranib+MEDI3617 (blue bars) therapy at days 3 or 8 after beginning treatment. Sections were stained for CD31, either desmin or collagen IV (BM), and DAPI. (A and C) Both MVD (A) and BM thickness (C) were significantly decreased at day 3 by both cediranib and dual therapy as compared with control treatment and remained low at day 8. (B) In the dual therapy-treated tumors, perivascular cell coverage (the percentage of the desmin/CD31 double-positive area in the CD31+ area) also was significantly higher on day 3 as compared with control tumors. (D) There was no significant difference in BM coverage among groups. (E) Representative images of CD31 (green)/desmin (red) staining in the normal brain (nl brain) and in control (cont)-, cediranib (cedi)-, and dual therapy (cedi+M3617)-treated tumors. (F) Representative images of CD31 (green)/collagen IV (red) staining in the normal brain and in control-, cediranib-, and dual therapy-treated tumors. Error bars represent the SEM. *P < 0.05 compared with control unless otherwise indicated. (Scale bars, 50 μm.)
Fig. S5.
Dual cediranib+MEDI3617 therapy promotes edema control in U87 tumors. U87 tumor-bearing mice (n = 5–8) were treated with control (green bars), MEDI3617 (orange bars), cediranib (red bars), or dual therapy (blue bars). Water content (edema) was measured on days 3, 5, and 10 after treatment start by the wet/dry weight ratio in the contralateral hemisphere (Contra) (A), ipsilateral hemisphere (Ipsi) (B), and tumor (C). Cediranib monotherapy significantly decreased water content compared with control in all tissues measured at day 3 (contralateral, *P = 0.0061; ipsilateral, **P = 0.0004; tumor, **P = 0.0002). Cediranib monotherapy also decreased edema on day 5 compared with control in both the contralateral (**P = 0.0002) and ipsilateral (**P = 0.0007) hemispheres. Dual therapy also decreased edema on day 3 (contralateral, *P = 0.0370; ipsilateral, **P = 0.0004; tumor, ***P = 0.0001) and day 5 (contralateral and ipsilateral, ***P < 0.0001) compared with control. There was no significant difference in edema in any of the tissues treated with dual therapy compared with those treated with cediranib except for an unexpected increase in the water content of the contralateral hemisphere at day 10 (*P = 0.013). Error bars represent the SEM. *P < 0.05 compared with control unless otherwise indicated.
Dual Therapy Alters TAM Polarization.
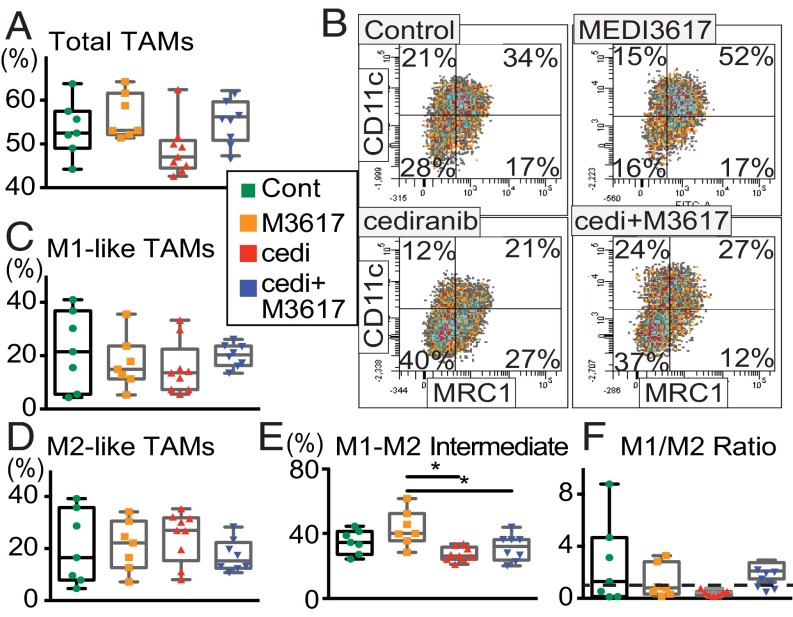
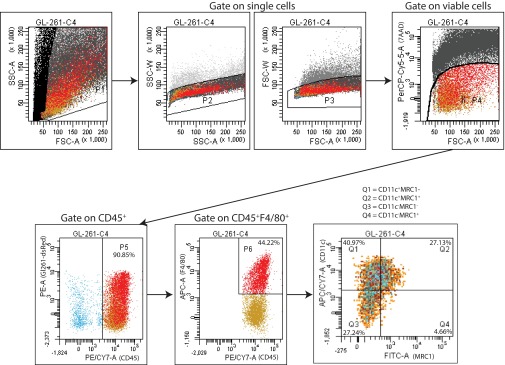
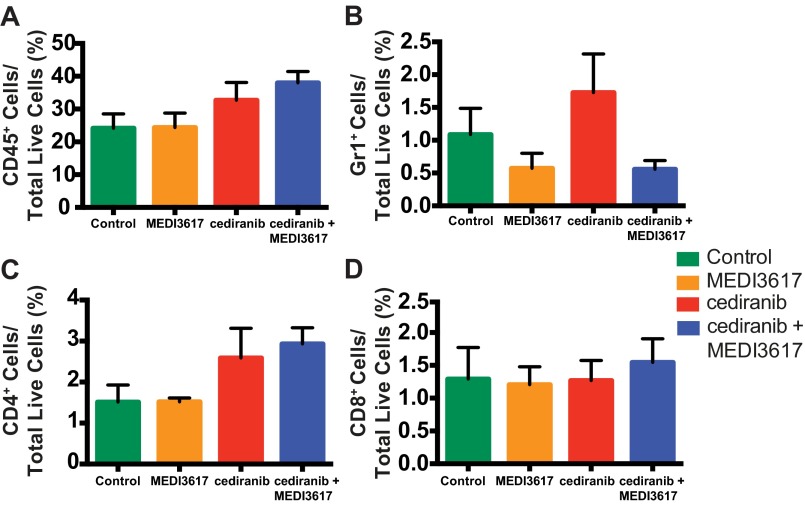
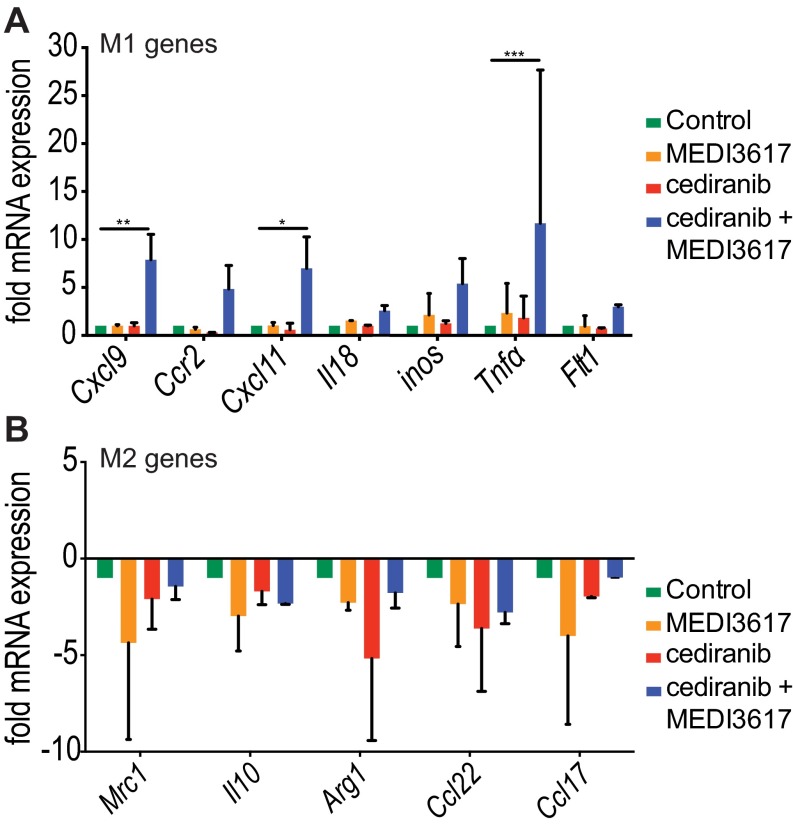
We recently demonstrated that the normalization of tumor vasculature leads to efficient immune cell recruitment and polarization of TAMs to antitumor phenotypes (30). Furthermore, Ang-2 blockade recently has been shown to improve tumor cell immune destruction (35). Based on these data we assessed whether immune cell infiltration or polarization might be altered by dual therapy in Gl261 tumors at day 10 (when tumors treated with dual therapy began to show significant delay in tumor growth as compared with tumors treated with cediranib monotherapy) (Fig. 1B). We found that the proportion of F4/80+ TAMs was significantly changed among treatment groups (Fig. 5A). We further determined the phenotype of the TAMs using the M1 activation marker CD11c and the M2 activation marker MRC1. Fig. 5B and Fig. S6 show representative images of the gating strategy used to define TAM phenotypes. Although