Significance
Fisheries management must avoid adverse impacts on the ecosystem. Doing so can be challenging in highly complex systems, particularly if the target species serves an important ecosystem function. Caribbean coral reefs provide a classic example in which herbivorous parrotfish are both an important fishery and key driver of ecosystem resilience. We developed and tested a multispecies fisheries model of parrotfish and linked it to a coral reef ecosystem experiencing climate change. We found that corals can remain resilient if less than 10% of the fishable parrotfish biomass is harvested and a minimum size of 30 cm is implemented. To our knowledge, this work is the first attempt to identify harvest levels that have little adverse effect on corals.
Keywords: sustainable fisheries, gear restriction, coral persistence, herbivory, climate change
Abstract
Many countries are legally obliged to embrace ecosystem-based approaches to fisheries management. Reductions in bycatch and physical habitat damage are now commonplace, but mitigating more sophisticated impacts associated with the ecological functions of target fisheries species are in their infancy. Here we model the impacts of a parrotfish fishery on the future state and resilience of Caribbean coral reefs, enabling us to view the tradeoff between harvest and ecosystem health. We find that the implementation of a simple and enforceable size restriction of >30 cm provides a win:win outcome in the short term, delivering both ecological and fisheries benefits and leading to increased yield and greater coral recovery rate for a given harvest rate. However, maintaining resilient coral reefs even until 2030 requires the addition of harvest limitations (<10% of virgin fishable biomass) to cope with a changing climate and induced coral disturbances, even in reefs that are relatively healthy today. Managing parrotfish is not a panacea for protecting coral reefs but can play a role in sustaining the health of reefs and high-quality habitat for reef fisheries.
Much effort in ecosystem-based fishery management has been directed to reducing the detrimental impacts of fishing gear on the ecosystem, including bycatch and habitat damage (1, 2). A more complex consideration is maintaining the ecosystem function of the target species. A well-known example is the importance of forage fish and krill to the diet of large fish, sea birds, and marine mammals (3–5), which generates a tradeoff between human and nonhuman (natural) consumption of the stock. Measuring such direct trophic tradeoffs is challenging enough (6), but some ecosystem functions act through complex ecological interactions that might involve multiple trophic levels and result in nonlinear impacts on the ecosystem (7). Perhaps not surprisingly, there has been little progress in modifying fishing activity to take account of these compound ecological processes (8).
The impacts of fishing are particularly severe in the most complex of marine ecosystems, coral reefs. In the Caribbean, parrotfish (Labridae, Scarinae) are the dominant herbivores on middepth (5–15 m) forereefs (9–11), helping keep large seaweeds in check and facilitating the recovery and growth of corals (12–14). However, parrotfish are an important fishery species (15), and their depletion can lead to a flip in coral population dynamics that locks reefs into a persistent degraded state (16). A shift to macroalgal dominance offers relatively little value to fisheries, because much of the primary production is lost to detrital pathways rather than fish-based consumption (17). Macroalgal dominance usually is associated with less complex coral habitats, which in turn are associated with lower biodiversity and fewer ecosystem functions (18). Although several countries [e.g., Belize, Bonaire, and Bermuda (19)] have implemented a ban on parrotfish harvest, most jurisdictions either have no fisheries restrictions or have set simple catch limits to maintain harvest (20, 21). Here, we work toward a more informed approach to Caribbean fisheries management that explicitly factors the functioning of parrotfishes in the ecosystem and reveals the tradeoff between parrotfish harvest and future resilience of coral populations. We then explore the degree to which simple, enforceable policy interventions can improve the tradeoff and increase the sustainability of both the fishery and the supporting ecosystem under climate change.
Results and Discussion
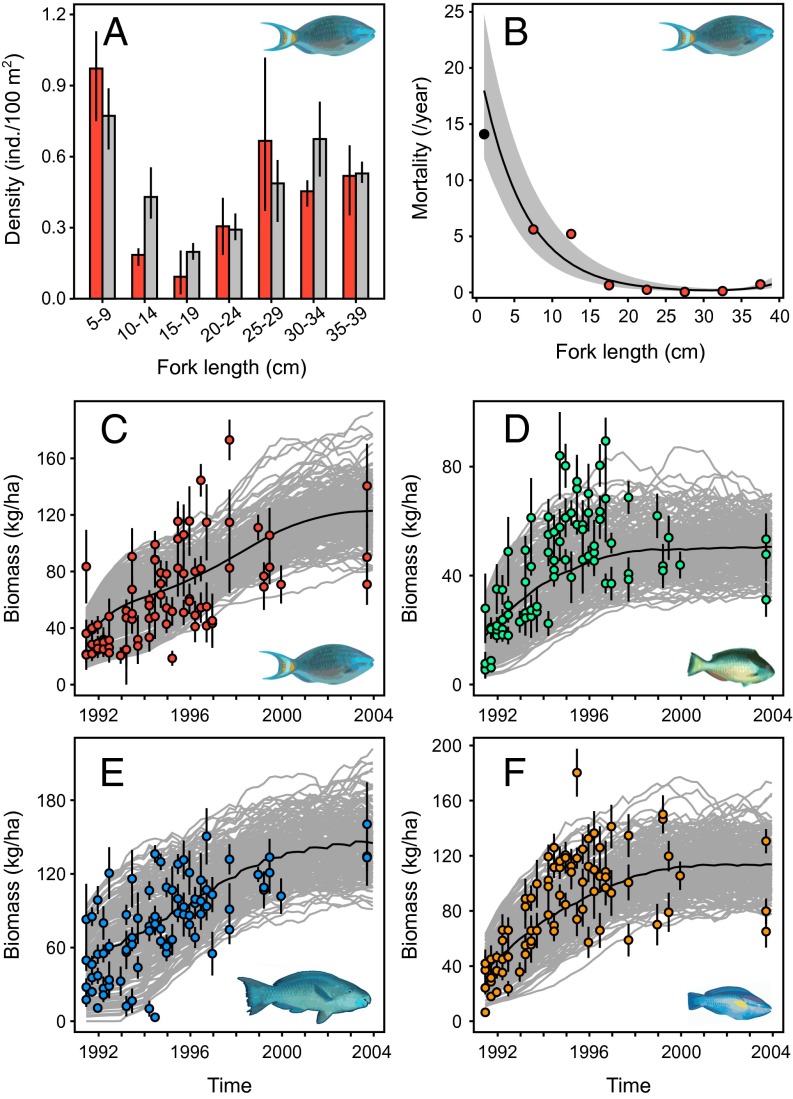
