Significance
Understanding controls on carbon storage in terrestrial ecosystems is essential to predict and mitigate human impacts on the global carbon cycle. This study identifies a general unifying mechanism to explain how photochemical mineralization of plant litter, photodegradation, affects subsequent biotic decomposition. Previous work has shown that photodegradation can be a major direct control of carbon loss in arid lands. We now demonstrate that, across a wide range of species, exposure to sunlight has large “photopriming” effects, enhancing microbial decomposition, especially due to blue–green light. Photodegradation increased availability of carbohydrates to hydrolytic enzymes, likely through reducing the bottleneck imposed by lignin in secondary cell walls. This mechanistic insight suggests that photodegradation is globally important for carbon turnover in terrestrial ecosystems.
Keywords: carbon cycle, plant litter decomposition, photodegradation, lignin, UV radiation
Abstract
A mechanistic understanding of the controls on carbon storage and losses is essential for our capacity to predict and mitigate human impacts on the global carbon cycle. Plant litter decomposition is an important first step for carbon and nutrient turnover, and litter inputs and losses are essential in determining soil organic matter pools and the carbon balance in terrestrial ecosystems. Photodegradation, the photochemical mineralization of organic matter, has been recently identified as a mechanism for previously unexplained high rates of litter mass loss in arid lands; however, the global significance of this process as a control on carbon cycling in terrestrial ecosystems is not known. Here we show that, across a wide range of plant species, photodegradation enhanced subsequent biotic degradation of leaf litter. Moreover, we demonstrate that the mechanism for this enhancement involves increased accessibility to plant litter carbohydrates for microbial enzymes. Photodegradation of plant litter, driven by UV radiation, and especially visible (blue–green) light, reduced the structural and chemical bottleneck imposed by lignin in secondary cell walls. In leaf litter from woody species, specific interactions with UV radiation obscured facilitative effects of solar radiation on biotic decomposition. The generalized effect of sunlight exposure on subsequent microbial activity, mediated by increased accessibility to cell wall polysaccharides, suggests that photodegradation is quantitatively important in determining rates of mass loss, nutrient release, and the carbon balance in a broad range of terrestrial ecosystems.
The carbon balance in terrestrial environments is primarily determined by inputs resulting from fixation of atmospheric carbon dioxide (CO2) in plant tissues and outputs from the mineralization of organic compounds (1). Due to the necessity to mitigate the increased atmospheric CO2 concentrations resulting from human activity, there has been an intense focus of interest on the controls of CO2 uptake by vegetation and their effects on carbon sequestration in terrestrial ecosystems (2). In contrast, there has been much less attention given to the factors and processes that control the dynamics of carbon losses, which can be equally important in determining the carbon balance at the ecosystem scale (3, 4).
Sunlight is clearly a key driver of CO2 capture by photosynthetic organisms, and solar radiation functions as the basis for energy flow and carbon cycling in all ecosystems (5). As such, the influence of solar radiation on carbon fixation and net primary production has been extensively studied, whereas the effects of solar radiation on factors controlling carbon loss from ecosystems are much less well understood. In aquatic ecosystems, solar radiation has been shown to affect carbon turnover as a result of photochemical reactions that change the optical and chemical characteristics of dissolved organic matter, particularly recalcitrant humic substances (6, 7). Moreover, exposure to sunlight stimulated biological degradation of biorefractory compounds (6, 8) and enhanced the production of dissolved inorganic carbon in water (9) and of gaseous carbon monoxide (CO) (10, 11). In terrestrial ecosystems, accumulating evidence has demonstrated the importance of photochemical mineralization of organic carbon compounds (photodegradation) as a control on organic matter turnover in arid and semiarid ecosystems (12–15). Losses of volatile carbon compounds, including CO2, CO, and methane (CH4), from recently senesced plant material exposed to natural or artificial radiation have been documented (16–18) and have been linked to photochemical degradation of cellulose (16) and partial or complete degradation of lignin (19–21). Recent studies have demonstrated stimulation of litter decomposition and respiration with prior exposure to ultraviolet (UV) (22, 23) or full solar radiation (24) in dry Mediterranean ecosystems. Nevertheless, the quantitative significance of photodegradation in terrestrial ecosystems that support greater microbial activity than arid-land ecosystems is generally considered to be minimal (25).
A major obstacle to our progress in understanding the functional significance of photodegradation in terrestrial ecosystems is that experiments evaluating the effects of solar radiation on litter decomposition are generally conducted under natural conditions where direct (i.e., abiotic) and indirect (biotically-mediated) contributions to the decomposition process are not distinguished (24, 26–28). In addition, for historical reasons related to studies of the effects of stratospheric ozone depletion (29, 30), there has been a strong emphasis on evaluating the effects of UV (200–400 nm) radiation (particularly UV-B, 280–315 nm) on litter decomposition, and these studies have yielded equivocal results with respect to the importance of this spectral region as a driver of photodegradation (31, 32). Recent evidence has demonstrated that the visible component of sunlight [blue and green wavelengths, 400–550 nm (BG)] can have a large impact on photodegradation (17, 20), suggesting that studies wholly focused on UV effects (27, 31–33) may have underestimated the quantitative importance of solar radiation as a control on carbon turnover.
We used a wide range of species from temperate ecosystems and appropriate sunlight attenuation filters (Fig. 1 A and B) to test the effects of solar radiation (UV and BG components) on litter decomposition and biodegradability. We separated the direct and indirect effects of photodegradation on plant litter decomposition and sequentially quantified their importance. Using this approach, we demonstrated that exposure to solar radiation increased microbial accessibility to labile carbon compounds and accelerated biotic litter decomposition across a wide range of plant species from a diversity of terrestrial ecosystems.
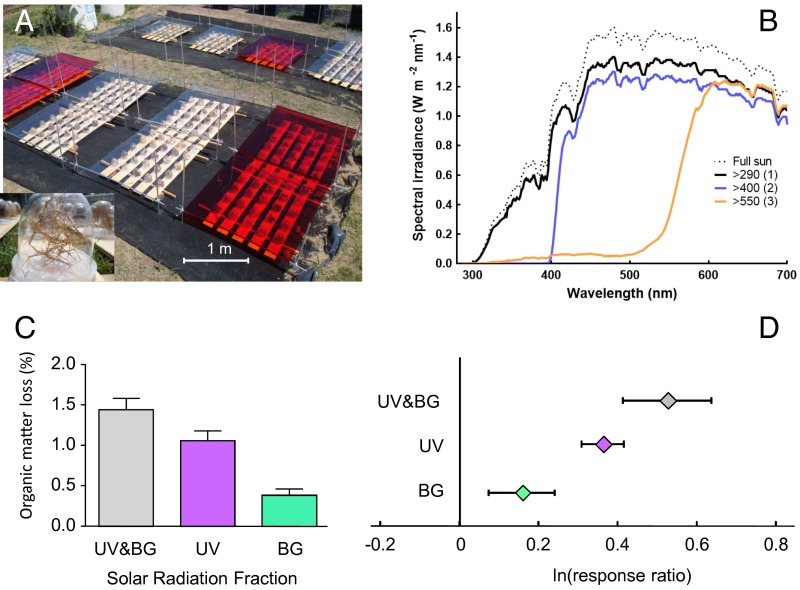
Fig. 1.
Solar radiation promotes direct losses of organic matter in leaf litter from a broad range of plant species. (A) Photo of experimental manipulations to test the effects of sunlight on leaf litter from 23 plant species under field conditions. (Inset) A close-up of the clear plastic cups used to hold litter samples. (B) Spectral distribution of summertime solar radiation at the field site and spectral irradiance under the filters used to create the radiation treatments: (filter 1) λ > 290 nm, with transparent polyethylene film that transmitted >95% of all solar radiation; (filter 2) λ > 400 nm, with attenuation of all UV radiation; and (filter 3) λ > 550 nm, with attenuation of all UV-and-BG (UV&BG) wavelengths (orange filter in A). (C) Effects of different wavelengths on average mass loss (+1 SEM) from litter of 23 species after 100 d of exposure to solar radiation. The effect of the UV&BG wavelengths of natural sunlight on organic matter (OM) loss (and all other evaluated variables) was calculated by subtracting the measured variable (i.e., OM loss) under filter 3 from the measured variable under filter 1; the effect of the UV component alone was calculated by subtracting the measured variable under filter 2 from the measured variable under filter 1 whereas the effect of the BG component alone was calculated by subtracting the measured variable under filter 3 from the measured variable under filter 2. (D) Mean effect sizes of the different wavelengths on organic matter loss across the 23 species. Details of metaanalysis can be found in Table S3. The graph shows the average natural log of the response ratio [±95% confidence intervals (CIs)]. If CIs do not cross the 0 line, the effect is considered to be significant.
Results
UV and BG Radiation Significantly Promote Organic Mass Loss from Litter Across a Broad Range of Species.
Senescent plant material (leaf litter) from 23 temperate species from South America (Table S1), which varied substantially in litter traits (Table S2), was exposed to different wavelengths of solar radiation in the absence of soil biota. Solar radiation had a detectable effect promoting the loss of organic matter from plant litter, with UV radiation and BG light having a significant role energizing photodegradation across all species (Fig. 1 C and D and Tables S3 and S4). In general, these results are quantitatively consistent with other studies that explored photodegradation of plant litter under field conditions (12, 16, 17, 20) although the breadth of species evaluated here, which ranged from annual herbaceous species to conifer and angiosperm tree species, establishes a general pattern for the spectral dependence of photodegradation of plant litter in terrestrial ecosystems.
Table S1.