there was no difference among the treatment groups in either CD11c+MRC1− M1-like TAMs (Fig. 5C) or CD11c−MRC1+ M2-like TAMs (Fig. 5D) alone, there were significantly more TAMs that did not display a clear M1- or M2-like activation state, i.e., CD11c+MRC1+ M1–M2 intermediate TAMs, in MEDI3617-treated tumors than in cediranib- and dual-treated tumors (Fig. 5E). We detected no significant change in the proportion of other immune cell populations [CD8+ T cells, CD4+ T cells, and CD11b+Gr1+ myeloid-derived suppressor cells (MDSCs)] (Fig. S7). Moreover, in vitro peritoneal macrophage stimulation studies showed enhanced expression of the M1-assocated genes such as Cxcl9, Cxcl11, and Tnfα with dual therapy (Fig. S8). Together, these results indicate that cediranib and MEDI3617 may have differential effects on TAM recruitment and phenotype.
Fig. 5.
Cediranib and MEDI3617 have differential effects on TAM recruitment and polarization in Gl261 tumors. Immune cell profiles in Gl261 tumors were analyzed by flow cytometry on day 10. (A) The percentage of F4/80+ TAMs was significantly different among treatment groups (ANOVA P = 0.046; n = 7 or 8). Cediranib monotherapy tended to reduce the percentage of TAMs compared with MEDI3617 monotherapy and dual therapy (P = 0.054 and P = 0.096, respectively). (B) Representative images of the gating strategy to define TAM phenotypes for each treatment group. TAMs were defined as one of four phenotypes: M1-like TAM (CD11c+MRC1−), M2-like (CD11c−MRC1+), M1–M2 intermediate (CD11c+MRC1+), and double-negative (CD11c−MRC1−) TAMs. (C) There was no significant difference among treatment groups in the percentage of M1-like TAMs. (D) There was no significant difference among any of the groups in the percentage of M2-like TAMs. (E) There was a significant difference among treatment groups in the percentage of M1–M2 intermediate TAMs that did not display clear M1- or M2-like activation (ANOVA P = 0.0031). Furthermore, both cediranib monotherapy and dual therapy significantly reduced the percentage of these TAMs compared with MEDI3617 monotherapy (*P = 0.0019 and *P = 0.029). (F) There was a trend toward a difference among treatment groups in the M1/M2 ratio (Kruskal–Wallis test, P = 0.098). Dual therapy tended to increase M1/M2 ratio compared with cediranib monotherapy (P = 0.053). The dashed line indicates an M1/M2 ratio of 1. Total TAMs were normalized to CD45+ cells for comparisons among treatment groups. All TAM phenotypes described were normalized to total F4/80+ cells for comparisons among treatment groups. Error bars represent the SEM.
Fig. S6.
The gating strategy used to determine TAM recruitment and phenotype in Gl261 tumors. Single-cell suspensions were stained for the following markers: 7AAD (cell viability), CD45, F4/80, CD11c, and MRC1. Cells were first gated according to side-scattered area (SSC-A) and forward-scattered area (FSC-A). Further gating according to SSC-A and side-scattered width (SSC-W) and FSC-A and forward-scattered width (FSC-W) was used to identify single cells. 7AAD was used to identify dead cells. Viable cells then were gated on CD45 to identify CD45+dsRed− tumor-infiltrating immune cells. CD45+ cells were further gated on F4/80 to identify CD45+F4/80+ TAMs. Finally, CD11c and MRC1 were used to identify specific TAM phenotypes: M1-like TAMs (CD11c+MRC1−), M2-like TAMs (CD11c−MRC1+), M1–M2 intermediate TAMs (CD11c+MRC1+), and double-negative TAMs (CD11c−MRC1−).
Fig. S7.