We first modeled the dynamics of multiple parrotfish species using a size-structured demographic model that integrates recruitment, growth, and size-specific mortality. The model was calibrated initially with demographic data of the stoplight parrotfish (Sparisoma viride) (Fig. 1 A and B) from Bonaire in the early 1990s (22, 23, 24). Because fishing for parrotfish was absent at this time (20, 23), and because the population of the stoplight parrotfish remained stable (25), we assumed that this dataset represents a relatively unfished demographic equilibrium. Maximum likelihood parameter estimates (see SI Appendix, SI Methods, section I for detailed parametrization) provided a realistic assessment of recruitment rate (2.5–8.0 fish/100 m2 every 3 mo) and total adult mortality (0.34–0.41/y). The observed bimodality in body-length distribution (Fig. 1A), indicative of differential survival among body sizes (26), was well reproduced by the size-dependent mortality function (Fig. 1B). Likelihood profile-based confidence intervals of parameters (SI Appendix, Fig. S1C) allowed the generation of random fluctuations in fish abundance that have a magnitude similar to those observed in situ.
Fig. 1.
Parrotfish population modeling. (A) Structure of the stoplight parrotfish (Sparisoma viride) population observed in Bonaire in 1989–1992 [red bars (22, 23)] and predicted by the best-fit model at equilibrium (gray bars). Error bars are SEs estimated by bootstrap. (B) Size-specific natural mortality of the stoplight parrotfish as estimated in Bonaire by the best-fit predictions. The gray envelope represents the normal 95% confidence interval of model estimates. Red dots indicate previous mortality estimates (24) obtained with the same survey data, and the black dot is an empirical estimate of recruit mortality of Sparisoma spp. from Barbados (45). (C−F) Biomass recovery of the stoplight (C), redband (Sparisoma aurofrenatum) (D), queen (Scarus vetula) (E), and princess (Scarus taeniopterus) (F) parrotfishes in Bermuda after cessation of trap fishing in April 1990. Dots represent observations averaged over 3-mo periods; error bars indicate the associated SEs. Gray lines show the individual (200 stochastic) simulations, and black lines show average simulated trajectories.
Model performance was evaluated further using an independent dataset, in this case for a nonstationary population. A long-term monitoring survey (1991–2003) performed in Bermuda after the closure of a trap fishery (27) revealed substantial and rapid recovery of most parrotfish populations (28). The model was able to reproduce the recovery dynamics observed in stoplight parrotfish (Fig. 1C and SI Appendix, Fig. S3) with realistic adjustments to recruitment and mortality. Simple species-specific adjustments to recruitment allowed us to reproduce the recovery of other dominant parrotfish species assuming that mortality was uniform across parrotfishes and was only a function of body length (Fig. 1 D–F and SI Appendix, Fig. S3). The model was finally able to predict population density and biomass for the most abundant parrotfishes observed in Bonaire (22) based on empirical estimates of recruitment (SI Appendix, Fig. S4). The resulting fishery model integrates the dynamics of the five dominant parrotfishes of Caribbean forereefs (29) that represent 80–95% of the total parrotfish abundance across the region (15, 20, 22, 27). The impact of fishing was simulated with the demographic parameters as estimated for Bonaire populations, therefore assuming that total parrotfish biomass in Bonaire in the early 1990s [∼470 kg/ha (22)] constitutes a representative unfished biomass (B0) for the Caribbean.
Two simple fisheries policies were implemented simultaneously when fishing mortality was added to the model. In the unregulated scenario, fishing mortality occurred for fish ≥15 cm fork length (FL) because 15 cm is approximately the minimum size of parrotfish caught in fish traps (30, 31), which are one of the most popular fishing gears in the Caribbean (31–33). We then simulated a ban on traps and imposed a minimum size limit of 30 cm FL, which essentially restricts fishing to the largest sizes. Note that the only impact of this policy in the model is to change the minimum size at which fish are vulnerable to fishing; this change represents a shift in gear, e.g., from traps to spears. Total biomass, yield (annual catch), and harvest rate (proportion of the exploitable biomass caught on an annual basis) were estimated at equilibrium for regular increments of fishing mortality.