List of species of leaf litter used in this study from temperate ecosystems of Argentina
| Genus | Species | Family | Life form | Ecosystem of origin | Code |
| Araucaria | auracana | Araucariaceae | Tree | Temperate forest | AA |
| Bromus | pictus | Poaceae | Perennial grass | Semiarid steppe | BP |
| Carduus | acanthoides* | Asteraceae | Annual herb | Mesic grassland | CA |
| Chusquea | culeou | Poaceae | Perennial grass | Temperate forest | ChC |
| Dactylis | glomerata* | Poaceae | Annual grass | Mesic grassland | DG |
| Fraxinus | americana* | Oleaceae | Tree | Temperate forest | FA |
| Glycine | max* | Fabaceae | Annual herb | Agroecosystem | GM |
| Helianthus | annuus* | Asteraceae | Annual herb | Agroecosystem | HA |
| Lolium | multiflorum* | Poaceae | Annual grass | Mesic grassland | LM |
| Maytenus | boaria | Celastraceae | Tree | Temperate forest | MB |
| Mulinum | spinosum | Apiaceae | Shrub | Semiarid steppe | MS |
| Nothofagus | obliqua | Nothofagaceae | Tree | Temperate forest | NO |
| Nothofagus | dombeyi | Nothofagaceae | Tree | Temperate forest | ND |
| Nothofagus | nervosa | Nothofagaceae | Tree | Temperate forest | NN |
| Nothofagus | antarctica | Nothofagaceae | Tree | Temperate forest | NA |
| Paspalum | quadrifarium | Poaceae | Perennial grass | Mesic Grassland | PQ |
| Pinus | ponderosa* | Pinaceae | Tree | Temperate forest | PP |
| Poa | ligularis | Poaceae | Perennial grass | Semiarid steppe | PL |
| Populus | nigra* | Salicaceae | Tree | Temperate forest | PN |
| Schizachyrium | scoparium | Poaceae | Perennial grass | Mesic grassland | ScS |
| Stipa | speciosa | Poaceae | Perennial grass | Semiarid steppe | SS |
| Triticum | aestivum* | Poaceae | Annual grass | Agroecosystem | TA |
| Zea | mays* | Poaceae | Annual grass | Agroecosystem | ZM |
Plant litter was collected from natural grassland and forest ecosystems as well as the four major agricultural species of Argentina. The final column is code for each species used in other tables and figures.
*Exotic species in ecosytem of origin.
Table S2.
Chemical and physical characteristics of initial leaf litter of 23 species used in photodegradation experiments
| Species code | OM, % | LMA, g⋅m−2 | Hemicellulose, % | Cellulose, % | Lignin, % | Polyphenols, % | Sunscreen, Abs305 |
| Herbaceous | |||||||
| BP | 79.5 ± 0.20 | 148 ± 8.6 | 56.8 ± 0.75 | 39.1 ± 0.63 | 4.08 ± 0.13 | 2.05 ± 0.09 | 0.22 ± 0.010 |
| CA | 86.8 ± 0.20 | 115 ± 2.7 | 56.3 ± 1.05 | 37.9 ± 0.48 | 5.78 ± 0.70 | 1.03 ± 0.20 | 0.18 ± 0.002 |
| ChC | 63.9 ± 1.23 | 59.2 ± 0.5 | 60.8 ± 0.08 | 31.1 ± 0.42 | 8.06 ± 0.42 | 1.90 ± 0.06 | 0.16 ± 0.010 |
| DG | 87.8 ± 0.47 | 44.5 ± 1.6 | 62.0 ± 0.64 | 33.0 ± 0.98 | 4.89 ± 0.40 | 1.54 ± 0.07 | 0.16 ± 0.004 |
| GM | 88.3 ± 0.44 | 36.0 ± 1.9 | 67.2 ± 3.26 | 20.5 ± 0.87 | 12.4 ± 3.97 | 4.41 ± 0.38 | 0.44 ± 0.009 |
| HA | 73.0 ± 0.89 | 58.1 ± 8.1 | 72.8 ± 4.70 | 16.2 ± 3.80 | 11.0 ± 3.93 | 1.43 ± 0.08 | 0.27 ± 0.018 |
| LM | 87.6 ± 0.34 | 49.4 ± 6.6 | 49.4 ± 3.09 | 47.6 ± 2.88 | 3.06 ± 0.80 | 1.46 ± 0.03 | 0.16 ± 0.005 |
| PQ | 91.7 ± 0.12 | 142 ± 10.7 | 56.6 ± 0.97 | 38.3 ± 0.82 | 5.11 ± 0.20 | 2.72 ± 0.10 | 0.20 ± 0.007 |
| PL | 92.3 ± 1.02 | 205 ± 8.8 | 50.2 ± 0.57 | 42.7 ± 0.42 | 7.14 ± 0.23 | 1.75 ± 0.07 | 0.15 ± 0.009 |
| ScS | 87.6 ± 0.50 | 59.2 ± 0.9 | 52.9 ± 7.04 | 37.6 ± 4.61 | 9.46 ± 2.45 | 3.70 ± 0.07 | 0.24 ± 0.004 |
| SS | 94.4 ± 0.18 | 281 ± 9.0 | 47.6 ± 0.82 | 45.9 ± 0.82 | 6.42 ± 0.02 | 1.44 ± 0.03 | 0.18 ± 0.009 |
| TA | 81.4 ± 0.27 | 77.4 ± 5.4 | 50.6 ± 1.61 | 43.1 ± 1.66 | 6.31 ± 0.25 | 1.09 ± 0.03 | 0.11 ± 0.005 |
| ZM | 84.5 ± 0.92 | 39.9 ± 1.8 | 51.4 ± 2.73 | 44.6 ± 2.55 | 3.96 ± 0.45 | 1.47 ± 0.05 | 0.17 ± 0.003 |
| Woody | |||||||
| AA | 94.7 ± 0.41 | 679 ± 28.3 | 53.0 ± 1.80 | 25.1 ± 0.59 | 21.9 ± 1.94 | 7.66 ± 1.11 | 0.22 ± 0.016 |
| FA | 87.6 ± 0.72 | 49.6 ± 3.6 | 64.7 ± 2.33 | 20.6 ± 0.65 | 14.8 ± 1.84 | 5.25 ± 0.32 | 0.23 ± 0.011 |
| MB | 92.3 ± 0.56 | 138 ± 5.5 | 52.3 ± 0.62 | 14.2 ± 0.04 | 33.5 ± 0.65 | 7.02 ± 0.20 | 0.27 ± 0.013 |
| MS | 98.2 ± 0.25 | 558 ± 31.8 | 53.6 ± 0.89 | 37.3 ± 0.55 | 9.16 ± 0.35 | 5.69 ± 0.06 | 0.50 ± 0.034 |
| NO | 93.1 ± 0.44 | 61.2 ± 1.9 | 56.3 ± 1.09 | 18.2 ± 1.77 | 25.4 ± 2.28 | 16.4 ± 0.21 | 0.77 ± 0.042 |
| ND | 95.6 ± 0.40 | 134 ± 0.5 | 58.8 ± 2.50 | 22.8 ± 2.19 | 18.4 ± 0.31 | 14.8 ± 0.31 | 0.61 ± 0.055 |
| NN | 95.7 ± 0.39 | 65.1 ± 2.8 | 52.3 ± 0.68 | 22.4 ± 0.92 | 25.3 ± 1.60 | 5.96 ± 0.22 | 0.19 ± 0.028 |
| NA | 91.3 ± 0.45 | 58.1 ± 1.3 | 53.1 ± 2.35 | 19.7 ± 1.45 | 27.1 ± 0.91 | 8.33 ± 0.50 | 0.27 ± 0.009 |
| PP | 95.7 ± 0.23 | 347 ± 14.6 | 52.9 ± 1.35 | 24.2 ± 0.87 | 22.9 ± 0.49 | 8.12 ± 0.22 | 0.28 ± 0.018 |
| PN | 82.7 ± 0.11 | 70.2 ± 3.3 | 59.1 ± 0.89 | 23.5 ± 0.36 | 17.4 ± 0.74 | 11.0 ± 0.55 | 0.41 ± 0.021 |
Mean (n = 3) and SE shown for organic matter (OM) content, leaf mass per area (LMA), hemicellulose, cellulose, lignin (from fiber extraction), total polyphenols, and sunscreen compounds (absorbance at 305 nm) for leaf litter from herbaceous and woody species used in the experiment. Descriptions of laboratory analyses can be found in Materials and Methods; species codes are from Table S1.
Table S3.
Results of metaanalysis for different variables in photodegradation experiments
| Variable and wavelength* | Mean, lnR | 95% CI | Qt | df | P | Categorical effects | Rosenthal´s fail-safe no. | ||||
| Min. | Max. | Qm | Qe | P | Category | ||||||
| Photodegradation | |||||||||||
| UV&BG | 0.5226 | 0.4355 | 0.6114 | 28.83 | 22 | 0.15 | 1,456 | ||||
| UV | 0.3617 | 0.2913 | 0.4383 | 20.87 | 22 | 0.52 | 707 | ||||
| BG | 0.1568 | 0.0947 | 0.2190 | 21.44 | 22 | 0.49 | 182 | ||||
| Photopriming | |||||||||||
| UV&BG | 0.2576 | 0.1665 | 0.3481 | 29.73 | 22 | 0.12 | 263 | ||||
| UV | 0.0536 | −0.0549 | 0.1408 | 36.76 | 22 | 0.025 | 14.28 | 22.44 | 0.0002 | Life form | — |
| BG | 0.2124 | 0.1513 | 0.2803 | 26.11 | 22 | 0.24 | 326 | ||||
| Lignin | |||||||||||
| UV&BG | −0.1611 | −0.2212 | −0.1132 | 67.74 | 22 | <0.0001 | 43.98 | 23.75 | <0.0001 | Life form | 944 |
| UV | −0.0328 | −0.0561 | −0.0093 | 21.44 | 22 | 0.20 | 70 | ||||
| BG | −0.1059 | −0.1332 | −0.0787 | 58.13 | 22 | <0.0001 | 38.25 | 19.88 | <0.0001 | Life form | 600 |
| Saccharification | |||||||||||
| UV&BG | 0.3847 | 0.2488 | 0.5415 | 45.81 | 22 | 0.002 | 12.66 | 33.14 | <0.0005 | Life form | 440 |
| UV | 0.1589 | 0.1069 | 0.2175 | 29.61 | 22 | 0.128 | 290 | ||||
| BG | 0.2299 | 0.1425 | 0.3278 | 36.15 | 22 | 0.029 | 10.15 | 26.01 | 0.001 | Life form | 277 |
Results of metaanalysis for all evaluated variables. All analyses were conducted as random effects models with the grouping variable of life form (woody vs. herbaceous). Resampling tests for boot-strapped confidence intervals were for 999 iterations. df, degrees of freedom; Mean, lnR, the mean effect size = ln(response ratio); QE, sample error variance not explained by the model; Qm, variance explained by the categorical model (performed in situations when QT was significant); Qt, total sample heterogeneity. Rosenthal´s fail-safe number is an evaluation of bias and demonstrates the number of species with no effect required to nullify the response. Calculations for light treatments and details of metaanalysis can be found in Materials and Methods. P values in bold indicate significant effects on life form categories on heterogeneity of variance.