Dual cediranib+MEDI3617 therapy has no effect on granulocyte or T-cell recruitment to Gl261 tumors. Gl261 tumors were collected from mice treated with control (n = 7), MEDI3617 (n = 7), cediranib (n = 7), or dual cediranib+MEDI3617 therapy (n = 8) at day 10 after the beginning of treatment. After disassociation, the resulting cell suspensions were stained with CD45, Gr1, CD4, and CD8 antibodies for flow cytometric analysis of myeloid and lymphoid populations within the tumors. There was no difference among the treatment groups in the percentages of CD45+ cells (A), CD45+Gr1+ granulocytes (B), CD4+ T lymphocytes (C), or CD8+ T lymphocytes (D). Error bars represent the SEM.
Fig. S8.
Peritoneal macrophage gene expression is altered by dual therapy. Peritoneal macrophages isolated from C57BL/6 mice were untreated (control; green bars) or were treated with MEDI3617 (100 nM) (orange bars), cediranib (0.5 μM) (red bars), or dual therapy (blue bars) for 24 h. (A) Gene-expression analysis showed that the M1-associated genes Cxcl9 (P = 0.011), Cxcl11 (P = 0.033), and Tnfα (P < 0.0001) were more highly expressed in dual therapy-treated macrophages than in untreated macrophages. Furthermore, dual therapy also increased the expression of Cxcl9 (P = 0.011), Cxcl11 (P = 0.020), and Tnfα (P = 0.0002) compared with MEDI3617 treatment. Finally, Cxcl9 (P = 0.011), Cxcl11 (P = 0.034), and Tnfα (P = 004) also were more highly expressed in dual therapy-treated macrophages than in cediranib-treated macrophages. (B) Gene expression analysis showed that the M2-associated genes did not change significantly in any of the treatments. Samples were measured in duplicate and experiments were repeated twice (n = 2 per treatment group). *P < 0.05, **P > 0.01, ***P > 0.001.
The Survival Benefit of Dual Anti-VEGFR/Ang-2 Therapy Is Mediated by TAMs.
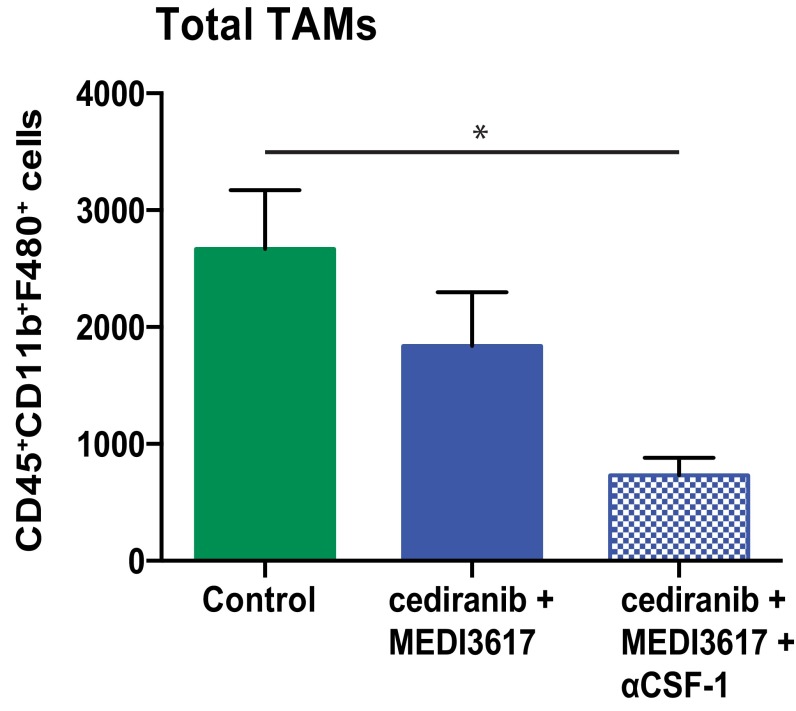
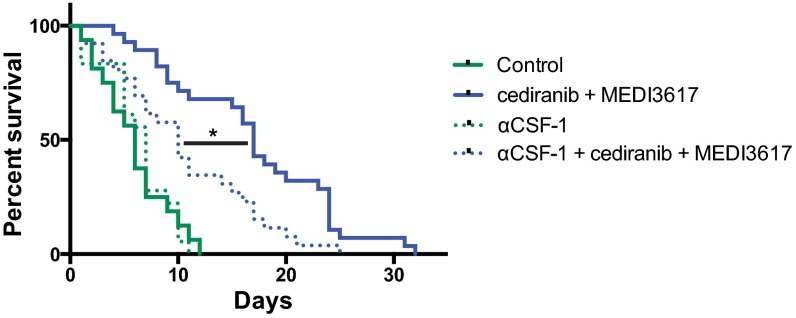
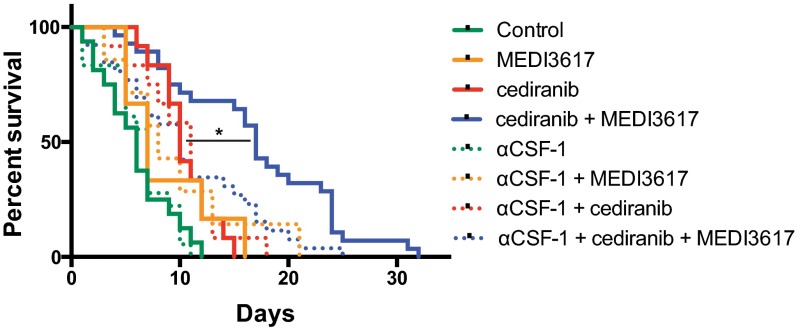
To assess a causal role of TAMs in response to dual therapy, we used an anti–colony-stimulating factor-1 (CSF-1)–neutralizing antibody to block the recruitment of TAMs in Gl261 tumors (Fig. S9). CSF-1 blockade as a monotherapy or in combination with MEDI3617 or cediranib had no significant effect on survival (Fig. 6 and Fig. S10). However, when combined with the dual therapy it significantly compromised the therapeutic benefits of dual therapy (median survival 10 d vs. 17 d). Interestingly, the survival benefit of dual therapy with anti–CSF-1 was comparable to that of cediranib monotherapy (Fig. S10), demonstrating that the additional survival benefit seen with dual therapy over cediranib monotherapy is mediated mainly by TAMs.
Fig. S9.