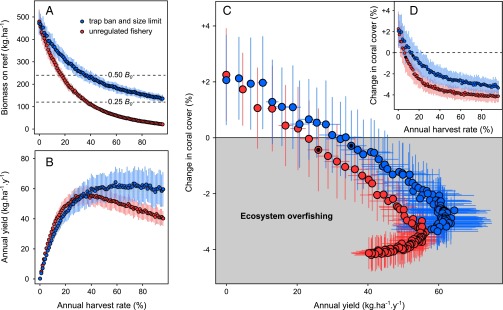
Model predictions for the unregulated trap fishery show that only a modest fishing intensity is required to cause a substantial decline in parrotfish biomass on the reef (Fig. 2A): Harvesting ∼20% of the exploitable biomass leaves around 50% of the virgin stock (0.50 B0) in the long term. A harvest rate of ∼40%, which constitutes the maximum sustainable yield for this fishery (∼55 kg of parrotfish⋅ha−1⋅y−1) (Fig. 2B), results in a 75% reduction in the unfished biomass (0.25 B0). More intensive harvesting leads to a drastic impoverishment of large parrotfish in the catch, thus reducing yield and causing growth overfishing. Implementing trap bans and a minimum size limit of 30 cm FL while maintaining an equivalent harvest rate (40%) leaves around 50% of virgin biomass (Fig. 2A) and allows a slightly higher yield (∼58 kg⋅ha−1⋅y−1) (Fig. 2B). The maximum sustainable yield for this policy culminates with much higher harvest rates, at 64 kg⋅ha−1⋅y−1, making this scenario advantageous for the fishery.
Fig. 2.
Effects of increasing exploitation rate on parrotfish and corals. (A and B) Average unharvested biomass (A) and average annual yield (B) of parrotfish at equilibrium for regular increments of annual harvest and two lengths at first capture (red, 15 cm; blue, 30 cm). Error bars denote SDs (100 simulations for each harvest rate). (C) Predicted changes in coral (in absolute units of percentage cover) for an average Caribbean reef that started at 15% coral cover and was subjected to fishing for a period of 5 y. Fishing is represented by the annual yield of parrotfish under the two harvest scenarios. (D) Predicted changes in coral for the corresponding annual harvest rates. Black filled circles outline harvest values at which a net coral decline occurs.
We next evaluated the ecological functioning of the surviving parrotfish populations on the coral reef. We first evaluated the grazing rate of each parrotfish individual as a function of parrotfish body-size distribution using species-specific allometric scaling relationships for bite size and bite rate (22, 34). Then a total grazing potential was estimated that accounts for differences in diet among parrotfish species and size. In a second step, we entered the parrotfish grazing potential into a spatially explicit simulation model of Caribbean coral reef dynamics that encompassed multiple species of corals and algae. The model has been tested against independent long-term data from Jamaica (35) and is able to represent long-term dynamics, including the impacts of disturbance, as well as predict observed changes in coral population trajectories as a function of grazing (16). Our first ecosystem simulations focused on short-term (5-y) reef responses to a parrotfish-harvesting strategy without climate-driven disturbances and were initialized with a representative total coral cover of 15% (19). The aim here was to understand the instantaneous responses of reefs to changes in fishing scenarios.
Model simulations predict that even a modest level of parrotfish harvest can impair the persistence of coral populations (Fig. 2 C and D). Ecosystem overfishing, which we define here as occurring when parrotfish harvest leads to net coral loss, occurs at a much lower annual catch (∼26 kg⋅ha−1⋅y−1) (Fig. 2C) than the estimated maximum sustainable yield, corresponding to an average 7% annual harvest rate (Fig. 2D). Banning traps and limiting minimum size to 30 cm FL prevents corals from declining for a similar yield. Net coral decline occurs at a higher harvest rate (14%) for an equivalent reduction of total biomass (Fig. 2D) but higher total catch (∼35 kg⋅ha−1⋅y−1) (Fig. 2C). The 30-cm threshold is beneficial for the reef as well as for the fishery yield because the fishery removes fewer, albeit larger, fishes, thus sustaining higher grazing rates for a similar catch. This benefit occurs even though larger-bodied fish have greater per capita grazing impacts (22, 34, 36), implying that the greater abundance of fish compensates for the targeting of the largest individuals.
Although a fisheries policy might facilitate slow levels of coral recovery, there is no guarantee that reefs will remain resilient under continued climate change. Indeed, one of the complexities of Caribbean coral reefs is their susceptibility to multiple ecosystem attractors (35, 37, 38). Since the regional die-backs of the branching coral Acropora spp. and sea urchin Diadema antillarum, which occurred because of disease outbreaks, Caribbean reefs have risked becoming embroiled in a reinforcing feedback that prevents coral replenishment and recovery even in the absence of external perturbations. Flipping to such reinforcing feedbacks constitutes a loss of ecological resilience (39). Therefore we sought to characterize fisheries policies that averted a loss of coral reef resilience.