Indicates the different light treatments for organic mass loss from direct photodegradation, organic mass loss due to photopriming (%), lignin concentration (mg/g), and saccharification (mg/g).
Table S4.
Organic mass loss of litter from direct photodegradation and subsequent biotic decomposition in second phase of photodegradation experiments
| Light treatment | Direct photodegradation, % organic matter lost | Biotic decomposition, % organic matter lost | ||||
| >290 nm | >400 nm | >550 nm | >290 nm | >400 nm | >550 nm | |
| Herbaceous | ||||||
| BP | 4.19 ± 0.66 | 2.70 ± 0.19 | 2.23 ± 0.13 | 27.92 ± 0.18 | 29.31 ± 2.01 | 23.72 ± 1.83 |
| CA | 3.48 ± 0.28 | 2.21 ± 0.25 | 2.16 ± 0.16 | 20.62 ± 2.02 | 18.20 ± 2.18 | 16.44 ± 1.52 |
| ChC | 3.36 ± 0.32 | 1.52 ± 0.33 | 1.49 ± 0.13 | 12.84 ± 0.98 | 8.65 ± 0.42 | 6.61 ± 0.59 |
| DG | 3.26 ± 0.29 | 2.92 ± 1.05 | 1.91 ± 0.17 | 29.51 ± 3.08 | 25.89 ± 2.91 | 24.06 ± 3.54 |
| GM | 3.91 ± 0.25 | 2.74 ± 0.22 | 2.37 ± 0.22 | 43.44 ± 1.80 | 48.68 ± 1.16 | 38.97 ± 2.57 |
| HA | 4.81 ± 0.49 | 3.15 ± 0.14 | 2.54 ± 0.41 | 76.16 ± 3.98 | 58.72 ± 7.22 | 65.15 ± 8.96 |
| LM | 3.40 ± 0.42 | 1.62 ± 0.27 | 1.35 ± 0.15 | 17.51 ± 2.46 | 13.38 ± 1.74 | 11.81 ± 1.05 |
| PQ | 4.66 ± 1.07 | 2.41 ± 0.22 | 2.17 ± 0.11 | 11.84 ± 1.00 | 9.39 ± 1.51 | 4.91 ± 0.87 |
| PL | 2.67 ± 0.11 | 2.27 ± 0.63 | 1.29 ± 0.07 | 9.22 ± 0.64 | 7.26 ± 0.16 | 4.91 ± 1.47 |
| ScS | 4.06 ± 0.33 | 2.50 ± 0.12 | 1.95 ± 0.17 | 14.35 ± 0.42 | 11.98 ± 0.31 | 9.46 ± 0.53 |
| SS | 2.13 ± 0.26 | 1.70 ± 0.37 | 1.48 ± 0.09 | 11.33 ± 0.75 | 9.39 ± 0.67 | 6.74 ± 0.72 |
| TA | 3.84 ± 0.23 | 2.65 ± 0.33 | 2.05 ± 0.32 | 22.55 ± 1.11 | 18.27 ± 0.38 | 14.17 ± 0.94 |
| ZM | 3.11 ± 0.30 | 2.41 ± 0.18 | 1.76 ± 0.10 | 18.02 ± 0.38 | 15.32 ± 0.60 | 14.65 ± 1.16 |
| Woody | ||||||
| AA | 2.36 ± 0.15 | 2.21 ± 0.05 | 2.11 ± 0.12 | 3.95 ± 0.69 | 4.40 ± 0.31 | 4.29 ± 0.42 |
| FA | 1.98 ± 0.14 | 1.08 ± 0.10 | 0.88 ± 0.12 | 32.90 ± 1.65 | 25.65 ± 1.95 | 24.54 ± 1.04 |
| MB | 4.36 ± 0.40 | 2.79 ± 0.29 | 2.32 ± 0.13 | 34.42 ± 5.36 | 56.78 ± 10.73 | 36.57 ± 2.65 |
| MS | 1.73 ± 0.13 | 1.14 ± 0.10 | 1.40 ± 0.11 | 20.63 ± 1.61 | 22.82 ± 1.17 | 21.01 ± 1.35 |
| NO | 3.91 ± 0.29 | 2.55 ± 0.10 | 2.21 ± 0.16 | 60.98 ± 2.09 | 79.35 ± 6.84 | 52.50 ± 5.22 |
| ND | 3.08 ± 0.33 | 2.26 ± 0.11 | 2.04 ± 0.20 | 17.16 ± 3.98 | 34.94 ± 4.78 | 20.62 ± 1.49 |
| NN | 2.75 ± 0.32 | 1.84 ± 0.07 | 1.48 ± 0.07 | 19.61 ± 3.57 | 36.32 ± 10.83 | 12.69 ± 1.28 |
| NA | 3.25 ± 0.17 | 2.77 ± 0.14 | 2.07 ± 0.07 | 30.52 ± 6.32 | 54.83 ± 8.33 | 26.30 ± 8.20 |
| PP | 2.56 ± 0.18 | 1.60 ± 0.30 | 2.26 ± 0.23 | 22.11 ± 6.17 | 30.56 ± 3.93 | 25.82 ± 4.26 |
| PN | 3.25 ± 0.18 | 3.03 ± 0.21 | 2.49 ± 0.18 | 45.52 ± 3.09 | 45.50 ± 5.08 | 40.29 ± 2.37 |
Columns show values for each of the three light attenuation treatments described in the experimental design (Materials and Methods). Mean (n = 5) and SE are shown. Calculations can be found in Materials and Methods; species codes are from Table S1.
Exposure to Solar Radiation Strongly Enhances Subsequent Microbial Decomposition of Plant Litter.
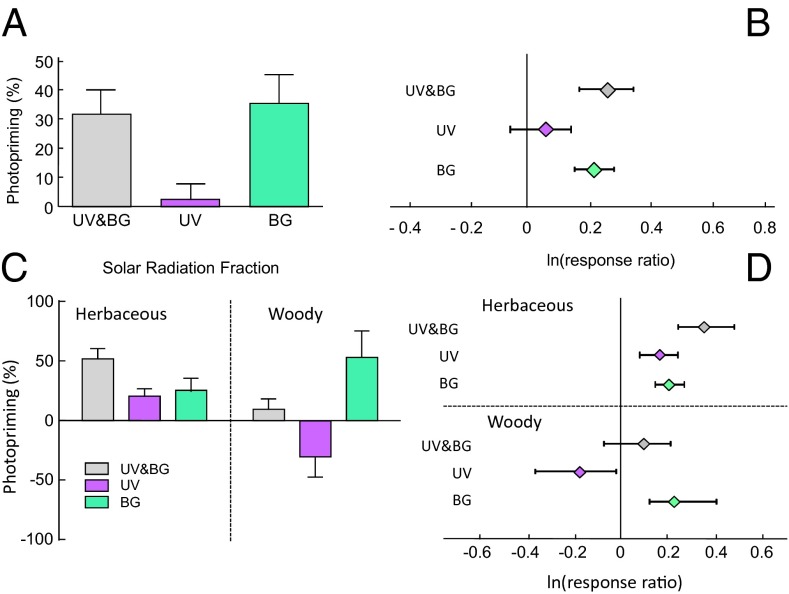
Leaf litter from the first phase of direct photodegradation experiments was subsequently placed on the soil surface in litterbags without light exposure under optimal field conditions for evaluation of biotic decomposition. Once plant litter was placed in contact with soil microorganisms, observed organic mass loss was significantly greater in litter previously exposed to solar radiation, suggesting that photodegradation primed the material for subsequent biological processing (“photopriming”) (Fig. 2 A and B). We evaluated the magnitude of photopriming (i.e., the relative increase in decomposition due to prior exposure to solar radiation) and observed that, across all species, previous exposure to visible (BG) light enhanced subsequent biotic decomposition by 30%, on average (Fig. 2 A and B). In contrast, the UV component alone had no significant photopriming effect when considered across all species (Fig. 2 A and B).
Fig. 2.
Exposure to solar radiation facilitates subsequent microbial decomposition. (A) Enhancement of microbial decomposition (photopriming) in a 45-d field soil incubation, evaluating the wavelength-dependent effects of prior exposure to solar radiation. Photopriming is defined as the percent increase for each species in biotic decomposition due to exposure to different wavelengths of solar radiation. Wavelength effects are calculated as indicated in Fig. 1. Each bar represents the average of 23 species (+1 SEM). (B) Mean effect sizes of the different wavelengths on photopriming across the 23 species (±95% CI). Details of metaanalysis can be found in Table S3. (C) Photopriming effects on decomposition, partitioned for herbaceous and woody species. Each bar represents the average of the group (n = 13 for herbaceous species; n = 10 for woody species, +1 SEM). (D) Mean effect sizes of solar radiation on photopriming, partitioned by herbaceous and woody species. Litter quality analyses and analysis of grouping for herbaceous and woody species can be found in Table S2 and Fig. S1.