CSF-1 blockade decreases TAMs. Mice bearing Gl261 tumors were treated with control (n = 5, green bar), dual cediranib+MEDI3617 (n = 5, blue bar), or dual therapy + anti–CSF-1 (n = 5, blue checkered bar) for 10 d before analysis with flow cytometry. The addition of anti–CSF-1 treatment led to a significant decrease in macrophage recruitment compared with control-treatment (*P = 0.0135). Error bars represent the SEM.
Fig. 6.
Inhibition of macrophage recruitment by CSF-1 blockade compromises the survival benefit of dual cediranib+MEDI3617 therapy. Mice bearing Gl261 tumors were treated with control (n = 16; solid green trace), anti–CSF-1 (αCSF-1; n = 18; dotted green trace), dual cediranib+MEDI3617 therapy (n = 28; solid blue trace), or anti–CSF-1 + dual therapy (n = 26; dotted blue trace). Anti–CSF-1 by itself had no effect on overall median survival compared with control treatment (7 d vs. 6 d). When combined with dual therapy, however, anti–CSF-1 significantly reduced overall median survival compared with dual therapy alone (10 d vs. 17 d; *P = 0.003). Dual therapy with or without combined anti–CSF-1 treatment significantly increased overall median survival compared with control treatment (P = 0.0035 and P < 0.0001, respectively). These data confirm the role of macrophages as essential mediators of the survival benefits observed with dual therapy.
Fig. S10.
Animal survival after treatment with cediranib and/or MEDI3617 with or without the inhibition of macrophage recruitment by CSF1-blockade. Mice bearing Gl261 tumors were treated with control (n = 16, solid green trace), anti–CSF-1 (n = 18, dotted green trace), MEDI3617 (n = 6, solid orange trace), anti–CSF-1+MEDI3617 (n = 7, dotted orange trace), cediranib (n = 12, solid red trace), anti–CSF-1+cediranib (n = 12, dotted red trace), dual therapy (n = 28, solid blue trace), or anti–CSF-1 + dual therapy (n = 26, dotted blue trace). Cediranib improved survival both as a monotherapy (P = 0.0032) and in combination with anti–CSF-1 (P = 0.0071). Tumor-bearing animals treated with dual therapy had significantly longer survival than the tumor-bearing animals treated with cediranib alone or with anti–CSF-1 (P = 0.0006 and P = 0.0013, respectively). The addition of anti–CSF-1 had no effect on overall median survival when combined with control, MEDI3617, or cediranib treatment as compared with each therapy alone. When combined with dual therapy, however, anti–CSF-1 significantly reduced overall median survival as compared with dual therapy alone (10 d vs. 17 d; *P = 0.003) and impaired the survival benefit of dual therapy over cediranib monotherapy (P = 0.4174). Dual therapy with and without combined anti–CSF-1 treatment led to significant increases in overall median survival compared with control treatment (P = 0.0035 and P < 0.0001, respectively). These data confirm the role of macrophages as essential mediators of the survival benefits observed with dual therapy.
Discussion
Here we report that combined inhibition of VEGFRs and Ang-2 significantly improves survival over monotherapy arms in murine GBM models. Dual therapy improved the extent of vascular normalization in two models of GBM, effectively reducing edema to a similar extent as cediranib monotherapy. Dual therapy delayed tumor growth in Gl261 tumors and increased tumor necrosis in U87 tumors. In the murine Gl261 model in immunocompetent mice, Ang-2/VEGFR blockade showed differential impacts on TAM recruitment and polarization. Further, blockade of TAM recruitment by an anti–CSF-1 antibody compromised the survival benefit of dual therapy to a level similar to that of cediranib monotherapy.
Our findings are consistent with previous preclinical reports of combined VEGF and Ang-2 signaling inhibition in various s.c. and orthotopic non-CNS tumor models (34, 36–41). However, in contrast to non-CNS models (34, 36, 38, 42–46), we observed little survival benefit of anti–Ang-2 monotherapy in GBM models. This result may reflect the greater dependence of GBM angiogenesis on VEGF. We show that Ang-2 inhibition alone leads to a modest improvement in vascular normalization and has no additional effect on intracranial edema or tumor growth. Additionally, in U87 tumors the ectopic overexpression of Ang-2 has little effect on the tumor vasculature or survival but is able to impair the normalization induced by blocking VEGF signaling (27). These data suggest that Ang-2 may be more relevant as a therapeutic target in GBM in the context of VEGF pathway inhibition.
Previously we have shown that the inhibition of VEGF during tumor growth leads to vascular normalization, the extent of which was associated with survival in preclinical models (10, 11, 27) and GBM patients (47–50). Recent clinical data suggest that improved tumor perfusion and oxygenation in both newly diagnosed and rGBM patients during anti-VEGF therapy correlates with significantly longer survival than seen in patients whose tumor blood perfusion does not increase or decreases (47–50). In both U87 and Gl261 tumors dual therapy resulted in a vasculature that more closely resembled that of the surrounding brain. In Gl261 tumors this effect occurred without a reduction in MVD, whereas the more vascularized U87 model required a reduction in MVD to attain the same end point (i.e., ∼300 vessels/mm2). The resulting extension of vascular normalization beyond that of cediranib monotherapy warrants further studies in combination with radiation, chemotherapy, or immunotherapy, because improvements in the tumor vasculature often improve the delivery and efficacy of other therapeutics (15).
Vascular normalization can enhance the influx of immune effector cells into tumors and prolong the survival of tumor-bearing mice receiving active immunotherapy (15, 51). TAM infiltration is an important determinant of tumor progression and response to therapy in GBM and other tumors (31–33). TAMs may be activated and polarized within a continuum of phenotypes, of which M1 and M2 macrophages represent two extreme phenotypes, depending on the specific cytokines and growth factors produced in the tumor microenvironment. CD11c and MRC1 are commonly used to identify M1- and M2-like TAMs, respectively (52). In many malignant tumor settings, including GBM, TAMs are skewed toward a protumoral M2-like phenotype and promote tumor angiogenesis and progression through the secretion of molecules such as VEGF and MMP-9 (32, 53–60). Both MEDI3617-treated tumors (P = 0.054) and dual therapy-treated tumors (P = 0.096) exhibited a trend toward increased TAMs compared with cediranib-treated tumors (Fig. 5A). These data suggest that the direct effects of blocking VEGFR or Tie-2 signaling in TAMs and the indirect effects via antivascular effects on immune cell populations are potentially independent. Furthermore, there was a trend toward a higher M1/M2 ratio in tumors treated with dual therapy than in tumors treated with cediranib alone (Kruskal–Wallis P = 0.098; P = 0.053 between the cediranib and dual-therapy arms) (Fig. 5E), suggesting that dual therapy may shift the balance of TAMs toward an antitumor phenotype. This observation is further supported by data in our companion paper showing that simultaneous blockade of Ang-2 and VEGF using a bispecific antibody significantly induces M1-like TAM polarization and increases the M1/M2 ratio compared with VEGF-inhibition alone in vivo (61).