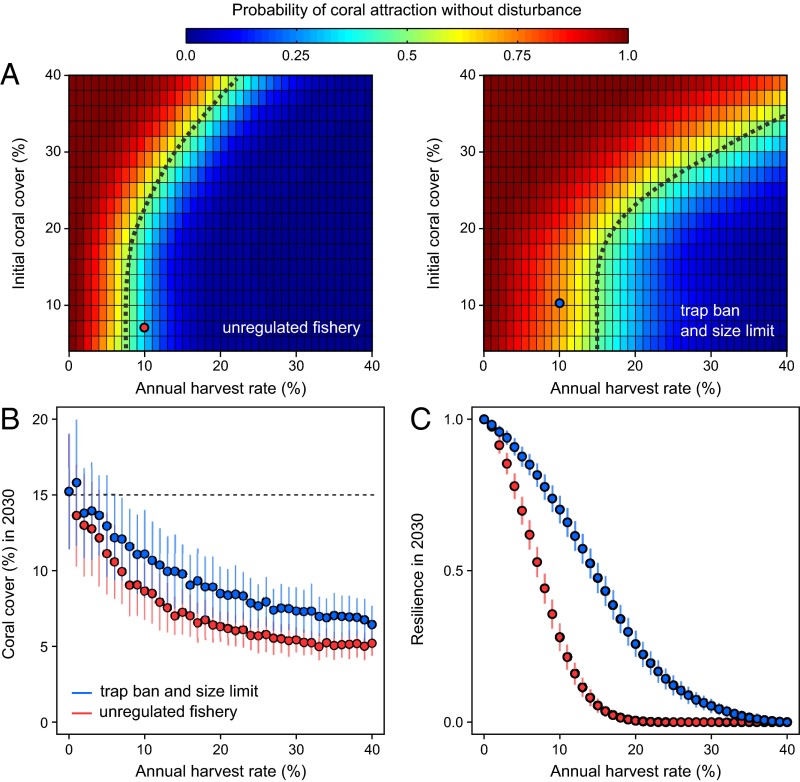
Resilience can be measured as the probability that a reef will cross an unstable equilibrium given its current state, the position of the unstable equilibrium (which is largely determined by the environment), and the disturbance regime the ecosystem experiences (35). Previous analyses have examined resilience as a function of overall fish grazing but have not been able to disaggregate grazing for application to fisheries; such operation requires modeling of fish population dynamics, fishing pressure, gear use, and catch limits. To study resilience, we first asked how our simple fishing policy alters the underlying dynamics of corals, i.e., the locations of thresholds that separate coral recovery from decline even in the absence of acute perturbations (Fig. 3A). Introducing the 30-cm size limit substantially increased the size of the coral basin of attraction, reducing the probability of an undesirable transition to a seaweed attractor.
Fig. 3.
Fishing impacts on coral reef resilience. (A) Reef phase portraits showing the probability of coral attraction (i.e., net coral growth) without external disturbances for the 15-cm (Left) and 30-cm (Right) size selectivity. The probability of coral attraction is quantified by the proportion of reef simulations (n =100) with an upward coral trajectory for various initial coral covers and harvest rates. The dotted line delineates the position of unstable equilibria where the probability of coral attraction is 0.50. (B) Projections of average coral cover in 2030 for incremental harvest rates with an initial 15% coral cover (dotted line) and a representative regime of hurricanes and coral bleaching for the Caribbean. (C) Reef resilience by 2030, estimated as the average probability of coral attraction at the end of the climate change scenario, based on the hysteresis plots and the projections of reef state and harvest rate. The limits of confidence envelops represent the SD of model outputs (n = 100 simulations). In A–C the red and blue dots indicate a projected reef in 2030 with a 10% harvest rate for the 15-cm (∼28 kg⋅ha−1⋅y−1) and 30-cm (∼34 kg⋅ha−1⋅y−1) size-selectivity scenarios, respectively.
The second step in calculating resilience subjected reefs to realistic disturbance regimes under climate change. Acute disturbances are likely to accelerate the decline of coral cover and precipitate shifts by pushing the system closer to the seaweed basin of attraction (35). Our modeling framework allows us to integrate climate-related disturbances and project future reef state (Fig. 3B) and resilience (Fig. 3C) under different fishery-management options. We considered the impact of hurricanes and coral bleaching, two acute mechanisms able to cause considerable coral mortality. Coral bleaching occurs when sea surface temperatures (SSTs) are abnormally high and can be predicted using global warming scenarios under different greenhouse gas emission trajectories (i.e., the Representative Concentration Pathway, RCP). Here we used the warming scenario RCP8.5 “business as usual,” which considers high greenhouse gas emissions (40); under this scenario bleaching events are severe and frequent. Hurricanes were simulated with a frequency and intensity representative of a moderate Caribbean storm regime (e.g., Belize). As we look to the future (Fig. 3B), ecosystem overfishing occurs with even lower harvest rates than needed in the short-term (Fig. 2D). Reef resilience was quantified as the probability that a reef still exhibits net coral growth after disturbance in 2030 (i.e., that it remains in the coral basin of attraction). Simulations reveal a drastic reduction in resilience with even a modest harvest (Fig. 3C). With a 10% harvest rate (∼28 kg⋅ha−1⋅y−1) in an unregulated trap fishery, reef resilience falls well below 0.5. Once again, however, implementation of a simple fishing restriction maintains reefs under coral attraction (i.e., above 0.5) (Fig. 3A), thus providing a considerable boost to resilience with a higher parrotfish catch (∼34 kg⋅ha−1⋅y−1). Manipulating reef conditions by simulating a range of initial coral covers (5–20%) and different hurricane regimes identified similar thresholds of ecosystem sustainability for the two management options. Our results suggest that even simple management measures are warranted for parrotfish, but for these measures to be most effective fisheries managers will need to consider combining them with low harvest rates (<10%).