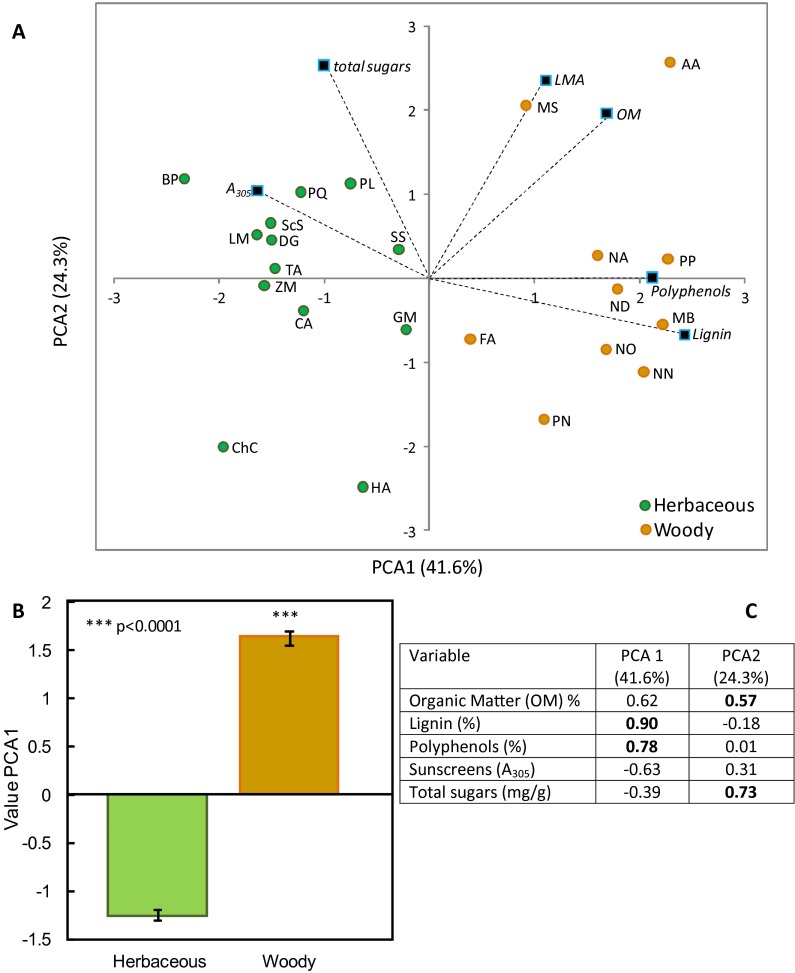
Upon further examination, we noticed that, in a subset of species (shrubs and trees), exposure to the UV component of sunlight not only failed to cause photopriming, but actually resulted in a significant inhibition of subsequent biotic decomposition when litter was placed in contact with the soil (seen as negative photopriming) (Fig. 2 C and D). When we partitioned the dataset to distinguish between herbaceous and woody species, we found a marked difference between these groups regarding the spectral dependence of the photopriming effect (Fig. 2 C and D). This grouping into woody and herbaceous species was supported by an evaluation of initial litter quality, which showed the woody species to be strongly associated with high lignin and polyphenol content of leaf litter (Fig. S1 and Table S2). Although UV and BG radiation contributed equally to photopriming of herbaceous litter (Fig. 2 C and D), for the woody species used in this study, BG promoted and UV inhibited subsequent biotic decomposition (Fig. 2 C and D). As a result of the seemingly antagonistic effects of UV and BG light, exposure to full solar radiation had almost no photopriming effect for the woody species' leaf litter used in these experiments (Fig. 2 C and D).
Fig. S1.
Principal components analysis (PCA) of initial litter quality demonstrates strong differences between woody and herbaceous litter. (A) PCA ordination of 23 species used in photodegradation experiments for six measured litter traits. (B) Average PCA score for axis 1 for groupings of herbaceous and woody species (±1 SEM). (C) Eigenvector scores for all traits on PCA axes 1 and 2. Marked in bold are the two highest eigenvector scores for each axis. Litter quality data can be found Table S2. Description of statistics can be found in Materials and Methods; species codes are from Table S1.
These data speak to two important insights into our understanding of the role of photodegradation on litter decomposition in terrestrial ecosystems. First, whereas it has been demonstrated that litter mass loss is directly caused by exposure to solar radiation (12, 17, 20), we suggest that the principal and quantitatively most important effect of photodegradation on the global carbon cycle may be the facilitation of biotically mediated carbon turnover. We demonstrate here, with a direct comparison of direct photodegradation and biotic decomposition (Table S4), that biotic decomposition overall had a much larger effect on organic matter loss for the same species (range 4–65%) than the direct effect of photodegradation, which was generally small (range 1.5–4.5%) (Table S4). Nevertheless, these relatively small rates of mass loss during the photodegradative phase had an inordinately large impact on total decomposition, by promoting microbially driven losses of carbon during biotic degradation [average photopriming effect across species ∼30% (Fig. 2A) and ranging from 0–185% (Table S4)]. If the relative magnitudes of photodegradation and biotic degradation found in our experiment are representative of other natural conditions (excluding extreme environments of arid lands), the contribution of photopriming as a facilitator of biotic decomposition is quantitatively much larger than the direct effects of photodegradation on carbon loss. Second, the observation that litter quality can affect the spectral dependence of photopriming (Fig. 2 C and D) provides an important element for conceptual and empirical models aimed to understand the role of photodegradation in litter decomposition in different ecosystems. In addition, the contrasting response of woody and herbaceous species to UV exposure may help to resolve the contradictory results reported for UV effects on photodegradation in terrestrial ecosystems (23, 27, 31–34).
Exposure to Solar Radiation Consistently Reduces Lignin Concentration of Plant Leaf Litter.
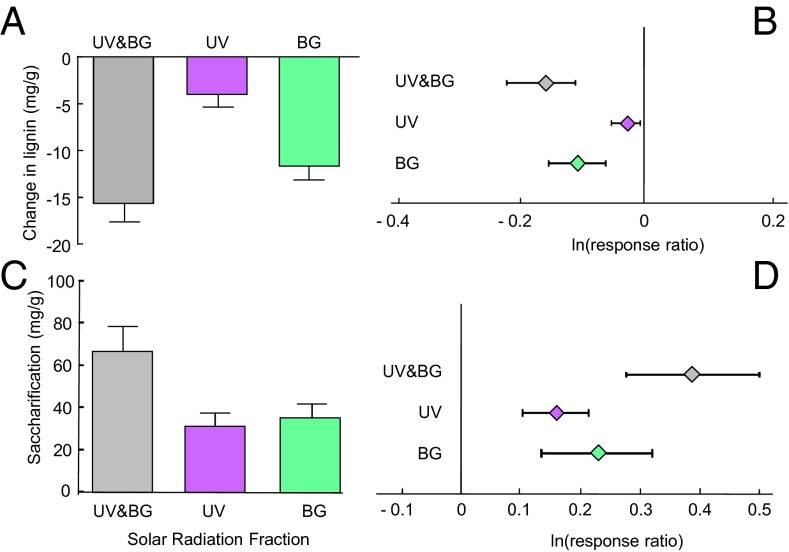
In the group of species used in our study, lignin concentration varied by a factor of nearly 10 (from 4% to 40%) (Table S2). Across this spectrum of species, the effects of solar radiation on litter lignin concentration were clear: lignin concentration was significantly reduced in litter exposed to full solar radiation, and the BG component of sunlight had a much larger effect than the UV component (Fig. 3 A and B). This reduction is noteworthy due to the fact that lignin in litter and soil generally increases over time with biotic degradation due to the preferential catabolism of labile compounds in the litter (4). The consistent and significant reduction in lignin concentrations (and thus lignin content) with exposure to BG, and to a lesser extent UV light, confirms the general response of degradation of lignin in leaf litter exposed to solar radiation (20, 21). The large impact of BG light in reducing lignin can be explained on the basis of the spectral properties of lignin, which, in contrast to cellulose, has strong light absorbance in the BG region (20), and the high relative proportion of BG with respect to UV quanta in solar radiation (BG:UV quantum ratio of ∼5) (Fig. 1B).
Fig. 3.
Lignin is reduced and saccharification increases when leaf litter is exposed to solar radiation. (A) Changes in lignin concentration (mg/g) due to solar radiation exposure in the field. Each bar represents the average of 23 species (+1 SEM). Wavelength effects are calculated as indicated in Fig. 1. (B) Mean effect sizes of different wavelengths on lignin concentration (mg/g) across the 23 species (±95% CI). Details of metaanalysis can be found in Table S3. (C) Effects of solar radiation on saccharification (the amount of sugars released by microbial enzymatic processing of cellulose as determined by an in vitro saccharification test using T. viride cellulose) (35) in plant litter. (D) Mean effect sizes of the different wavelengths on saccharification across the 23 species (±95% CI).
Photodegradation Facilitates Access of Microbial Enzymes to Cell Wall Polysaccharides Through Lignin Degradation.
We were interested in the potential mechanisms that could explain the biotic facilitation (photopriming) observed in our results. We reasoned that the positive effect of previous exposure to solar radiation on microbial decomposition of litter in the soil (Fig. 2 A and B) may be associated with the alleviation of lignin inhibition on cellulose–enzyme interactions. Although lignin is most often considered to inhibit microbial degradation due to its poor energy yield, it is also important to recognize that lignin functions as a structural and hydrophobic barrier impeding access of microbial enzymes to plant cell wall polysaccharides, such as cellulose (35), making cell walls virtually impermeable to enzymatic degradation (36). Moreover, it is widely accepted that lignin inhibits cellulose hydrolysis by binding active sites of cellulolitic enzymes (37) and reducing enzymatic efficiency (38).