We also found that the majority of TAMs did not show clear M1- or M2-like activation and that the proportion of these cells was significantly altered after dual therapy. The significance of these populations remains unclear and should be defined in future studies; however, we speculate that they may represent TAMs within the M1–M2 continuum that have the plasticity to differentiate into either protumor or antitumor TAMs depending on the cytokine milieu. In our previous study vascular normalization increased the production of immune-stimulatory factors while also reducing immune-suppressive factors in TAMs compared with control-treated tumors (30). Because dual therapy normalizes the vasculature, TAMs in dual therapy-treated tumors are adapted to an antitumor phenotype, as shown in our companion manuscript, and thus are able to affect survival positively. However, additional interventions may be necessary to decrease the MDSCs and further activate cytotoxic T lymphocytes—for example by immune checkpoint inhibition—to enhance antitumor adaptive immunity further (30, 62, 63). Taken together, our data warrant further studies to assess the therapeutic potential of the anti-VEGF/Ang-2 combination with novel immunotherapeutics in GBM (51, 64).
Reprogramming the tumor immune environment is a promising therapeutic strategy; preclinical studies blocking TAM function or repolarizing TAMs show promising survival benefits in multiple tumor models (58, 65–67). We previously have shown that anti-VEGF therapy can enhance the recruitment of TAMs in preclinical GBM models and that the number of TAMs is negatively correlated with survival in GBM patients, confirming their potential as a therapeutic target in GBM (11, 68). A recent preclinical study in GBM found that survival could be enhanced by shifting the TAM phenotype away from an M2-like phenotype (33). Here we show that dual blockade of Ang-2 and VEGFR leads to improved vascular normalization and tends to alter the TAM phenotype and/or recruitment compared with anti-VEGFR monotherapy. These changes are associated with delayed GBM progression and increased survival. This dual targeting approach may be an effective way to overcome the resistance to anti-VEGF monotherapy that develops rapidly in patients with GBM.
Materials and Methods
All animal procedures followed Public Health Service Policy on Humane Care of Laboratory Animals guidelines and were approved by the Massachusetts General Hospital (MGH) Institutional Animal Care and Use Committee. MGH is accredited with the Association for Assessment and Accreditation of Laboratory Animal Care and the NIH Office of Laboratory Animal Welfare (A3596-01).
Gl261 and U87 cells were orthotopically implanted into C57BL/6 and nude mice, respectively, and were monitored for tumor growth using intravital fluorescence microscopy or optical frequency domain imaging (OFDI) (69). Treatment with IgG control (10 mg/kg) (MedImmune), MEDI3617 (10 mg/kg), cediranib (6 mg/kg), or dual therapy was initiated at tumor diameters of 2.5 mm in U87 tumors or 2.0 mm in Gl261 tumors. MRI was used to determine tumor size at the time points described. Tissue was harvested and frozen or paraffin-embedded for histological analyses. Ki67, cleaved caspase 3, CD31, collagen IV, and desmin stainings were assessed using MATLAB or Image J. Edema was assessed using dry/wet weight analysis (11). Tumors were harvested at day 10 for flow cytometry, stained for TAM markers, and analyzed with FACSDiva (BD Biosciences) or FlowJo (Tree Star) software. Data are expressed as mean ± SEM. The t tests were two-sided. For comparisons among more than two groups ANOVA or Kruskal–Wallis tests were followed by post hoc multiple comparisons. P < 0.05 was considered significant. Experimental procedures are described in detail in SI Materials and Methods.
SI Materials and Methods
Animal Models and Cell Lines.
Gl261 cells (National Cancer Institute Repository) were cultured in NeuroCult NS-A Proliferation Medium (Stemcell Technologies) as nonadherent neurospheres. U87 cells (ATCC) were cultured in DMEM with 10% (vol/vol) FBS. Cell lines were authenticated by IDEXX Laboratories. Both cell lines were stably transduced with GFP or Discosoma red fluorescent protein (dsRed) using a retroviral construct.
For most studies, cranial windows were implanted into nude or C57BL/6 mice as previously described (70), and mice were allowed to recover for 1 wk before tumor implantation. Briefly, U87-GFP fragments (0.2–0.3 mm in diameter) then were implanted into the left cerebral hemisphere at a depth of 0.4–1 mm. A Gl261-dsRed cell suspension (500,000 cells) in NeuroCult was injected stereotactically at a depth of 1.5 mm.
For the anti–CSF-1 survival experiments, Gl261-GFP tumors were implanted into C57BL/6 mice through a small cranial door (made by cutting a three-sided square in the skull to allow temporary access to the brain during injection) instead of using cranial windows.
In Vitro Cell Viability Assay.
U87 or Gl261 cells were treated with increasing concentrations of cediranib, MEDI3617, or the combination and were incubated for 72 h. Cediranib concentrations ranged from 1–106 nM, and MEDI3617 concentrations ranged from 0.00113–1.13 nM. Dual therapy combined cediranib at concentrations of 102 nM, 103 nM, or 106 nM with MEDI3617 at concentrations of 0.113 nM or 0.00113 nM. Cell viability was measured using a 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide] (MTT) assay.
Pretreatment Tumor Size Monitoring.
For survival, growth, histological, and edema studies, U87-GFP and Gl261-dsRed tumors were monitored daily by fluorescent intravital microscopy and OFDI (69) until the tumors reached an average diameter of 2.5 mm or 2.0 mm, respectively, at which point treatment began (defined as day 0). For the anti–CSF-1 survival experiments, Gl261-GFP tumors were allowed to grow for 21 d, at which point the tumors were ∼2.0 mm in diameter, before treatment started.
Treatment Protocols.