We argue that simple size limits, combined with harvest rates, could allow policy makers to evaluate and implement an appropriate tradeoff between fishery yield and reef resilience. A focus on resilience is important because many policies, including the United Nations Convention on Biological Diversity and Climate Change, explicitly require ecosystems to be “managed for resilience.” Implementation of this approach ideally requires knowing the virgin biomass of parrotfish for a jurisdiction, monitoring the parrotfish stock, and being able to enforce catch and size limits. We believe that this goal is feasible because well-enforced, protected marine areas can provide an estimate of virgin biomass, many jurisdictions routinely monitor parrotfish stocks for other ecological purposes (e.g., ref. 29), and managers in St. Croix, the location of a major parrotfish fishery, are currently implementing size limits and setting annual catch limits. Rather than placing an additional monitoring burden on fisheries managers, this approach uses a wide network of coral reef surveyors who monitor fish stocks for recreational or conservation purposes. We also stress that, to be fully efficient, the implementation of parrotfish size and harvest limits must ensure that the fishing effort does not shift to surgeonfish, whose feeding complementarity with parrotfish contributes to the control of macroalgal blooms (12, 41). An effective ecosystem-based management of reef fisheries requires full consideration of all the species that contribute to coral persistence, and future models may need to integrate the dynamics of other herbivores following the foundation presented here. Finally, our multimodel approach could be adapted for other ecosystems where a fishery impacts ecological resilience. Examples include kelp habitats in New England (42) and Tasmania (43) where overfishing drives algal deforestation by depleting predators of herbivorous urchins. Failure to prevent ecosystem overfishing will lead to a long-term reduction in habitat quality, which in turn will reduce biodiversity and the productivity of the fishery for future generations (44).
Methods
Parrotfish populations were modeled using a length-structured matrix model that accounts for size-dependent growth, mortality, and settlement. Model equations, parametrization, sensitivity to parameters, and performance tests are described in details in SI Appendix, SI Methods, section I and SI Appendix, Figs. S1–S4 and therefore are described only briefly here. The model follows the fate of fish numbers in predefined size classes (1-cm FL) with a 3-mo time step. Recruitment occurs at 1 cm and is independent of adult stock size, following empirical evidence from Bermuda (28). At each time step, fish either grow or stay in their current size class following transition probabilities derived from the von Bertalanffy growth equation. Growth coefficients were estimated from the maximum length of each parrotfish species based on a relationship established with empirical data (SI Appendix, Eq. 6 and SI Appendix, Fig. S1A). Survival after growth is driven by size-specific functions of mortality caused by predation, senescence, and fishing (F). A predation mortality function imposes high mortality of settlers (45) and allows an observed size-escape from predation in adult parrotfishes (34). Mortality resulting from senescence increases exponentially with body size. With constant rates of recruitment, growth, and survival, the population structure asymptotically reaches a deterministic equilibrium with a stable size distribution when, within every size class, the arrival of fish as the result of growth equals the loss of fish as the result of mortality.
Parameters of the mortality functions and settlement rates were calibrated by optimizing model outputs against 2-y observations of a stationary population of the stoplight parrotfish in Bonaire (22, 23), which is assumed to be representative of an unfished population (F = 0 for every size class). Likelihood profiles were computed to determine a 95% confidence interval for each parameter (SI Appendix, Fig. S1C) for the generation of random fluctuations in fish abundance.
The virtual effects of fishing on parrotfish populations were investigated using the best-fit parameter values relative to empirical observations from Bonaire. We considered a knife-edge recruitment into the fishery at 15 cm and 30 cm FL (sizes at first capture) for the unregulated (trap) and regulated (trap ban and size limit) fishery scenarios, respectively. After a burn-in period of 20 y to achieve an unfished equilibrium state (F = 0), a total of 100 stochastic simulations were run for incremental F values over an additional 20-y period to reach the corresponding fished equilibrium. Stock biomass (expressed in kilograms per hectare) and annual yield (expressed in kilograms per hectare per year) were estimated for every fished equilibrium (SI Appendix, SI Methods, section I). Annual harvest rate (% per year), defined as the proportion of exploitable stock biomass harvested by the fishery, was subsequently used as the primary descriptor of fishing intensity on parrotfish populations. Being evaluated at equilibrium (i.e., 20 y after the application of fishing), stock biomass, yield, and harvest rate must be considered as long-term metrics of fishery stocks.
At every time step of the demographic model we estimated a rate of instantaneous grazing (GR) for the whole parrotfish assemblage using a previously published model of parrotfish grazing (34, 46). The model (SI Appendix, SI Methods, section II) calculates the proportion of the 2D reef area that is grazed per hour by combining fish density with species- and phase-specific bite rates and bite size as functions of body length (22, 34, 36). This estimate of grazing is disaggregated among three algal types (short algal turf and the upright macroalgae Dictyota and Lobophora) based on observations of feeding preferences of different parrotfish species and life phases (46). As a result, parrotfish grazing at every time step integrates complementarity in feeding among parrotfish species and sizes (12, 41) and its functional importance for the control of algal development (47).
The ecosystem impacts of grazing reductions caused by fishing were quantified by using a spatially explicit model of coral-reef dynamics (35, 46, 48). Model parameters and assumptions are fully described in SI Appendix, SI Methods, section III. Briefly, the model is individual-based and implements, over a regular square lattice of 400 0.25-m2 cells of reef substrate, the rates of recruitment, growth, and mortality of four groups of corals representative of a typical middepth (5–15 m) Caribbean forereef. The model has a discrete time scale of 6 mo, which does not capture the rapid turnover of algae resulting from the balance between continuous algal production and instantaneous grazing. Rather, the model requires an estimate of the net impact of grazing (GI) over a 6-mo period: the surface area of the reef that is grazed sufficiently often that algae are maintained in a cropped state (i.e., short turf) rather than developing into a macroalgal (i.e., Dictyota or Lobophora) canopy (46, 49). If not grazed during 6 mo, the reef substrate gives rise to macroalgae that pre-empt the space available for coral recruitment and growth and reduce postsettlement coral survival (50).