Our first approach was to measure the effect of prior exposure to sunlight on cellulose availability in plant litter for microbial enzymes. We conducted an in vitro test for saccharification (the amount of sugars released during cellulase digestion) (35), to evaluate the magnitude of the effect, and role of different wavelengths in affecting carbohydrate availability. For all species, previous exposure of leaf litter to solar radiation significantly increased in vitro saccharification—in some cases up to 150% above nonexposed litter (Fig. 3 C and D and Table S5). The relative contributions of UV and BG light to increased saccharification were similar (Fig. 3 C). These results suggest that the proximate mechanism for the observed photopriming effect is the increased microbial accessibility to cellulose (and presumably other polysaccharides) in leaf litter. These changes were almost certainly not due to thermal effects during photodegradation, because extended exposure of plant litter to high temperatures (60 °C) had no demonstrable effect on these variables (Table S6). For woody species litter, increased saccharification was more modest than for herbaceous species (Table S5), which is consistent with the lack of photopriming in this group of species (Fig. 2 C and D).
Table S5.
Lignin concentration, total carbohydrates, and saccharification for all litter types decomposed under different light treatments
| Species code | Lignin concentration, mg/g | Total carbohydrates, mg/g | Saccharification, mg/g | ||||||
| >290 nm | >400 nm | >550 nm | >290 nm | >400 nm | >550 nm | >290 nm | >400 nm | >550 nm | |
| Herbaceous | |||||||||
| BP | 30.9 ± 2.5 | 29.2 ± 1.3 | 38.8 ± 3.35 | 350.4 ± 4.91 | 354.4 ± 5.3 | 336.1 ± 9.9 | 274.2 ± 4.1 | 249.6 ± 8.9 | 186.5 ± 3.8 |
| CA | 73.7 ± 2.1 | 78.4 ± 2.2 | 88.4 ± 1.4 | 246.2 ± 11.6 | 266.8 ± 12.5 | 248.8 ± 12.7 | 286.2 ± 5.0 | 260.7 ± 6.0 | 226.0 ± 7.0 |
| ChC | 53.5 ± 3.2 | 63.5 ± 3.8 | 78.3 ± 2.8 | 242.6 ± 2.8 | 244.3 ± 7.8 | 242.1 ± 3.3 | 234.0 ± 9.5 | 156.6 ± 10.1 | 97.9 ± 4.1 |
| DG | 37.2 ± 2.2 | 44.0 ± 3.8 | 54.6 ± 2.9 | 300.8 ± 2.3 | 307.0 ± 3.6 | 308.8 ± 3.4 | 286.3 ± 10.2 | 219.8 ± 15.1 | 165.6 ± 15.1 |
| GM | 88.3 ± 3.4 | 96.6 ± 7.3 | 110.1 ± 7.4 | 235.7 ± 2.8 | 240.2 ± 3.3 | 244.3 ± 2.4 | 192.8 ± 9.0 | 165.0 ± 6.2 | 152.4 ± 5.7 |
| HA | 66.6 ± 7.9 | 79.3 ± 8.0 | 83.7 ± 8.4 | 187.7 ± 4.5 | 189.3 ± 3.4 | 193.7 ± 4.4 | 132.1 ± 8.9 | 146.5 ± 14.0 | 122.7 ± 1.6 |
| LM | 27.0 ± 3.1 | 30.4 ± 3.2 | 48.2 ± 6.0 | 303.3 ± 4.2 | 305.8 ± 3.7 | 299.2 ± 3.5 | 372.1 ± 10.5 | 308.2 ± 9.0 | 252.5 ± 11.1 |
| PQ | 54.9 ± 1.3 | 61.0 ± 5.0 | 71.5 ± 2.2 | 300.3 ± 3.2 | 292.5 ± 3.0 | 300.6 ± 6.6 | 207.0 ± 3.0 | 150.0 ± 12.6 | 104.8 ± 4.0 |
| PL | 52.5 ± 1.8 | 58.7 ± 3.6 | 82.0 ± 5.8 | 297.4 ± 31.4 | 274.5 ± 17.9 | 301.2 ± 13.6 | 262.5 ± 7.3 | 154.6 ± 9.4 | 58.6 ± 5.8 |
| ScS | 69.8 ± 3.1 | 70.4 ± 5.1 | 91.2 ± 4.4 | 289.1 ± 4.6 | 294.2 ± 6.7 | 284.5 ± 5.2 | 165.8 ± 4.3 | 133.9 ± 10.8 | 100.6 ± 5.6 |
| SS | 51.3 ± 1.4 | 50.9 ± 0.6 | 67.1 ± 2.0 | 218.0 ± 15.1 | 208.3 ± 9.5 | 224.0 ± 14.3 | 273.5 ± 10.5 | 199.4 ± 4.0 | 86.1 ± 6.5 |
| TA | 33.8 ± 1.8 | 37.0 ± 1.3 | 46.6 ± 0.8 | 299.4 ± 7.6 | 313.9 ± 5.8 | 308.5 ± 8.9 | 392.8 ± 44.0 | 346.0 ± 18.9 | 279.6 ± 17.4 |
| ZM | 38.1 ± 4.2 | 38.3 ± 4.6 | 39.4 ± 4.3 | 275.7 ± 4.7 | 280.0 ± 1.4 | 273.2 ± 4.4 | 219.9 ± 29.6 | 215.7 ± 23.5 | 174.0 ± 15.7 |
| Woody | |||||||||
| AA | 243.9 ± 13.0 | 263.9 ± 1.1 | 252.2 ± 11.3 | 315.4 ± 8.5 | 318.4 ± 9.7 | 311.3 ± 4.5 | 121.3 ± 8.2 | 103.0 ± 10.6 | 101.9 ± 10.9 |
| FA | 91.2 ± 1.7 | 99.9 ± 2.9 | 106.4 ± 5.0 | 259.3 ± 7.2 | 258.8 ± 9.1 | 252.5 ± 8.9 | 165.8 ± 2.5 | 146.0 ± 4.7 | 145.6 ± 9.0 |
| MB | 358.9 ± 7.1 | 373.2 ± 7.2 | 373.7 ± 3.9 | 246.1 ± 2.3 | 244.5 ± 1.6 | 247.2 ± 2.5 | 134.5 ± 4.7 | 120.2 ± 6.6 | 121.7 ± 9.9 |
| MS | 131.0 ± 4.3 | 139.1 ± 3.7 | 148.9 ± 3.5 | 261.6 ± 12.8 | 264.3 ± 9.5 | 254.7 ± 17.1 | 248.8 ± 8.5 | 228.0 ± 9.1 | 235.6 ± 14.2 |
| NO | 286.3 ± 5.3 | 288.0 ± 8.5 | 315.8 ± 6.1 | 245.2 ± 10.8 | 230.5 ± 7.4 | 238.1 ± 9.5 | 112.8 ± 7.1 | 105.0 ± 6.8 | 88.1 ± 5.5 |
| ND | 191.9 ± 3.7 | 199.8 ± 5.3 | 207.7 ± 7.5 | 241.4 ± 7.1 | 246.2 ± 6.1 | 238.5 ± 8.5 | 159.7 ± 9.2 | 174.4 ± 9.1 | 152.1 ± 8.0 |
| NN | 145.2 ± 3.6 | 138.8 ± 3.0 | 146.6 ± 3.0 | 236.7 ± 5.8 | 231.2 ± 3.4 | 228.0 ± 6.6 | 156.5 ± 8.7 | 125.2 ± 5.7 | 99.1 ± 5.0 |
| NA | 243.4 ± 9.5 | 229.3 ± 3.7 | 238.0 ± 8.1 | 258.7 ± 17.7 | 270.4 ± 22.0 | 257.0 ± 13.6 | 171.8 ± 2.7 | 164.9 ± 5.4 | 149.6 ± 4.0 |
| PP | 286.2 ± 8.4 | 282.1 ± 3.4 | 285.7 ± 8.9 | 270.1 ± 13.1 | 284.6 ± 9.2 | 249.7 ± 12.7 | 232.6 ± 6.2 | 220.8 ± 9.2 | 183.1 ± 7.2 |
| PN | 140.3 ± 6.0 | 149.8 ± 5.6 | 171.8 ± 7.2 | 208.2 ± 9.7 | 206.6 ± 5.2 | 207.2 ± 9.1 | 119.8 ± 4.8 | 106.5 ± 11.7 | 103.0 ± 6.3 |
Columns show values for each of the three light attenuation treatments described in the experimental design (Materials and Methods). Mean (n = 5) and SE are shown. Species codes are from Table S1.
Table S6.
Evaluation of potential thermal effects on litter chemistry and available sugars in Populus nigra (PN) litter used in photodegradation experiments
| Populus nigra litter (PN) | |||
| Leaf litter characteristics | Control | 60 °C | Significance |
| OM, % | 84 ± 0.1 | 84 ± 0.4 | n.s. |
| Lignin, mg/g | 163 ± 10 | 157 ± 7 | n.s. |
| Total sugars, mg/g | 253 ± 12.6 | 259 ± 7.9 | n.s. |
| Available sugars, mg/g | 108 ± 4.3 | 113 ± 3.8 | n.s. |
Five samples of 1.500 g were subjected to two treatments: (i) control, where ground samples were stored at ambient temperature in the dark, and (ii) high temperature, where ground samples were placed in a 60 °C drying oven. Both treatments lasted for 4 mo. Description of all laboratory analyses can be found in Materials and Methods. None of the evaluated variables demonstrated a significant difference for a Student's t test (n.s. in last column). Values are means of five samples ±1 SEM.