Mice were treated with control IgG (10 mg/kg) (MedImmune) + 1% Tween; MEDI3617 (10 mg/kg) + 1% Tween; control IgG + cediranib (6 mg/kg); cediranib + MEDI3617; anti–CSF-1 (20 mg/kg) (557858; BD Biosciences); or MEDI3617 + cediranib + anti–CSF-1. Cediranib dissolved in 1% Tween was administered by oral gavage daily. MEDI3617 was administered by i.p. injection twice weekly. Anti–CSF-1 was administered i.p weekly. For survival studies, mice were killed humanely when they became moribund or displayed severe neurological symptoms.
OFDI Analysis of Tumor Growth.
Mice bearing cranial windows were assessed every other day beginning on day 0 of treatment using a custom-built OFDI microscope as previously described (69). Tumor growth was assessed from structural images generated using ImageJ.
MRI-Based Measurement of Tumor Volume.
T2-weighted Rapid Acquisition with Relaxation Enhancement (RARE) images were acquired on a 9.4-Tesla MRI scanner (Bruker Biospin) as previously described (11). Tumor volumes were determined from the T2 hyperintense regions of the brain.
Histology and Immunostaining.
Brains with tumors were collected from U87-bearing mice at days 3 and 8 after treatment initiation and from Gl261-bearing mice at days 14 and 21. Samples were snap-frozen and were embedded in optimum cutting temperature (OCT) compound (Tissue-Tek). Samples were sectioned every 10 μm, incubated with primary antibodies (see below) overnight at 4 °C before incubation with fluorescently conjugated secondary antibodies (1:200) (Jackson ImmunoResearch), and imaged using an Olympus FV1000 Laser Scanning Confocal Microscope (Olympus America, Inc.). Necrotic areas were excluded from analysis. MVD, perivascular cell coverage of vessels, BM coverage, proliferation, apoptosis, and macrophage densities were assessed using a semiautomatic in-house MATLAB (MathWorks) segmentation algorithm. BM thickness was assessed using ImageJ (NIH).
Primary antibodies included CD31 (endothelial cells; 1:200; MAB1398Z; EMD Millipore), desmin (perivascular cells; 1:500; AF3844; R&D Systems), collagen IV (BM; 1:2,000; AB7569; EMD Millipore), Ki67 (proliferation; 1:200; 15580; Abcam), cleaved caspase 3 (apoptosis; 1:200; 9664; Cell Signaling Technology), and Alexa Fluor 647-conjugated F4/80 (macrophages; 1:50; MCA497A647; AbD Serotec).
Additional tissue for histological analyses was collected on day 6 from mice bearing U87 tumors or on day 20 from mice bearing Gl261 tumors and was embedded in paraffin; 5-μm sections were stained with H&E to determine the extent of necrosis.
Edema Analysis by Dry/Wet Weight Analysis.
Edema in the tumor, ipsilateral hemisphere, and contralateral hemisphere was assessed, immediately after mice were killed, using dry/wet weight analysis as previously described to determine the water content of the tumor (11).
Flow Cytometry.
On day 10 of treatment animals were anesthetized and PBS-perfused, and tumors were collected. Single-cell suspensions were blocked with rat anti-mouse CD16/CD32 mAB (BD Biosciences) and were stained with the following monoclonal anti-mouse antibodies: FITC-CD4, PE-Cy7-CD4, FITC-CD8a, PE-CD8a, APC-CD8a, PE-CD45, PE-Cy7-CD45, APC-Gr1, APC-Cy7-CD11b, FITC-CD11c, APC-CD11c, and APC-Cy7-CD11c (BD Biosciences); FITC-F4/80, PE-F4/80, and APC-F4/80 (eBioscience); and FITC-MRC1 and PE-MRC1 (AbD Serotec). 7-Amino-actinomycin D (7AAD) (eBioscience) was used to assess viability. Appropriate fluorochrome-conjugated isotype-matched control IgGs were used. Flow cytometry data were acquired on an LSR II flow cytometer (BD Biosciences) and were analyzed with FACSDiva software (BD Biosciences).
To determine the effect of the addition of anti–CSF-1 therapy, Gl261 tumor-bearing mice were treated for 10 d before tumor collection. Single-cell suspensions were blocked as described above and were stained with the monoclonal anti-mouse antibodies CD45-PercP-Cy5 (eBioscience) and CD11b-BV510 and F4/80-PE (BioLegend). Flow cytometry data were acquired on an LSR II flow cytometer and were analyzed with FlowJo software.
In Vitro Peritoneal Macrophage Polarization Assay.
To study the direct effect of treatments on macrophages, peritoneal macrophages were isolated and treated according to Xu et al. (71) with modifications as follows. Brewer thioglycollate (1 mL, 3% wt/vol) (Sigma-Aldrich) was injected into the peritoneal cavities of C57BL/6 mice. Four days later, mice were killed, and peritoneal fluid containing macrophages was harvested via syringe. Macrophages (5 × 106) were plated in poly-l-lysine–coated dishes in DMEM/F12-10 medium and were allowed to attach to the surface overnight. Adherent macrophages then were treated with MEDI3617 (100 nM), cediranib (0.5 μM), or dual therapy (100 nM MEDI3617 + 0.5 μM cediranib) for 24 h before RNA was collected and the expression of common M1 and M2 genes was analyzed by quantitative PCR. LPS (100 ng/mL) was added as a positive control for stimulation of M1 activity. The following genes were analyzed: Cxcl9, Ccr2, Cxcl11, Il18, inos, Tnfα, Flt1, Mrc1, Il10, Arg1, Ccl22, and Ccl17.
Statistical Analysis.
Data are expressed as mean ± SEM. All statistical tests were two-sided, and results were considered statistically significant at P < 0.05. ROUT was used to determine if outliers were present. Survival studies were assessed using nonparametric log-rank tests with Bonferroni post hoc correction for multiple comparisons. Tumor growth curves were analyzed by linear regression linearity holds because the residuals were randomly distributed around the zero. Statistical analyses for all other experiments were performed using unpaired t tests or, when more than two groups were assessed, by ANOVA followed by Holm–Sidak post hoc tests for multiple comparisons or by nonparametric Kruskal–Wallis test followed by Dunn’s post hoc test for multiple comparisons as indicated. Statistical analyses were performed using the statistical software Prism (GraphPad Software, Inc.).