For each parrotfish demographic equilibrium associated with incremental F values, the corresponding instantaneous GR was converted into the longer-term GI with the assumption that fishing affects GI in similar proportions to GR relative to an unfished condition. Earlier model applications (35, 46) suggested that unfished parrotfish may maintain up to 40% of the reef substratum in a cropped state. For every fished equilibrium, GI therefore was estimated as 0.4 × GR/GRpristine, where GRpristine corresponds to the mean GR obtained at equilibrium with F = 0 (n = 100 replicate simulations). The resulting percentage of grazed area was apportioned to algal types relative to the feeding preferences of the current parrotfish assemblage.
Coral populations were simulated for every resulting GI value over a period of 5 y. All simulations were run for both thresholds of gear selectivity and without external disturbances (i.e., hurricanes and coral bleaching) to isolate the net impact of fishing on reef dynamics. Initial coral cover was fixed at 15% (all coral groups equally represented), which is representative of the average coral cover on present-day Caribbean reefs (19). Initial macroalgal cover and ungrazable substratum (e.g., sand) were fixed at 10% each. At the end of 100 replicate reef simulations, absolute change in coral cover was averaged, and SDs were calculated for every F increment.
Reef resilience can be defined as the ability of a reef to exhibit coral recovery after acute disturbances, indicating that the reef remained in its coral-based stability domain (16, 35, 48). Whether a reef is likely to exhibit net coral growth, stasis, or decline can be determined by modeling its phase portrait. The phase portrait represents the trajectories of coral cover in the absence of acute disturbances for different initial covers and different values of an environmental driver. Here, combining models allowed us to represent reef trajectories as a function of parrotfish harvest rate. Phase portraits were built by running 100 stochastic simulations for every combination of initial coral cover (5–40%) and equilibrial GI associated with incremental harvest rates (0–40%). Simulations were run for 50 y without external disturbances. For every simulated reef, the proportion of all 100 simulations exhibiting net coral growth gives the probability of attraction to a coral-dominated state. Changes in system behavior from coral to macroalgal attraction delineate a set of unstable equilibria (dotted lines in Fig. 3A) that represent threshold levels of parrotfish harvest for any given reef state. Theoretically, a reef can remain in stasis indefinitely if it sits precisely on its unstable equilibrium (48). Reefs that clearly fell below or to the right of the unstable equilibrium would never experience a net coral growth. In contrast, reefs lying above and to the left of the unstable equilibrium have sufficient grazing to exhibit a net increase in coral cover.
Phase portraits were subsequently used to evaluate the impact of incremental harvest rates on future (2015–2030) reef resilience. First, 100 stochastic simulations of parrotfish demographics were run over 15 y for different fished equilibriums (harvest rate varying from 0 to 40%). Equilibrial GR values were converted into community grazing GI thus producing 15-y series of stochastic GI, from which the coral-reef model was run with a 15% initial coral cover and realistic hurricane and bleaching regimes. Hurricanes were scheduled in summer with a storm frequency and intensity (Saffir–Simpson scale) derived from hurricane tracks during the past century in Belize [Atlantic hurricane database (51)]. To obtain temporally realistic hurricane regimes, each simulation started at a random year of the historical time series and proceeded forward for 15 y. Coral-bleaching events were projected according to future SST as predicted by the Hadley Centre Global Environmental Model HadGEM1 (52) assuming high greenhouse gases emissions (scenario RCP8.5) (40). Caribbean basin mean monthly SSTs were calculated from 2015 to 2030 to estimate degree heating weeks, which determine the probability of coral bleaching (details are given in SI Appendix, SI Methods, section III). Coral cover in 2030 was averaged, and SDs were calculated for every increment of harvest rate. Reef resilience in 2030 was estimated as the average probability of net coral growth (extracted from the appropriate phase portrait) based on the projections of reef state and harvest rate.
Supplementary Material
Acknowledgments
We thank Jules van Rooij for providing the data in his PhD dissertation, collected by him and J.H.B. on the reefs of Bonaire; H. Choat for providing raw data on somatic growth; C. Baille for early model developments; and N. Krueck for fruitful discussions related to the parrotfish fishery model. This research received funding from the European Union 7th Framework Programme (P7/2007-2013) under Grant Agreement No. 244161 (FORCE project). P.J.M. received funding from the Natural Environment Research Council, the Australian Research Council, and the Pew Charitable Trusts.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1601529113/-/DCSupplemental.