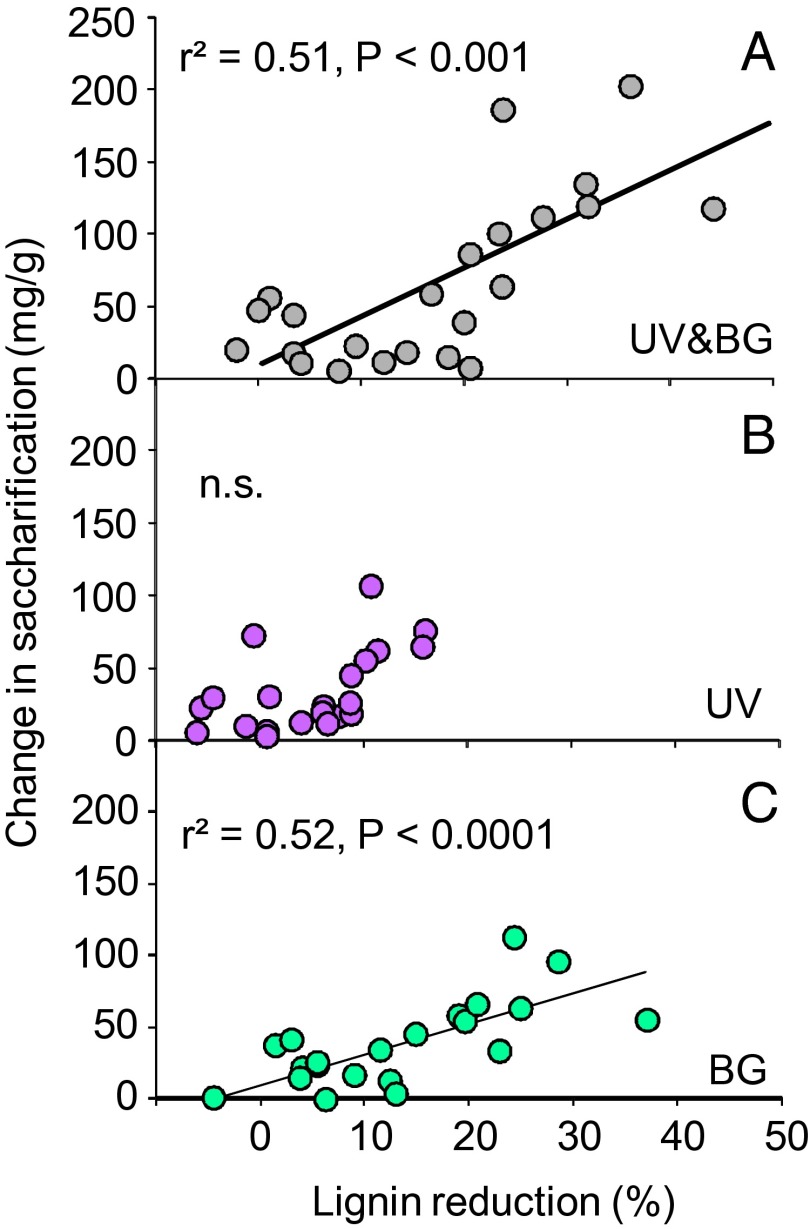
Next, we evaluated the relationship between the observed changes in saccharification and the direct effects of light exposure on lignin concentration. A comparison between lignin reduction and changes in saccharification demonstrated a highly significant linear relationship for UV-and-BG light, and for BG light (Fig. 4 A and C), but not for UV light alone (Fig. 4B), which overall had very little effect in reducing litter lignin concentration (Fig. 3 A and B). Based on these results, we conclude that the increase in carbohydrate availability is most likely caused by the reduction in lignin concentration during photodegradation and that this increased accessibility to leaf litter carbohydrates is the most parsimonious explanation for the observed photopriming of biotic decomposition (Fig. 4).
Fig. 4.
Photopriming depends on the effectiveness of lignin reduction due to photodegradation. Each symbol corresponds to the average of an individual species. (A) Relationship between lignin reduction (the relative decrease in lignin concentration due to exposure to different wavelengths of solar radiation) in plant litter and changes in saccharification (absolute difference between saccharification due to exposure to different wavelengths of solar radiation) for (A) UV&BG, (B) UV, and (C) BG wavelengths of solar radiation. Wavelength effects are calculated as indicated in Fig. 1. Only the relationships for UV&BG and BG light are significant, as indicated in the graph.
Discussion
Soil microorganisms oxidize large quantities of plant carbohydrates as a source of energy and as a way to access scarce nutrients. Limited accessibility to labile carbon compounds is a fundamental stumbling block to microbial degradation of plant litter (39). Lignin in secondary cell walls is the major obstacle affecting the velocity of microbial carbon turnover because enzymes to degrade lignin are energetically costly and nonspecific, and the amorphous nature of lignin makes it resistant to enzymatic degradation (40). In fact, a flurry of recent biotechnological research has focused on opportunities for altering lignin composition and content in second generation biofuel crops, to increase cell wall digestibility and thus improve the efficiency of cellulosic ethanol production (41, 42). We show that, in litter of a broad range of plant species, photodegradation can directly reduce lignin concentration (Fig. 3 A and B), which adds generality to the results of previous studies (19, 20, 43). In addition, an important implication of the present experiments is that the degradation of litter lignin caused by sunlight exposure may increase the digestibility of labile carbon compounds locked in lignin linkages within the cell wall matrix (Fig. 4). Interestingly, the increased saccharification due to exposure to sunlight is quantitatively similar to changes in saccharification achieved through genetic manipulation of plant lignin content (35, 44, 45). Taken together, our results suggest that photodegradation of plant litter essentially reduces the bottleneck imposed by lignin in secondary cell walls and greatly facilities microbial access to carbohydrates (Fig. 4) and biotic decomposition (Fig. 2 A and B). The lack of photopriming in litter of woody species (Fig. 2 C and D) may be related to the optical shielding of lignin molecules by other cell wall components, as woody species' leaf litters are characterized by high leaf mass per unit area (LMA) and high abundance of other aromatic compounds such as polyphenols (Table S2). An alternative, intriguing hypothesis is that the interaction between UV radiation and components of woody leaf litter leads to the formation of photoproducts such as quinones (46), which are known to have inhibitory effects on a broad group of microorganisms (47).
The effects of photodegradation on biotic decomposition of plant litter and carbon turnover in terrestrial ecosystems have been underappreciated until now. Our data suggest that the importance of photodegradation extends well beyond its demonstrated direct effects in arid lands (12–14, 17, 24). In addition, our results are consistent with a recent study in which previous light exposure was a determining factor in the subsequent biotic degradability of terrestrial organic matter in the aquatic environment at the watershed scale (48). The mechanistic model for photopriming outlined here suggests that this process can be of importance in many highly productive terrestrial ecosystems where senescent plant material may be exposed to solar radiation for some period during the year, including tundra, grasslands, savannah, agroecosystems, drought-deciduous forests, and alpine ecosystems. This implication challenges the estimation of a modest contribution of photodegradation to the global carbon cycle (25). The full nature of the interactions between photodegradation, subsequent microbial decomposition, and climate controls has yet to be determined although it seems that the way in which photodegradation affects the enzymatic accessibility to carbohydrates in plant cell walls, and thus litter decomposability, is an underestimated factor determining plant carbon turnover in a wide range of terrestrial ecosystems.
Materials and Methods
Experimental Design and Litter Collection.
The experimental approach was divided into two phases in the field: (i) an evaluation of mass loss from photodegradation under light attenuation treatments in the absence of soil biota; and (ii) an evaluation of mass loss from subsequent biotic decomposition in samples contained in fiberglass mesh litterbags placed in contact with soil. Additionally, we measured the physical and chemical characteristics of litter before and after the first phase (direct photodegradation). We installed the experiment at the experimental field site of the Instituto de Investigaciones Fisiológicas y Ecológicas Vinculadas a la Agricultura (IFEVA)-Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), at the School of Agronomy campus of the University of Buenos Aires (34°36'S 58°22'W) (20). Climate is temperate and subhumid, with an average temperature of 16.9 °C and year-round rainfall of 986 mm mean annual precipitation (49).
We collected senescent leaf material from 23 species across a range of life forms, families, and functional types from temperate ecosystems of South America (Table S1). Leaf litter came from the Pampas grassland region of central Argentina (native mixed C3–C4 prairie and agroecosystems) (50), the Patagonian semiarid steppe (51), and temperate mixed deciduous–evergreen forest (52). All leaf litter was carefully collected from the field sites at the natural time of senescence or, in the case of evergreen species, brought to the laboratory and separated for only recently senesced, fully intact litter. All litter was pooled for each species, and, in all cases, litter came from multiple individuals (>10) collected from the field. Litter was air dried and stored indoors without light exposure until the experiment was ready to begin.
Field Phase 1: Direct Photodegradation.
We constructed filter “roofs” in the field to have replicated light exposure treatments for all 23 species. The roofs consisted of aluminum frames of 1.5 m × 2 m, which were held above the ground using aluminum poles. Plastic filters were then suspended tautly across these frames to create a homogenous light environment in the area beneath the filters (Fig. 1A). Three different plastic filters were used to achieve the light attenuation treatments: (i) >290 nm, with fully transparent 30-µm polyethylene film, which transmitted >95% of all solar radiation; (ii) >400 nm, with attenuation of all UV radiation [280–400 nm; Costech 226 UV filter (equivalent to Rosco UV Filter)]; and (iii) >550 nm, with attenuation of all UV and blue–green (BG) wavelengths (280–580 nm; Rosco E-Color 135 Deep Golden Amber). Spectral measurements in the field were obtained at midday as described in ref. 53. Transmittance of filters was evaluated using a UV-VIS spectrophotometer (Shimadzu Scientific Instruments). In total, we constructed 15 frames, (n = 5 for each light treatment).
Beneath each filter, we constructed wooden pallets of 1.2 m × 1 m placed on the soil surface and inclined at an angle of 25 degrees toward the north to maximize light exposure. Samples ranging from 1 g to 2 g (depending on the species characteristics and litter availability) were replicated for each of the 23 species and light treatments. Litter was placed in dome-shaped clear plastic cups (10 cm diameter × 15 cm high, Venecia Cáliz; La Papelera) (see Inset in Fig. 1A). The cups were sealed at the bottom with a plastic lid and fixed to the wooden pallet using Velcro tape attached to the base of the cup. The cups were transparent to all wavelengths of solar radiation (similar to the plastic filters used for the full sun treatment) (Fig. 1B, filter 1) and did not interfere with light attenuation treatments. In addition, the cups were largely impervious to water condensation and were protected from rainfall penetration by the plastic filters placed above each aluminum frame. Transmittance of the plastic cups was checked periodically with the spectroradiometer to avoid artifacts due to photo-aging of the plastic. As a precaution, the entire set of plastic cups was replaced midway through the experiment. Leaf litter was arranged in the cups to mimic as much as possible a 3D exposure of field conditions (Fig. 1A, Inset). All samples (23 species × 3 light treatments × 5 replicates = 345 in total) were placed beneath the three light treatments on December 31 (midsummer in the Southern Hemisphere) and incubated for 100 d. At the end of the incubation, litter samples were removed from the field and placed in a 50 °C drying oven for 48 h and weighed for determination of mass loss. Ash-free dry mass was determined for all samples (54) for calculations of organic matter loss.