Acknowledgments
We thank S. Roberge for outstanding technical support for mouse studies; P. Huang for managing the mouse colony and generating mice for the paper; and E. Ager, S. Goel, and C. Kesler for valuable discussions and experimental assistance. The investigators’ research was supported by NIH Grants P01-CA080124 (to R.K.J., D.G.D., and D.F.), P50CA165962 (to T.T.B. and R.K.J.), R01CA126642 and R35CA197743 (to R.K.J.), R01-CA096915 and S10-RR027070 (to D.F.), and R01-CA159258 (to D.G.D.); by National Cancer Institute/Federal Share Proton Beam Program Income (R.K.J. and D.F.); by Department of Defense Breast Cancer Research Innovator Award W81XWH-10-1-0016 (to R.K.J.); by grants from MedImmune and the National Foundation for Cancer Research (to R.K.J.); by a Children’s Tumor Foundation (CTF) Clinical Research Award, an American Cancer Society Research Scholar Award, and an Ira Spiro Award (all to L.X.); and by the CTF Drug Discovery Initiative (L.X.). Additional support was provided by German Research Foundation and Solidar-Immun Foundation Fellowships (to J.K.); an Aid for Cancer Research Foundation Fellowship (to Z.A.); a Susan G. Komen Postdoctoral Fellowship (to G.S.); a Tosteson & Fund for Medical Discovery Fellowship (to T.V.); Affymetrix (M.S.); a CTF Clinical Research Award, an American Cancer Society Research Scholar Award, and an Ira Spiro Award (all to L.X.); and the CTF Drug Discovery Initiative (L.X.).
Footnotes
Conflict of interest statement: R.K.J. received consultant fees from Ophthotech, SPARC, SynDevRx, and XTuit. R.K.J. owns equity in Enlight, Ophthotech, SynDevRx, and XTuit and serves on the Board of Directors of XTuit and on the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund, and Tekla World Healthcare Fund. No reagents or funding from these companies was used in these studies. T.T.B. received consultant fees from Merck, Roche, Kirin Pharmaceuticals, Spectrum Pharmaceuticals, Novartis, and Champions Biotechnology. C.C.L. is an employee of MedImmune.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1525349113/-/DCSupplemental.
References
- 1.Lu-Emerson C, et al. Lessons from anti-vascular endothelial growth factor and anti-vascular endothelial growth factor receptor trials in patients with glioblastoma. J Clin Oncol. 2015;33(10):1197–1213. doi: 10.1200/JCO.2014.55.9575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman HS, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 3.Kreisl TN, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vredenburgh JJ, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13(4):1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 5.Vredenburgh JJ, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 6.Chinot OL, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert MR, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wick W, et al. LB-05 phase III trial exploring the combination of bevacizumab and lomustine in patients with first recurrence of a glioblastoma: The EORTC 26101 trial. Neuro-oncol. 2015;17(Suppl 5):v1. [Google Scholar]
- 9.Batchelor TT, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–3218. doi: 10.1200/JCO.2012.47.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winkler F, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: Role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6(6):553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Kamoun WS, et al. Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J Clin Oncol. 2009;27(15):2542–2552. doi: 10.1200/JCO.2008.19.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batchelor TT, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28(17):2817–2823. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batchelor TT, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 15.Jain RK. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of 6angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holash J, Wiegand SJ, Yancopoulos GD. New model of tumor angiogenesis: Dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene. 1999;18(38):5356–5362. doi: 10.1038/sj.onc.1203035. [DOI] [PubMed] [Google Scholar]
- 18.Hu B, et al. Angiopoietin-2 induces human glioma invasion through the activation of matrix metalloprotease-2. Proc Natl Acad Sci USA. 2003;100(15):8904–8909. doi: 10.1073/pnas.1533394100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee OH, et al. Sustained angiopoietin-2 expression disrupts vessel formation and inhibits glioma growth. Neoplasia. 2006;8(5):419–428. doi: 10.1593/neo.06109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stratmann A, Risau W, Plate KH. Cell type-specific expression of angiopoietin-1 and angiopoietin-2 suggests a role in glioblastoma angiogenesis. Am J Pathol. 1998;153(5):1459–1466. doi: 10.1016/S0002-9440(10)65733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zagzag D, et al. In situ expression of angiopoietins in astrocytomas identifies angiopoietin-2 as an early marker of tumor angiogenesis. Exp Neurol. 1999;159(2):391–400. doi: 10.1006/exnr.1999.7162. [DOI] [PubMed] [Google Scholar]
- 22.Hu B, Cheng SY. Angiopoietin-2: Development of inhibitors for cancer therapy. Curr Oncol Rep. 2009;11(2):111–116. doi: 10.1007/s11912-009-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daly C, et al. Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells. Proc Natl Acad Sci USA. 2006;103(42):15491–15496. doi: 10.1073/pnas.0607538103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim I, et al. Angiopoietin-2 at high concentration can enhance endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Oncogene. 2000;19(39):4549–4552. doi: 10.1038/sj.onc.1203800. [DOI] [PubMed] [Google Scholar]
- 25.Teichert-Kuliszewska K, et al. Biological action of angiopoietin-2 in a fibrin matrix model of angiogenesis is associated with activation of Tie2. Cardiovasc Res. 2001;49(3):659–670. doi: 10.1016/s0008-6363(00)00231-5. [DOI] [PubMed] [Google Scholar]
- 26.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10(3):165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 27.Chae SS, et al. Angiopoietin-2 interferes with anti-VEGFR2-induced vessel normalization and survival benefit in mice bearing gliomas. Clin Cancer Res. 2010;16(14):3618–3627. doi: 10.1158/1078-0432.CCR-09-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.di Tomaso E, et al. Glioblastoma recurrence after cediranib therapy in patients: Lack of “rebound” revascularization as mode of escape. Cancer Res. 2011;71(1):19–28. doi: 10.1158/0008-5472.CAN-10-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sie M, et al. The angiopoietin 1/angiopoietin 2 balance as a prognostic marker in primary glioblastoma multiforme. J Neurosurg. 2009;110(1):147–155. doi: 10.3171/2008.6.17612. [DOI] [PubMed] [Google Scholar]
- 30.Huang Y, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci USA. 2012;109(43):17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mantovani A, Sica A. Macrophages, innate immunity and cancer: Balance, tolerance, and diversity. Curr Opin Immunol. 2010;22(2):231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Pyonteck SM, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leow CC, et al. MEDI3617, a human anti-angiopoietin 2 monoclonal antibody, inhibits angiogenesis and tumor growth in human tumor xenograft models. Int J Oncol. 2012;40(5):1321–1330. doi: 10.3892/ijo.2012.1366. [DOI] [PubMed] [Google Scholar]