References
- 1.Link J. Ecosystem-Based Fisheries Management: Confronting Tradeoffs. Cambridge Univ Press; Cambridge, UK: 2010. [Google Scholar]
- 2.Pikitch EK, et al. Ecology. Ecosystem-based fishery management. Science. 2004;305(5682):346–347. doi: 10.1126/science.1098222. [DOI] [PubMed] [Google Scholar]
- 3.Cury PM, et al. Global seabird response to forage fish depletion--one-third for the birds. Science. 2011;334(6063):1703–1706. doi: 10.1126/science.1212928. [DOI] [PubMed] [Google Scholar]
- 4.Smith AD, et al. Impacts of fishing low-trophic level species on marine ecosystems. Science. 2011;333(6046):1147–1150. doi: 10.1126/science.1209395. [DOI] [PubMed] [Google Scholar]
- 5.Essington TE, et al. Fishing amplifies forage fish population collapses. Proc Natl Acad Sci USA. 2015;112(21):6648–6652. doi: 10.1073/pnas.1422020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan IC, et al. Impacts of depleting forage species in the California current. Environ Conserv. 2013;40(04):380–393. [Google Scholar]
- 7.Estes JA, et al. Trophic downgrading of planet Earth. Science. 2011;333(6040):301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 8.Travis J, et al. Integrating the invisible fabric of nature into fisheries management. Proc Natl Acad Sci USA. 2014;111(2):581–584. doi: 10.1073/pnas.1305853111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis SM, Wainwright PC. Herbivore abundance and grazing intensity on a Caribbean coral reef. J Exp Mar Biol Ecol. 1985;87(3):215–228. [Google Scholar]
- 10.Morrison D. Comparing fish and urchin grazing in shallow and deeper coral reef algal communities. Ecology. 1988;69(5):1367–1382. [Google Scholar]
- 11.Williams I, Polunin N. Large-scale associations between macroalgal cover and grazer biomass on mid-depth reefs in the Caribbean. Coral Reefs. 2001;19(4):358–366. [Google Scholar]
- 12.Burkepile DE, Hay ME. Herbivore species richness and feeding complementarity affect community structure and function on a coral reef. Proc Natl Acad Sci USA. 2008;105(42):16201–16206. doi: 10.1073/pnas.0801946105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mumby PJ, Harborne AR. Marine reserves enhance the recovery of corals on Caribbean reefs. PLoS One. 2010;5(1):e8657. doi: 10.1371/journal.pone.0008657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steneck RS, Arnold SN, Mumby PJ. Experiment mimics fishing on parrotfish: Insights on coral reef recovery and alternative attractors. Mar Ecol Prog Ser. 2014;506:115–127. [Google Scholar]
- 15.Vallès H, Oxenford HA. Parrotfish size: A simple yet useful alternative indicator of fishing effects on Caribbean reefs? PLoS One. 2014;9(1):e86291. doi: 10.1371/journal.pone.0086291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mumby PJ, Steneck RS, Hastings A. Evidence for and against the existence of alternate attractors on coral reefs. Oikos. 2013;122(4):481–491. [Google Scholar]
- 17.Carpenter RC. Mass mortality of Diadema antillarum. II: Effects on population densities and grazing intensity of parrotfishes and surgeonfishes. Mar Biol. 1990;104(1):79–86. [Google Scholar]
- 18.Done TT, Ogden JJ, Wiebe W, Rosen B. Biodiversity and ecosystem function of coral reefs. In: Mooney HA, Cushman JH, Medina E, Sala OE, Schulze E-D, editors. Functional Roles of Biodiversity, UKA Global Perspective. John Wiley and Sons; Chichester, UK: 1996. pp. 393–429. [Google Scholar]
- 19.Jackson JBC, Donovan MK, Cramer KL, Lam VV. 2014. Status and Trends of Caribbean Coral Reefs: 1970-2012 (Global Coral Reef Monitoring Network, International Union for the Conservation of Nature, Gland, Switzerland)
- 20.Hawkins JP, Roberts CM. Effects of fishing on sex-changing Caribbean parrotfishes. Biol Conserv. 2003;115(2):213–226. [Google Scholar]
- 21.Mumby PJ. Stratifying herbivore fisheries by habitat to avoid ecosystem overfishing of coral reefs. Fish Fish. 2016;17:266–278. [Google Scholar]
- 22.Bruggemann JH, Van Kessel AM, Van Rooij JM, Breeman AM. Bioerosion and sediment ingestion by the Caribbean parrotfish Scarus vetula and Sparisoma viride: Implications of fish size, feeding mode and habitat use. Mar Ecol Prog Ser. 1996;134(1):59–71. [Google Scholar]
- 23.van Rooij JM, Kroon FJ, Videler JJ. The social and mating system of the herbivorous reef fish Sparisoma viride: One-male versus multi-male groups. Environ Biol Fishes. 1996;47(4):353–378. [Google Scholar]
- 24.van Rooij JM. 1996 Behavioural energetics of the parrotfish Sparisoma viride. Flexibility in a coral reef setting. PhD dissertation (University of Groningen, The Netherlands). Available at irs.ub.rug.nl/ppn/331416387. Accessed January 14, 2016. [Google Scholar]
- 25.van Rooij JM, Videler JJ. Mortality estimates from repeated visual censuses of a parrotfish (Sparisoma viride) population: Demographic implications. Mar Biol. 1997;128(3):385–396. [Google Scholar]
- 26.Huston MA, DeAngelis DL. Size bimodality in monospecific populations: A critical review of potential mechanisms. Am Nat. 1987;129(5):678–707. [Google Scholar]