Field Phase 2: Biotic Decomposition.
The second phase of the experiment involved the biotic decomposition of all litter that had been previously exposed to light attenuation treatments described above in phase 1. Each sample was divided approximately in half, and between 0.500 and 0.750 g was placed in a 10 × 15-mm, 2-mm mesh litterbag for field incubation. The other half of the sample was ground to pass a 40-µm mesh and evaluated for lignin content, total carbohydrates, and saccharification (described in Litter Chemical and Morphological Characteristics). Litterbags were placed outside on October 1 (early spring, Southern Hemisphere) in an area of 20 × 20 m from which all aboveground vegetation had been removed. The litterbags were randomly located and held in place, in direct contact with the surface soil with small aluminum stakes. A heavy shadecloth, doubled (light transmittance of <5% total radiation), was placed above all litterbags to block exposure to solar radiation. After 45 d in the field (November 15), litterbags were collected, carefully cleaned to remove any extraneous debris, placed in a 50 °C drying oven for 48 h, and weighed for determination of mass loss. Ash-free dry mass was determined for all samples to correct for soil contamination from the field (54), and for calculations of organic matter loss.
Litter Chemical and Morphological Characteristics.
Characteristics of initial litter quality were assessed including leaf mass per area (LMA), total soluble polyphenols, lignin, cellulose and hemicellulose concentrations, sunscreens, and organic matter content. For exposed litter from phase 1, we also measured lignin, cellulose and hemicellulose concentrations, total carbohydrates, and saccharification (n = 5 in all cases). LMA was evaluated by scanning five to six leaves at 600 dpi resolution (ScanJet 2400; Hewlett Packard), with area calculated using Adobe Photoshop cs2 (Adobe Systems Software Ltd). Litter was then placed in a 50 °C drying oven for 48 h and weighed, with LMA (g⋅m−2) calculated as total area/total dry mass. We determined total soluble polyphenols by extraction with a 50% methanol and analyzed by the Folin–Ciocalteu method (55). Absorbance was measured at λ = 760 nm. For quantification, a calibration curve was made using gallic acid (0.1 mg/L) as a standard (52). Sunscreens present in plant litter were measured using a methanol extraction (53). Absorbance was measured at λ = 305 nm using a UV-1700 PharmaSpec UV-VIS spectrophotometer (Shimadzu Corporation). Lignin, hemicelluloses, and celluloses were evaluated using sequential extraction, based on fiber analysis (56), using an automated Ankom 220 Fiber Analyzer (Ankom Technology), including sulphuric acid digestion and ash determination. Total carbohydrates in litter were extracted from a hot acid digestion (57). Absorbance of extract was measured at λ = 490 nm. Saccharification (the amount of sugars released by microbial enzymatic processing of cellulose) (35) was estimated by the action of the cellulase enzyme (Trichoderma viride cellulase, C9422; Sigma Aldrich), which degrades accessible cellulose (35, 58). Fifty milligrams of ground litter was incubated with 50 U/mL enzyme in 10 mL of pH 5.5 acetate buffer and 0.2 mL of toluene. Samples were incubated at 50 °C with constant agitation for 72 h. The product (sugar) was quantified using the dinitrosalicylic acid method (59), and absorbance was measured at λ = 575 nm.
Calculations and Statistics.
Average values (±1 SEM) for litter of each species and from each of the three light treatments [(i) >290 nm; (ii) >400 nm; (iii) >550 nm] can be found in Tables S1–S4. For figures in the main text, the effect of the UV-and-BG component on organic matter (OM) loss was calculated by subtracting the measured variable (i.e., OM loss) under filter iii from the measured variable under filter i; the UV component alone was calculated by subtracting the measured variable under filter ii from the measured variable under filter i whereas the BG component alone was calculated by subtracting the measured variable under filter iii from the measured variable under filter ii (see Fig. 1B for details of filter transmittance). Photopriming was calculated as the percent change for each species when comparing different wavelength components (UV and BG, UV alone and BG alone). Statistical evaluation of wavelength effects on organic matter loss, photopriming, lignin concentration, and saccharification, was carried out using a metaanalysis (60): in this case, using the natural log of the response ratios between light treatments as described above. All metaanalyses were conducted as random effects models with the grouping variable of life form (woody vs. herbaceous). Resampling tests of 999 iterations were conducted to calculate boot-strapped confidence intervals. The random effects model with boot-strapped confidence intervals is the most conservative approach for assessing effects of treatments using a metaanalytical approach, and this evaluation was ideal for our experimental design, given the wide range of species and without the complication of differences in experimental protocol across species (60). All metaanalyses were completed using MetaWin 2.1 software, (MRSsoft). Correlative relationships between lignin reduction (the relative decline in lignin concentrations due to exposure to different wavelengths of solar radiation) and changes in saccharification (absolute difference between saccharification due to exposure to different wavelengths of solar radiation) for each of the light treatments were evaluated using least-squares linear regression. Initial litter quality measurements were evaluated with principal components analysis (PCA); a t test for differences in PCA axes was evaluated with a Student’s t test. For all parametric analyses, α = 0.05. PCA analyses were completed with Infostat/Professional (Version 1.1; National University of Córdoba, Statistics and Design).
SI Text
The supplementary information file contains Fig. S1 and Tables S1–S6, all of which are cited in the main text. Details on methodology and statistics can be found in Materials and Methods in the main text of the manuscript.
Acknowledgments
We thank M. Laura Martínez for technical assistance in the field and the laboratory; L. Yahdjian, L. Vivanco, and P. Araujo for donation of litter for use in the experiments; M. Tinaro, A. Tornese, M. Pendergast, and W. De Nicolo for field assistance and sample preparation; and Julia Koricheva for advice on metaanalysis techniques and interpretation. We thank L. Vivanco and two anonymous reviewers for helpful comments on a previous version of this manuscript. The Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) provided funding support with grants Proyectos de Investigación Científica y Tecnológica (PICT) 2008-108, 2010-147, 2011-041, 2011-558, and 2013-0148.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1516157113/-/DCSupplemental.
References
- 1.Schlesinger WH, Andrews JA. Soil respiration and the global carbon cycle. Biogeochemistry. 2000;48(1):7–20. [Google Scholar]
- 2.Jackson RB, et al. Trading water for carbon with biological carbon sequestration. Science. 2005;310(5756):1944–1947. doi: 10.1126/science.1119282. [DOI] [PubMed] [Google Scholar]
- 3.Adair EC, et al. Simple three-pool model accurately describes patterns of long-term litter decomposition in diverse climates. Glob Change Biol. 2008;14(11):2636–2660. [Google Scholar]
- 4.Schmidt MW, et al. Persistence of soil organic matter as an ecosystem property. Nature. 2011;478(7367):49–56. doi: 10.1038/nature10386. [DOI] [PubMed] [Google Scholar]
- 5.Chapin FS, Matson PA, Mooney HA. Principles of Terrestrial Ecosystem Ecology. Springer; New York: 2002. [Google Scholar]
- 6.Kieber DJ, McDaniel J, Mopper K. Photochemical source of biological substrates in sea water: Implications for carbon cycling. Nature. 1989;341:637–639. [Google Scholar]
- 7.Mopper K, et al. Photochemical degradation of dissolved organic carbon and its impact on the oceanic carbon cycle. Nature. 1991;353:60–62. [Google Scholar]
- 8.Amon RW, Benner R. Photochemical and microbial consumption of dissolved organic carbon and dissolved oxygen in the Amazon River system. Geochim Cosmochim Acta. 1996;60:1783–1792. [Google Scholar]
- 9.Graneli W, Lindell M, Tranvik L. Photo‐oxidative production of dissolved inorganic carbon in lakes of different humic content. Limnol Oceanogr. 1996;41(4):698–706. [Google Scholar]
- 10.Zuo Y, Jones RD. Photochemistry of natural dissolved organic matter in lake and wetland waters: Production of carbon monoxide. Water Res. 1997;31(4):850–858. [Google Scholar]
- 11.Stubbins A, et al. Open-ocean carbon monoxide photoproduction. Deep Sea Res Part II Top Stud Oceanogr. 2006;53(14):1695–1705. [Google Scholar]
- 12.Austin AT, Vivanco L. Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature. 2006;442(7102):555–558. doi: 10.1038/nature05038. [DOI] [PubMed] [Google Scholar]
- 13.Rutledge S, Campbell DI, Baldocchi D, Schipper LA. Photodegradation leads to increased CO2 losses from terrestrial organic matter. Glob Change Biol. 2010;16:3065–3074. [Google Scholar]
- 14.King JY, Brandt LA, Adair EC. Shedding light on plant litter decomposition: advances, implications and new directions in understanding the role of photodegradation. Biogeochemistry. 2012;111(1):57–81. [Google Scholar]