- 35.Grenga I, Kwilas AR, Donahue RN, Farsaci B, Hodge JW. Inhibition of the angiopoietin/Tie2 axis induces immunogenic modulation, which sensitizes human tumor cells to immune attack. J Immunother Cancer. 2015;3:52. doi: 10.1186/s40425-015-0096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hashizume H, et al. Complementary actions of inhibitors of angiopoietin-2 and VEGF on tumor angiogenesis and growth. Cancer Res. 2010;70(6):2213–2223. doi: 10.1158/0008-5472.CAN-09-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koh YJ, et al. Double antiangiogenic protein, DAAP, targeting VEGF-A and angiopoietins in tumor angiogenesis, metastasis, and vascular leakage. Cancer Cell. 2010;18(2):171–184. doi: 10.1016/j.ccr.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Brown JL, et al. A human monoclonal anti-ANG2 antibody leads to broad antitumor activity in combination with VEGF inhibitors and chemotherapy agents in preclinical models. Mol Cancer Ther. 2010;9(1):145–156. doi: 10.1158/1535-7163.MCT-09-0554. [DOI] [PubMed] [Google Scholar]
- 39.Daly C, et al. Angiopoietin-2 functions as a Tie2 agonist in tumor models, where it limits the effects of VEGF inhibition. Cancer Res. 2013;73(1):108–118. doi: 10.1158/0008-5472.CAN-12-2064. [DOI] [PubMed] [Google Scholar]
- 40.Kienast Y, et al. Ang-2-VEGF-A CrossMab, a novel bispecific human IgG1 antibody blocking VEGF-A and Ang-2 functions simultaneously, mediates potent antitumor, antiangiogenic, and antimetastatic efficacy. Clin Cancer Res. 2013;19(24):6730–6740. doi: 10.1158/1078-0432.CCR-13-0081. [DOI] [PubMed] [Google Scholar]
- 41.Keskin D, et al. Targeting vascular pericytes in hypoxic tumors increases lung metastasis via angiopoietin-2. Cell Reports. 2015;10(7):1066–1081. doi: 10.1016/j.celrep.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang H, et al. Specifically targeting angiopoietin-2 inhibits angiogenesis, Tie2-expressing monocyte infiltration, and tumor growth. Clin Cancer Res. 2011;17(5):1001–1011. doi: 10.1158/1078-0432.CCR-10-2317. [DOI] [PubMed] [Google Scholar]
- 43.Oliner J, et al. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell. 2004;6(5):507–516. doi: 10.1016/j.ccr.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 44.Falcón BL, et al. Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels. Am J Pathol. 2009;175(5):2159–2170. doi: 10.2353/ajpath.2009.090391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holopainen T, et al. Effects of angiopoietin-2-blocking antibody on endothelial cell-cell junctions and lung metastasis. J Natl Cancer Inst. 2012;104(6):461–475. doi: 10.1093/jnci/djs009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mazzieri R, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19(4):512–526. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Sorensen AG, et al. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 2012;72(2):402–407. doi: 10.1158/0008-5472.CAN-11-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gerstner ER, Batchelor TT. Antiangiogenic therapy for glioblastoma. Cancer J. 2012;18(1):45–50. doi: 10.1097/PPO.0b013e3182431c6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emblem KE, et al. Vessel architectural imaging identifies cancer patient responders to anti-angiogenic therapy. Nat Med. 2013;19(9):1178–1183. doi: 10.1038/nm.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Batchelor TT, et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci USA. 2013;110(47):19059–19064. doi: 10.1073/pnas.1318022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kwilas AR, Donahue RN, Tsang KY, Hodge JW. Immune consequences of tyrosine kinase inhibitors that synergize with cancer immunotherapy. Cancer Cell Microenviron. 2015;2(1):e677. doi: 10.14800/ccm.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med. 2015;212(4):435–445. doi: 10.1084/jem.20150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86(5):1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 54.De Palma M, Naldini L. Role of haematopoietic cells and endothelial progenitors in tumour angiogenesis. Biochim Biophys Acta. 2006;1766(1):159–166. doi: 10.1016/j.bbcan.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 55.Lewis JS, Landers RJ, Underwood JC, Harris AL, Lewis CE. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol. 2000;192(2):150–158. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH687>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 56.Lin EY, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66(23):11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 57.Hanahan D, Coussens LM. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 58.DeNardo DG, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1(1):54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216(1):15–24. doi: 10.1002/path.2370. [DOI] [PubMed] [Google Scholar]
- 60.Noy R, Pollard JW. Tumor-associated macrophages: From mechanisms to therapy. Immunity. 2014;41(1):49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kloepper J, et al. Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. Proc Natl Acad Sci USA. 2016;113:4476–4481. doi: 10.1073/pnas.1525360113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yasuda S, et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo. Clin Exp Immunol. 2013;172(3):500–506. doi: 10.1111/cei.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hamzah J, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453(7193):410–414. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]
- 64.Meisen WH, et al. The Impact of Macrophage- and Microglia-Secreted TNFα on Oncolytic HSV-1 Therapy in the Glioblastoma Tumor Microenvironment. Clin Cancer Res. 2015;21(14):3274–3285. doi: 10.1158/1078-0432.CCR-14-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stockmann C, et al. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 2008;456(7223):814–818. doi: 10.1038/nature07445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beatty GL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Palma M, et al. Tumor-targeted interferon-alpha delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer Cell. 2008;14(4):299–311. doi: 10.1016/j.ccr.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 68.Lu-Emerson C, et al. Increase in tumor-associated macrophages after antiangiogenic therapy is associated with poor survival among patients with recurrent glioblastoma. Neuro-oncol. 2013;15(8):1079–1087. doi: 10.1093/neuonc/not082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vakoc BJ, et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat Med. 2009;15(10):1219–1223. doi: 10.1038/nm.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yuan F, et al. Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial windows. Cancer Res. 1994;54(17):4564–4568. [PubMed] [Google Scholar]
- 71.Xu L, Xie K, Fidler IJ. Therapy of human ovarian cancer by transfection with the murine interferon beta gene: Role of macrophage-inducible nitric oxide synthase. Hum Gene Ther. 1998;9(18):2699–2708. doi: 10.1089/hum.1998.9.18-2699. [DOI] [PubMed] [Google Scholar]