- 27.Luckhurst BE. A fishery-independent assessment of Bermuda’s coral reef fish stocks by diver census following the fish pot ban–a progress report. Proc Gulf Caribb Fish Inst. 1994;46:309–323. [Google Scholar]
- 28.O’Farrell S, Harborne AR, Bozec Y-M, Luckhurst BE, Mumby PJ. Protection of functionally important parrotfishes increases their biomass but fails to deliver enhanced recruitment. Mar Ecol Prog Ser. 2015;522:245–254. [Google Scholar]
- 29.Kramer PA. Synthesis of coral reef health indicators for the western Atlantic: Results of the AGRRA program(1997-2000) Atoll Res Bull. 2003;496:1–57. [Google Scholar]
- 30.Rakitin A, Kramer DL. Effect of a marine reserve on the distribution of coral reef fishes in Barbados. Mar Ecol Prog Ser. 1996;131:97–113. [Google Scholar]
- 31.Hawkins JP, Roberts CM, Gell FR, Dytham C. Effects of trap fishing on reef fish communities. Aquat Conserv Mar Freshw Ecosyst. 2007;17(2):111–132. [Google Scholar]
- 32.Gobert B. Comparative assessment of multispecies reef fish resources in the Lesser Antilles. Fish Res. 2000;44(3):247–260. [Google Scholar]
- 33.Mahon R, Hunte W. Trap mesh selectivity and the management of reef fishes. Fish Fish. 2001;2(4):356–375. [Google Scholar]
- 34.Mumby PJ, et al. Fishing, trophic cascades, and the process of grazing on coral reefs. Science. 2006;311(5757):98–101. doi: 10.1126/science.1121129. [DOI] [PubMed] [Google Scholar]
- 35.Mumby PJ, Hastings A, Edwards HJ. Thresholds and the resilience of Caribbean coral reefs. Nature. 2007;450(7166):98–101. doi: 10.1038/nature06252. [DOI] [PubMed] [Google Scholar]
- 36.Bruggemann JH, Kuyper MW, Breeman AM. Comparative analysis of foraging and habitat use by the sympatric Caribbean parrotfish Scarus vetula and Sparisoma viride (Scaridae) Mar Ecol Prog Ser. 1994;112(1-2):51–66. [Google Scholar]
- 37.Knowlton N. Thresholds and multiple stable states in coral reef community dynamics. Am Zool. 1992;32(6):674–682. [Google Scholar]
- 38.Scheffer M, Carpenter SR. Catastrophic regime shifts in ecosystems: Linking theory to observation. Trends Ecol Evol. 2003;18(12):648–656. [Google Scholar]
- 39.Holling CS. Resilience and stability of ecological systems. Annu Rev Ecol Syst. 1973;4:1–23. [Google Scholar]
- 40.Riahi K, et al. RCP 8.5 – A scenario of comparatively high greenhouse gas emissions. Clim Change. 2011;109(1-2):33–57. [Google Scholar]
- 41.Burkepile DE, Hay ME. Feeding complementarity versus redundancy among herbivorous fishes on a Caribbean reef. Coral Reefs. 2011;30(2):351–362. [Google Scholar]
- 42.Steneck RS, Leland A, McNaught DC, Vavrinec J. Ecosystem flips, locks, and feedbacks: The lasting effects of fisheries on Maine’s kelp forest ecosystem. Bull Mar Sci. 2013;89(1):31–55. [Google Scholar]
- 43.Ling SD, Johnson CR. Marine reserves reduce risk of climate-driven phase shift by reinstating size- and habitat-specific trophic interactions. Ecol Appl. 2012;22(4):1232–1245. doi: 10.1890/11-1587.1. [DOI] [PubMed] [Google Scholar]
- 44.Rogers A, Blanchard JL, Mumby PJ. Vulnerability of coral reef fisheries to a loss of structural complexity. Curr Biol. 2014;24(9):1000–1005. doi: 10.1016/j.cub.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 45.Vallès H, Kramer DL, Hunte W. 2008. Differential effect of early post-settlement processes on the abundance of two concurrently settling coral reef fishes. Proc 11th Int Coral Reef Symp Ft Lauderdale Fla:335–339.
- 46.Mumby PJ. The impact of exploiting grazers (Scaridae) on the dynamics of Caribbean coral reefs. Ecol Appl. 2006;16(2):747–769. doi: 10.1890/1051-0761(2006)016[0747:tioegs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 47.Bellwood DR, Hughes TP, Folke C, Nyström M. Confronting the coral reef crisis. Nature. 2004;429(6994):827–833. doi: 10.1038/nature02691. [DOI] [PubMed] [Google Scholar]
- 48.Mumby PJ, Wolff NH, Bozec Y-M, Chollett I, Halloran P. Operationalizing the resilience of coral reefs in an era of climate change. Conserv Lett. 2014;7(3):176–187. [Google Scholar]
- 49.Williams ID, Polunin NV, Hendrick VJ. Limits to grazing by herbivorous fishes and the impact of low coral cover on macroalgal abundance on a coral reef in Belize. Mar Ecol Prog Ser. 2001;222:187–196. [Google Scholar]
- 50.Arnold SN, Steneck RS, Mumby PJ. Running the gauntlet: Inhibitory effects of algal turfs on the processes of coral recruitment. Mar Ecol Prog Ser. 2010;414:91–105. [Google Scholar]
- 51.Jarvinen BR, Neuman CJ, Davis MAS. 1984. A tropical cyclone data tape for the North Atlantic Basin, 1886–1983: Contents, limitations and uses. (National Hurricane Center, Miami), pp 1–24, National Oceanic and Atmospheric Administration Technical Memorandum NWS NHC 22.
- 52.Johns TC, et al. The new Hadley Centre climate model (HadGEM1): Evaluation of coupled simulations. J Clim. 2006;19(7):1327–1353. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.