- 15.Day TA, Guénon R, Ruhland CT. Photodegradation of plant litter in the Sonoran Desert varies by litter type and age. Soil Biol Biochem. 2015;89:109–122. [Google Scholar]
- 16.Schade GW, Hormann RM, Crutzen PJ. CO emissions from degrading plant matter. Tellus. 1999;51B:899–908. [Google Scholar]
- 17.Brandt LA, Bohnet C, King JY. Photochemically induced carbon dioxide production as a mechanism for carbon loss from plant litter in arid ecosystems. J Geophys Res-Biogeosci. 2009;114:G02004. [Google Scholar]
- 18.Lee H, Rahn T, Throop H. An accounting of C-based trace gas release during abiotic plant litter degradation. Glob Change Biol. 2012;18(3):1185–1195. [Google Scholar]
- 19.Henry HAL, Brizgys K, Field CB. Litter decomposition in a California annual grassland: Interactions between photodegradation and litter layer thickness. Ecosystems (N Y) 2008;11(4):545–554. [Google Scholar]
- 20.Austin AT, Ballaré CL. Dual role of lignin in plant litter decomposition in terrestrial ecosystems. Proc Natl Acad Sci USA. 2010;107(10):4618–4622. doi: 10.1073/pnas.0909396107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Y, King J, Karlen S, Ralph J. Using 2D NMR spectroscopy to assess effects of UV radiation on cell wall chemistry during litter decomposition. Biogeochemistry. 2015;125(3):427–436. [Google Scholar]
- 22.Gaxiola A, Armesto JJ. Understanding litter decomposition in semiarid ecosystems: Linking leaf traits, UV exposure and rainfall variability. Front Plant Sci. 2015;6:140. doi: 10.3389/fpls.2015.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker NR, Allison SD. Ultraviolet photodegradation facilitates microbial litter decomposition in a Mediterranean climate. Ecology. 2015;96(7):1994–2003. doi: 10.1890/14-1482.1. [DOI] [PubMed] [Google Scholar]
- 24.Yanni SF, Suddick EC, Six J. Photodegradation effects on CO2 emissions from litter and SOM and photo-facilitation of microbial decomposition in a California grassland. Soil Biol Biochem. 2015;91:40–49. [Google Scholar]
- 25.Foereid B, Rivero MJ, Primo O, Ortiz I. Modelling photodegradation in the global carbon cycle. Soil Biol Biochem. 2011;43(6):1383–1386. [Google Scholar]
- 26.Gallo ME, Porras-Alfaro A, Odenbach KJ, Sinsabaugh RL. Photoacceleration of plant litter decomposition in an arid environment. Soil Biol Biochem. 2009;41(7):1433–1441. [Google Scholar]
- 27.Brandt LA, King JY, Hobbie SE, Milchunas DG, Sinsabaugh RL. The role of photodegradation in surface litter decomposition across a grassland ecosystem precipitation gradient. Ecosystems (N Y) 2010;13:765–781. [Google Scholar]
- 28.Barnes PW, Throop HL, Hewins DB, Abbene ML, Archer SR. Soil coverage reduces photodegradation and promotes the development of soil-microbial films on dryland leaf litter. Ecosystems (N Y) 2012;15(2):311–321. [Google Scholar]
- 29.Williamson CE, et al. Solar ultraviolet radiation in a changing climate. Nat Clim Chang. 2014;4(6):434–441. [Google Scholar]
- 30.Ballaré CL, Caldwell MM, Flint SD, Robinson SA, Bornman JF. Effects of solar ultraviolet radiation on terrestrial ecosystems: Patterns, mechanisms, and interactions with climate change. Photochem Photobiol Sci. 2011;10(2):226–241. doi: 10.1039/c0pp90035d. [DOI] [PubMed] [Google Scholar]
- 31.Kirschbaum MUF, Lambie SM, Zhou H. No UV enhancement of litter decomposition observed on dry samples under controlled laboratory conditions. Soil Biol Biochem. 2011;43(6):1300–1307. [Google Scholar]
- 32.Uselman SM, Snyder KA, Blank RR, Jones TJ. UVB exposure does not accelerate rates of litter decomposition in a semi-arid riparian ecosystem. Soil Biol Biochem. 2011;43:1254–1265. [Google Scholar]
- 33.Wang J, Liu L, Wang X, Chen Y. The interaction between abiotic photodegradation and microbial decomposition under ultraviolet radiation. Glob Change Biol. 2015;21(5):2095–2104. doi: 10.1111/gcb.12812. [DOI] [PubMed] [Google Scholar]
- 34.Lin Y, Scarlett RD, King JY. Effects of UV photodegradation on subsequent microbial decomposition of Bromus diandrus litter. Plant Soil. 2015;395(1-2):263–271. [Google Scholar]
- 35.Chen F, Dixon RA. Lignin modification improves fermentable sugar yields for biofuel production. Nat Biotechnol. 2007;25(7):759–761. doi: 10.1038/nbt1316. [DOI] [PubMed] [Google Scholar]
- 36.Gressel J. Transgenics are imperative for biofuel crops. Plant Sci. 2008;174(3):246–263. [Google Scholar]
- 37.Sewalt V, Glasser W, Beauchemin K. Lignin impact on fiber degradation. 3. Reversal of inhibition of enzymatic hydrolysis by chemical modification of lignin and by additives. J Agric Food Chem. 1997;45(5):1823–1828. [Google Scholar]
- 38.Várnai A, Siika-aho M, Viikari L. Restriction of the enzymatic hydrolysis of steam-pretreated spruce by lignin and hemicellulose. Enzyme Microb Technol. 2010;46(3):185–193. [Google Scholar]
- 39.Himmel ME, et al. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science. 2007;315(5813):804–807. doi: 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- 40.Sinsabaugh RL, Antibus RK, Linkins AE. An enzymic approach to the analysis of microbial activity during plant litter decomposition. Agric Ecosyst Environ. 1991;34(1):43–54. [Google Scholar]
- 41.Weng JK, Li X, Stout J, Chapple C. Independent origins of syringyl lignin in vascular plants. Proc Natl Acad Sci USA. 2008;105(22):7887–7892. doi: 10.1073/pnas.0801696105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lockhart J. Altering lignin composition to improve biofuel production. Plant Cell. 2015;27(8):2082. doi: 10.1105/tpc.15.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foereid B, Bellarby J, Meier-Augenstein W, Kemp H. Does light exposure make plant litter more degradable? Plant Soil. 2010;333(1):275–285. [Google Scholar]
- 44.Van Acker R, et al. Improved saccharification and ethanol yield from field-grown transgenic poplar deficient in cinnamoyl-CoA reductase. Proc Natl Acad Sci USA. 2014;111(2):845–850. doi: 10.1073/pnas.1321673111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson NA, et al. Manipulation of guaiacyl and syringyl monomer biosynthesis in an Arabidopsis cinnamyl alcohol dehydrogenase mutant results in atypical lignin biosynthesis and modified cell wall structure. Plant Cell. 2015;27(8):2195–2209. doi: 10.1105/tpc.15.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Müller U, Rätzsch M, Schwanninger M, Steiner M, Zöbl H. Yellowing and IR-changes of spruce wood as result of UV-irradiation. J Photochem Photobiol B. 2003;69(2):97–105. doi: 10.1016/s1011-1344(02)00412-8. [DOI] [PubMed] [Google Scholar]
- 47.Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol. 2006;57(1):233–266. doi: 10.1146/annurev.arplant.57.032905.105159. [DOI] [PubMed] [Google Scholar]
- 48.Cory RM, Ward CP, Crump BC, Kling GW. Carbon cycle: Sunlight controls water column processing of carbon in arctic fresh waters. Science. 2014;345(6199):925–928. doi: 10.1126/science.1253119. [DOI] [PubMed] [Google Scholar]
- 49.Vivanco L, Austin AT. Intrinsic effects of species on leaf litter and root decomposition: A comparison of temperate grasses from North and South America. Oecologia. 2006;150(1):97–107. doi: 10.1007/s00442-006-0495-z. [DOI] [PubMed] [Google Scholar]
- 50.Spirito F, Yahdjian L, Tognetti PM, Chaneton EJ. Soil ecosystem function under native and exotic plant assemblages as alternative states of successional grasslands. Acta Oecol. 2014;54:4–12. [Google Scholar]
- 51.Golluscio RA, Oesterheld M. Water use efficiency of twenty-five co-existing Patagonian species growing under different soil water availability. Oecologia. 2007;154(1):207–217. doi: 10.1007/s00442-007-0800-5. [DOI] [PubMed] [Google Scholar]
- 52.Vivanco L, Austin AT. Tree species identity alters forest litter decomposition through long-term plant and soil interactions in Patagonia, Argentina. J Ecol. 2008;96(4):727–736. [Google Scholar]
- 53.Mazza CA, et al. Functional significance and induction by solar radiation of ultraviolet-absorbing sunscreens in field-grown soybean crops. Plant Physiol. 2000;122(1):117–126. doi: 10.1104/pp.122.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harmon ME, Nadelhoffer KJ, Blair JM. Measuring decomposition, nutrient turnover, and stores in plant litter. In: Robertson GP, Coleman DC, Bledsoe CS, Sollins P, editors. Standard Soil Methods for Long-Term Ecological Research. Oxford Univ Press; Oxford: 1999. pp. 202–240. [Google Scholar]
- 55.Palm CA, Rowland AP. A minimum dataset for characterization of plant quality for decomposition. In: Cadish G, Giller KE, editors. Driven by Nature: Plant Litter Quality and Decomposition. 1997. pp. 379–392. (CAB International, Wallingford, UK) [Google Scholar]
- 56.Van Soest PJ. Use of detergents in analysis of fibrous feeds. II. A rapid method for the determination of fiber and lignin. J Assoc Off Anal Chem. 1963;46:829–835. [Google Scholar]
- 57.Dubois M, Gilles KA, Hamilton JK, Rebers P, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350–356. [Google Scholar]
- 58.Ghose T. Measurement of cellulase activities. Pure Appl Chem. 1987;59(2):257–268. [Google Scholar]
- 59.Breuil C, Saddler J. Comparison of the 3, 5-dinitrosalicylic acid and Nelson-Somogyi methods of assaying for reducing sugars and determining cellulase activity. Enzyme Microb Technol. 1985;7(7):327–332. [Google Scholar]
- 60.Koricheva J, Gurevitch J, Mengersen J, editors. Handbook of Meta-analysis in Ecology and Evolution. Princeton Univ Press; Princeton: 2013. [Google Scholar]